Abstract
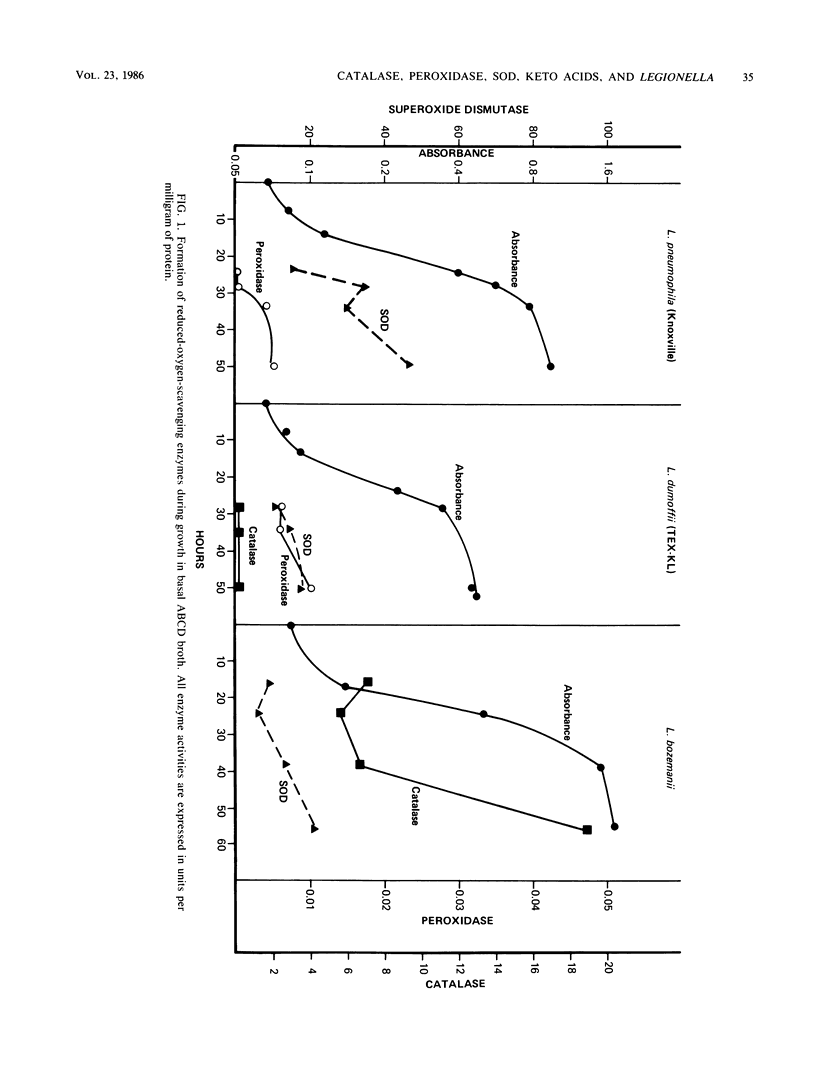
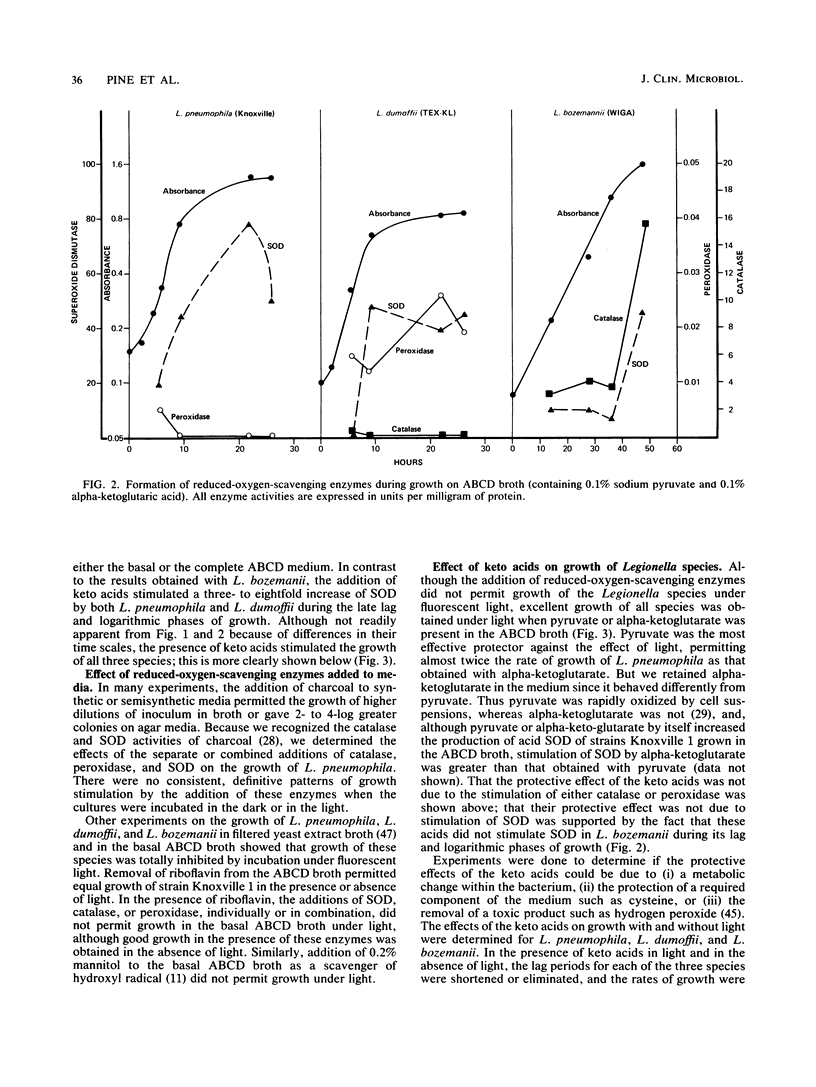
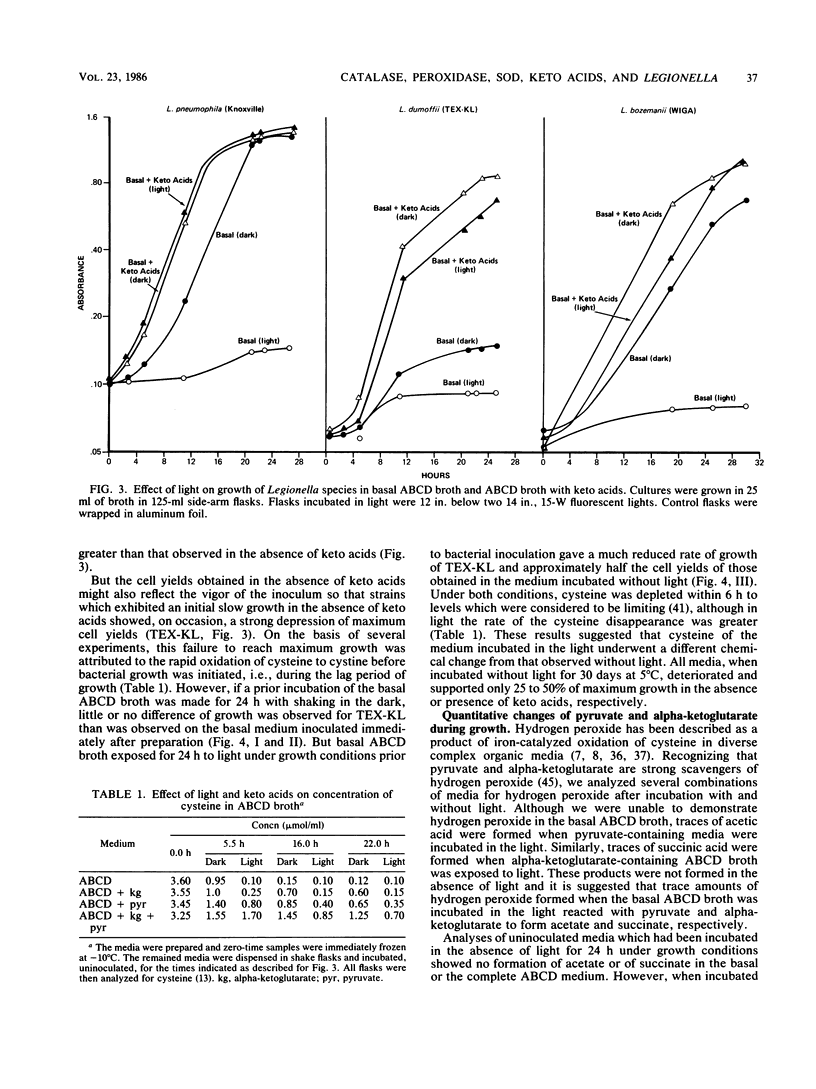
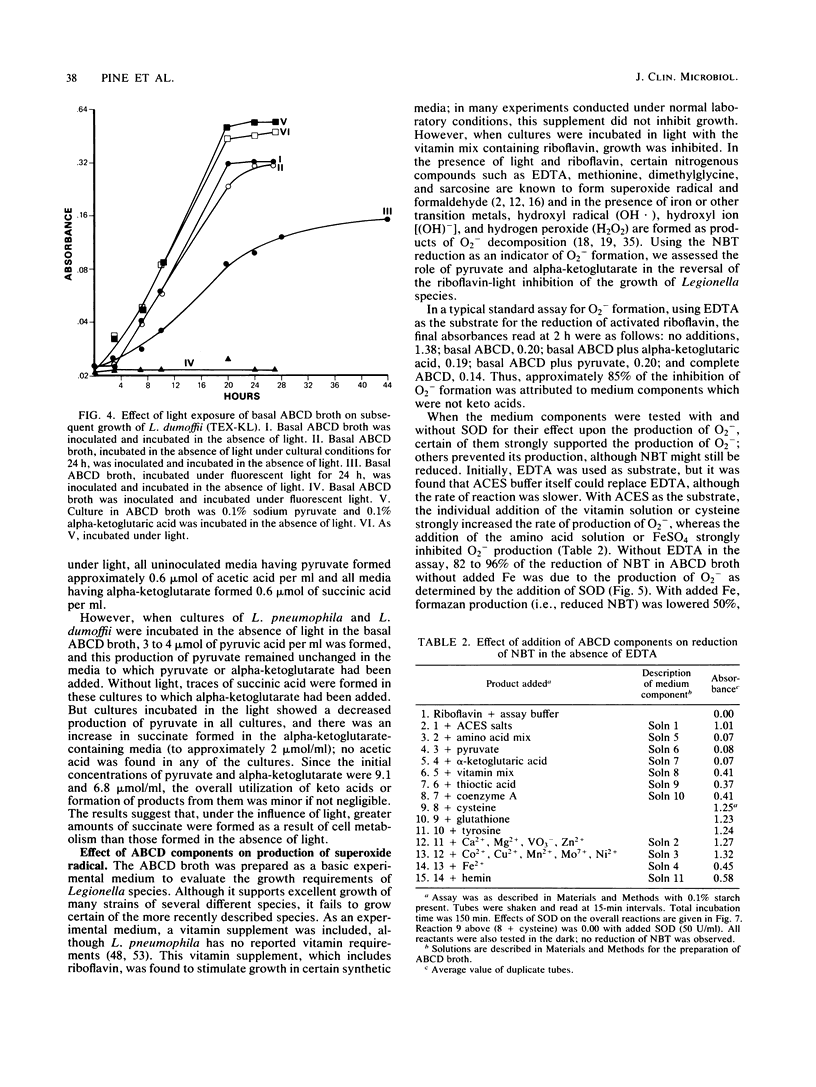
Keto acids and reduced-oxygen-scavenging enzymes were examined for their roles in supporting the growth of Legionella species and for their potential reactions between the chemical components of the media. When grown in an experimental ACES (2-[(2-amino-2-oxoethyl)-amino] ethanesulfonic acid)-buffered chemically defined (ABCD) broth, the presence of keto acids shortened the lag periods, increased the rates of growth, and gave maximum cell yields. In addition, keto acids affected the specific activities of reduced-oxygen-scavenging enzymes determined during growth. The specific activities of superoxide dismutase of Legionella pneumophila (Knoxville) and L. dumoffii (TEX-KL) were increased three- to eightfold, while that of L. bozemanii (WIGA) was not affected. All strains appeared to be equally sensitive to the effects of superoxide anion (O2-) generated by light-activated riboflavin, and all were equally protected by the presence of keto acids in the ABCD broth. Production of trace amounts of acetate and succinate in pyruvate- and alpha-ketoglutarate-containing media exposed to light suggested that hydrogen peroxide was formed. Pyruvate and alpha-ketoglutarate were products of growth on amino acids, and there was no quantitative evidence that these keto acids were metabolized when they were added to the medium. The rate of cysteine oxidation in ABCD broth was increased by the presence of ferric ion or by exposure to light or by both, and keto acids reduced the rate of this oxidation. ACES buffer was a substrate for the production of O2- in the presence of light, and the combined addition of Fe2+ ions, cysteine, and either keto acid to the medium strongly inhibited the production of O2-. Thus, keto acids inhibited the rate of cysteine oxidation, they stimulated rapid growth by an unknown process, and, in combination with added Fe2+ ions and cysteine, they reversed the toxic effects of light by inhibiting O2- production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Ballou D., Palmer G., Massey V. Direct demonstration of superoxide anion production during the oxidation of reduced flavin and of its catalytic decomposition by erythrocuprein. Biochem Biophys Res Commun. 1969 Sep 10;36(6):898–904. doi: 10.1016/0006-291x(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Butler J., Halliwell B. Reaction of iron-EDTA chelates with the superoxide radical. Arch Biochem Biophys. 1982 Oct 1;218(1):174–178. doi: 10.1016/0003-9861(82)90333-2. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Granberg G. P., Nyberg G. K., Edlund M. B. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl Environ Microbiol. 1979 Mar;37(3):383–390. doi: 10.1128/aem.37.3.383-390.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Nyberg G., Wrethén J. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl Environ Microbiol. 1978 Aug;36(2):223–229. doi: 10.1128/aem.36.2.223-229.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Wrethén J., Beckman G. Superoxide dismutase in Bacteroides fragilis and related Bacteroides species. J Clin Microbiol. 1977 Sep;6(3):280–284. doi: 10.1128/jcm.6.3.280-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini D., De Marco C., Dupré S. Luminol chemiluminescence studies of the oxidation of cysteine and other thiols to disulfides. Arch Biochem Biophys. 1968 Mar 20;124(1):18–26. doi: 10.1016/0003-9861(68)90299-3. [DOI] [PubMed] [Google Scholar]
- FRISELL W. R., CHUNG C. W., MACKENZIE C. G. Catalysis of oxidation of nitrogen compounds by flavin coenzymes in the presence of light. J Biol Chem. 1959 May;234(5):1297–1302. [PubMed] [Google Scholar]
- Feeley J. C., Gorman G. W., Weaver R. E., Mackel D. C., Smith H. W. Primary isolation media for Legionnaires disease bacterium. J Clin Microbiol. 1978 Sep;8(3):320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers R. S., Martin S. E., Brewer D. G., Ordal Z. J. Catalase and enumeration of stressed Staphylococcus aureus cells. Appl Environ Microbiol. 1977 May;33(5):1112–1117. doi: 10.1128/aem.33.5.1112-1117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fanning D. D. Effect of heme on Bacteroides distasonis catalase and aerotolerance. J Bacteriol. 1983 Dec;156(3):1012–1018. doi: 10.1128/jb.156.3.1012-1018.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen metabolism in Lactobacillus plantarum. J Bacteriol. 1974 Jan;117(1):166–169. doi: 10.1128/jb.117.1.166-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of iron in oxygen radical reactions. Methods Enzymol. 1984;105:47–56. doi: 10.1016/s0076-6879(84)05007-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B. The superoxide dismutase activity of iron complexes. FEBS Lett. 1975 Aug 1;56(1):34–38. doi: 10.1016/0014-5793(75)80105-0. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Hoffman P. S., George H. A., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979 Jan;25(1):8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. I. Physiological aspects of enhanced aerotolerance. Can J Microbiol. 1979 Jan;25(1):1–7. doi: 10.1139/m79-001. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Pine L., Bell S. Production of superoxide and hydrogen peroxide in medium used to culture Legionella pneumophila: catalytic decomposition by charcoal. Appl Environ Microbiol. 1983 Mar;45(3):784–791. doi: 10.1128/aem.45.3.784-791.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner J. E., Friedman R. L., Bigley R. H., Iglewski B. H. Effect of oxygen-dependent antimicrobial systems on Legionella pneumophila. Infect Immun. 1983 Jan;39(1):487–489. doi: 10.1128/iai.39.1.487-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Jacobs R. F., Wilson C. B., Weaver W. M., Klebanoff S. J. Susceptibility of Legionella pneumophila to oxygen-dependent microbicidal systems. J Immunol. 1982 Nov;129(5):2192–2197. [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Chignell C. F. Free radicals in pharmacology and toxicology--selected topics. Pharmacol Rev. 1981 Dec;33(4):189–211. [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Norrod E. P., Morse S. A. Presence of hydrogen peroxide in media used for cultivation of Neisseria gonorrhoeae. J Clin Microbiol. 1982 Jan;15(1):103–108. doi: 10.1128/jcm.15.1.103-108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg G. K., Granberg G. P., Carlsson J. Bovine superoxide dismutase and copper ions potentiate the bactericidal effect of autoxidizing cysteine. Appl Environ Microbiol. 1979 Jul;38(1):29–34. doi: 10.1128/aem.38.1.29-34.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasculle A. W., Feeley J. C., Gibson R. J., Cordes L. G., Myerowitz R. L., Patton C. M., Gorman G. W., Carmack C. L., Ezzell J. W., Dowling J. N. Pittsburgh pneumonia agent: direct isolation from human lung tissue. J Infect Dis. 1980 Jun;141(6):727–732. doi: 10.1093/infdis/141.6.727. [DOI] [PubMed] [Google Scholar]
- Picker S. D., Fridovich I. On the mechanism of production of superoxide radical by reaction mixtures containing NADH, phenazine methosulfate, and nitroblue tetrazolium. Arch Biochem Biophys. 1984 Jan;228(1):155–158. doi: 10.1016/0003-9861(84)90056-0. [DOI] [PubMed] [Google Scholar]
- Pine L., Hoffman P. S., Malcolm G. B., Benson R. F., Gorman G. W. Whole-cell peroxidase test for identification of Legionella pneumophila. J Clin Microbiol. 1984 Feb;19(2):286–290. doi: 10.1128/jcm.19.2.286-290.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Hoffman P. S., Malcolm G. B., Benson R. F., Keen M. G. Determination of catalase, peroxidase, and superoxide dismutase within the genus Legionella. J Clin Microbiol. 1984 Sep;20(3):421–429. doi: 10.1128/jcm.20.3.421-429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle C. T., Gregory E. M. Superoxide dismutase and O2 lethality in Bacteroides fragilis. J Bacteriol. 1979 Apr;138(1):139–145. doi: 10.1128/jb.138.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. W., Pine L., Hutner S. H., George J. R., Harrell W. K. Metal requirements of Legionella pneumophila. J Clin Microbiol. 1981 Apr;13(4):688–695. doi: 10.1128/jcm.13.4.688-695.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Allen R. G. Liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1980 Jan;11(1):19–21. doi: 10.1128/jcm.11.1.19-21.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Gowda S. Chemically defined medium for Legionella pneumophila growth. J Clin Microbiol. 1981 Jan;13(1):115–119. doi: 10.1128/jcm.13.1.115-119.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Goldin B. R., Jacobus N. V., Gorbach S. L. Superoxide dismutase in anaerobic bacteria of clinical significance. Infect Immun. 1977 Apr;16(1):20–25. doi: 10.1128/iai.16.1.20-25.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. E., Yan J. F., Wang J. L. The iron(3)-catalyzed oxidation of cysteine by molecular oxygen in the aqueous phase. An example of a two-thirds-order reaction. J Am Chem Soc. 1966 Apr 20;88(8):1663–1667. doi: 10.1021/ja00960a016. [DOI] [PubMed] [Google Scholar]
- Tesh M. J., Morse S. A., Miller R. D. Intermediary metabolism in Legionella pneumophila: utilization of amino acids and other compounds as energy sources. J Bacteriol. 1983 Jun;154(3):1104–1109. doi: 10.1128/jb.154.3.1104-1109.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W. J., Miller R. D. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J Clin Microbiol. 1979 Jul;10(1):50–55. doi: 10.1128/jcm.10.1.50-55.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth P. M. The action of light on culture media. J Clin Pathol. 1969 May;22(3):273–277. doi: 10.1136/jcp.22.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R. B., Lorenz J. R. Toxicity of irradiated medium for repair-deficient strains of Escherichia coli. J Bacteriol. 1972 Oct;112(1):649–652. doi: 10.1128/jb.112.1.649-652.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Westfall H. N. Substrate utilization by Legionella cells after cryopreservation in phosphate buffer. Appl Environ Microbiol. 1984 Aug;48(2):380–385. doi: 10.1128/aem.48.2.380-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Hawkins R. E., Brian M., Carrell R. W. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975 Feb;85(2):337–341. [PubMed] [Google Scholar]


