Abstract
Here we show that ouabain-induced cell growth regulation is intrinsically coupled to changes in the cellular amount of Na/K-ATPase via the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway. Ouabain increases the endocytosis and degradation of Na/K-ATPase in LLC-PK1, human breast (BT20), and prostate (DU145) cancer cells. However, ouabain stimulates the PI3K/Akt/mTOR pathway and consequently up-regulates the expression of Na/K-ATPase in LLC-PK1 but not BT20 and DU145 cells. This up-regulation is sufficient to replete the plasma membrane pool of Na/K-ATPase and to stimulate cell proliferation in LLC-PK1 cells. On the other hand, ouabain causes a gradual depletion of Na/K-ATPase and an increased expression of cell cycle inhibitor p21cip, which consequently inhibits cell proliferation in BT20 and DU145 cells. Consistently, we observe that small interfering RNA-mediated knockdown of Na/K-ATPase is sufficient to induce the expression of p21cip and slow the proliferation of LLC-PK1 cells. Moreover, this knockdown converts the growth stimulatory effect of ouabain to growth inhibition in LLC-PK1 cells. Mechanistically, both Src and caveolin-1 are required for ouabain-induced activation of Akt and up-regulation of Na/K-ATPase. Furthermore, inhibition of the PI3K/Akt/mTOR pathway by rapamycin completely blocks ouabain-induced expression of Na/K-ATPase and converts ouabain-induced growth stimulation to growth inhibition in LLC-PK1 cells. Taken together, we conclude that changes in the expression of Na/K-ATPase dictate the growth regulatory effects of ouabain on cells.
The Na/K-ATPase, a member of P-type ATPase family, was discovered as an energy transducing ion pump. It transports Na+ and K+ across the cell membrane and maintains ion homeostasis in animal cells (1, 2). Recent studies indicate that the Na/K-ATPase is also an important receptor that can transduce ligand binding into the activation of protein kinase cascades (3). Specifically, the Na/K-ATPase interacts with Src, which provides at least two important cellular regulations (4, 5). First, association with Na/K-ATPase keeps Src in an inactive state. Thus, the Na/K-ATPase serves as a native negative Src regulator (4). Second, this interaction forms a functional receptor complex for cardiotonic steroids (CTS)3 (3), a group of well characterized ligands of the Na/K-ATPase. Cardiotonic steroids include cardenolides (e.g. ouabain) and bufadienolides (e.g. marinobufagenin) (6). Although CTS are known cardiac drugs, some of them have now been identified as endogenous steroid hormones (6–8). Binding of CTS to the receptor complex activates the Na/K-ATPase-associated Src. Subsequently, the activated Src transactivates other tyrosine kinases, and together they recruit and further phosphorylate multiple membrane and soluble proteins, which results in the activation of protein kinase cascades and the generation of second messengers (3, 4, 6). Ultimately, this chain of signaling events would alter cellular functions and cell growth in a cell-specific manner (5, 9–12). For instance, we and others have demonstrated that ouabain-induced activation of ERK and PI3K/Akt/mTOR pathways are responsible for cell growth stimulation in transformed cell lines, in primary cultures, as well as in vivo (13–18).
It has also been recognized for a long time that CTS inhibit cell growth in many cancer cells (19–24). Of particular significance are studies that indicate the beneficial effects of CTS therapy in women with breast cancer (25–29). Consistently, recent in vitro and in vivo studies have identified several new CTS compounds that exhibit anti-cancer activities (30–32). Oleandrin, for example, is in clinical trials in the United States as an anti-cancer remedy for human cancers (31, 33). Although ouabain inhibits the pumping function of the Na/K-ATPase, it is important to note that the growth inhibitory effect of ouabain can occur at doses that neither cause significant changes in intracellular Na+ and K+ nor affect cell viability. Rather, much like its effect on cell growth stimulation, ouabain induces cell growth inhibition through the activation of protein kinases and the generation of second messengers (19–23, 34). For example, a recent report showed that these nontoxic concentrations of ouabain stimulated Src, resulting in activation of the epidermal growth factor receptor/ERK pathway and induction of the expression of cell cycle inhibitor p21cip and cell growth arrest (34). Thus, it becomes important to understand the molecular mechanisms that govern different fates of cells in response to CTS stimulation.
Prior studies have demonstrated that CTS induce endocytosis of the Na/K-ATPase and regulate its cellular expression via receptor-mediated signal transduction (35, 36). Because the Na/K-ATPase has both pumping and signaling functions, it is conceivable that changes in the amount of cellular Na/K-ATPase could have significant consequences on cell growth. Therefore, we have conducted the following experiments to reveal the role of cellular Na/K-ATPase in ouabain-induced cell growth regulation.
EXPERIMENTAL PROCEDURES
Materials—Cell culture media, fetal bovine serum, and trypsin were purchased from Invitrogen; Optitran nitrocellulose membrane was from Schleicher & Schuell; the ECL Supersignal kit was purchased from Pierce; and the monoclonal anti-α1 antibody (α6F) was obtained from the Developmental Studies Hybridoma Bank at the University of Iowa. The anti-α2 antibody was from Millipore (Billerica, MA), and the anti-α3 was from Affinity Bioreagents (Golden, CO). The anti-p21cip antibody, anti-Src monoclonal antibody, and all the secondary horseradish peroxidase-conjugated antibodies were from Santa Cruz (Santa Cruz, CA). The polyclonal anti-Akt and anti-pAkt at Ser473 antibodies were from Cell Signaling (Danvers, MA). The MTT assay kit was purchased from American Type Culture Collection (Manassas, VA). [3H]Ouabain was from PerkinElmer Life Sciences.
Cell Culture—The pig kidney epithelia cells (LLC-PK1 cells), human breast cancer cells (BT-20 cells and MDA-MB-435s), and human prostate cancer cells (DU145 cells) were obtained from American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium or RPMI 1640 medium (for DU145 cells) in the presence of 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2 humidified incubator (37). The Na/K-ATPase knockdown (A4–11 and PY-17), rat α1-rescued PY-17 cells (AAC-19), and caveolin-1 knockdown (C2-9) cells were derived from LLC-PK1 cells and cultured in Dulbecco's modified Eagle's medium as described (38). To eliminate the confounding effect of growth factors in the serum, the cells were serum-starved for 24 h before experiments unless otherwise indicated.
Western Blot Analysis—The cells were washed with phosphate-buffered saline and solubilized in ice-cold radioimmune precipitation assay buffer containing 1% Nonidet P-40, 1% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, 1 mm NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 50 mm Tris-HCl, pH 7.4, as previously described (37). The cell lysates were then centrifuged at 14,000 rpm, and the supernatants were used for protein assay and subjected to Western blot analysis. The samples were separated on SDS-PAGE (50 μg/lane) and transferred to a cellulose membrane. The membranes were blocked with 4% nonfat dried milk in TBS-T (10 mm Tris-HCl, 150 mm NaCl, 0.05% Tween 20, pH 8.0) for 1 h at room temperature and probed with specific antibodies. The protein signals were detected using an ECL kit and quantified using a Bio-Rad GS-670 imaging densitometer.
MTT Assay for Measuring Cell Proliferation—The cells were subcultured in 96-well plates at the density of 10,000 cells/well. After 12 h of serum starvation, the cells were exposed to different concentrations of ouabain for 24 h. Ten μl of MTT reagent was added to each well for 90 min. The formazan crystal was dissolved with detergent, and the OD value was measured at 570 nm as instructed by the manufacturer.
Cell Growth Curve and Cell Cycle Measurement—The cells were subcultured in 12-well plates at 50,000/well for 24 h, serum-starved for 24 h, and then treated with different concentrations of ouabain for indicated time periods. At each indicated time point, three wells of control or treated cells were trypsinized and counted. For cell cycle measurement, the cells were trypsinized and resuspended in citrate buffer (8.55% sucrose, 1.18% sodium citrate, 5% Me2SO, pH 7.6) and stained with propidium iodide as described by Konski et al. (39). The stained cells were then analyzed by flow cytometry.
[3H]Ouabain Binding—To measure the effects of ouabain on cell surface expression of Na/K-ATPase, serum-starved cells were cultured in 12-well plates and treated with ouabain for 72 h. Afterward, the cells were washed with serum-free medium and subjected to [3H]ouabain binding assay as described (40). Briefly, the cells were incubated in K+-free Krebs solution (137 mm NaCl, 2.8 mm CaCl2, 0.6 mm NaH2PO4, 1.2 mm MgSO4, 10 mm dextrose, 15 mm Tris, pH 7.4) for 15 min and then exposed to 100 nm [3H]ouabain for 30 min at 37 °C in the presence of 20 μm monensin. At the end of incubation, the cells were washed three times with ice-cold K+-free Krebs solution, solubilized in 0.1 m NaOH-0.2% SDS, and counted in a scintillation counter for [3H]ouabain. Nonspecific binding was measured in the presence of 5 mm unlabeled ouabain and subtracted from total binding. Parallel plates were used for counting cell numbers. [3H]Ouabain binding data were calculated as binding sites/cell.
Confocal Imaging and Immunocytochemistry—LLC-PK1, BT20, and DU145 cells were serum-starved for 24 h and treated with ouabain (10 nm) for 6 h on coverslips. The cells were fixed with ice-cold methanol for 15 min and blocked with Signal Enhancer from Invitrogen. A monoclonal anti-Na/K-ATPase α1 antibody was then added and incubated overnight at 4 °C. After three washes, a secondary Alexa 488-conjugated anti-mouse antibody was added and incubated for 2 h at room temperature. The coverslip was washed, mounted, and imaged using a Leica confocal microscope as described (5).
Quantitative RT-PCR—The cells were seeded in 6-cm dishes and serum-starved for 24 h after the confluence reached 90%. The cells were then treated with 10 nm ouabain for 24 h. Total RNA was extracted using TRIzol and subjected to quantitative RT-PCR analysis. The assays were performed on the AB 7500 real time PCR system with the following protocol: 1 cycle (6 min at 95 °C), 40 cycles (30 s at 95 °C, 30 s at 52 °C, 30 s at 72 °C, and 1 min at 85 °C), followed by a dissociation stage to ensure a single product. The reactions are in a total volume of 25 μl, including 1.25 μl of Sybr Green (Invitrogen), 0.125 μl of DNA polymerase (Denville), 2.5 μl of dNTP (Invitrogen), 10 ng of cDNA. Primer pairs were used at 5 μm concentration, and the sequences are: human GAPDH, 5′-GGGAAGGTGAAGGTCGGAGT and 3′-TCCACTTTACCAGAGTTAAAAGCAG; human Na/K-ATPase α1, 5′-TGTCCAGAATTGCAG and 3′-TGCCCGCTTAAGAATAGGTAGGT (41); pig GAPDH, 5′-ATGCTGGTGCTGAGTTCGTGG and 3′-AGATGATGACCCTTTTGGCTCC (42); and pig Na/K-ATPase α1, 5′-ATCTCTGCTTCGTTGGGCTCATCT and 3′-AATGGCTTTGGCTGTGATGGGATG. The relative Na/K-ATPase α1 gene expression was calculated by comparative threshold (CT) method (ΔΔCT). To normalize the difference amounts of cDNA, GAPDH was used as an internal control.
Statistics—The data are presented as the means ± S.E. and compared using Student's t test. Significance was accepted at p < 0.05.
RESULTS
Ouabain Regulates Cell Growth and Na/K-ATPase Expression in a Cell-specific Manner—It is known that ouabain can either stimulate or inhibit cell proliferation in a cell-specific manner (16, 20, 34, 43–46). To understand the molecular mechanism of these opposing effects, we first compared the effects of ouabain on cell growth in two human epithelia-derived cancer cell lines (BT20 and DU145) and a pig kidney epithelial cell line (LLC-PK1). As shown in Fig. 1A, MTT assays indicated that ouabain at 1–50 nm stimulated cell proliferation in LLC-PK1 cells. These findings are consistent with the observed effects of ouabain on other renal epithelial cells (47, 48). In contrast, we failed to detect any stimulatory effect of ouabain on either breast cancer BT20 or prostate cancer DU145 cells. Moreover, 10–50 nm ouabain caused a significant inhibition of cell proliferation in these cells (Fig. 1A). To further confirm these observations, we counted cell numbers in the presence or absence of ouabain as a function of time. As depicted in Fig. 1B, ouabain increased the number of LLC-PK1 cells in a time-dependent manner, confirming that ouabain stimulates the proliferation of these cells. On the contrary, the same treatment significantly inhibits the proliferation of BT20 cells and DU145 cells. These findings are in accordance with what has been reported in the literature (22, 34). Because ouabain inhibited cell proliferation by stimulating the expression of cell cycle inhibitor p21cip in human breast cancer MDA-MB-435s cells (34), we measured the effects of ouabain on p21cip expression. As depicted in Fig. 1C, whereas 10 nm ouabain stimulated the expression of cell cycle inhibitor p21cip in BT20 and DU145 cells, it failed to do so in LLC-PK1 cells.
FIGURE 1.
Effects of ouabain on cell growth in different cells. A, LLC-PK1 cells, BT20 cells, and DU145 cells were subcultured in 96-well plates (10,000 cells/well) and serum-starved. After exposure to different concentrations of ouabain for 24 h, the cells were subjected to MTT assay as described under “Experimental Procedures.” The values are mean ± S.E. The quantification data are from three independent experiments. *, p < 0.05. B, the above three cell lines were subcultured in 12-well plates (50,000 cells/well) and serum-starved. After ouabain treatment, three wells of control (Con) and ouabain-treated cells were trypsinized and counted at indicated time points. The same experiments were repeated four times. C, LLC-PK1, BT20, and DU145 cells were serum-starved overnight and treated with ouabain for 24 h. Equal amount of cell lysates were assayed for p21cip using Western blot.
Because the Na/K-ATPase is the receptor for ouabain, we next tested whether ouabain affects the expression of Na/K-ATPase. Western blot analyses indicated that all three cell lines expressed a ouabain-sensitive α1 isoform of Na/K-ATPase. Under the same experimental conditions, the expression of α2 or α3 isoform was undetectable (data not shown). Interestingly, the effects of ouabain on α1 expression were also cell-specific. Although ouabain caused induction of α1 expression in LLC-PK1 cells after either 24 h or 72 h exposure, it reduced the cellular amount of α1 in both BT20 and DU145 cells (Fig. 2A). Because ouabain also inhibited the proliferation of MDA-MB-435s cells (34), we measured the effects of ouabain on α1 expression after these cells were exposed to 5 nm ouabain for 24 h. A significant reduction in α1 expression was detected (82 ± 2% of control, n = 3, p < 0.05). Thus, ouabain-induced changes in α1 expression were clearly correlated with its effects on cell proliferation.
FIGURE 2.
Effects of ouabain on Na/K-ATPase expression in different cells. A, LLC-PK1, BT20, and DU145 cells were treated with different concentrations of ouabain for either 24 or 72 h. Equal protein amount of cell lysates were analyzed for Na/K-ATPase α1 expression. The upper panel shows representative Western blots of 24 h treatment, and the lower panel shows quantitative data from four or five independent experiments presented in the bar graphs. B, LLC-PK1 and BT20 cells were treated with 10 nm ouabain for 24 h, and the cell lysates (50 μg/lane) were assayed for Na/K-ATPase β1 using Western blot. C, LLC-PK1, BT20, and DU145 cells were exposed to different concentrations of ouabain for 72 h and then subjected to [3H]ouabain binding assays as described under “Experimental Procedures.” *, p < 0.05; **, p < 0.01. Con, control; Oua, ouabain.
It is known that the β1 subunit plays an important role not only in the formation of a functional Na/K-ATPase but also in the formation of tight junctions and in the suppression of tumor cell growth (49–51). Thus, we assessed whether ouabain affects β1 expression in LLC-PK1 and cancer cell lines. The cells were treated with 10 nm ouabain for 24 h, and the amount of β1 was measured in the cell lysates by Western blot. As depicted in Fig. 2B, ouabain caused a modest increase in β1 expression in LLC-PK1 but not BT-20 cells.
To assess the functional consequence of these changes in α1 expression, we measured the number of active Na/K-ATPase in the plasma membrane. The cells were exposed to different concentrations of ouabain for 72 h and then subjected to [3H]ouabain binding. It is important to note that this assay measured the number of functional Na/K-ATPase that was not occupied by 5 or 10 nm ouabain after 72 h treatment. As depicted in Fig. 2C, ouabain up to 10 nm barely changed the number of active Na/K-ATPase in LLC-PK1 cells, indicating that the ouabain-induced up-regulation of α1 is just sufficient to replete the plasma membrane pool of active Na/K-ATPase. In contrast, ouabain caused a significant decrease in the number of active plasma membrane Na/K-ATPase in both BT20 and DU145 cells (Fig. 2C). Taken together, the data suggest that the decrease in the plasma membrane Na/K-ATPase may be involved in the ouabain-induced growth inhibition in cancer cells.
Reduction of the Na/K-ATPase Slows Cell Proliferation and Enables Ouabain to Inhibit Cell Proliferation in LLC-PK1 Cells—LLC-PK1 cells and the two cancer cell lines are derived from different organs and species. To further test the proposal that a decrease in cellular Na/K-ATPase is involved in ouabain-induced inhibition of cell proliferation, we measured the effects of a graded knockdown of the Na/K-ATPase on cell proliferation in LLC-PK1 cells. The generation of the knockdown cell lines has been previously reported (4, 52). A4–11 and PY-17 cells expressed about 40 and 10% of the Na/K-ATPase in comparison with the control vector-transfected LLC-PK1 (P-11) cells, respectively (4). As shown in Fig. 3, P-11 cells behaved similarly to the parent LLC-PK1 cells in response to ouabain stimulation. Ouabain, at concentrations from 1 to 10 nm, stimulated cell proliferation. In contrast, A4–11 and PY-17 cells behaved like BT20 and DU145 cells. No growth stimulation was detected. Instead, reduction of Na/K-ATPase expression by small interfering RNA-mediated mechanism sensitized these cells to ouabain-induced cell growth inhibition (Fig. 3). For example, 10 nm ouabain caused more than a 80% reduction in cell proliferation in PY-17 cells in comparison with that in P-11 cells. Moreover, the growth inhibitory effects of ouabain were clearly correlated with the cellular amount of Na/K-ATPase.
FIGURE 3.
Effects of Na/K-ATPase knockdown on ouabain-induced cell growth regulation. The control (P-11) and Na/K-ATPase knockdown cells (A4–11 and PY-17) were treated with different concentrations of ouabain for 24 h and subjected to MTT assays. n = 10–15 measurements. *, p < 0.05; **, p < 0.01.
To test whether this growth inhibitory effect of ouabain applies to other cardiotonic steroids, we measured the effects of digoxin, digitoxin, and marinobufagenin on cell proliferation in PY-17 cells. We observed that these compounds caused a growth inhibition similar to that of ouabain (data not shown).
To further examine the consequences of Na/K-ATPase reduction in cell growth regulation, we compared the cell proliferation curves of stable cell lines P-11 and PY-17 as well as AAC-19, a rat α1-rescued PY-17 cell line (4, 52). As shown in Fig. 4A, PY-17 cells proliferated much slower than P-11 cells, whereas reintroduction of rat Na/K-ATPase α1 restored cell growth in AAC-19 cells.
FIGURE 4.
Effects of Na/K-ATPase knockdown on cell growth. A, P-11, PY-17, and AAC-19 cells were subcultured in 12-well plates (50,000 cells/well), and three wells of each were trypsinized and counted at the indicated time points. The data were from three independent experiments. B, P-11, PY-17, and AAC-19 cells were lysed in radioimmune precipitation assay buffer and analyzed by Western blot using an anti-p21cip antibody. A representative Western blot and the quantification data are shown. *, p < 0.05 compared with P11 cells. C, cell cycle distribution was measured as described under “Experimental Procedures.” The same experiments were repeated three times.
Because ouabain stimulated the expression of p21cip and inhibited cell proliferation in human cancer cells (Fig. 1) (34), we tested whether knockdown of cellular Na/K-ATPase affects the expression of this cell cycle inhibitor. As expected, the expression of p21cip was quite low in the control P-11 cells (Fig. 4B). Knockdown of the Na/K-ATPase caused a significant induction of this cell cycle inhibitor in PY-17 cells. Moreover, rescuing PY-17 cells by knocking in rat α1 was sufficient to reduce the expression of p21cip in AAC-19 cells. Consistently, we also detected more G0/G1 cells in PY-17 cells than that in both P-11 and AAC-19 cells (Fig. 4C).
Ouabain Has No Effect on α1 mRNA—To probe the molecular mechanism of ouabain-induced cell-specific regulation of α1, we first measured α1 mRNA using a quantitative RT-PCR after the cells were treated with 10 nm ouabain. As depicted in Fig. 5, ouabain had no effect on the levels of α1 mRNA in LLC-PK1, BT20, and DU145 cells. Thus, the ouabain-induced changes in the α1 protein are likely via translational/post-translational mechanisms.
FIGURE 5.
Effects of ouabain on α1 mRNA levels. LLC-PK1, BT20, and DU145 cells were treated with 10 nm ouabain for 24 h. Total RNA was extracted and RT-PCR was performed to probe the α1 mRNA as described under “Experimental Procedures”. Data were from four independent experiments. oua, ouabain; con, control.
Ouabain Stimulates Endocytosis and Degradation of Na/K-ATPase—We showed that ouabain stimulated Na/K-ATPase endocytosis in LLC-PK1 cells (35). If ouabain also stimulates the endocytosis in BT20 and DU145 cells, this could result in an increased degradation of Na/K-ATPase and a decrease in the α1 protein in these cells. To test this postulation, the cells were exposed to 10 nm ouabain and then immunostained for α1. As expected, most of α1 resided in the plasma membrane in BT20 and DU145 cells as in LLC-PK1 cells (Fig. 6A). After 6 h of ouabain exposure, many α1-positive vesicles were accumulated in BT20 and DU145 cells.
FIGURE 6.
Ouabain increases endocytosis and degradation of the Na/K-ATPase. A, LLC-PK1 cells, BT20 cells, and DU145 cells were treated with 10 nm ouabain (Oua) for 6 h and fixed with cold methanol. Na/K-ATPase α1 was immunostained and visualized using a Leica confocal microscope. The red arrows indicate the Na/K-ATPase α1-positive vesicles. B and C, LLC-PK1 or BT20 cells were serum-starved for 24 h, exposed to 10 μg/ml cycloheximide (CHX) for 1 h, and then treated with 10 nm ouabain for different times as indicated. The cell lysates were subjected to Western blot analyses of Na/K-ATPase α1. Tubulin was used as an internal control. A representative Western blot, and the quantitative data from four experiments are shown. *, p < 0.05. Con, control.
To determine whether ouabain-induced endocytosis also leads to increased degradation of Na/K-ATPase, LLC-PK1 cells and BT-20 cells were pretreated with cycloheximide, a protein synthesis inhibitor, for 1 h, and then exposed to 10 nm ouabain for 6, 16, and 24 h. As depicted in Fig. 6 (B and C), ouabain treatment produced more degradation of the α1 subunit in both BT-20 and LLC-PK1 cells.
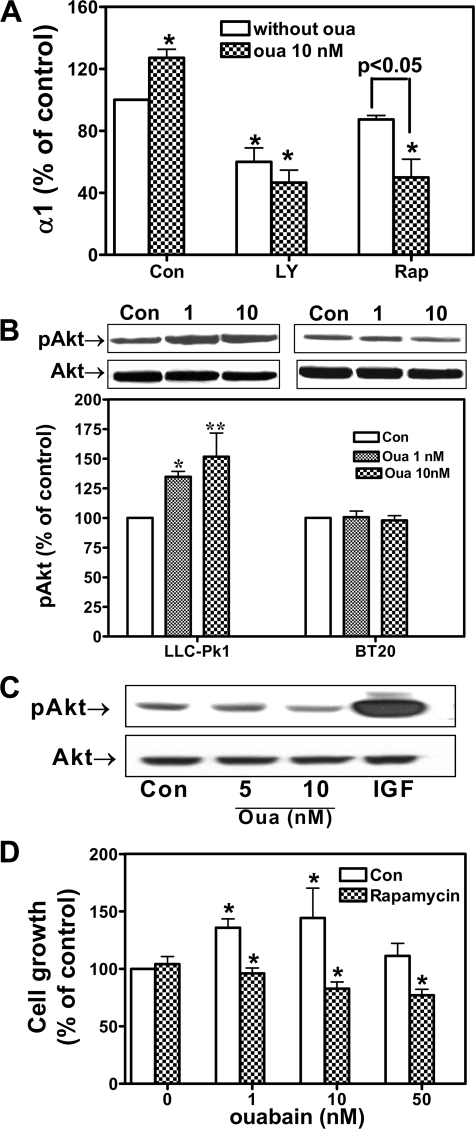
Ouabain Activates Akt in LLC-PK1 but Not BT-20 and DU145 Cells—We and others have demonstrated that ouabain stimulates the PI3K/Akt/mTOR pathway. It is known that activation of mTOR could increase the translation of many mRNAs. Because ouabain did not increase α1 mRNA in LLC-PK1 cells, we tested whether ouabain-induced up-regulation of α1 is sensitive to inhibitors of PI3K and mTOR. As depicted in Fig. 7A, pretreatment of cells with either PI3K inhibitor LY294002 or mTOR inhibitor rapamycin completely abolished ouabain-induced up-regulation of α1 subunit. Interestingly, both LY294002 and rapamycin reduced the basal expression of α1. Moreover, in the presence of rapamycin, ouabain treatment actually resulted in a further decrease in the expression of α1. This is not surprising, because rapamycin could theoretically block ouabain-induced α1 expression but not ouabain-induced α1 endocytosis and degradation.
FIGURE 7.
Ouabain up-regulates Na/K-ATPase expression and cell growth through activation of the PI3K/Akt/mTOR pathway. A, LLC-PK1 cells were pretreated with 10 nm rapamycin (Rap) or 50 μm LY294002 for 30 min. Ouabain (Oua, 10 nm) was then added into the medium for an additional 24 h. The cell lysates were analyzed for Na/K-ATPase α1 using Western blot. The data were from four separate experiments. *, p < 0.05 in comparison with control. B, LLC-PK1 and BT20 cells were incubated with ouabain for 15 min, and the cell lysates were analyzed for phosphorylated Akt (pAkt). The values are means ± S.E. of three experiments. *, p < 0.05. C, DU145 cells were serum-starved and treated with ouabain or 10 nm IGF for 15 min. The pAkt was probed by Western blot, and a representative Western blot from three repeats is shown. D, LLC-PK1 cells were pretreated with 10 nm rapamycin for 30 min and then incubated with ouabain for an additional 24 h. The cell growth was then measured using MTT assays. The quantitative data were from three to five independent experiments. *, p < 0.05 in comparison with the control (Con).
To test whether activation of the Akt/mTOR pathway is involved in ouabain-induced cell-specific regulation of α1 expression, we subsequently measured the effect of ouabain on Akt in three different cell lines. As shown in Fig. 7 (B and C), whereas ouabain stimulated Akt phosphorylation in LLC-PK1 cells, it failed to do so in both BT20 and DU145 cells. To be sure that the Akt pathway is intact in cancer cells, we exposed DU145 cells to IGF and measured for Akt activation. As shown in Fig. 7C, IGF caused a remarkable stimulation of Akt in DU145 cells. Consistently, when the same experiments were repeated in PY-17 cells, ouabain showed no effect on Akt phosphorylation (data not shown).
Rapamycin Sensitizes LLC-PK1 Cells to Ouabain-induced Cell Growth Inhibition—The above data indicate that activation of PI3K/Akt/mTOR is required for ouabain to increase the expression of α1. Because repletion of α1 is essential for ouabain-induced cell growth stimulation, we reasoned that inhibition of mTOR by rapamycin would convert ouabain-induced cell growth into growth inhibition in LLC-PK1 cells. Indeed, as shown in Fig. 7D, the addition of rapamycin was sufficient to block ouabain-induced cell proliferation in LLC-PK1 cells. Moreover, it also sensitized the cell to ouabain-induced cell growth inhibition. For example, 10 nm ouabain was sufficient to cause significant inhibition of cell proliferation in LLC-PK1 cells pretreated with the inhibitor.
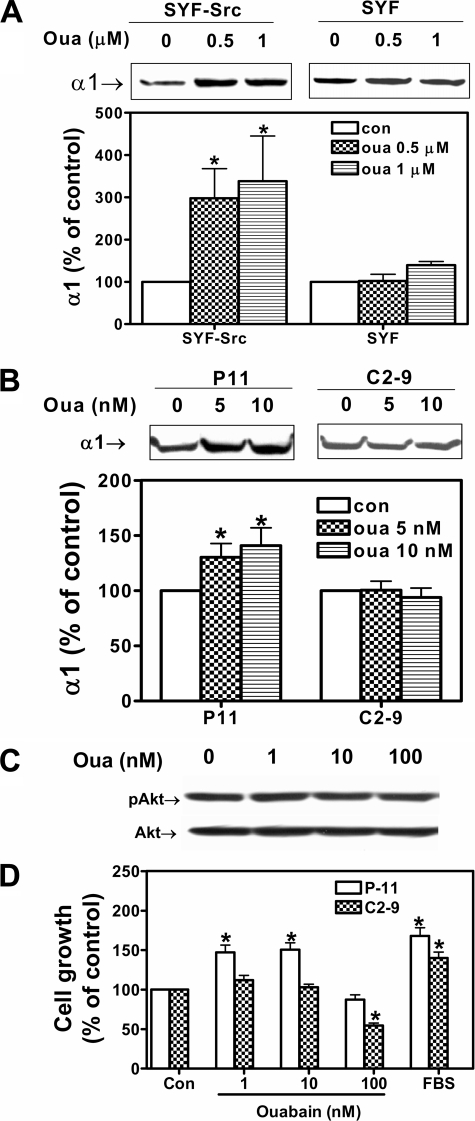
Involvement of Src and Caveolin-1—We have shown that the Na/K-ATPase signals through caveolae by forming a receptor complex with Src. To further understand the molecular mechanism of ouabain-induced up-regulation of α1, we first addressed the role of Src. SYF cells (in which the Src family kinases Src, Yes, and Fin were knocked out) were exposed to ouabain for 24 h. Because these cells were from mice and express a ouabain-resistant Na/K-ATPase, 0.5 and 1.0 μm ouabain were used in the experiments. As depicted in Fig. 8A, knock-out of Src completely blocked ouabain-induced α1 expression. Consistently, when SYF cells were rescued by c-Src, ouabain was able to cause a 3-fold induction of α1 expression.
FIGURE 8.
Involvement of Src and caveolin-1 in ouabain-induced growth regulation. A, SYF cells and SYF-Src cells were treated with different concentrations of ouabain (Oua) for 72 h and measured for α1 expression. A representative Western blot and quantitative data from four separate experiments are shown. B, P-11 and C2-9 cells were treated with different concentrations of ouabain for 72 h and probed for α1 expression. A representative Western blot and quantitative data from four separate experiments are shown. C, C2-9 cells were treated with different concentrations of ouabain for 15 min and probed for pAkt. A representative Western blot of three independent experiments is shown. D, P-11 and C2-9 cells were exposed to different concentrations of ouabain, and cell growth was measured by MTT assays. Fetal bovine serum was used as a positive control. The data are from five separate experiments. *, p < 0.05 in comparison with untreated control (Con) cells.
To test the involvement of caveolin-1 and caveolae, we utilized caveolin-1-specific small interfering RNA and established a caveolin-1 knockdown LLC-PK1 cell line (C2-9 cells) that expressed about 20% of caveolin-1 in comparison with the parent LLC-PK1 cells (36). As shown in Fig. 8B, knockdown of caveolin-1 completely abolished ouabain-induced increases in α1 expression. Consistently, ouabain failed to stimulate Akt in these cells (Fig. 8C). Moreover, knockdown of caveolin-1 not only abolished ouabain-induced cell proliferation but also sensitized the cells to ouabain-induced cell growth inhibition (Fig. 8D).
DISCUSSION
We have made the following major observations in this report. First, the stimulatory or inhibitory effect of ouabain on cell proliferation depends on whether ouabain can activate the Akt/mTOR pathway and replete cellular Na/K-ATPase against ouabain-induced endocytosis and subsequent degradation of the enzyme. Second, inhibition of the Akt/mTOR pathway by rapamycin increases ouabain-induced depletion of Na/K-ATPase and sensitizes cells to ouabain-induced growth inhibition. Finally, reduction of cellular Na/K-ATPase by means other than ouabain can also stimulate the expression of cell cycle inhibitor p21cip, resulting in cell growth inhibition.
Although ouabain exhibited both stimulatory and inhibitory effects on cell proliferation in a cell-specific manner, the activation of proximal signaling events (e.g., Src and ERK) has been recorded in both types of cells (34). Thus, the ouabain-activated downstream events must significantly diverge in cells whose growth response to ouabain went in opposite directions. In accordance, we observed that ouabain stimulated the Akt/mTOR pathway, resulting in translational up-regulation of Na/K-ATPase and stimulation of cell proliferation in LLC-PK1 cells. In contrast, ouabain failed to activate the Akt/mTOR pathway and replete the Na/K-ATPase in human cancer cells such as BT20 and DU145 cells as well as in the Na/K-ATPase knockdown cells (Fig. 7). Interestingly, ouabain-induced activation of the PI3K/Akt/mTOR pathway has been reported in several cell lines and primary cultures (13, 15, 35). Consistently, this activation appears to be required for ouabain to stimulate growth in these cells (13).
It is important to note that our experiments were conducted in serum-free cultures. Because serum is required for promoting and maintaining normal cell growth, the observed ouabain effects on cell signaling and growth might be due to serum starvation-induced changes in cell cycle. Thus, future studies will be required to resolve whether ouabain affects cell signaling and growth differently when cells are in normal logarithmic growth with serum.
In many types of cells, binding of ouabain and other cardiotonic steroids to the Na/K-ATPase is known to induce endocytosis and degradation of the enzyme (35, 53–55). Functionally, ouabain-induced endocytosis, even by low concentrations of ouabain, could lead to a significant reduction of the Na/K-ATPase over time if it is not compensated by up-regulation of α1 expression (Fig. 2). In principle, this down-regulation may be sufficient to account for ouabain-induced cell growth arrest (34). Consistently, we found that blocking ouabain-induced repletion of the Na/K-ATPase by rapamycin sensitized these cells to ouabain-induced cell growth inhibition. Moreover, we observed that knockdown of the Na/K-ATPase by small interfering RNA was equally effective in stimulating the expression of p21cip and inhibiting cell proliferation in LLC-PK1 cells. Taken together, it is clear that the amount of cellular Na/K-ATPase is important for cell growth control.
Needless to say, the molecular mechanism that prevents ouabain from stimulating the PI3K/Akt/mTOR pathway in BT20 and DU145 cells remains to be revealed. Nevertheless, it is important to discuss the potential role of caveolin-1, the Na/K-ATPase, and caveolae in this regulation. It is known that caveolae compartmentalize signaling events (56). Specifically, disruption of caveolae by depletion of either caveolin-1 or cholesterol can significantly alter insulin/IGF-induced activation of PI3K/Akt/mTOR pathways (57, 58). Interestingly, expression of caveolin-1 and/or Na/K-ATPase is significantly reduced in many cancer cell lines. Specifically, the expression of caveolin-1 is almost absent in BT20 cells, whereas expression of the Na/K-ATPase is significantly reduced in DU145 cells. Because both caveolin-1 and Na/K-ATPase are important for the formation of caveolae (59), it is possible that reduction of either caveolin-1 or the Na/K-ATPase may contribute to the uncoupling of ouabain binding to the activation of PI3K/Akt/mTOR pathway in these cells. Consistently, as shown in Fig. 8, we found that knockdown of caveolin-1 indeed abolished ouabain-induced activation of Akt. Similarly, ouabain also failed to stimulate Akt in the Na/K-ATPase knockdown PY-17 cells.
A large increase in intracellular Na+ is known to stimulate transcription of αl mRNA (60–62). This occurs when cells are exposed to low extracellular K+ or ouabain concentrations that produce more than 50% inhibition of the Na/K-ATPase (60–62). Apparently, this is unlikely the molecular mechanism by which 1–10 nm ouabain increased expression of the Na/K-ATPase in LLC-PK1 cells. First, ouabain at such low concentrations does not increase the cellular Na+ concentration in LLC-PK1 cells even after 24 h of exposure because of coordinated down-regulation of both Na/K-ATPase and Na/H exchange in these cells (35, 63). Second, the ouabain-induced up-regulation is different from that of low K+ because no change in α1 mRNA was detected (64). Finally, it is known that activation of the PI3K/Akt/mTOR pathway can stimulate the translation of many mRNAs. Consistently, we found that inhibition of the PI3K/Akt/mTOR pathway by either LY294002 or rapamycin was sufficient to block ouabain-induced up-regulation of the Na/K-ATPase.
Epidemiological studies in the late 1970s and early 1980s indicated the potential benefits of using digitalis drugs in patients with breast cancer (26, 27). Phase I and II clinical trials are underway in the United States and in Europe to test several new digitalis compounds as anti-cancer drugs. Taking the liberty of extrapolating in vitro to in vivo conditions, our new findings suggest that expression of the Na/K-ATPase may be used to assess the effectiveness of digitalis therapy on certain cancers or individual patients. Moreover, they suggest the potential use of rapamycin in the digitalis therapy of cancer. To this end, it is of interest to note that rapamycin is currently in clinical trials as an anti-cancer agent. It remains to be tested whether rapamycin-induced down-regulation of the Na/K-ATPase contributes to its effectiveness as an anti-cancer drug.
Acknowledgments
We thank Martha Heck for invaluable assistance in the preparation and editing of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-36573, HL-67963, and GM 78565.
Footnotes
The abbreviations used are: CTS, cardiotonic steroid(s); IGF, insulin-like growth factor; mTOR, mammalian target of rapamycin; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PI3K, phosphoinositide 3-kinase; RT, reverse transcription; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ERK, extracellular signal-regulated kinase.
References
- 1.Skou, J. C. (1965) Physiol. Rev. 45 596-617 [DOI] [PubMed] [Google Scholar]
- 2.Lingrel, J. B., Orlowski, J., Shull, M. M., and Price, E. M. (1990) Prog. Nucleic Acids Res. Mol. Biol. 38 37-89 [DOI] [PubMed] [Google Scholar]
- 3.Li, Z., and Xie, Z. (2009) Pfluegers Arch. Eur. J. Physiol. 457 635-644 [DOI] [PubMed] [Google Scholar]
- 4.Liang, M., Cai, T., Tian, J., Qu, W., and Xie, Z. J. (2006) J. Biol. Chem. 281 19709-19719 [DOI] [PubMed] [Google Scholar]
- 5.Tian, J., Cai, T., Yuan, Z., Wang, H., Liu, L., Haas, M., Maksimova, E., Huang, X. Y., and Xie, Z. J. (2006) Mol. Biol. Cell 17 317-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoner, W. (2008) Nat. Med. 14 16-17 [DOI] [PubMed] [Google Scholar]
- 7.Hamlyn, J. M., Blaustein, M. P., Bova, S., DuCharme, D. W., Harris, D. W., Mandel, F., Mathews, W. R., and Ludens, J. H. (1991) Proc. Natl. Acad. Sci. U. S. A. 88 6259-6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagrov, A. Y., Fedorova, O. V., Dmitrieva, R. I., French, A. W., and Anderson, D. E. (1996) Cardiovasc. Res. 31 296-305 [PubMed] [Google Scholar]
- 9.Haas, M., Wang, H., Tian, J., and Xie, Z. (2002) J. Biol. Chem. 277 18694-18702 [DOI] [PubMed] [Google Scholar]
- 10.Haas, M., Askari, A., and Xie, Z. (2000) J. Biol. Chem. 275 27832-27837 [DOI] [PubMed] [Google Scholar]
- 11.Xie, Z., and Cai, T. (2003) Mol. Interv. 3 157-168 [DOI] [PubMed] [Google Scholar]
- 12.Kotova, O., Al-Khalili, L., Talia, S., Hooke, C., Fedorova, O. V., Bagrov, A. Y., and Chibalin, A. V. (2006) J. Biol. Chem. 281 20085-20094 [DOI] [PubMed] [Google Scholar]
- 13.Liu, L., Zhao, X., Pierre, S. V., and Askari, A. (2007) Am. J. Physiol. Cell Physiol. 293 1489-1497 [DOI] [PubMed] [Google Scholar]
- 14.Elkareh, J., Kennedy, D. J., Yashaswi, B., Vetteth, S., Shidyak, A., Kim, E. G., Smaili, S., Periyasamy, S. M., Hariri, I. M., Fedorova, L., Liu, J., Wu, L., Kahaleh, M. B., Xie, Z., Malhotra, D., Fedorova, O. V., Kashkin, V. A., Bagrov, A. Y., and Shapiro, J. I. (2007) Hypertension 49 215-224 [DOI] [PubMed] [Google Scholar]
- 15.Zhou, X., Jiang, G., Zhao, A., Bondeva, T., Hirszel, P., and Balla, T. (2001) Biochem. Biophys. Res. Commun. 285 46-51 [DOI] [PubMed] [Google Scholar]
- 16.Aydemir-Koksoy, A., Abramowitz, J., and Allen, J. C. (2001) J. Biol. Chem. 276 46605-46611 [DOI] [PubMed] [Google Scholar]
- 17.Khundmiri, S. J., Metzler, M. A., Ameen, M., Amin, V., Rane, M. J., and Delamere, N. A. (2006) Am. J. Physiol. Cell Physiol. 291 1247-1257 [DOI] [PubMed] [Google Scholar]
- 18.Ferrandi, M., Molinari, I., Barassi, P., Minotti, E., Bianchi, G., and Ferrari, P. (2004) J. Biol. Chem. 279 33306-33314 [DOI] [PubMed] [Google Scholar]
- 19.Chueh, S. C., Guh, J. H., Chen, J., Lai, M. K., and Teng, C. M. (2001) J. Urol. 166 347-353 [PubMed] [Google Scholar]
- 20.Huang, Y. T., Chueh, S. C., Teng, C. M., and Guh, J. H. (2004) Biochem. Pharmacol. 67 727-733 [DOI] [PubMed] [Google Scholar]
- 21.Jing, Y., Ohizumi, H., Kawazoe, N., Hashimoto, S., Masuda, Y., Nakajo, S., Yoshida, T., Kuroiwa, Y., and Nakaya, K. (1994) Jpn. J. Cancer Res. 85 645-651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh, J. Y., Huang, W. J., Kan, S. F., and Wang, P. S. (2001) J. Urol. 166 1937-1942 [PubMed] [Google Scholar]
- 23.Taguchi, K., Kumanogoh, H., Nakamura, S., and Maekawa, S. (2007) Neurosci. Lett. 413 42-45 [DOI] [PubMed] [Google Scholar]
- 24.Watabe, M., Ito, K., Masuda, Y., Nakajo, S., and Nakaya, K. (1998) Oncogene 16 779-787 [DOI] [PubMed] [Google Scholar]
- 25.Stenkvist, B., Bengtsson, E., Eklund, G., Eriksson, O., Holmquist, J., Nordin, B., and Westman-Naeser, S. (1980) Anal. Quant. Cytol. 2 49-54 [PubMed] [Google Scholar]
- 26.Stenkvist, B., Bengtsson, E., Eriksson, O., Holmquist, J., Nordin, B., and Westman-Naeser, S. (1979) Lancet 1 563. [DOI] [PubMed] [Google Scholar]
- 27.Stenkvist, B. (1999) Oncol. Rep. 6 493-496 [PubMed] [Google Scholar]
- 28.Stenkvist, B., Pengtsson, E., Dahlqvist, B., Eriksson, O., Jarkrans, T., and Nordin, B. (1982) N. Engl. J. Med. 306 484. [PubMed] [Google Scholar]
- 29.Haux, J. (1999) Med. Hypotheses 53 543-548 [DOI] [PubMed] [Google Scholar]
- 30.Newman, R. A., Yang, P., Pawlus, A. D., and Block, K. I. (2008) Mol. Interv. 8 36-49 [DOI] [PubMed] [Google Scholar]
- 31.Ye, M., Qu, G., Guo, H., and Guo, D. (2004) J. Steroid Biochem. Mol. Biol. 91 87-98 [DOI] [PubMed] [Google Scholar]
- 32.Mijatovic, T., Van Quaquebeke, E., Delest, B., Debeir, O., Darro, F., and Kiss, R. (2007) Biochim. Biophys. Acta 1776 32-57 [DOI] [PubMed] [Google Scholar]
- 33.Newman, R. A., Yang, P., Hittelman, W. N., Lu, T., Ho, D. H., Ni, D., Chan, D., Vijjeswarapu, M., Cartwright, C., Dixon, S., Felix, E., and Addington, C. (2006) J. Exp. Ther. Oncol. 5 167-181 [PubMed] [Google Scholar]
- 34.Kometiani, P., Liu, L., and Askari, A. (2005) Mol. Pharmacol. 67 929-936 [DOI] [PubMed] [Google Scholar]
- 35.Liu, J., Kesiry, R., Periyasamy, S. M., Malhotra, D., Xie, Z., and Shapiro, J. I. (2004) Kidney Int. 66 227-241 [DOI] [PubMed] [Google Scholar]
- 36.Liu, J., Liang, M., Liu, L., Malhotra, D., Xie, Z., and Shapiro, J. I. (2005) Kidney Int. 67 1844-1854 [DOI] [PubMed] [Google Scholar]
- 37.Wang, H., Haas, M., Liang, M., Cai, T., Tian, J., Li, S., and Xie, Z. (2004) J. Biol. Chem. 279 17250-17259 [DOI] [PubMed] [Google Scholar]
- 38.Liang, M., Cai, T., Tian, J., Qu, W., and Xie, Z. J. (2006) J. Biol. Chem [DOI] [PubMed]
- 39.Konski, A. A., Myles, J. L., Sawyer, T., Neisler, J., Phibbs, G., Leininger, S., Kim, K., and Dobelbower, R. R., Jr. (1991) Int. J. Radiat. Oncol. Biol. Phys. 21 1033-1039 [DOI] [PubMed] [Google Scholar]
- 40.Baker, P. F., and Willis, J. S. (1972) J. Physiol. 224 441-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy, K. T., Snow, R. J., Petersen, A. C., Murphy, R. M., Mollica, J., Lee, J. S., Garnham, A. P., Aughey, R. J., Leppik, J. A., Medved, I., Cameron-Smith, D., and McKenna, M. J. (2004) J. Physiol. 556 507-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeshima, A., Nojima, Y., and Kojima, I. (2002) Kidney Int. 62 446-454 [DOI] [PubMed] [Google Scholar]
- 43.Liu, L., Abramowitz, J., Askari, A., and Allen, J. C. (2004) Am. J. Physiol. Heart Circ. Physiol. 287 H2173-H2182 [DOI] [PubMed] [Google Scholar]
- 44.Murata, Y., Matsuda, T., Tamada, K., Hosoi, R., Asano, S., Takuma, K., Tanaka, K., and Baba, A. (1996) Jpn. J. Pharmacol. 72 347-353 [DOI] [PubMed] [Google Scholar]
- 45.Kaplan, J. G. (1978) Annu. Rev. Physiol. 40 19-41 [DOI] [PubMed] [Google Scholar]
- 46.McConkey, D. J., Lin, Y., Nutt, L. K., Ozel, H. Z., and Newman, R. A. (2000) Cancer Res. 60 3807-3812 [PubMed] [Google Scholar]
- 47.Dmitrieva, R. I., and Doris, P. A. (2003) J. Biol. Chem. 278 28160-28166 [DOI] [PubMed] [Google Scholar]
- 48.Nguyen, A. N., Wallace, D. P., and Blanco, G. (2007) J. Am Soc. Nephrol. 18 46-57 [DOI] [PubMed] [Google Scholar]
- 49.Inge, L. J., Rajasekaran, S. A., Yoshimoto, K., Mischel, P. S., McBride, W., Landaw, E., and Rajasekaran, A. K. (2008) Histol. Histopathol 23 459-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajasekaran, S. A., Ball, W. J., Jr., Bander, N. H., Liu, H., Pardee, J. D., and Rajasekaran, A. K. (1999) J. Urol. 162 574-580 [PubMed] [Google Scholar]
- 51.Rajasekaran, S. A., Palmer, L. G., Quan, K., Harper, J. F., Ball, W. J., Jr., Bander, N. H., Peralta Soler, A., and Rajasekaran, A. K. (2001) Mol. Biol. Cell 12 279-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang, M., Tian, J., Liu, L., Pierre, S., Liu, J., Shapiro, J., and Xie, Z. J. (2007) J. Biol. Chem. 282 10585-10593 [DOI] [PubMed] [Google Scholar]
- 53.Cook, J. S., Karin, N. J., Fishman, J. B., Tate, E. H., Pollack, L. R., and Hayden, T. L. (1985) Soc. Gen. Physiol. Ser. 39 3-19 [PubMed] [Google Scholar]
- 54.Pollack, L. R., Tate, E. H., and Cook, J. S. (1981) Am. J. Physiol. Cell Physiol 241 173-183 [Google Scholar]
- 55.Pollack, L. R., Tate, E. H., and Cook, J. S. (1982) Prog. Clin. Biol. Res. 91 71-87 [PubMed] [Google Scholar]
- 56.Anderson, R. G. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 10909-10913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Podar, K., Tai, Y. T., Cole, C. E., Hideshima, T., Sattler, M., Hamblin, A., Mitsiades, N., Schlossman, R. L., Davies, F. E., Morgan, G. J., Munshi, N. C., Chauhan, D., and Anderson, K. C. (2003) J. Biol. Chem. 278 5794-5801 [DOI] [PubMed] [Google Scholar]
- 58.Cohen, A. W., Razani, B., Wang, X. B., Combs, T. P., Williams, T. M., Scherer, P. E., and Lisanti, M. P. (2003) Am. J. Physiol. Cell Physiol 285 222-235 [DOI] [PubMed] [Google Scholar]
- 59.Cai, T., Wang, H., Chen, Y., Liu, L., Gunning, W. T., Quintas, L. E., and Xie, Z. J. (2008) J. Cell Biol. 182 1153-1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cayanis, E., Russo, J. J., Wu, Y. S., and Edelman, I. S. (1992) J. Membr. Biol. 125 163-170 [DOI] [PubMed] [Google Scholar]
- 61.Lescale-Matys, L., Hensley, C. B., Crnkovic-Markovic, R., Putnam, D. S., and McDonough, A. A. (1990) J. Biol. Chem. 265 17935-17940 [PubMed] [Google Scholar]
- 62.Qin, X., Liu, B., and Gick, G. (1994) Am. J. Hypertens. 7 96-99 [DOI] [PubMed] [Google Scholar]
- 63.Cai, H., Wu, L., Qu, W., Malhotra, D., Xie, Z., Shapiro, J. I., and Liu, J. (2008) Am. J. Physiol. Cell Physiol 294 555-563 [DOI] [PubMed] [Google Scholar]
- 64.Pollack, L. R., Tate, E. H., and Cook, J. S. (1981) J. Cell. Physiol. 106 85-97 [DOI] [PubMed] [Google Scholar]