Abstract
The BLM helicase associates with the telomere structural proteins TRF1 and TRF2 in immortalized cells using the alternative lengthening of telomere (ALT) pathways. This work focuses on identifying protein partners of BLM in cells using ALT. Mass spectrometry and immunoprecipitation techniques have identified three proteins that bind directly to BLM and TRF2 in ALT cells: telomerase-associated protein 1 (TEP1), heat shock protein 90 (HSP90), and topoisomerase IIα (TOPOIIα). BLM predominantly co-localizes with these proteins in foci actively synthesizing DNA during late S and G2/M phases of the cell cycle when ALT is thought to occur. Immunoprecipitation studies also indicate that only HSP90 and TOPOIIα are components of a specific complex containing BLM, TRF1, and TRF2 but that this complex does not include TEP1. TEP1, TOPOIIα, and HSP90 interact directly with BLM in vitro and modulate its helicase activity on telomere-like DNA substrates but not on non-telomeric substrates. Initial studies suggest that knockdown of BLM in ALT cells reduces average telomere length but does not do so in cells using telomerase.
Bloom syndrome (BS)4 is a genetic disease caused by mutation of both copies of the human BLM gene. It is characterized by sun sensitivity, small stature, immunodeficiency, male infertility, and an increased susceptibility to cancer of all sites and types. The high incidence of spontaneous chromosome breakage and other unique chromosomal anomalies in cells from BS patients indicate an increase in homologous recombination in somatic cells (1). Another notable feature of non-immortalized and immortalized cells from BS individuals is the presence of telomeric associations (TAs) between homologous chromosomes (2). Work from our group and others have suggested a role for BLM in recombination-mediated mechanisms of telomere elongation or ALT (alternative lengthening of telomeres), processes that maintain/elongate telomeres in the absence of telomerase (3–5). However, the exact mechanism by which BLM contributes to telomere stability is unknown.
Several proteins interact with and regulate BLM helicase activity, including two telomere-specific proteins, TRF1 and TRF2 (6, 7). Although TRF2 stimulates BLM unwinding of telomeric and non-telomeric 3′-overhang substrates, TRF1 inhibits BLM unwinding of telomeric substrates. TRF2-mediated stimulation of BLM helicase activity on a telomeric substrate is observed when TRF2 is present in excess or with equimolar amount of TRF1 but not when TRF1 is present in molar excess. Both proteins associate with BLM specifically in ALT cells in vivo, suggesting their involvement in the ALT pathways. In addition to TRF1 and TRF2, the telomere single-strand DNA-binding protein POT1 strongly stimulates BLM helicase activity on long telomeric forked duplexes and D-loop structures (8). Other proteins also play an important role in telomere maintenance in telomerase-negative cells, including RAD50, NBS1, and MRE11, which co-localize with TRF1 and TRF2 in specialized ALT-associated promyelocytic leukemia (PML) nuclear bodies (APBs) (9–11). Thus, we hypothesize that BLM complex formation may be essential for the ALT mechanism, and its modification may occur dynamically during the specific nucleic acid transactions required to protect the telomere in cells using the ALT pathways.
This study has identified previously unknown protein partners of BLM and TRF2 in ALT cells using double immunoprecipitation and mass spectrometry (MS). These include telomerase-associated protein 1 (TEP1), heat shock protein 90 (HSP90), and topoisomerase IIα (TOPOIIα). These proteins associate with BLM and TRF2 in cells using ALT but not in cells using telomerase and directly interact with BLM in vitro. This complex of proteins localizes to sites of new DNA synthesis in vivo in ALT cells, suggesting a role in telomere maintenance. We also identified HSP90 and TOPOIIα in another ALT-specific complex consisting of BLM, TRF1, and TRF2 but not TEP1. In vitro analyses demonstrate that HSP90 inhibits BLM helicase activity using both telomeric and non-telomeric substrates, whereas TEP1 and TOPOIIα initially slow the kinetics of BLM unwinding only using telomeric substrates. These findings suggest the presence of dynamic BLM-associated ALT complexes that include previously unidentified interacting proteins. The function of TEP1 in the BLM·TRF2 complex remains unclear, although its previously described interaction with the RNA subunit of telomerase (12) suggests an interesting hypothesis of cross-talk between mechanisms of telomere elongation.
EXPERIMENTAL PROCEDURES
Cell Lines—Two immortalized and telomerase-negative cell lines, Saos2 and WI-38-VA13/2RA, two immortalized and telomerase-positive cell lines, MCF7 and HeLa, and a non-immortalized primary cell line of diploid human fibroblasts, WI-38, were obtained from ATCC. The SV-40 transformed BS fibroblast cell line GM08505C was obtained from the Coriell Cell Repository. MCF7, Saos2, and GM08505C cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum (Hyclone). WI-38 and WI-38-VA13/2RA were cultured in minimal essential medium (Invitrogen) containing 10% fetal bovine serum. All cell lines were grown at 37 °C and 5% CO2.
Cell Cycle Synchronization, Bromodeoxyuridine (BrdUrd) Pulse Labeling, and Flow Cytometry—Cells were prepared for synchronization in G1/S phases as described previously (7). BrdUrd pulse labeling (11) and cell cycle analysis were performed on propidium iodide-labeled cells using a Coulter Epics XL flow cytometer.
In Vivo Protein Coimmunoprecipitation—In vivo immunoprecipitations from nuclear extracts were performed as previously described (7). For double immunoprecipitations, the immunoprecipitates from the first round of immunoprecipitations were washed with lysis buffer and eluted with 100 mm sodium citrate, pH 3.0, followed by neutralization of the pH and the addition of the second antibody.
MALDI-TOF and Protein Identification—SDS-gel sample bands were cut and submitted to the OSU Proteomics Core. Samples were transferred to the MassPrep station for automated in-gel protein digestion. Briefly, gel pieces were destained with ammonium bicarbonate/acetonitrile and reduced with dithiothreitol. The reducing mixture was removed, and iodoacetamide in ammonium bicarbonate was added and incubated 20 min at 37 °C. Alkylation solution was removed followed by washing with ammonium bicarbonate/water and dehydration with acetonitrile. In-gel digestion of the extracted proteins was carried out with 6-ng/μl trypsin in 50 mm ammonium bicarbonate for 5 h at 37 °C. Digested peptides were extracted with a mixture of 1% formic acid, 2% acetonitrile and applied onto a stainless steel MALDI plate (Micromass). Mass spectra of resulting peptides were recorded on the MALDI-TOF spectrometer in reflectron mode. Calibration was performed with angiotensin I (average molecular mass 1296.5 Da), renin (average molecular mass 1759.0 Da), and ACTH 18–39 clip (adenocorticotropic hormone clip 18–39, average molecular mass 2465.199 Da). The resulting peptides were matched with their corresponding proteins with ProFound by searching the non-redundant data base maintained at the NCBI (www.ncbi.nlm.nih.gov). At least six peptides were used to identify each protein within the band. The following parameters were used for the search at ProFound: taxa Homo sapiens complete modifications iodoacetamide (Cys), partial modification oxidation (M), allowed incomplete cleavages 1, monoisotopic masses, and a mass tolerance of 50 ppm.
Immunostaining and Antibodies—Western blotting was done using standard procedures (7). Antibodies were purchased from Accurate Scientific (BrdUrd, OBT 0030), Bethyl Laboratories (BLM, A300-110A for immunoprecipitations and Western blots; TOPOIIα, A300-054A for immunoprecipitations and Western blots), Calbiochem (TOPOIIα, NA14 for immunofluorescence), Imgenex (TRF2, IMG-124A for immunofluorescence), and Santa Cruz Biotechnology (HSP90, sc-1057; PML, PG-M3; TEP1, sc-13052). Secondary antibodies included fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit, FITC-conjugated goat anti-mouse, rhodamine-conjugated goat anti-mouse, rhodamine-conjugated goat anti-rabbit, and rhodamine-conjugated donkey anti-rat (Jackson ImmunoResearch Laboratories).
Transfection and Immunofluorescence—Cells were transfected with the pEGFP-BLM expression vector using Effectene transfection kit (Qiagen) following the manufacturers' protocol. For the siRNA experiments pBLMsiRNA was generated by ligating DNA sequences corresponding to pre-validated siRNA sequences (Ambion) into the pSilencer 4.1 CMV puro expression vector (Ambion). Oligonucleotides containing siRNA sequences were synthesized by IDT (Coralville, IA), purified by SDS-PAGE, and annealed before ligation (top strand, 5′-GATCCGGAAGTTGTATGCACTACCTTCAAGAGAGGTAGTGCATACAACTTCCTTA-3′; bottom strand, 5′-AGCTTAAGGAAGTTGTATGCACTACCTCTCTTGAAGGTAGTGCATACAACTTCCG-3′). DH5α cells were transformed with the ligation product and grown in LB plus ampicillin. All products were sequenced. For controls, the pSCsiRNA vector was used (Ambion). The cells were viewed using an Axiovert Zeiss microscope. Images were captured on a Zeiss AxioCam MRm camera using AxioVision (Release 4.5) imaging software.
Protein Purification—Jel1 yeast (protease deficient) were transformed with the pYES-BLM vector using the Bio 101 Systems yeast transformation protocol (Bio 101 Systems). Transformed colonies were selected on plates lacking uracil, and starter cultures were used to inoculate SD minimal base (plus -Ura dropout powder) containing 2% glucose. Cultures were grown to an A600 of 1.5–2.0 in an orbital shaker at 30 °C. Yeast cells were pelleted and resuspended in an equal volume of S.D. minimal base (plus -Ura dropout powder) containing 2% galactose and grown for an additional 20–24 h. Yeast were then pelleted, resuspended in buffer A (50 mm Tris, pH 8.0, 500 mm NaCl, 10% glycerol, and 1:500 mammalian protease inhibitors (Sigma), and lysed using a French press cell by applying 20,000 pounds/square inch. Lysates were bound to 1.0 ml of nickel-nitrilotriacetic acid resin (Novagen) charged with NiSO4 in binding buffer (buffer A + 50 mm imidazole) at 4 °C for 4 h. The protein-bound resin was applied to a column and washed with 50 ml of binding buffer, 2.5 ml of binding buffer + 70 mm imidazole (120 mm total) and eluted with 4.0 ml of binding buffer + 450 mm imidazole (500 mm total). The eluted protein was dialyzed in buffer Z (60 mm Tris, pH 7.5, 100 mm NaCl, 10% glycerol, 1 mm EDTA, 1 mm dithiothreitol) at 4 °C for 4 h and then snap-frozen in small aliquots using liquid nitrogen and stored at -80 °C. Purity was confirmed by staining with Biosafe Coomassie (Bio-Rad). The maximum velocity (Vmax) for the pure enzyme was determined to be 0.26362 fmol unwound per min, and the Michaelis-Menten constant (Km) was 27.118 nm.
Baculovirus containing Myc-tagged TEP1 was amplified and used to infect SF9 cells. Cells were harvested 72 h after infection, pelleted at 500 × g for 10 min, washed, and re-suspended in 30 ml of binding buffer (50 mm Tris, pH 7.5, 300 mm NaCl, 20% glycerol, protease inhibitor (Sigma, P8340). Cells were sonicated 3 × 10 s and incubated with DNase 1 for 1 h at 4 °C. Lysate was clarified at 20,000 × g for 30 min and bound to agarose-conjugated anti-Myc antibodies (1-ml bed volume) for 4 h. A chromatography column (Bio-Rad, 731-1550) was packed with lysate and washed with 2 × 10 ml of PBS. Myc-tagged TEP1 was eluted with 7 × 1 ml of 100 mm NH4OH. Fractions containing TEP1 were dialyzed into buffer containing 60 mm Tris, pH 7.5, 100 mm NaCl, 1 mm EDTA, 1 mm dithiothreitol, and 10% glycerol, snap-frozen in liquid nitrogen and stored at -80 °C. Protein purity was ascertained by SDS-PAGE (supplemental Fig. 1). TEP1 cDNA was sequenced, and 8 individual base pair discrepancies were found by comparison to an NCBI published sequence (nucleotides 346, 1658, 1860, 2733, 3163, 3210, 3464, and 3583). These discrepancies were confirmed by sequencing a set of human genomic DNAs. Two alterations, at 3163 and 3583, are polymorphic, whereas the others remain unclear in origin or effect. Topoisomerase IIα was obtained from United States Biochemicals; HSP90 was a generous gift from Dr. David Toft, Mayo Clinic, Rochester, MN, and pure UvrD helicase was a gift from Dr. Tim Lohman, Washington University, St. Louis, MO.
Enzyme-linked Immunosorbent Assay Detection for Protein-Protein Interaction—Enzyme-linked immunosorbent assay was conducted as described previously (7). Briefly, wells were coated with 50 μl of purified BLM protein (diluted to 2.5 ng/μl in carbonate buffer) or with 50 μl of BSA as a background control and incubated overnight at 4 °C. Wells were washed and incubated with 300 μl of blocking buffer (PBS, 3% BSA, 0.1% Tween 20) for 1 h at 37 °C. After blocking, various concentrations of proteins were added (50 μl) and incubated for 2 h at 37 °C. After washing, primary antibody (1:1000) was added (50 μl) and incubated for 1 h at 37 °C. Wells were washed, and secondary antibody (1:10,000) was added (50 μl) and incubated for 1 h at 37 °C. After washing, cells were incubated with 50 μl of TACS-sapphire detection reagent (Trevigen, Inc.) for 10 min in the dark. Reactions were stopped by adding equal volumes of 0.2 n HCl. Absorbance was read at 490 nm, and all values were corrected for background in reactions without primary and secondary antibodies.
DNA Substrate—The telomeric 3′-overhang substrate was generated by annealing a 38-mer (5′-CCCTAACCCTAACCCTAACCCTAGAGGGAAAGGAAGAA-3′) with a 68-mer (5′-TTCTTCCTTTCCCTCTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGGTTAGGG-3′). The non-telomeric 3′-overhang substrate was created by annealing a 38-mer (5′-ATGAGAAGCAGCCGTATCAGGAAGAGGGAAAGGAAGAA-3′) with a 68-mer (5′-TTCTTCCTTTCCCTCTTCCTGATACGGCTGCTTCTCATCTACAACGTGATCCGTCATTCGGAGTG-3′). Single-stranded oligonucleotides were purified using denaturing polyacrylamide gels before use. The shorter of the two strands was end-labeled with γ-32P using T4 polynucleotide kinase (New England Biolabs) according to the manufacturer's protocol. The labeled strand was mixed at equimolar concentrations with the unlabeled complement, heated at 95 °C for 5 min, and slowly cooled to room temperature to generate the partial double-stranded DNA.
Helicase Assays—Helicase assays were performed as described (7) with some modifications. These assays measure the unwinding of 32P-labeled DNA from a partial duplex DNA molecule. In a 50-μl reaction volume, the DNA substrate (0.5 nm) was incubated with BLM in 1× helicase buffer (20 mm Tris-HCl, pH 7.5, 0.1 mg/ml BSA, 2 mm MgCl2, 2 mm ATP, and 1 mm dithiothreitol) at 37 °C. In reactions containing additional proteins, the substrate was preincubated with these proteins for 5 min before adding BLM. Reactions were terminated after the desired time with helicase stop buffer (30% glycerol, 50 mm EDTA, 0.9% SDS, 0.25% bromphenol blue, and 0.25% xylene cyanol). The reaction products were separated, resolved by 12% native PAGE, and analyzed using a Storm 860 PhosphorImager and ImageQuant software. All assays were performed at least three times.
Telomere Restriction Fragment Length Analysis—Genomic DNA was isolated using the Genomic DNA Isolation kit (Trevigen). Briefly, 10 μg of DNA was digested with RsaI and HinfI overnight at 37 °C. Digested DNA was electrophoresed on 0.8% agarose gel in 1× Tris-buffered EDTA and transferred overnight to a positively charged nylon membrane (Immobilon-NY+). DNA was cross-linked to the membrane by Stratalinker, and southern hybridization was performed using a γ-32P-labeled telomere-specific probe (CCCTAAA)3. Blots were hybridized in 5× SSC (1× SSC = 0.15 m NaCl and 0.015 m sodium citrate), 5× Denhardt's solution, 40% formamide, 0.5% SDS overnight at 42 °C, washed 3 times for 15 min in 1× SSC, 0.1% SDS at 65 °C, and exposed to Biomax MS autoradiographic film for 2 days at -80 °C.
RESULTS
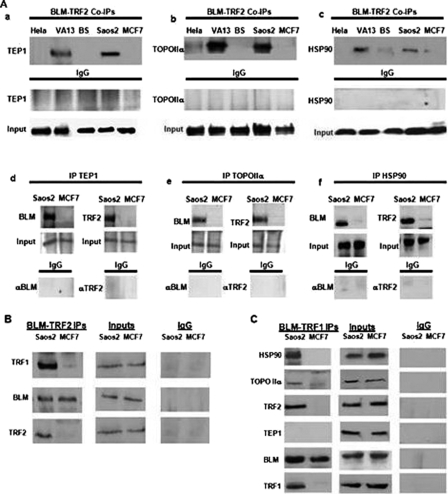
Identification of Three Protein Partners of BLM in a BLM-associated ALT Complex—BLM is expressed in all tissues with active cell proliferation. Its expression peaks during late S and the G2 phases of the cell cycle, when ALT is thought to occur (13). BLM is capable of resolving stalled replication forks and is part of a multiprotein complex formed dynamically during replication (14–16). The interaction of BLM with telomere-specific proteins TRF1 and TRF2 is observed in immortalized cells that use ALT mechanisms to maintain telomeres (6, 7, 17); BLM also co-localizes with TRF2 in foci actively synthesizing DNA (6, 7). These observations prompted our attempts to isolate a BLM·TRF2 complex from ALT cells using double immunoprecipitation and MS techniques. Whole cell lysates were prepared from immortalized cell lines using ALT (Saos2 and VA13) or telomerase (HeLa and MCF7) to maintain telomeres and BS cell line GMO8505C. Antibodies specific for both BLM and TRF2 were used to enrich for BLM complexes, which were then separated by 10% SDS-PAGE and evaluated by silver staining (Fig. 1). Unique protein bands and those expressed in higher amounts in ALT cells compared with telomerase-positive cells were selected for identification by MS analysis. In total, five proteins were identified that included BLM, TOPOIIα, TEP1, and HSP90. The presence of each protein in the complex was validated by immunoprecipitation and Western analysis of lysates from the ALT and telomerase cells (Fig. 2A). Antibodies specific for TEP1, TOPOIIα, and HSP90 were used to detect the presence of each protein in the immunocomplex. The Western blot results demonstrate the above three proteins in significant amounts in immunoprecipitates from ALT cells in comparison to those from telomerase-positive cells (Fig. 2A, a–c). They were undetected in IgG controls, indicating that the pulldown assays were specific for the antibodies used.
FIGURE 1.
Identification of BLM- and TRF2-associated proteins by mass spectrometry. BLM-associated proteins were immunoprecipitated from immortalized telomerase-positive (HeLa and MCF7, lanes 3 and 5) and telomerase-negative ALT cells (WI38-VA13 and Saos2, lanes 4 and 6) using anti-BLM antibody. Lysates were subjected to a second round of immunoprecipitation using anti-TRF2 to enrich for BLM-associated proteins in cells that use ALT. Lanes 1 and 2 are IgG controls for MCF7 and Saos2 cells. Immunoprecipitates were resolved by 10% SDS-PAGE and silver-stained. Proteins identified by mass spectrometry are labeled.
FIGURE 2.
Detection of BLM and TRF2-associated proteins by Western analysis. A, BLM and TRF2 were coimmunoprecipitated sequentially from nuclear extracts of ALT (WI38-VA13 and Saos2) and telomerase-positive (HeLa and MCF7) or BS cells (GM08508) (panels a–c). Immunoprecipitations (IP) were carried out with goat polyclonal BLM and TRF2 antibodies or normal goat IgG (negative control). Immunoprecipitates were resolved by 6% SDS-PAGE, Western-blotted with anti-TEP1 (a), anti-TOPOIIα (b), and anti-HSP90 (c) antibodies. TEP1 (d), TOPOIIα (e), and HSP90 (f) were also immunoprecipitated from nuclear extracts of Saos2 and MCF7 cells, resolved, and probed with anti-BLM and anti-TRF2. IgG controls and the inputs are also shown. B, TRF1 associates with BLM and TRF2. BLM·TRF2 immunocomplex from Saos2 and MCF7 were subjected to Western analysis using anti-TRF1 antibody. TRF1 immunoprecipitated from the ALT cell line Saos2. BLM was detected in the immunocomplex from both cell lines, whereas TRF2 was present in complex with BLM and TRF1 in Saos2. C, TEP1 is not a component of the ALT complex containing TRF1. Whole cell lysates from Saos2 and MCF7 were subjected to sequential immunoprecipitation using anti-BLM and anti-TRF1 antibodies. HSP90, TOPOIIα, and TRF2 were detected in complex with BLM and TRF2 in Saos2 but not in MCF7. TEP1 was not included in this specific complex in either cell line. The Input lanes show the presence of the proteins in whole cell lysates.
Single immunoprecipitations were performed using anti-TEP1, anti-TOPOIIα, and anti-HSP90 antibodies (Fig. 2A, d–f). The immunocomplexes with anti-TEP1, anti-HSP90, and anti-TOPOIIα were resolved by denaturing SDS-PAGE as before. Western analysis confirmed the coimmunoprecipitation of BLM and TRF2 with TEP1, TOPOIIα, and HSP90 in ALT cell lysates only. We repeated these experiments with another ALT (VA13) and telomerase cell line (HeLa) (data not shown) at least three times to ascertain reproducibility. In all immunoprecipitation experiments, nuclear extracts were pretreated with micrococcal nuclease, suggesting that DNA tethering is not required for the interactions between these proteins (data not shown).
As both TRF1 and TRF2 are telomere-specific proteins binding to telomere repeats, it was important to determine whether TRF1 was also present in the same complex with BLM and TRF2. Western analysis of the immunoprecipitates from the ALT (Saos2) and telomerase-positive cells (MCF7) showed that TRF1 was present in the BLM·TRF2 immunocomplex from the ALT cells but not in the telomerase-positive cells (Fig. 2B). The presence of overlapping bands/signals in the gel most likely prevented detection by MS analysis. These results were confirmed using different ALT and telomerase cells lines (data not shown). BLM was immunoprecipitated from both cell lines as indicated by Western blot, but TRF2 was only present in a complex with BLM and TRF1 in Saos2 lysates.
Next, to determine the specificity of the complex, we performed double immunoprecipitation using anti-BLM and anti-TRF1 antibodies and immunoprecipitated the BLM-TRF1 immunocomplex from the ALT and telomerase-positive cells (Fig. 2C). The previously identified HSP90 and TOPOIIα were detected along with TRF2 in the complex by Western analysis; however, we were unable to detect TEP1 in this specific complex. This experiment was repeated several times using a different ALT cell line (VA13) with similar negative results. To rule out any effects of antibody specificity, anti-TEP1 and anti-TRF1 were used for immunoprecipitation, and the anti-BLM was used for Western analyses; similar negative results were generated (data not shown). These complexed proteins were undetected in MCF7. These data suggest a dynamic nature of the interactions between BLM, TRF1, and TRF2.
BLM-associated Proteins Co-localize with BLM and TRF2 in Vivo—Immunofluorescence studies were performed to determine whether the three proteins identified by immunoprecipitation and MS co-localize with BLM and TRF2 in ALT cells. As TRF2 is a telomere-specific protein that associates with BLM in ALT cells (6, 7), co-localization of the BLM-associated proteins with TRF2 and BLM would suggest a role in telomere maintenance. ALT and telomerase cells were grown in a 6-well plate to 60% confluency and fixed for immunofluorescence. All three proteins, TEP1, TOPOIIα, and HSP90, co-localize with BLM in two different ALT cell lines tested, Saos2 (Fig. 3A) and VA13/2RA (data not shown), as indicated by the merger of the individual red and green foci to form distinct yellow foci. Similarly, these proteins also co-localize with TRF2 in the ALT cell lines Saos2 (Fig. 3B) and VA13 (data not shown). Strong co-localization was not observed in the telomerase-positive cell lines MCF7 (Fig. 3, A and B) and HeLa, or in the primary fibroblast cell line WI-38 (data not shown). The immunofluorescence data were quantified by determining the number of cells with co-localized foci in ALT and telomerase-positive cells (Fig. 3C). Results suggest a significant number of cells with co-localized foci in the ALT cell line Saos2. Co-localization with BLM and TRF2 is observed in 80% to 90% of the ALT cells analyzed; co-localization in the telomerase-positive cells varied between 15 and 30%. These data show that co-localization of these BLM-associated proteins is increased in ALT cells and further suggest a role for them in telomere elongation.
FIGURE 3.
TEP1, TOPOIIα, and HSP90 co-localize with BLM and TRF2 in ALT cells in vivo. Three proteins co-localize with BLM (A) and TRF2 (B) in immortalized ALT cells Saos2. A, for generating immunofluorescence with BLM, cells were transfected with pEGFP-BLM, fixed, and immunostained using rabbit polyclonal anti-TEP1, anti-TOPOIIα, and anti-HSP90, and rhodamine-labeled secondary. Negative controls with rabbit IgG are shown. DAPI, 4′,6-diamidino-2-phenylindole. B, immunofluorescence with TRF2- and BLM-associated ATL complex proteins were performed as described under “Experimental Procedures.” Immunostaining was performed using mouse monoclonal anti-TRF2 and rabbit polyclonal antibodies against the BLM-associated proteins. Mouse IgG was used for negative controls. BLM (A) and TRF2 (B) merge with TEP1 or TOPOIIα or HSP90 to give yellow foci in ALT cell line Saos2. Strong co-localization was not observed in the telomerase-positive cell line MCF7. C, quantitation of A and B. % of cells showing co-localization of BLM or TRF2 with the BLM-associated ALT complex proteins were calculated from three independent experiments to obtain averages and S.D.
BLM-associated ALT Proteins Associate with APBs and Co-localize at Sites of New DNA Synthesis in ALT Cells—A consistent feature of ALT cells is the presence of ALT-associated PML nuclear bodies or APBs (9, 10) in which telomeric DNA, telomeric proteins (TRF1 and TRF2), and homologous recombination proteins (RMN complex, RAD51, RAD52, and RPA) reside. The PML nuclear bodies derive their name from promyelocytic leukemia or PML protein, a putative tumor suppressor. Previous studies have shown that both BLM and TRF2 co-localize with PML in ALT cells, suggesting that BLM is a component of APBs (7). Hence, we asked whether TOPOIIα, TEP1, and HSP90 associate with the PML bodies similarly to BLM and TRF2 (7). Additionally, if the BLM-associated ALT complex proteins are involved in ALT, their localization in APBs will increase as the cell progresses into the late S and G2 phases of the cell cycle when ALT is thought to occur. TOPOIIα, TEP1, and HSP90 co-localize with APBs in the ALT cell line Saos2 (Fig. 4A). Distinct co-localized foci were absent in the telomerase-positive cell line MCF7. Co-localization occurs in a cell cycle-dependent manner (Fig. 4B). During G1 and early S phases (2–5 h post-release from aphidicolin treatment), 10–15% of the ALT cells contain co-localized foci for TEP1 and PML, 20–30% contain co-localized foci for TOPOIIα and PML, and 25–35% contain co-localized foci of HSP90 and PML. As cells progress into late S and G2/M phases (10–12 h post-release), cells with co-localized foci increase to 45–65% for TEP1 and PML, 55–70% for TOPOIIα and PML, and 50–80% for HSP90 and PML. The cell cycle-specific increases in cells with co-localized foci suggest that not only do these proteins associate with APBs but that they also may be involved in the specific DNA-protein transactions required for telomere elongation during ALT. MCF7, the telomerase-positive cell line, did not show similar protein localization nor did they demonstrate any cell cycle-specific dynamic of co-localization. The identical cell cycle distributions of Saos2 (Fig. 4C) and MCF7 (data not shown) were confirmed by flow cytometry. All experiments were repeated with another ALT (VA13) and telomerase (HeLa) cell line to assure the consistency of the findings. Similar results were also obtained with nocodazole-treated cells (data not shown).
FIGURE 4.
BLM-associated proteins co-localize with APBs. A, co-localization of ALT-associated PML bodies with the BLM-associated ALT complex proteins was observed in the synchronized ALT cell line Saos2 enriched in late S and G2 phases but not in the telomerase-positive cell line MCF7. Cells were fixed and immunostained with anti-TOPOIIα, anti-TEP1, and anti-HSP90 rabbit antibodies (fluorescein isothiocyanate-labeled secondary antibody) and anti-PML mouse monoclonal antibody (rhodamine-labeled secondary antibody). BLM-associated proteins foci and PML foci were present in all cell lines analyzed. Merged images indicate the association of the proteins in vivo. Mouse IgG was used for negative controls. DAPI, 4′,6-diamidino-2-phenylindole. B, cells were blocked at the G1/S-phase boundary with aphidicolin and analyzed at different times post-release by co-staining for PML and TEP1 or TOPOIIα or HSP90. Co-localization was scored with digital photography and repeated at least three times to obtain averages and S.D. C, flow cytometry data show the cell-cycle staging of the Saos2 cells at different times post-release.
Proteins implicated in ALT, including BLM and TRF2, co-localize with sites of BrdUrd incorporation during late S and G2 phases of the cell cycle (7). To test whether the newly identified proteins similarly co-localize with foci of BrdUrd incorporation, cells were blocked at the G1/S-phase boundary by aphidicolin treatment, pulse-labeled with BrdUrd 12 h post-release, and analyzed by immunofluorescence with anti-BrdUrd antibodies as described under “Experimental Procedures.” Cell cycle distribution was confirmed by flow cytometry (data not shown). Immunofluorescence data show that the three proteins co-localize with BrdUrd foci exclusively in ALT cells (Saos2) but not in telomerase-positive cells (MCF7) (Fig. 5). These data suggest that the three proteins are present at the sites of active DNA synthesis, occurring during late S and G2 phases of the cell cycle when telomere elongation is thought to occur.
FIGURE 5.
BLM-associated proteins co-localize with foci of DNA synthesis in ALT cells. Saos2 (A) and MCF7 (B) cells were synchronized at G1/S by aphidicolin block as described before. Cells were pulse-labeled with BrdUrd at 10 h post-release from the block and subsequently fixed for immunostaining. Cell-cycle stage was confirmed by flow cytometry (data not shown). Cells were immunostained with anti-BrdUrd antibodies (rhodamine-labeled secondary) and either anti-TOPOIIα, anti-TEP1, or anti-HSP90 (fluorescein isothiocyanate-labeled secondary). Negative controls were done with rat IgG. DAPI, 4′,6-diamidino-2-phenylindole.
BLM-associated Proteins Directly Interact with BLM in Vitro—The above immunoprecipitation and immunofluorescence results suggest that TEP1, TOPOIIα, and HSP90 associate with BLM and TRF2 in vivo in cells using ALT. Direct physical interactions between these proteins and purified BLM were then examined using enzyme-linked immunosorbent assays to determine whether these proteins directly interact with BLM in vitro. BLM was bound to the wells of 96-well microtiter plates, and after blocking, purified TEP1, TOPOIIα, or HSP90 was added at different concentrations. Bound proteins were then detected using specific primary antibodies, horseradish peroxidase-conjugated secondary, and colorimetric substrate. All three proteinsinteractwithBLMinadose-dependent manner (Fig. 6). DNase treatment does not interfere with binding (data not shown), suggesting the interactions of proteins are not facilitated by the presence of DNA. None bind to BSA.
FIGURE 6.
BLM interacts with TOPOIIα, HSP90, and TEP1 in vitro. Interactions between BLM and TOPOIIα (A), BLM and HSP90 (B), and BLM and TEP1 (C) were assayed by enzyme-linked immunosorbent assay. Microtiter plate wells were prebound with BLM or BSA and incubated with increasing concentrations of TOPOIIα or HSP90 or TEP1 as indicated. Interactions were determined using anti-TOPOIIα, anti-HSP90, and anti-TEP1 antibodies and a colorimetric substrate (absorbance at 450 nm). Shaded and black bars indicate the values for BLM, whereas the white bars are BSA. Wells were also coated with TOPOIIα, HSP90, and TEP1 for positive controls. No primary antibodies and No secondary antibodies reactions were done for negative controls and background corrections.
Effects of BLM-associated Proteins on BLM Helicase Activity—BLM unwinding activity in vitro can be regulated by the telomere repeat-binding proteins TRF1 and TRF2 (7). As three new BLM-interacting proteins have been identified in vivo and in vitro, their effects on BLM helicase activity were examined using telomeric and non-telomeric DNA substrates (7). The telomeric substrate was generated to mimic a native linearized telomere DNA with a 3′-single-stranded overhang and 23-base pair telomere sequence consisting of five 5′-TTAGGG-3′ repeats within the 38-base pair duplex region (7). The non-telomeric substrate, devoid of telomere sequence, was used as a control to determine whether BLM showed any sequence specificity toward the telomeric substrate during unwinding. We find that BLM at a concentration of 30 nm effectively unwinds both types of substrates in the absence of other proteins in an ATP-dependent manner (Fig. 7) yet unwinds telomeric substrates more efficiently than the conventional 3′-overhang non-repetitive control substrate. We observe almost a 2–6-fold enhancement in unwinding of the telomere-like substrate compared with the control substrate. This kinetic advantage on the telomeric substrate was not observed at lower concentrations of BLM. These assays have been performed with various BLM concentrations ranging from 0.5 to 50 nm; more efficient unwinding of the telomeric substrate than the control substrate by BLM was only apparent at concentrations of 30 nm and above. In the presence of equimolar TOPOIIα (Fig. 7A), there are subtle differences in unwinding of the telomeric substrate, especially at early time points (0.5–2.5 min), at which TOPOIIα slows the kinetics of BLM unwinding. A concentration of 30 nm TOPOIIα does not alter the ability of BLM to unwind the non-telomeric substrate. In contrast, HSP90 strongly inhibits BLM unwinding on both telomeric and non-telomeric substrates (Fig. 7B). Under similar conditions, TEP1 slows down BLM unwinding of the telomeric substrate during the initial 2 min of unwinding (Fig. 7C). The telomeric substrate is unwound 10–30% within the first 2 min of unwinding compared with 60–80% with BLM alone. This effect of TEP1 on BLM helicase activity is lost when the reactions proceed beyond 2 min. Almost 70% of the substrate is unwound by 2.5 min, and after 5 min the unwinding activity of BLM in the presence of TEP1 is almost indistinguishable from that of BLM alone. Control experiments with Myc-tagged proteins purified from uninfected cells did not show an effect on BLM helicase activity (data not shown), suggesting that the effect of TEP1 on BLM unwinding function is not because of the presence of unknown endogenous proteins co-purified by the anti-Myc antibody. None of the three proteins alone was capable of unwinding either substrate (data not shown). A non-specific bacterial helicase UvrD was evaluated similarly to examine the specificity of these effects on BLM (Fig. 7, D–F). None of the three had any effect on UvrD helicase activity.
FIGURE 7.
Effects of TOPOIIα, HSP90, and TEP1on BLM helicase activity. BLM unwinds both telomeric and non-telomeric DNA substrates (Non-Tel Subs)in the absence of any other proteins. 30 nm TOPOIIα (A) or HSP90 (B) or TEP1 (C) was preincubated with the DNA substrates (2 nm) for 5 min followed by the addition of 30 nm BLM. Reactions were quenched at various times, and the products of these assays were analyzed as described under “Experimental Procedures.” Unwinding was determined by monitoring the formation of single-stranded oligos from duplexed substrates, and quantitation was done using the ImageQuant software. Product formed (% unwinding) was plotted against time to show the kinetics of reactions with each protein. The effects of TOPOIIα, HSP90, and TEP1 were also monitored in the presence of a nonspecific bacterial UvrD helicase (D–F).
DISCUSSION
BS cells exhibit a high incidence of chromosomal abnormalities, including elevated levels of homologous TAs. High frequencies of TAs are observed in metaphase spreads prepared from all types of BS cells, which suggests a role for BLM in telomere maintenance. TAs also suggest that telomeres from homologous chromosome arms potentially undergo recombination. Thus, BLM may be required to process telomere ends by facilitating T-loop formation or by resolving recombinant or entangled ends formed by exposed chromosome ends during replication. The association of BLM with TRF2 (18) suggests that BLM may be particularly important for telomere length and structure maintenance when recombination is up-regulated, such as in cells using ALT.
Data from our laboratory and others have suggested a role for BLM in telomere maintenance in cells using ALT (6, 7, 17, 19, 20). BLM interacts both physically and functionally with telomere-binding proteins TRF1 and TRF2, and these interactions modify the enzymatic activity of BLM (7). To further our understanding of a precise role for BLM in telomere maintenance in the absence of telomerase, we used double immunoprecipitation and MS to identify BLM·TRF2-associated proteins in ALT cells. TOPOIIα, TEP1, and HSP90 associate with BLM and TRF2 in cells using ALT (Figs. 1 and 2A) and are present within APBs in late S and G2 phases where new telomere synthesis most likely occurs (Figs. 4 and 5). BLM, TRF2, TOPOIIα, HSP90, and TRF1 are also components of a more specific complex that does not include TEP1 (Figs. 2, B and C). Taken together, our results demonstrate interactions of proteins that are unique and perhaps essential for telomere dynamics in immortalized cells lacking the telomere-replicating enzyme telomerase. We have not yet investigated the function of a fifth protein identified in these experiments, the spliceosome-associated protein 145, SF3B2. SF3B2 is a component of the splicing factor 3b protein complex (21). Future studies will determine whether SF3B2 interacts directly with BLM or regulates its unwinding activity.
The yeast homolog of BLM, Sgs1 (22–24), when mutated confers a hyper-recombination phenotype that may be reminiscent of the chromosome anomalies of BS cells. Sgs1 interacts in vivo with both topoisomerase IIα and topoisomerase IIIα, suggesting a conserved interaction between RecQ-like helicases and topoisomerases (25–27). DNA topoisomerases are nuclear enzymes necessary for topological interconversions of DNA during transcription, replication, and chromosome segregation during both meiosis and mitosis (28, 29); they may also influence telomere stability. In somatic and meiotic human cells, BLM associates with topoisomerase IIIα (30), but to date no such interaction between BLM and TOPOIIα has been reported. BLM co-localizes with topoisomerase IIIα in PML protein nuclear bodies, and this localization is disrupted in BS cells (30). The interaction of topoisomerase IIIα and BLM occurs in the N-terminal region of BLM and is important for maintaining genomic stability in human cells (31). Another recent study has suggested a role for topoisomerase IIIα in ALT (32), as a direct interaction between TOPOIIIα and BLM was demonstrated in cells using ALT as well as in in vitro experiments. The interaction of TOPOIIα with BLM in cells using ALT supports the idea that topoisomerases are critically involved in pathways of telomere length maintenance in the absence of telomerase, most likely during the resolution of entangled telomere ends. Telomeres co-purify with nuclear matrix at places where TOPOIIα is present (33), suggesting that TOPOIIα may be associated with telomeres and provide a mechanism for untangling chromosome ends. Additionally, telomere repeat sequences define a consensus sequence for TOPOIIα, which is catalytically active in cleaving telomere DNA repeats in vitro and associates with chromosomal telomeric DNA in vivo (34). Although in vitro helicase assays show that TOPOIIα slightly slows the kinetics of BLM unwinding of telomeric substrates initially (Fig. 7A), it has no effect on non-telomeric substrates at these concentrations. Unlike the telomeric substrate, TOPOIIα stimulates BLM unwinding of non-telomeric substrates at lower concentrations in vitro.5 At 30 nm, TOPOIIα slightly reduces the advantage of BLM in unwinding the telomere-like substrate initially, but the reduced helicase activity is still significantly higher than that with non-telomeric substrate (Fig. 7A). Hence, BLM maintains its preference for telomeric DNA over that of non-telomeric DNA at high concentrations. Although there is a subtle effect of TOPOIIα on BLM helicase activity with a 3′-overhang telomeric substrate, inhibition of RecQ helicase activity by a topoisomerase is not unusual. Topoisomerase I inhibits WRN helicase activity on a forked duplex substrate (35); this inhibition is attributed to TOPO I binding to DNA or inhibition of WRN ATPase activity by TOPO I. Thus, it would be interesting to test whether TOPOIIα has any effect on BLM unwinding activity with other types of telomeric DNA substrates, including those with telomeric D-loops. In contrast, HSP90 inhibits BLM unwinding of both telomeric and non-telomeric substrates.
Neither TOPOIIα nor HSP90 affect the activity of UvrD, another 3′-5′ helicase. This suggests that the effects of TOPOIIα and HSP90 are specific to the enzymatic activity of BLM and are not universally applicable to all helicases. The effects of TOPOIIα and HSP90 are specifically dependent on the helicase activity of BLM. When these experiments were repeated with a K795E BLM “helicase dead” mutant, unwinding of the DNA substrates was not observed (data not shown).
HSP90 is a chaperone protein required for efficient telomerase assembly in vitro and in vivo (36). HSP90 remains stably associated with the telomerase complex and is a critical component for telomere length maintenance in telomerase-positive cells (37). Here, we find that HSP90 is not only a component of BLM-associated complex but that it may be involved in telomere DNA synthesis in ALT cells, as it co-localizes with BLM and TRF2 specifically at sites of BrdUrd incorporation during late S and G2 phases of the cell cycle (Fig. 5). The precise role of HSP90 is unclear. It may be required to maintain the integrity of the associated proteins. As a component of the telomerase complex, HSP90 functions in the telomerase translocation step during telomere elongation, perhaps holding the complex together or assisting in a change of conformation to promote telomere lengthening (36). The high expression level of HSP90 in cells using ALT and its direct association with BLM suggests a novel role of the protein in assembly/stability of an ALT-specific complex. The strong inhibition of BLM helicase activity observed using both control and telomere-like DNA substrates in the presence of HSP90 (Fig. 7B) suggests that HSP90 may not be directly involved during the telomere unwinding stages of the ALT pathway but may act upstream of BLM-mediated telomere replication/recombination events.
Previous studies from our laboratory have shown that TRF2 stimulates BLM helicase activity on non-telomeric and telomeric substrates including a 3′ overhang and a telomeric D-loop structure in vitro (7). In contrast to TRF2, TRF1 inhibits BLM helicase activity on both types of telomeric substrates but not on non-telomeric substrates. Equimolar TRF1, TRF2, and BLM in vitro enhance telomere-like substrate unwinding (7). In vitro studies have also shown that TRF1 interacts directly with the helicase domain of BLM and may, therefore, represent an important mechanism to shut down helicase activity during invasion or in repackaging of the newly synthesized and disentangled telomeres of the homologous chromosomes during the ALT mechanism.
The precise role of TEP1 in the ALT pathway is uncertain. Although TEP1 interacts with BLM and TRF2, we were unable to detect it in the BLM-TRF1 immunoprecipitates (Fig. 2C) or to detect BLM in TEP1-TRF1 immunoprecipitate (data not shown). We have also carried out double immunoprecipitation using anti-BLM and anti-TEP1; TRF1 was still undetected in the complex (supplemental Fig. 2). Thus, TEP1 may not be a part of a complex that contains TRF1 and TRF2 together. We cannot exclude the possibility that the lack of TEP1 detection was due to antibody accessibility. We have repeated these experiments several times using antibodies from two different sources, each generated against a different epitopes of TEP1 (both the N and C terminus), but have obtained similar negative results. In vitro data suggest a strong interaction between BLM and TEP1 independent of DNA or RNA (Fig. 6). TEP1 also affects BLM helicase activity during the initial stages of unwinding of the telomeric DNA (Fig. 7C). DNA binding assays with TEP1 have determined that the slow unwinding of BLM in presence of TEP1 is a consequence of TEP1 binding to DNA. The binding assays show that TEP1 can effectively bind human telomere RNA and telomeric DNA substrate but not non-telomeric or control DNA (supplemental Fig. 3). TEP1 binding may cause a delay in efficient unwinding of the telomeric substrate by BLM. More studies are under way to understand the binding properties of TEP1 with different DNA and RNA substrates. Although TEP1 is associated with telomerase activity in human, mice, and rat immortalized cell extracts (38), its precise role in telomerase-positive cells is not clearly understood. Murine studies indicate that TEP1 is not essential for mouse telomere length maintenance (38). TEP1 is also a component of a large cytoplasmic ribonucleoprotein known as the vault particle (39). The association and co-localization of TEP1 with BLM and TRF2 suggest that TEP1 may have an important function in ALT pathways.
Future studies will determine the precise role of each protein in ALT pathways and their effects on the BLM helicase activities. How these proteins come together to carry out telomere elongation is unknown. Our work suggests that dynamic BLM-associated ALT complex proteins may be involved in facilitating ALT in the absence of telomerase in immortalized cells. Also, we cannot exclude the possibility that there are other proteins in this ALT-specific complex including one or more shelterin components. We identified the shelterin proteins POT1, RAP1, and TIN2 in the BLM-associated ALT-specific complex with TRF2 and TRF1 (data not shown) by immunoprecipitation and Western analysis but not in telomerase-positive cells. The presence of overlapping protein bands of similar molecular sizes in the gel most likely prevented their detection by MS. These proteins, including TRF1, TRF2, and TOPOIIα, may determine the structure of the uncapped telomere ends in ALT cells by facilitating the formation of T-loops and D-loops and subsequently promoting elongation of the telomeres.
Purified BLM is substantially more active in unwinding telomere-like DNA substrates than non-telomere-like substrates. This selectivity of BLM helicase activity in vitro may correlate with its function pertaining to telomeres in vivo. We have conducted a preliminary telomere restriction fragment (TRF) length assay to determine whether disruption of BLM expression alters telomere length in ALT cells (Figs. 8, A and B). We find that the telomere length attrition occurs rapidly in cells using ALT, at a rate of ∼750 bp/population doubling, which is almost 10 times faster than what would be expected by the DNA end replication problem. This may be due to replication fork stalling and breaks within the telomere DNA. In general, telomere DNA is difficult to replicate and replication forks within telomeres often stall (40). This may be more likely in ALT cells where the telomeres are much longer and thereby prone to replication fork stalling. BLM helicase may function at the stalled replication forks in addition to mediating ALT-specific recombination events. No significant effect on the TRF length was observed in the telomerase-positive cell line MCF7. BLM knockdown also reduces the co-localization of the BLM-associated proteins with TRF2 and APBs in ALT cells (Fig. 8C) but not their level of expression, as determined by Western analysis (Fig. 8D). No significant changes in the co-localization pattern of the BLM-associated proteins with TRF2 and APBs were observed in the telomerase-positive cell lines HeLa and MCF7 (data not shown). This may suggest a functional role of the BLM-associated proteins in the ALT pathway. Studies are currently under way to validate these observations using additional cell lines.
FIGURE 8.
BLM knockdown and TRF length analysis in cells using ALT (Saos2) or telomerase (MCF7) to maintain telomeres demonstrate the requirement for BLM in ALT. A, Saos2 and MCF7 cells were transfected with pBLMsiRNA and control pSCsiRNA (negative or untreated control) and cultured for 3 weeks in the presence of the selective antibiotic puromycin (6 ng/μl). Stably transfected clones were harvested and analyzed by Western analysis using rabbit anti-BLM (Bethyl Laboratories). B, genomic DNAs were isolated and analyzed by Southern blot. TRF lengths are decreased in average size in Saos2 cells after siRNA treatment against BLM, in comparison to the treated controls. This decrease was not observed in MCF7 cells treated similarly. Size markers are shown to the left. Kbp, kilobase pairs. C, BLM knockdown affects the co-localization of the BLM-associated proteins with APBs and TRF2 in ALT cells. Co-localization of TEP1, TOPOIIα, and HSP90 with APBs or TRF2 was monitored by immunofluorescence in the ALT cell line Saos2 transiently transfected with control or pBLMsiRNA as described under “Experimental Procedures.” Percent co-localization was determined as before (Fig. 3C). D, BLM knockdown does not affect the expression level of TEP1, HSP90, and TOPOIIα. Total cell lysates prepared from control or pBLMsiRNA-transfected Saos2 and MCF7 cell lines were resolved by 10% SDS-PAGE and Western-analyzed using anti-TEP1, anti-HSP90, and anti-TOPOIIα. α-Actin was used for the loading control.
Certainly, our data collectively suggest that the BLM helicase is critical to regulating telomere length in ALT cells. Further studies with the BLM-associated ALT-specific proteins will aid in understanding mechanisms that contribute to telomere maintenance.
Supplementary Material
Acknowledgments
We thank Dr. Arun Tiwari and Jeffrey Cotrill at the OSU Proteomics Core for help with generation of the mass spectrometry data, Drs. Carolyn Price and Rick Fishel for critical review and helpful discussions, Dr. Samir Acharya, Sara Javaid, Cathy Ebert, and Qi Wang for laboratory support. We also thank Drs. David Toft (Mayo Clinic, Rochester, MN), Tim Lohman (Washington University, St. Louis, MO), and Lea Harrington (Ontario Cancer Institute, Toronto, Canada) for providing pure UvrD helicase, HSP90, and TEP1 vectors, respectively.
This work was supported, in whole or in part, by National Institutes of Health Grant CA-117898. This work was also supported by The Bloom's Syndrome Foundation.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
Footnotes
The abbreviations used are: BS, bloom syndrome; PML, promyelocytic leukemia; APB, ALT-associated PML nuclear bodies; TEP1, telomerase-associated protein 1; HSP90, heat shock protein 90; TOPOIIα, topoisomerase IIα; BrdUrd, bromodeoxyuridine; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; TRF, telomere restriction fragment; TA, telomeric association; MS, mass spectroscopy; siRNA, small interfering RNA; PBS, phosphate-buffered saline; BSA, bovine serum albumin.
B. Russell and J. Groden, unpublished results.
References
- 1.German, J. (1995) Dermatol. Clin. 13 7-18 [PubMed] [Google Scholar]
- 2.German, J., and Crippa, L. P. (1966) Ann. Genet. 9 143-154 [Google Scholar]
- 3.Lundblad, V., and Blackburn, E. H. (1993) Cell 73 347-360 [DOI] [PubMed] [Google Scholar]
- 4.Bryan, T. M., Englezou, A., Gupta, J., Bacchetti, S., and Reddel, R. R. (1995) EMBO J. 14 4240-4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunham, M. A., Neumann, A. A., Fasching, C. L., and Reddel, R. R. (2000) Nat. Genet. 26 447-450 [DOI] [PubMed] [Google Scholar]
- 6.Stavropoulos, D. J., Bradshaw, P. S., Li, X., Pasic, I., Truong, K., Ikura, M., Ungrin, M., and Meyn, M. S. (2002) Hum. Mol. Genet. 11 3135-3144 [DOI] [PubMed] [Google Scholar]
- 7.Lillard-Wetherell, K., Machwe, A., Langland, G. T., Combs, K. A., Behbehani, G. K., Schonberg, S. A., German, J., Turchi, J. J., Orren, D. K., and Groden, J. (2004) Hum. Mol. Genet. 13 1919-1932 [DOI] [PubMed] [Google Scholar]
- 8.Opresko, P. L., Mason, P. A., Podell, E. R., Lei, M., Hickson, I. D., Cech, T. R., and Bohr, V. A. (2005) J. Biol. Chem. 280 32069-32080 [DOI] [PubMed] [Google Scholar]
- 9.Yeager, T. R., Neumann, A. A., Englezou, A., Huschtscha, L. I., Noble, J. R., and Reddel, R. R. (1999) Cancer Res. 59 4175-4179 [PubMed] [Google Scholar]
- 10.Grobelny, J. V., Godwin, A. K., and Broccoli, D. (2000) J. Cell Sci. 113 4577-4585 [DOI] [PubMed] [Google Scholar]
- 11.Wu, G., Lee, W. H., and Chen, P. L. (2000) J. Biol. Chem. 275 30618-30622 [DOI] [PubMed] [Google Scholar]
- 12.Harrington, L., McPhail, T., Mar, V., Zhou, W., Oulton, R., Bass, M. B., Arruda, I., and Robinson, M. O. (1997) Science 275 973-977 [DOI] [PubMed] [Google Scholar]
- 13.Dutertre, S., Ababou, M., Onclercq, R., Delic, J., Chatton, B., Jaulin, C., and Amor-Gueret, M. (2000) Oncogene 19 2731-2738 [DOI] [PubMed] [Google Scholar]
- 14.Karow, J. K., Constantinou, A., Li, J. L., West, S. C., and Hickson, I. D. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 6504-6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohaghegh, P., Karow, J. K., Brosh, R. M., Jr., Bohr, V. A., and Hickson, I. D. (2001) Nucleic Acids Res. 29 2843-2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchitto, A., and Pichierri, P. (2002) J. Cell Biol. 157 19-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lillard-Wetherell, K., Combs, K. A., and Groden, J. (2005) Cancer Res. 65 5520-5522 [DOI] [PubMed] [Google Scholar]
- 18.Zhu, X. D., Kuster, B., Mann, M., Petrini, J. H., and Lange, T. (2000) Nat. Genet. 25 347-352 [DOI] [PubMed] [Google Scholar]
- 19.Li, J. L., Harrison, R. J., Reszka, A. P., Brosh, R. M., Jr., Bohr, V. A., Neidle, S., and Hickson, I. D. (2001) Biochemistry 40 15194-15202 [DOI] [PubMed] [Google Scholar]
- 20.Opresko, P. L., von Kobbe, C., Laine, J. P., Harrigan, J., Hickson, I. D., and Bohr, V. A. (2002) J. Biol. Chem. 277 41110-41119 [DOI] [PubMed] [Google Scholar]
- 21.Igel, H., Wells, S., Perriman, R., and Ares, M., Jr. (1998) RNA 4 1-10 [PMC free article] [PubMed] [Google Scholar]
- 22.Watt, P. M., Hickson, I. D., Borts, R. H., and Louis, E. J. (1996) Genetics 144 935-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamagata, K., Kato, J., Shimamoto, A., Goto, M., Furuichi, Y., and Ikeda, H. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 8733-8738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myung, K., Datta, A., Chen, C., and Kolodner, R. D. (2001) Nat. Genet. 27 113-116 [DOI] [PubMed] [Google Scholar]
- 25.Shor, E., Gangloff, S., Wagner, M., Weinstein, J., Price, G., and Rothstein, R. (2002) Genetics 162 647-662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ui, A., Satoh, Y., Onoda, F., Miyajima, A., Seki, M., and Enomoto, T. (2001) Mol. Genet. Genomics 265 837-850 [DOI] [PubMed] [Google Scholar]
- 27.Ui, A., Seki, M., Ogiwara, H., Onodera, R., Fukushige, S., Onoda, F., and Enomoto, T. (2005) DNA Repair 4 191-201 [DOI] [PubMed] [Google Scholar]
- 28.Wang, J. C. (1996) Annu. Rev. Biochem. 65 635-692 [DOI] [PubMed] [Google Scholar]
- 29.Champoux, J. J. (2001) Annu. Rev. Biochem. 70 369-413 [DOI] [PubMed] [Google Scholar]
- 30.Johnson, F. B., Lombard, D. B., Neff, N. F., Mastrangelo, M. A., Dewolf, W., Ellis, N. A., Marciniak, R. A., Yin, Y., Jaenisch, R., and Guarente, L. (2000) Cancer Res. 60, 1162-1167 [PubMed] [Google Scholar]
- 31.Wu, L., Davies, S. L., North, P. S., Goulaouie, H., Riou, J. F., Turley, H., Gatter, K. C., and Hickson, I. D. (2000) J. Biol. Chem. 275 9636-9644 [DOI] [PubMed] [Google Scholar]
- 32.Temime-Smaali, N., Guittat, L., Wenner, T., Bayart, E., Douarre, C., Gomez, D., Giraud-Panis, M., Londono-Vallejo, A., Gilson, E., Amor-Gueret, M., and Riou, J. F. (2008) EMBO J. 27 1523-1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lange, T. (1992) EMBO J. 11 717-724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon, H. J., Choi, I. Y., Kang, M. R., Kim, S. S., Muller, M. T., Spitzner, J. R., and Chung, I. K. (1998) Biochim Biophys Acta 1395 110-120 [DOI] [PubMed] [Google Scholar]
- 35.Laine, J.-P., Opresko, P. L., Indig, F. E., Harrigan, J. A., von Kobbe, C., and Vohr, V. A. (2003) Cancer Res. 63 7136-7146 [PubMed] [Google Scholar]
- 36.Holt, S. E., Aisner, D. L., Baur, J., Tesmer, V. M., Dy, M., Ouellette, M., Trager, J. B., Morin, G. B., Toft, D. O., Shay, J. W., Wright, W. E., and While, M. A. (1999) Genes Dev. 13 817-826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forsythe, H. L., Jarvis, J. L., Turner, J. W., Elmore, L. W., and Holt, S. E. (2001) J. Biol. Chem. 276 15571-15574 [DOI] [PubMed] [Google Scholar]
- 38.Liu, Y., Snow, B. E., Hande, M. P., Baerlocher, G., Kickhoefer, V. A., Yeung, D., Wakeham, A., Itie, A., Siderovski, D. P., Landorp, P. M., Robinson, M. O., and Harrington, L. (2000) Mol. Cell. Biol. 20 8178-8184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kickhoefer, V. A., Stephen, A. G., Harrington, L., Robinson, M. O., and Rome, L. H. (1999) J. Biol. Chem. 274 32712-32717 [DOI] [PubMed] [Google Scholar]
- 40.Ohki, R., and Ishikawa, F. (2004) Nucleic Acids Res. 32 1627-1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.