Abstract
We investigated the role of G protein-coupled receptor kinase (GRK)-mediated phosphorylation in agonist-induced desensitization, arrestin association, endocytosis, and intracellular trafficking of the D2 dopamine receptor (DAR). Agonist activation of D2 DARs results in rapid and sustained receptor phosphorylation that is solely mediated by GRKs. A survey of GRKs revealed that only GRK2 or GRK3 promotes D2 DAR phosphorylation. Mutational analyses resulted in the identification of eight serine/threonine residues within the third cytoplasmic loop of the receptor that are phosphorylated by GRK2/3. Simultaneous mutation of these eight residues results in a receptor construct, GRK(-), that is completely devoid of agonist-promoted GRK-mediated receptor phosphorylation. We found that both wild-type (WT) and GRK(-) receptors underwent a similar degree of agonist-induced desensitization as assessed using [35S]GTPγS binding assays. Similarly, both receptor constructs internalized to the same extent in response to agonist treatment. Furthermore, using bioluminescence resonance energy transfer assays to directly assess receptor association with arrestin3, we found no differences between the WT and GRK(-) receptors. Thus, phosphorylation is not required for arrestin-receptor association or agonist-induced desensitization or internalization. In contrast, when we examined recycling of the D2 DARs to the cell surface, subsequent to agonist-induced endocytosis, the GRK(-) construct exhibited less recycling in comparison with the WT receptor. This impairment appears to be due to a greater propensity of the GRK(-) receptors to down-regulate once internalized. In contrast, if the receptor is highly phosphorylated, then receptor recycling is promoted. These results reveal a novel role for GRK-mediated phosphorylation in regulating the post-endocytic trafficking of a G protein-coupled receptor.
Dopamine receptors (DARs)3 are members of the GPCR superfamily and consist of five structurally distinct subtypes (1, 2). These can be divided into two subfamilies on the basis of their structure and pharmacological and transductional properties (3). The “D1-like” subfamily includes the D1 and D5 receptors, which couple to the heterotrimeric G proteins GS or GOLF to stimulate adenylyl cyclase activity and raise intracellular levels of cAMP. The D2-like subfamily includes the D2, D3, and D4 receptors, which couple to inhibitory Gi/o proteins to reduce adenylyl cyclase activity as well as modulate voltage-gated K+ or Ca2+ channels. Within the central nervous system, these receptors modulate movement, learning and memory, reward and addiction, cognition, and certain neurendocrine functions. As with other GPCRs, the DARs are subject to a wide variety of regulatory mechanisms, which can either positively or negatively modulate their expression and functional activity (4).
Upon agonist activation, most GPCRs undergo desensitization, a homeostatic process that results in a waning of receptor response despite continued agonist stimulation (5, 6). Desensitization is believed to involve the phosphorylation of receptors by either G protein-coupled receptor kinases (GRKs) and/or second messenger-activated kinases such as PKA or PKC. Homologous forms of desensitization involve only agonist-activated receptors and appear to be primarily mediated by GRKs. In many cases, GRK-mediated phosphorylation has been shown to decrease receptor/G protein interactions and also initiate arrestin binding, which further promotes endocytosis of the receptor through clathrin-coated pits (7–9). Once internalized, GPCRs can engage additional signaling pathways (10), be sorted for recycling to the plasma membrane, or targeted for degradation (7–9). Among the DARs, the D2 receptor is arguably one of the most validated drug targets in neurology and psychiatry. For instance, all receptor-based anti-parkinsonian drugs work via stimulating the D2 DAR (11), whereas all Food and Drug Administration-approved antipsychotic agents are antagonists of this receptor subtype (12, 13). The D2 DAR is also therapeutically targeted in other disorders such as restless legs syndrome, tardive dyskinesia, Tourette syndrome, and hyperprolactinemia. As such, more knowledge concerning the regulation of the D2 DAR could be helpful in improving current therapies or devising new treatment strategies.
In comparison with other GPCRs, however, detailed mechanistic information concerning regulation of the D2 DAR is mostly lacking, although some progress has recently been made. For instance, we (14) and others (15) have found that PKC-mediated phosphorylation can regulate both D2 receptor desensitization and trafficking. In our PKC study, we mapped out all of the PKC phosphorylation sites within the third intracellular loop (IC3) of the receptor, and we determined the existence of two PKC phosphorylation domains. Both of these domains were found to regulate receptor sequestration, whereas only one domain regulated functional uncoupling in response to PKC activation (14). In response to agonist activation, the D2 DAR has also been shown to undergo functional desensitization (4), although this has not been intensively investigated. More thoroughly examined is the observation that agonist stimulation of the D2 DAR promotes its sequestration from the cell surface into vesicular compartments that appear distinct from those harboring internalized D1 DARs or β-adrenergic receptors (16–21). In addition to uncertainty over the endocytic pathway involved, controversy also exists as to whether or not D2 DAR internalization is dynamin-dependent and whether the internalized receptors can partially or completely recycle to the cell surface or, alternatively, if they undergo degradation (19, 21–24). The D2 DAR does appear to undergo GRK-mediated phosphorylation upon agonist activation, which has been suggested to promote arrestin association and receptor sequestration (16, 19, 25), although this process has not been studied in detail and its relationship to functional receptor desensitization is unknown.
In this study, we have further characterized GRK-mediated phosphorylation of the D2 DAR and determined its role in agonist-induced receptor desensitization, internalization, and recycling. Using site-directed mutagenesis, we have mapped out all of the GRK phosphorylation sites within the D2 DAR and determined that these are distinct from the PKC phosphorylation sites. Using a GRK phosphorylation-null mutant receptor, we found, surprisingly, that GRK-mediated phosphorylation is not actually required for agonist-induced receptor desensitization, arrestin association, or internalization. In contrast, we found that the GRK phosphorylation-null receptor was impaired in its ability to recycle to the cell surface subsequent to internalization and was degraded to a greater extent in comparison with the wild-type receptor. These results suggest that GRK-mediated phosphorylation of the D2 DAR regulates its intracellular trafficking or sorting once internalized, a novel mechanism for GRK-mediated regulation of GPCR function.
EXPERIMENTAL PROCEDURES
Materials—HEK293-tsa201 (HEK293T) cells were a gift of Dr. Vanitha Ramakrishnan. [3H]Sulpiride (69–77.7 Ci/mmol), [3H]methylspiperone (80–85.5 Ci/mmol), [32P]orthophosphate (carrier-free), and [3H]cAMP (25–40 Ci/mmol) were purchased from PerkinElmer Life Sciences. Nonradioactive cAMP was from Diagnostic Products Corp. (Los Angeles, CA). Dulbecco's modified Eagle's medium (DMEM) was from Cellgro® Mediatech, Inc. (Herndon, VA). Fetal calf serum and other cell culture reagents were purchased from Invitrogen. Calcium-phosphate transfection kits were from Clontech. MiniComplete™ protease inhibitor mixture was purchased from Roche Applied Science. Site-directed mutagenesis kits were obtained from Stratagene (La Jolla, CA). Anti-FLAG M2 affinity gel and all other reagents were purchased from Sigma. Anti-GRK antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Plasmids and Mutagenesis—For receptor phosphorylation assays, an amino terminus FLAG-tagged rat D2L DAR (pSF-D2L) was used (14). For other studies a nontagged rat D2L in pcDNA was used. For BRET assays, amino-terminally FLAG-tagged rat D2LDAR WT and the GRK null mutant (GRK(-)) were fused at their carboxyl termini to a full-length RLuc8 (26) subcloned into pcDNA3.1(+). mVenus was amplified by PCR using sense and antisense primers containing unique NheI and BspEI restriction sites, respectively, and cloned in-frame into bovine β-arrestin2 (arrestin3) N1-Zeocin vector, kindly provided by H. Paris (INSERM, Toulouse, France). Site-directed mutagenesis was performed using the QuikChange® XL kit from Stratagene (La Jolla, CA). Single or multiple point mutations were created to replace serine residues by alanines or threonine residues by valines. All constructs were verified by DNA sequencing prior to use. Constructs for GRKs (GRK2, GRK3, GRK5, and GRK6), arrestin2, arrestin3, and dynamin K44A were a kind gift from Dr. Jeffrey L. Benovic. The GRK4 construct was a gift from Dr. Richard Premont.
Cell Culture and Transfections—HEK293T cells were cultured in DMEM supplemented with 10% fetal calf serum, 1 mm sodium pyruvate, 50 units/ml penicillin, 50 μg/ml streptomycin. Cells were grown at 37 °C in 5% CO2 and 90% humidity. HEK293T cells were transfected using the calcium-phosphate precipitation method (Clontech). Cells were seeded in 100- or 150-mm plates, and transfection was carried out at ∼50% confluency according to the manufacturer's instructions. After 18 h of transfection, the media were replaced, and the cells were aliquoted for subsequent experiments. Dopamine treatments always included an antioxidant, 0.2 mm sodium metabisulfite.
Whole Cell Phosphorylation Assays—Metabolic labeling of cells and subsequent immunoprecipitation of the D2L DAR was carried out as described previously (14). Briefly, HEK293T cells were transfected with pSF-D2L using the calcium-phosphate method. One day after transfection, cells were seeded at 1–1.5 × 106 per well of a poly-d-lysine-coated 6-well plate for phosphorylation assays and ∼2 × 106 cells in a 100-mm dish for radioligand binding assays to quantify the level of receptor expression. The next day, the cells were washed once with Earle's balanced salt solution (EBSS) and incubated for 1 h in phosphate-free DMEM with 10% fetal calf serum. Media were removed and replaced with 1 ml of fresh media supplemented with 200 μCi/ml [32P]H3PO4. After 45 min at 37 °C, the cells were then challenged with 10 μm DA or other agents in media supplemented with 0.2 mm sodium metabisulfite. Cells were then transferred to ice, washed twice with ice-cold EBSS, and solubilized for 1 h at 4 °C in 1 ml of solubilization buffer (50 mm HEPES, 1 mm EDTA, 10% glycerol, 1% Triton X-100, pH 7.4, 4 °C) + 150 mm NaCl supplemented with Complete protease inhibitor mixture and phosphatase inhibitors (40 mm sodium pyrophosphate, 50 mm NaF). The samples were cleared by centrifugation in a Microfuge, and the protein concentration was determined by bicinchoninic acid protein assay (Pierce). The level of D2 DAR expression for each transfection was quantified via radioligand binding assays using the cells from the same transfection. After receptor/protein quantification, equal amounts of receptor protein were then transferred to fresh tubes with 40 μl of washed M2-agarose and incubated overnight with mixing at 4 °C. The samples were then washed once with solubilization buffer and 500 mm NaCl, once with solubilization buffer and 150 mm NaCl, and once with Tris-EDTA, pH 7.4, at 4 °C. Samples were then incubated 2× SDS-PAGE loading buffer for 1 h at 37 °C before being resolved by 4–20% Tris-glycine SDS-PAGE. The gels were dried and subjected to auto-radiography. After developing, the band intensities were quantitated by LabWorks™ software (UVP Inc., Upland, CA).
Intact Cell [3H]Sulpiride Binding Assays—HEK293T cells expressing rat D2L DAR were seeded 1 day after transfection at a density of 2 × 105 cells/well in poly-d-lysine-coated 24-well plates. The following day, cells were incubated in the presence of either 0.2 mm sodium metabisulfite (control) or 0.2 mm sodium metabisulfite plus 10 μm dopamine in DMEM-H (DMEM with 20 mm HEPES) for 1 h. For recycling experiments, after stimulating the cells with dopamine for 1 h, the cells were washed three times with ice-cold EBSS and then incubated with pre-warmed DMEM-H for 1 h at 37 °C. Stimulation or recovery was terminated by quickly cooling the plates on ice and washing the cells three times with ice-cold EBSS. Cells were then incubated with 0.5 ml of [3H]sulpiride in EBSS (final concentration, 6.4 nm) at 4 °C for 3 h, 30 min. Nonspecific binding was determined in the presence of 5 μm (+)-butaclamol. Cells were washed three times with ice-cold EBSS, and ∼0.5 ml of 1% Triton X-100, 5 mm EDTA was added. Samples were mixed with 4.5 ml of liquid scintillation mixture and counted with a Beckman LS6500 scintillation counter.
Membrane [3H]Methylspiperone Binding Assays—HEK293T cells were harvested by incubation with 5 mm EDTA in EBSS (without Ca2+ and Mg2+) and collected by centrifugation at 300 × g for 10 min. The cells were resuspended in lysis buffer (5 mm Tris, pH 7.4, at 4 °C; 5 mm MgCl2) and were disrupted using a Dounce homogenizer followed by centrifugation at 34,000 × g for 30 min. The resulting membrane pellet was resuspended in binding buffer (50 mm Tris, pH 7.4) by homogenization. The membrane suspension was then added to assay tubes containing [3H]methylspiperone in a final volume of 1.0 ml. (+)-Butaclamol was added at a final concentration of 3 μm to determine nonspecific binding. The assay tubes were incubated at room temperature for 1.5 h, and the reaction was terminated by rapid filtration through GF/C filters pretreated with 0.3% polythyleneimine. Radioactivity bound to the filters was quantitated by liquid scintillation spectroscopy.
Determination of cAMP Production—HEK293T cells were seeded into 24-well plates 1 day before the assay at a density of 2 × 105 cells per well. The cells were washed once with prewarmed EBSS, and then the cells were further incubated with various concentrations of dopamine in a total volume of 0.4 ml at 37 °C for 10 min in the presence of 3 μm forskolin, 30 μm Ro-20-1724 (phosphodiesterase inhibitor), 0.2 mm sodium metabisulfite (to prevent oxidation of dopamine), and 10 μm propranolol (to block endogenous β-adrenergic receptors) in 20 mm HEPES-buffered DMEM. To terminate the reaction, the supernatant was aspirated, and 3% perchloric acid (200 μl/well) was added. 80 μl of 15% KHCO3 was then added to neutralize the acid. The plates remained on ice for an additional 10 min and were then centrifuged at 1,300 × g for 20 min. The accumulation of cAMP was measured by a competitive binding assay described previously (27) with modifications. Briefly, 50 μl of the supernatant from each well was transferred to a 1.2-ml reaction tube containing 50 μl of cAMP-binding protein (PKA lysate prepared from bovine adrenals), 50 μl of [3H]cAMP, and 150 μl of Tris-EDTA buffer. The reaction was incubated for at least 90 min at 4 °C. After the incubation, 250 μl of charcoal solution (2% carbon, 0.5% bovine serum albumin) was added to each tube and vortexed gently. Tubes were then incubated at 4 °C for 10 min followed by centrifugation (1,300 × g) for 20 min. Radioactivity in the supernatant was then quantified by liquid scintillation spectroscopy at a counting efficiency of 58%. The cAMP concentrations were determined using a standard curve from 0.1 to 10 pmol of cAMP.
[35S]GTPγS Binding Assays—The [35S]GTPγS binding assays were carried out as described by Gardner et al. (28) with modifications. Briefly, HEK293T cells cultured in 100-mm dishes were washed twice with ice-cold membrane preparation (MP) buffer (50 mm Tris, 10 mm sodium pyrophosphate, 10 mm NaF, 5 mm EDTA, pH 7.4). The MP buffer was removed and replaced with a further 8 ml of MP buffer. The cells were then scraped and homogenized with a Dounce homogenizer followed by centrifugation at 34,000 × g for 30 min. After removing the supernatant, 10 ml of ice-cold HEPES buffer (20 mm HEPES, 6 mm MgCl2, and 100 mm NaCl, pH 7.4) was added, and the pellet was again centrifuged at 34,000 × g for 30 min at 4 °C. The resulting pellet was stored at -80 °C until used. The frozen membrane pellet was thawed and resuspended with 4.5 ml of ice-cold HEPES buffer. Membrane suspension (∼20–40 μg of protein) was incubated with HEPES buffer with 0.1 mm dithiothreitol, 0.2 mm sodium metabisulfite, 5 μm GDP, 0.1 nm [35S]GTPγS, and various concentrations of dopamine at 30 °C for 30 min in a final volume of 1 ml. Basal binding was determined in the absence of agonist, and nonspecific binding was determined in the presence of 10 μm nonradioactive GTPγS. The reaction was terminated by rapid filtration through GF/C filters with four washes of 4 ml of ice-cold 50 mm Tris-HCl, pH 7.4. Radioactivity bound to the filters was quantified by liquid scintillation spectroscopy. Free (0.1 fmol) [35S]GTPγS was counted to calculate the counts/min to fmol conversion. The protein amount was measured with bicinchoninic acid protein assay (Pierce). DA-stimulated [35S]GTPγS binding was calculated by subtracting the basal binding and normalized with the amount of membrane protein.
Western Blotting—Cells in 100-mm dishes were washed twice with ice-cold EBSS and solubilized for 1 h at 4 °C in 1 ml of solubilization buffer (50 mm HEPES, 1 mm EDTA, 10% glycerol, 1% Triton X-100, pH 7.4, 50 mm NaF, 40 mm sodium pyrophosphate, and 150 mm NaCl) supplemented with Complete protease inhibitor mixture. The samples were cleared by centrifugation, and the protein concentration was determined using the BCA protein assay kit from Pierce. Cell lysates (20 μg) were resolved by 4–12% SDS-PAGE and transferred to nitrocellulose membrane. Blots were incubated with membrane blocking buffer (Zymed Laboratories Inc.) for 1 h at room temperature. For detection of the expression of each GRK construct, the blots were incubated with antibodies (1:1000 dilution in blocking buffer) to GRK2, GRK3, GRK4, GRK5, and GRK6 for 1 h at room temperature. Membranes were washed with TBST (1× Tris-buffered saline, 0.1% Tween 20) and then incubated with secondary antibodies coupled to horseradish peroxidase in TBST for 30 min at room temperature. Membranes were washed, and immunoreactive proteins were visualized by chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate; Pierce).
BRET Analyses—The tetracycline-regulated expression of the HEK293 (T-Rex-293) (Invitrogen) cell line was cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum and 5 μg/ml blasticidin at 37 °C with 5% CO2. Bovine GRK2 cDNA (a gift from J. Benovic) was subcloned into pcDNA4/TO (Invitrogen). pcDNA4-GRK2 was transfected into T-Rex-293 cells using Lipofectamine (Invitrogen), and a Zeocin-resistant clone was selected, and tetracycline-inducible expression was verified by immunoblotting following overnight tetracycline induction (0.1 μg/μl). T-Rex HEK293-GRK2 cells (8 × 105 cells/ml) were transiently transfected using polyethyleneimine in a 1:3 ratio (Polysciences Inc.), with a constant amount of FLAG-D2R-RLuc8 as donor and increasing amounts of the mVenus-arrestin3 as the acceptor for the titration experiments and with a fixed amount of donor and acceptor for the concentration-effect studies. BRET experiments were performed 48 h after transfection. The cells were harvested, washed, and resuspended in Dulbecco's phosphate-buffered saline. Approximately 200,000 cells per well, in duplicate, were distributed in 96-well plates, and the fluorescence (excitation at 510 nm and emission at 540 nm, 1-s recording) and luminescence in the presence of 5 μm coelenterazine H (no filters, 5-min post-coelenterazine addition, and 1-s recording) were quantified (Polarstar and Pherastar; BMG). BRET titration curves were determined in the presence or absence of quinpirole (final 5 μm), and data were collected using a BMG Pherastar. The BRET signal was determined by calculating the ratio of the light emitted by Venus (510–540 nm) over that emitted by RLuc8 (485 nm). The net BRET values were obtained by subtracting the background given by RLuc8 expressed alone. For the titration experiments, the expression level of each protein was determined by measuring the total level of fluorescence and luminescence, respectively, and net-BRET was plotted against the relative expression ratio.
Data Analysis—All binding assays were routinely performed in triplicate and were repeated three to four times. Cyclic AMP experiments were performed in duplicate and were repeated three to four times. Estimation of the radioligand binding parameters, KD and Bmax, as well as the EC50 values for dopamine inhibition of cAMP accumulation, was calculated using the GraphPad Prism curve-fitting program (GraphPad Software Inc., San Diego). The curves presented throughout this study, representing the best fits to the data, were also generated using this software program.
RESULTS
Identification of GRK-mediated Phosphorylation Sites within the D2 DAR—When expressed in HEK293T cells, the D2 DAR is primarily phosphorylated in response to either PKC or GRK activation. Fig. 1A shows that the D2 DAR is phosphorylated under basal (control) conditions, but its phosphate content increases ∼4-fold when the cells are treated with the PKC activator, PMA, or ∼2-fold when treated with the agonist dopamine (DA). We have previously characterized the PKC-mediated phosphorylation of the D2 DAR in detail and found that PKC does not mediate the agonist-induced receptor phosphorylation response (14). Interestingly, despite possessing several canonical PKA phosphorylation sites, the D2 DAR does not appear to be phosphorylated by PKA when expressed in HEK293T cells (14). The DA-stimulated D2 DAR phosphorylation was found to be blocked by co-treatment with a D2 antagonist and was dose- and time-dependent, as well as augmented by overexpression of GRK2 (supplemental Fig. 1). Notably, the agonist-induced receptor phosphorylation occurs quite rapidly, achieving maximal levels within 8 min of stimulation (supplemental Fig. 1, lower panel). Fig. 1B shows that pretreatment of the HEK293T cells with pertussis toxin, which completely uncouples the D2 DAR from G protein activation (data not shown), does not affect the DA-induced receptor phosphorylation with or without GRK2 overexpression. Thus, agonist occupancy, rather than second messenger activation, appears to be sufficient for the DA-stimulated phosphorylation, as expected for a GRK-mediated process (29).
FIGURE 1.
Agonist- and PKC-mediated phosphorylation of the D2 DAR expressed in HEK293T cells. Transfected HEK293T cells were metabolically labeled with [32P]H3PO4 for 45 min prior to stimulation with the indicated drugs for 20 min. The cells were then solubilized, and the samples were subjected to immunoprecipitation as described under “Experimental Procedures.” Receptors were quantified in each transfection, and equal amounts of receptor protein were loaded in each gel lane followed by SDS-PAGE resolution. The extent of receptor phosphorylation was visualized by autoradiography and quantified as described below. A, upper panel shows a representative autoradiogram in which the cells were treated with the indicated drugs: 1st lane, vehicle (Control); 2nd lane, 1 μm PMA; 3rd lane, 10 μm DA. In the lower panel, the receptor phosphorylation was quantified by scanning the autoradiograms followed by analysis with the LabWorks™ software. The data are presented as the percentage of the basal phosphorylation and expressed as the mean ± S.E. values from more than four independent experiments. B, HEK293T cells were transfected with FLAG-tagged D2L DAR only (upper panel) or with a GRK2 expression construct (lower panel). Transfected cells were cultured in the presence or absence of 200 ng/ml of pertussis toxin (PTX) for ∼18 h and then labeled with [32P]H3PO4 before stimulating the cells with or without DA as described in A. This experiment was performed twice with identical results.
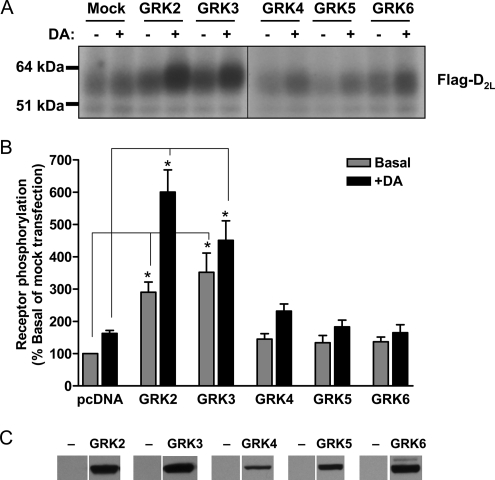
In Fig. 2, we explored the GRK specificity of the D2 DAR phosphorylation in HEK293T cells. In these experiments, all of the nonvisual GRKs (GRKs2–6) were overexpressed followed by assessment of basal and DA-stimulated receptor phosphorylation. Strikingly, only overexpression of GRK2 or GRK3 led to increased D2 DAR phosphorylation, whereas GRKs4–6 were without effect. To ascertain that the lack of effect of GRKs4–6 was not because of a lack of GRK expression, we performed immunoblots for each of the GRKs after their overexpression in the HEK293T cells (Fig. 2C). Notably, all of the GRKs were expressed at high levels following their cellular transfection. Interestingly, no endogenous GRK was detected via immunoblotting in untransfected cells, suggesting relatively low GRK expression. Previously, however, Iwata et al. (18) were able to show that HEK293 cells express low levels of GRK2, GRK5, and GRK6. Taken together, these results suggest that endogenous GRK2 mediates the agonist-induced D2 DAR phosphorylation in HEK293T cells.
FIGURE 2.
Effect of overexpressing nonvisual GRKs on basal and agonist-induced D2 DAR phosphorylation. HEK293T cells were transiently transfected with the FLAG-tagged D2L DAR along with the following expression constructs: pcDNA (Mock), GRK2, GRK3, GRK4, GRK5, or GRK6. [32P]H3PO4-labeled cells were stimulated with 1 μm DA for 20 min and processed as described in Fig. 1. A, autoradiogram from a single experiment, representative of four, is shown. B, receptor phosphorylation was quantified as described in Fig. 1. Data are expressed as the mean ± S.E. values from at least three independent experiments. *, p < 0.005, compared with the basal or DA-stimulated values of the mock transfection, respectively, unpaired Student's t test. C, analysis of GRK isozyme expression in transfected HEK293T cells. Cell lysates were prepared from cells transfected with the indicated GRKs and used to determine the expression of each GRK isozyme by immunoblotting with specific anti-GRK antibodies. A representative experiment is shown, which was performed twice with identical results.
We have previously used site-directed mutagenesis to map out the sites for PKC-mediated phosphorylation of the D2 DAR (14). These consist of three serine and two threonine residues present in two distinct clusters within the third intracellular loop (IC3) of the receptor (Fig. 3). To map out the GRK-mediated phosphorylation sites within the receptor, we performed a series of iterative mutagenesis experiments in which we selectively targeted cytoplasmic serine or threonine residues and evaluated the effects of mutating these residues on DA-stimulated receptor phosphorylation. As a guide toward selecting which residues to target, we took note of the fact that GRKs frequently, but not always, phosphorylate serine or threonine residues with acidic amino acids in close physical proximity (30, 31). These experiments led to the identification of six serine and two threonine residues within the IC3 loop of the receptor, which appear to represent all of the GRK phosphorylation sites on the D2 DAR (Fig. 3). Fig. 4 shows the effect of mutating one, two, three, five, or all eight of these residues simultaneously on both basal and DA-stimulated receptor phosphorylation in cells co-transfected with GRK2. As can be seen, progressive mutation results in a gradual decline in DA-stimulated phosphorylation until no stimulation over basal is observed when all eight residues are simultaneously mutated. Hereafter, we will refer to the receptor with all eight of these residues simultaneously mutated as GRK(-). Notably, the basal phosphorylation is also reduced in the GRK(-) receptor but only by ∼40%. Most importantly, Fig. 4 illustrates that we can detect the effects of mutating a single residue on DA-stimulated phosphorylation of the receptor. This leads us to conclude that the eight IC3 residues indicated in Figs. 3 and 4 represent all of the GRK2 phosphorylation sites within the D2 DAR. DA stimulation of D2 DAR phosphorylation is also abolished in the GRK(-) receptor when co-expressed with GRK3 (data not shown), suggesting redundancy between these GRK isozymes on D2 DAR phosphorylation.
FIGURE 3.
D2 DAR contains multiple potential phosphorylation sites for GRK and PKC. The rat D2L DAR sequence is shown. Black residues represent those absent in the D2S isoform. The PKC phosphorylation sites are shown as red residues, and the blue residues represent the GRK phosphorylation sites (see text).
FIGURE 4.
Identification of GRK phosphorylation sites in the D2 DAR via site-directed mutagenesis. Cells were transfected with the WT D2 DAR or the indicated Ser/Thr mutant receptors, along with GRK2. Serine and threonine residues were mutated to alanine and valine, respectively. [32P]H3PO4-labeled cells were stimulated with 1 μm DA for 20 min and processed as described in Fig. 1. A representative autoradiogram is shown in the top panel. Autoradiograms from multiple experiments were scanned and quantified as described in Fig. 1. These data are presented as a percentage of the basal phosphorylation of the WT D2 DAR and expressed as mean ± S.E. values from at least four independent experiments.
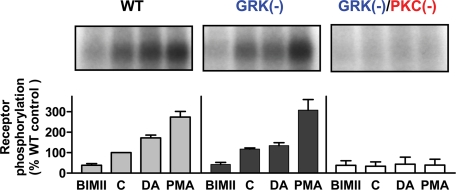
To further investigate the relationship between the PKC and GRK phosphorylation sites, we created a receptor in which all five PKC sites plus all eight GRK sites were simultaneously mutated (cf. Fig. 3), and this construct is referred to as GRK(-)/PKC(-). This construct along with the wild-type and GRK(-) constructs were subsequently evaluated using in situ phosphorylation assays. Fig. 5, top panel, presents results for DA- and PMA-stimulated phosphorylation of the wild-type receptor, which are similar to those shown in Fig. 1A, but additionally illustrates that treatment of the cells with the PKC inhibitor, BIMII, significantly reduces the basal phosphorylation state of the receptor, as we described previously (14). We also previously showed that BIMII treatment abolishes PMA-stimulated receptor phosphorylation while having no effect on that stimulated by DA (14). Fig. 5, middle panel, shows that elimination of the GRK phosphorylation sites has no effect on the PMA-stimulated phosphorylation, whereas the DA-stimulated phosphorylation is abolished, as shown previously in Fig. 4. Simultaneous elimination of all GRK and PKC phosphorylation sites results in a receptor construct (GRK(-)/PKC(-)) whose phosphorylation is not detectable above background levels, including that in the basal state (Fig. 5, lower panel). These results support our contention that the PKC and GRK phosphorylation sites are distinct (Fig. 3) and that these kinases are primarily involved in D2 DAR phosphorylation in the HEK293T cells. Furthermore, it appears as if the D2 DAR is phosphorylated by both GRK and PKC in the basal state, although PKC predominates in this reaction as exemplified by the BIMII results (Fig. 5).
FIGURE 5.
GRK and PKC phosphorylation sites are distinct. Cells were transfected with FLAG-tagged WT (left), GRK(-)(center), or GRK(-)/PKC(-) (right) mutant D2 DARs. For the GRK(-)/PKC(-) mutant, all of the red and blue residues shown in Fig. 3 were simultaneously mutated as follows: serines to alanines and threonines to valines. [32P]H3PO4-labeled cells were treated with the indicated drugs for 20 min, except BIMII where the cells were pretreated for 40 min. Following solubilization, the samples were subjected to immunoprecipitation as described under “Experimental Procedures” and resolved by 4–20% SDS-PAGE. Representative autoradiograms are shown. 1st lane, 10 μm BIMII; 2nd lane, vehicle (control); 3rd lane, 10 μm DA; 4th lane, 1 μm PMA. This experiment was performed twice with identical results. Autoradiograms were scanned and quantified as described in Fig. 1. These data are presented as a percentage of the basal phosphorylation of the WT D2 DAR and expressed as mean ± S.D. values from two independent experiments.
Effect of GRK-mediated Phosphorylation on Agonist-induced Receptor Desensitization—Having identified a GRK-mediated phosphorylation-null receptor, we wished to use this construct to investigate the role of GRK-mediated phosphorylation in agonist-induced desensitization of the receptor. In preliminary experiments, we first determined that the GRK(-) receptor expresses to the same extent as the wild-type receptor in HEK293T cells (Fig. 6A). We also utilized two assays for D2 DAR function for comparing the functional activity of the wild-type and GRK(-) receptors. Fig. 6B shows that the GRK(-) construct supports DA-mediated inhibition of cAMP accumulation to the same degree as the wild-type receptor. We also examined agonist-stimulated [35S]GTPγS binding to membranes prepared from transfected HEK293T cells (Fig. 6, C and D). Notably, there were no differences between the wild-type and GRK(-) receptors in mediating this functional response. These data imply that simply eliminating the GRK-mediated phosphorylation sites from the D2 DAR has no effect on either receptor expression or functional coupling to G proteins.
FIGURE 6.
Effect of mutating the GRK phosphorylation sites on D2 DAR expression and function. HEK293T cells were transiently transfected with either WT or GRK(-) mutant D2L DAR. A, total cellular D2L DAR expression was measured by [3H]methylspiperone (2 nm) binding to HEK293T cell membranes. The data represent the mean ± S.E. values from 12 experiments. B, dopamine inhibition of cAMP accumulation was measured in intact HEK293T cells. Cells were incubated with various concentrations of DA for 10 min in the presence of 3 μm forskolin. cAMP accumulation was then assessed as described under “Experimental Procedures.” The data shown represent the mean ± S.E. values from six experiments and are expressed as a percentage of forskolin-stimulated cAMP accumulation in the absence of DA. C, DA-stimulated [35S]GTPγS binding was determined in membranes prepared from HEK293T cells transiently expressing WT or GRK(-) mutant D2L DARs. Dose-response data are expressed as agonist-stimulated binding over basal. D, average values for 100 μm DA-stimulated [35S]GTPγS binding are shown. Data represent the means ± S.E. from 12 experiments.
Our initial plan was to use the cAMP accumulation assay as a functional readout for agonist-induced receptor desensitization; however, preliminary experiments revealed that pretreatment of the cells with D2 agonists led to an overall sensitization of cAMP accumulation by multiple effectors, including forskolin (data not shown). The mechanism(s) for this sensitization response, which is frequently observed with Gi/Go-linked GPCRs, is not completely understood, but it is cell-dependent and believed to lie downstream of the receptor proteins (32). Because this sensitization of downstream components will obscure receptor desensitization, we decided to use the [35S]GTPγS assay, which directly assesses the ability of the receptor to “activate” G proteins, as the functional readout for D2 DAR activation. Fig. 7 shows that pretreatment of the cells with DA leads to a time-dependent decline in the ability of the wild-type or GRK(-) receptors to mediate DA-stimulated [35S]GTPγS binding. A maximal ∼30% desensitization of the response was observed after ∼3 h of DA pretreatment (Fig. 7C). The t½ for the DA-induced decline in receptor activity was ∼1 h. This decline in responsiveness appeared to be limited to the Vmax of the reaction rather than to a decrease in agonist potency (Fig. 7, A and B). Agonist-induced desensitization of the GRK(-) construct appeared to be very similar to that of the wild-type receptor in terms of extent and rate, with the possible exception of a slight lag at the 1-h pretreatment time point (Fig. 7). These results suggest that GRK-mediated phosphorylation of the receptor is not required for agonist-induced receptor desensitization.
FIGURE 7.
Role of receptor phosphorylation in D2 DAR desensitization. HEK293T cells were transfected with either WT or GRK(-) mutant D2L DARs. Cells were preincubated in the absence or presence of 10 μm DA for the indicated times followed by membrane preparation and [35S]GTPγS binding assays as described under “Experimental Procedures.” A and B, amount of DA-stimulated [35S]GTPγS binding is expressed as the percent maximum observed in the control (no DA pretreatment) group. Single representative experiments are shown, which were replicated three times with identical results. C, maximum binding for each DA treatment group was plotted versus preincubation time. The data are expressed as a percentage of the control (no DA pretreatment) and represent the mean ± S.E. values from three independent experiments. *, p < 0.03 compared with WT at the time of 60 min of preincubation, unpaired Student's t test.
Because GRK-mediated phosphorylation is believed to promote arrestin association with GPCRs, leading to their desensitization and internalization (9, 29, 33), we wondered what the effect(s) of overexpressing arrestins would be on agonist-induced desensitization of the wild-type and GRK(-) receptors. Fig. 8 shows that overexpression of either of the nonvisual arrestins, arrestin2 (Fig. 8A) or arrestin3 (Fig. 8B), has no effect on agonist-induced desensitization of the wild-type D2 DAR. Similar results were observed with the GRK(-) receptor (data not shown). This lack of effect of arrestin overexpression on desensitization is especially interesting given that arrestin overexpression was found to augment agonist-induced receptor internalization (see below). These results question whether or not arrestin association with the receptor is necessary for the agonist-induced desensitization response.
FIGURE 8.
Effect of overexpressing arrestin2 or arrestin3 on D2 DAR desensitization. HEK293T cells were transfected with the wild-type D2L DAR along with either empty vector (pcDNA), arrestin2 (arr2) (A), or arrestin3 (arr3) (B) expression constructs. Cells were preincubated in the absence or presence of 10 μm DA for 3 h followed by membrane preparation and [35S]GTPγS binding assays as described under “Experimental Procedures.” The amount of DA-stimulated [35S]GTPγS binding is expressed as the percent maximum observed in the control (no DA pretreatment) group. Single representative experiments are shown, which were replicated two times with identical results.
Effect of GRK-mediated Phosphorylation on Agonist-induced Receptor Internalization—As noted above, GRK-mediated phosphorylation of GPCRs is believed to lead to their internalization by promoting their association with arrestins (9, 29, 33). We were thus interested in using the GRK(-) construct to examine the role of receptor phosphorylation in agonist-induced internalization of the D2 DAR. Previously, we have used an intact cell radioligand binding assay to assess the number of cell surface receptors (14). This assay employs [3H]sulpiride, a hydrophilic D2-selective antagonist, which does not permeate cells and only labels receptors on the cell surface. The supplemental Fig. 2A shows that a 1-h pretreatment of the cells with DA results in an ∼25% loss of cell surface receptors, whereas the total cellular complement of receptors measured with the hydrophobic antagonist, [3H]methylspiperone, remains unchanged. This agonist-induced receptor internalization is blocked by co-expression with the dominant-negative K44A mutant of dynamin and is augmented by co-expression with arrestin2 or arrestin3 (supplemental Fig. 2B). The arrestin-promoted receptor internalization is further augmented by co-expression with GRK2 or GRK3, but not with GRK5 or GRK6 (supplemental Fig. 2B). Notably, the agonist-induced receptor internalization is not blocked by pertussis toxin treatment indicating that receptor-G protein coupling is not required for this response (supplemental Fig. 2C).
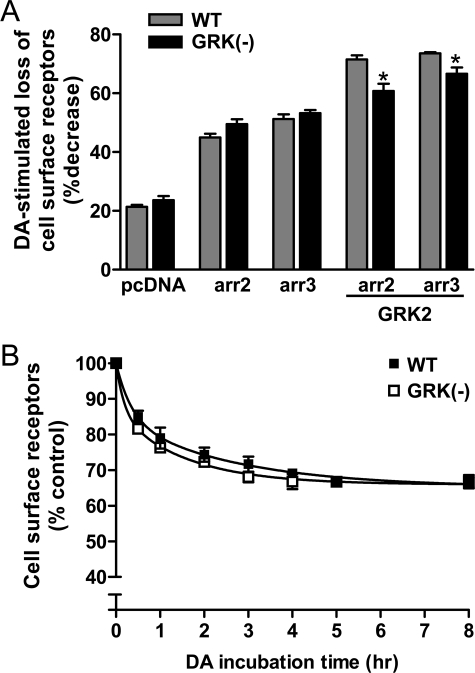
In Fig. 9A, we compare agonist-induced internalization of the wild-type and GRK(-) receptors and find that there is no difference in response between these two constructs. Moreover, co-expression with either arrestin2 or arrestin3 augments the internalization of the GRK(-) construct to the same degree as the wild-type receptor (Fig. 9A). Overexpression of GRK2 further augments the agonist-induced receptor internalization for both WT and GRK(-) constructs in the presence of arrestin2/3, although this effect is more pronounced for the WT receptor (Fig. 9A). These differences might suggest that receptor phosphorylation plays a facilitating role in internalization, but only at very high levels of arrestin and GRK2 expression. The mechanism for this is not clear, but it does not appear to result from phosphorylation-enhanced receptor-arrestin interactions (see below). The observation that GRK2 overexpression can augment internalization of the phosphorylation-null GRK(-) receptor implies a unique mechanism of action by GRK2, which is currently under investigation.
FIGURE 9.
Effect of eliminating GRK phosphorylation sites on DA-induced D2 DAR internalization. A, HEK293T cells were transfected with either WT or GRK(-) mutant D2L DAR along with empty vector (pcDNA), arrestin 2 (arr2), arrestin 3 (arr3), or in combination with GRK2. The cells were incubated in the absence or presence of 10 μm DA for 1 h and then subjected to intact cell [3H]sulpiride-binding assays as described under “Experimental Procedures.” The internalization of the D2 receptor was assessed by measuring the decrease in cell surface [3H]sulpiride-binding sites, and the data are expressed as the percent loss of cell surface receptors. The values shown represent the means ± S.E. of at least three independent experiments. *, p < 0.05 compared with WT in the presence of arrestin and GRK2 overexpression. B, HEK293T cells were transfected with either WT or GRK(-) mutant D2L DARs. Cells were incubated in the absence or presence of 10 μm DA for indicated times and then subjected to intact cell [3H]sulpiride binding assays. The amount of cell surface receptor is expressed as the percentage of [3H]sulpiride binding observed in the control (no DA treatment) group. Data are represented as the means ± S.E. from at least three experiments (0–3 h). Data for 4, 5, and 8 h are from two independent experiments.
The time course for agonist-induced internalization of the wild-type and GRK(-)D2 DARs was investigated in the experiment shown in Fig. 9B. The t½ of the agonist-induced receptor internalization response occurs at about 1–2 h and is maximal after 4–5 h of pretreatment time. Notably, the GRK(-) construct internalizes at the same rate and to the same extent as the wild-type receptor (Fig. 9B). Taken together, these results argue that GRK-mediated receptor phosphorylation is not required for agonist-induced receptor internalization.
Although GRK-mediated receptor phosphorylation appears dispensable for both agonist-induced desensitization and internalization, we were curious about the relationship of these two functional responses, especially given that arrestin overexpression augments internalization but not desensitization. We thus used co-expression of the K44A dominant-negative mutant of dynamin to block receptor internalization and see if this had any effect on receptor desensitization. The supplemental Fig. 2B shows that co-expression of this DynK44A mutant completely blocks agonist-induced receptor internalization elicited by 1 h of DA pretreatment. In contrast, a longer 3-h pretreatment of DA does lead to some receptor internalization in the presence of the DynK44A mutant, but this is attenuated by ∼60% (Table 1). Fig. 10 shows the effects of co-expressing the DynK44A mutant on DA-induced desensitization as assessed using the [35S]GTPγS binding assay. In this case, a 3-h pretreatment period with DA was employed as this paradigm resulted in maximal DA-induced desensitization (Fig. 7C). As can be seen, co-expression of the DynK44A mutant had no effect on the agonist-induced desensitization despite the significant impairment in receptor internalization (Fig. 10 and Table 1). These results suggest that internalization of the D2 DAR from the cell surface per se does not explain the agonist-induced desensitization response.
TABLE 1.
Effect of overexpressing dominant-negative dynamin mutant on agonist-induced D2 DAR internalization and desensitization HEK293T cells were transfected with D2L DAR along with pcDNA (mock) or the dynamin K44A mutant (DynK44A). Cells were incubated in the absence or presence of 10 μm DA for 3 h followed by intact-cell [3H]sulpiride-binding assays or membrane preparation and [35S]GTPγS binding assays as described under “Experimental Procedures.” Receptor sequestration is assessed by the loss of [3H]sulpiride binding, and desensitization is calculated by the decrease in [35S]GTPγS binding at 100 μm DA stimulation. Data are represented as mean ± S.E. from four independent experiments.
| [3H]Sulpiride binding | [35S]GTPγS binding | |
|---|---|---|
| % internalization | % desensitization | |
| pcDNA | 27.7 ± 3.2 | 23.7 ± 4.8 |
| DynK44A | 12.1 ± 2.3a | 23.6 ± 2.7 |
Values are p < 0.01, compared with the mock transfection, unpaired Student's t test
FIGURE 10.
Effect of blocking internalization on agonist-induced D2 DAR desensitization. HEK293T cells were transfected with D2L DAR along with pcDNA vector or the dynamin K44A expression construct. Cells were preincubated in the absence or presence of 10 μm DA for 3 h followed by membrane preparation and [35S]GTPγS binding assays as described in Figs. 7 and 8 and under “Experimental Procedures.” The amount of DA-stimulated [35S]GTPγS binding is expressed as the percent maximum observed in the control (no DA pretreatment) group. Values shown are means ± S.E. of five independent experiments.
Direct Assessment of Receptor/Arrestin Interactions using BRET—Given our current results, and the notion that GRK-mediated phosphorylation critically regulates GPCR-arrestin association (9, 29, 33), we wished to directly assess the D2 DAR interactions with a nonvisual arrestin using BRET. We have previously described a BRET-based method for relative quantification of arrestin3 recruitment to the human D2S DAR (34). As shown in Fig. 11, both WT and GRK(-)D2 DARs recruited arrestin3 in an agonist-specific manner, and their association was rapid (nearly maximal at 1 min) and stable over time (at least 10 min; data not shown). Interestingly, the BRET titration curve shown in Fig. 11B revealed an even more robust arrestin3 recruitment by the GRK(-) receptor in comparison with that of the WT receptor. This was further confirmed by the higher potency and efficacy of quinpirole-induced association of the GRK(-) receptor with arrestin3 in comparison with that of WT (Fig. 11C). Similar results were observed with DA as the stimulating agonist (data not shown). Taken together, these data show that the GRK-mediated D2 DAR phosphorylation neither promotes nor is required for arrestin3 recruitment to the receptor.
FIGURE 11.
Direct assessment of D2 DAR-arrestin3 association using BRET assays. A, ligand-induced arrestin3 recruitment by the WT or the GRK(-)D2 DARs was studied by a well characterized BRET-based biosensor. B, titration experiments were performed in T-Rex HEK293-GRK2 cells in the presence (filled symbols and solid lines) and absence (open symbols and dashed lines)of 5 μm quinpirole for 5 min as described under “Experimental Procedures.” Values are expressed as means ± S.D. of two independent experiments. C, constant amount of WT-(▪) or GRK(-)(▴)-RLuc8 fusions (0.3 μg) and mVenus-arrestin3 (30 μg) were expressed in T-Rex HEK293-GRK2 cells, and BRET was recorded as described under “Experimental Procedures” after incubation with the indicated concentration of quinpirole for 5 min. Values are expressed as means ± S.E. of three independent experiments.
Effect of GRK-mediated Phosphorylation on Receptor Recycling—Although GRK-mediated phosphorylation appears to be dispensable for D2 DAR desensitization and internalization, we wondered if intracellular trafficking of the receptor might be affected by this post-translational modification. Specifically, we wished to evaluate the effects of GRK-mediated phosphorylation on receptor recycling to the cell surface subsequent to agonist-induced internalization. Fig. 12 shows experiments in which the wild-type and GRK(-) receptors were pretreated with DA for 2 h followed by agonist washout and recovery for 1 h (Fig. 12A) or for up to 2 h (Fig. 12B). These experiments were also performed using GRK2 overexpression to maximally phosphorylate the wild-type receptor. Cell surface receptor numbers were monitored using intact cell [3H]sulpiride binding assays. After 2 h of DA pretreatment, ∼30% of the receptors were internalized from the cell surface (Fig. 9B). However, after washout and a 1-h recovery period, about half of those internalized wild-type receptors have recycled to the cell surface (Fig. 12A). In contrast, only about 25% of the internalized GRK(-) receptors have recycled to the cell surface within this time period (Fig. 12A). Fig. 12B shows a detailed time course for the recycling of the wild-type and GRK(-) receptors to the cell surface subsequent to agonist-induced internalization. As can be seen, although the rates of recovery of both receptors appear to be similar, the maximal recovery of the GRK(-) receptor is significantly blunted by ∼50% in comparison with the wild-type receptor. Extended recovery times beyond 2 h did not result in any further increase in cell surface receptor binding (data not shown). These results suggest that GRK-mediated phosphorylation facilitates the intracellular recycling of the D2 DAR subsequent to agonist-induced internalization.
FIGURE 12.
Role of receptor phosphorylation in D2 DAR recycling. Receptor recycling was assessed by measuring the recovery of cell surface [3H]sulpiride binding 1 h after dopamine removal. HEK293T cells were transfected with either WT or GRK(-)D2 DARs along with pcDNA or GRK2. After a 2-h pretreatment with 10 μm DA, the cells were incubated in the absence of DA for an additional hour, and the surface receptor number was then assessed. A, recovery of D2 DAR was assessed by the percent increase in [3H]sulpiride binding 1 h after DA washout. All values are expressed as means ± S.E. of four independent experiments. *, p < 0.02; **, p < 0.005 compared with WT. B, time course of cell surface receptor recovery. Cells were incubated in the absence of DA for the indicated times after a 1-h DA pretreatment, and then surface receptor number was assessed. All values are expressed as means ± S.E. of three independent experiments. *, p < 0.03. C, effect of protein synthesis inhibition on post-endocytic D2 DAR recycling. HEK293T cells were transfected with either WT or GRK(-) mutant D2L DARs along with GRK2. 50 μg/ml of cycloheximide or vehicle (DMSO, control) was applied 1 h before 10 μm DA stimulation and was present throughout the rest of the experiment. After a 1-h stimulation with 10 μm DA, the cells were washed and then incubated in the absence of DA for 1 h, and then the surface receptor number was assessed by cell surface [3H]sulpiride binding. Recovery of D2 DAR was assessed by the percent increase in [3H]sulpiride binding 1 h after DA washout. All values are expressed as means ± S.E. of three independent experiments. D, agonist-induced down-regulation of wild-type and GRK(-)D2 DARs. HEK293T cells were transfected with either WT or GRK(-) mutant D2L DARs along with GRK2. Cells were incubated in the absence or presence of 10 μm DA for the indicated times and then membranes were prepared for [3H]methylspiperone binding. The level of total cellular receptor expression is expressed as the percentage of [3H]methylspiperone binding (∼2 nm) observed in the control (no DA pretreatment) group. Data are represented as the means ± S.E. from at least four experiments. *, p < 0.05 compared with GRK(-) mutant.
To determine that the recovery of the cell surface receptors was indeed because of receptor recycling and not due to new receptor synthesis, we repeated the experiment shown in Fig. 12A in the presence of the protein synthesis inhibitor cycloheximide. Pretreatment of the cells with cycloheximide had no effect on agonist-induced internalization and, more importantly, had no effect on the ability of the receptors to recover to the cell surface upon agonist washout (Fig. 12C). This was true for both the wild-type and GRK(-) receptors. These results suggest that the observed cell surface receptor recovery is indeed due to recycling of internalized receptors.
Because the degree of receptor recovery/recycling was less than 100% for both the wild-type and GRK(-) receptors, it appears that a fraction of the internalized D2 DARs is down-regulated, presumably through degradative pathways. We thus examined the rate of agonist-induced down-regulation of the D2 DARs using [3H]methyspiperone, which will label the total complement of receptors in the cellular membranes. Fig. 12D shows that prolonged treatment of the cells with dopamine results in an ∼15–30% loss of the total number of WT D2 receptors. It should be noted that the agonist-induced receptor phosphorylation is quite stable in nature, at least to 100 min of stimulation (supplemental Fig. 1). Interestingly, the GRK(-) receptor appears to down-regulate in response to agonist treatment to a greater extent than the WT D2 DAR receptor. These results suggest that the impaired post-endocytic recycling of the GRK(-) receptor is probably due to differential sorting leading to an enhanced rate of receptor degradation once the receptor is internalized.
DISCUSSION
In this study, we investigated the role of GRK-mediated receptor phosphorylation in regulating D2 DAR signaling. In an initial approach, we screened for GRK specificity for phosphorylating the D2 DAR followed by mapping the GRK phosphorylation sites using site-directed mutagenesis. Our results show that, when expressed in HEK293 cells, only GRK2 and GRK3 are active in phosphorylating the D2 DAR. Interestingly, we found that GRK4 does not phosphorylate the D2 DAR despite prior observations that GRK4 constitutively phosphorylates the D1 DAR when expressed in these cells (35). Also of note is our observation that GRK6 was inactive in phosphorylating the D2 DAR, either in the absence or presence of agonist. Recently, Gainetdinov et al. (36) showed that GRK6-deficient mice exhibited enhanced central nervous system dopaminergic neurotransmission leading to their speculation that the D2 DAR is a physiological substrate for GRK6. Our current results, however, suggest that GRK6 does not directly phosphorylate the D2 DAR but perhaps another protein that may be involved in regulating dopaminergic signaling in the central nervous system.
Our mutagenesis experiments revealed the existence of eight unique GRK2/3 phosphorylation sites, six serine and two threonine residues, all contained with the IC3 loop of the receptor. Although our study exclusively employed the D2L isoform of the receptor (1, 2), all the identified GRK sites are present in the D2S isoform as well suggesting that both isoforms may be regulated similarly by GRK phosphorylation, Notably, the D2 DAR GRK sites were found to be distinct from the five PKC phosphorylation sites that we previously identified (14). These GRK and PKC sites also appear to be functionally distinct in that PKC phosphorylation appears to promote receptor uncoupling and internalization (14) whereas GRK phosphorylation does not (this study). Mutagenizing both the GRK and PKC sites simultaneously results in a completely phosphorylation-null receptor suggesting that the D2 DAR is only phosphorylated by GRK and PKC in HEK293 cells.
The availability of a GRK phosphorylation-null mutant receptor, GRK(-), allowed examination of the role of this post-translational modification in regulating D2 DAR signaling, particularly with respect to desensitization phenomena. Agonist-induced desensitization of D2 DAR function has not been extensively studied, although it has been reported to occur in cells endogenously expressing D2 DARs (37, 38) as well as in heterologous expression systems (39, 40). As noted previously, the role of GRK-mediated phosphorylation in agonist-induced desensitization of D2 DAR functional signaling has not been investigated. Using receptor-stimulated [35S]GTPγS binding as a functional readout, we observed that agonist pretreatment of HEK293 cells resulted in a time-dependent decline in receptor-G protein coupling. This decline in responsiveness was because of a reduction in the maximal [35S]GTPγS binding elicited by agonist. Interestingly, this contrasts with PKC-mediated desensitization of the D2 DAR, which produces a reduction in agonist potency but not efficacy (14). Surprisingly, the GRK(-) receptor desensitized in a similar fashion as the wild-type receptor in response to agonist pretreatment indicating that GRK phosphorylation is not actually required for this regulatory response. These results contrast with the widely spread notion that agonist-induced desensitization of GPCRs is primarily mediated by GRK phosphorylation (5–9) and further add to a growing body of literature suggesting that GPCR desensitization and phosphorylation can be uniquely separate events (33, 41, 42).
If GRK-mediated phosphorylation is not required for agonist-induced desensitization of the D2 DAR, then what is? One possibility is receptor internalization away from the cell surface, which would prevent the contact of agonist with receptor. Interestingly, the time course and extent of agonist-induced desensitization (Fig. 7B) are similar to those for agonist-induced receptor internalization (Fig. 9B). However, effective blockade of receptor internalization using a dominant-negative mutant of dynamin did not inhibit the agonist-induced desensitization response (Fig. 10). It is conceivable, however, that the D2 DARs are translocated from their normal membrane location in response to agonist activation, but are blocked from being removed from the cell surface in the presence of the dynamin mutant. Thus, functional uncoupling of the receptors may be due to their physical translocation away from active signaling complexes and/or membrane microdomains. These possibilities are currently under investigation.
A related question is whether or not association of the receptor with arrestin is required for agonist-induced desensitization. Interestingly, overexpression of arrestin2 or arrestin3 enhanced agonist-induced receptor internalization (Fig. 9) without enhancement of functional desensitization (Fig. 8). These results might suggest that arrestin association is not involved in the functional uncoupling of the receptor. In contrast, a recent study by Neve and co-workers (43), using a mutant D2 DAR deficient in arrestin binding, suggests that arrestin association is indeed required for agonist-induced desensitization. Clearly, more work will be required to resolve this particular issue.
With respect to agonist-induced receptor internalization, the role of arrestin association appears to be clearer. As noted, we found that overexpression of arrestin2 or arrestin3 increased agonist-induced receptor internalization as detected using cell surface receptor binding assays (Fig. 9) or confocal fluorescence microscopy (data not shown). Both arrestin2 and arrestin3 appeared to be equally efficacious in this regard, although we have recently found, using arrestin3-deficient mice, that it is arrestin3 that mediates D2 DAR internalization in the brain (44). Similarly, Neve and co-workers (45) found that a mutant D2 DAR deficient in arrestin3 binding exhibited impaired agonist-induced receptor internalization. These results argue strongly for a role of arrestins in D2 DAR internalization. Notably, however, GRK-mediated receptor phosphorylation appears dispensable for agonist-induced receptor internalization as the GRK(-) mutant receptor internalized at the same rate and to the same extent as the wild-type D2 DAR at physiological levels of arrestin/GRK2 expression. Thus, although agonist-induced receptor internalization appears to be dependent on arrestins, this process seems to be independent from GRK-mediated receptor phosphorylation.
An important question raised by our data is the importance and role of GRK-mediated receptor phosphorylation in arrestin binding and activation by D2 DARs. A general model for arrestin activation by GPCRs involves the interaction of arrestin with two regions on the receptor. One region is provided by at least two phosphorylated residues in close proximity that interact with the “phosphate sensor” on arrestin. The other is a region of the receptor that is conformationally changed upon receptor activation (33). Recent studies have suggested that, for many GPCRs, the activation-dependent regions of arrestin binding include the IC2 and IC3 loops (33, 43, 45, 46). Indeed, Neve and co-workers (45) have found that a region in the IC3, near the fifth transmembrane-spanning domain of the D2 DAR, is absolutely critical for arrestin association. Our current results, which show that GRK-mediated phosphorylation neither enhances nor is required for arrestin association with the receptor, suggest that, in the case of the D2 DAR, an agonist-induced conformational change is the primary, if not only, driver for arrestin binding and association.
A remaining question, however, is what structural element within the receptor activates the phosphate sensor within arrestin. One possibility is that negatively charged acidic residues located within the intracellular domains of the receptor serve this function. Such a role has been suggested for acidic regions of the chemokine receptor D6 (47) and the lutropin receptor (48). In preliminary experiments, we tested this hypothesis by simultaneously mutating glutamic acid residues 278, 280, and 282, which are in close proximity to four of the identified GRK phosphorylation sites (cf. Fig. 3); however, these mutations had no effect on agonist-induced receptor internalization (data not shown). There are multiple other acidic residues in the IC3 of the receptor, however, that could possibly serve this role, or alternatively, the D2 DAR may activate arrestin through a unique mechanism possibly involving conformational alterations. In this regard, it is interesting that Shukla et al. (49) have shown that phosphorylation-null mutants of the AT1a angiotensin or β2-adrenergic receptors are still capable of promoting conformational changes in arrestin3 despite a large reduction in receptor-arrestin affinities. Taken together, our current results suggest that the D2 DAR is an extreme example of a GPCR that associates with and activates arrestin in a completely phosphorylation-independent manner.
If GRK-mediated phosphorylation of the D2 DAR is not important for agonist-induced arrestin association and internalization/desensitization, then an obvious question is what is the function of this post-translational modification? Our current results suggest that GRK-mediated phosphorylation regulates the post-endocytic trafficking of the D2 DAR. Previous studies have found that the D2 DAR is endocytosed through a clathrin-dependent pathway, but it is not present within the same endocytic vesicles as internalized D1 dopamine or β2-adrenergic receptors (19, 21, 22). Once endocytosed, the D2 DAR also appears to exhibit variable recycling with some investigators reporting complete degradation (22) whereas others report robust, albeit slow recycling (21). Our results appear to be intermediate in nature such that the agonist-induced internalized wild-type D2 DAR recovers to about 75% of control levels with a t½ of ∼30 min (Fig. 12). In contrast, the phosphorylation-null GRK(-) receptor only recovers to about 30% of control levels and appears to be biased toward down-regulation/degradation.
In general, endocytosed GPCRs can be sorted into various endosomal recycling and/or lysosomal degradation pathways with different GPCRs exhibiting different degrees of each (7–9). It is now recognized that the sorting of endocytosed GPCRs into these various pathways can be an active process, which is regulated by sequence-specific determinants on the GPCR (7, 8). These sequence motifs in turn regulate the interaction of GPCRs with specific sorting proteins that can promote the entry of the receptor into one pathway or another. Our current data now provide a novel mechanism is this regard whereby the phosphorylation state of the D2 DAR can bias the receptor toward either recycling or degradation. For instance, when the D2 DAR is heavily phosphorylated in response to agonist activation, which might occur in cell types expressing high levels of GRK2/3 and/or in synaptic locations that experience transient high levels of transmitter, the receptor is biased toward recycling pathways. In contrast, if the receptor is not heavily phosphorylated during the process of agonist-induced internalization (which is a phosphorylation-independent event), then receptor degradation is promoted. We have recently provided data suggesting that D2 DARs can indeed be trafficked to lysosomes where, presumably, they undergo degradation (50). Interestingly, the D2 DAR is also known to be ubiquitinated (51), which has been shown to target some GPCRs to lysosomes (7, 8); however, we found that the WT and GRK(-)D2 DARs do not differ in their ubiquitination state (data not shown). A similar model has recently been suggested for μ-opioid receptors in which receptor phosphorylation was shown to regulate receptor recycling through either Rab4- or Rab11-mediated pathways (52). Thus, phosphorylation may provide a common mechanism for regulating the intracellular trafficking of many GPCRs.
An outstanding question is how GRK-mediated phosphorylation regulates endocytic sorting of the D2 DAR. The most parsimonious explanation is that the phosphorylation state of the D2 DAR regulates its association with one or more proteins, which in turn regulate receptor trafficking through either recycling or degradative pathways. A growing number of such “sorting” proteins are in the process of being identified (7, 8). Recently, Whistler and co-workers (22, 53, 54) have identified a “pro-degradation” protein termed GASP (GPCR-associated sorting protein), which appears to promote the sorting of certain GPCRs to lysosomes and their subsequent degradation. These investigators have also shown that GASP can directly interact with the D2 DAR to promote its degradation and down-regulation (22). This suggests an attractive hypothesis whereby GRK phosphorylation regulates D2 DAR-GASP interactions and thus biases the ability of the receptor to recycle or down-regulate. However, although we were able to verify that GASP directly interacts with the D2 DAR, using co-immunoprecipitation analyses, we did not observe any differences between the WT and GRK(-) receptors.4 Similarly, von Zastrow and co-workers (55) have described a “pro-recycling” endosome-associated protein, Hrs, that facilitates the recycling of the β2-adrenergic receptor. Although we also found that Hrs affects recycling of the D2 DAR, this phenomenon appears not to be regulated by receptor phosphorylation (data not shown). Despite these negative results, we strongly favor the hypothesis that the phosphorylation state of the receptor regulates its association with a sorting protein that directs the intracellular trafficking of the receptor. Future experiments directed at identifying additional intracellular sorting mechanisms for GPCRs in general, and the D2 DAR in particular, should shed light on this issue.
Supplementary Material
Acknowledgments
We thank Dr. Vanitha Ramakrishnan for the HEK293tsa201 cells and David Cabrera for laboratory management. Dr. Eneko Urizar is also thanked for the helpful interpretation of the BRET data. We thank Dr. Jennifer Whistler for contributing the GASP expression construct. We also thank the NINDS DNA sequencing facility at the National Institutes of Health for assistance.
This work was supported, in whole or in part, by a National Institutes of Health grant from NINDS (Intramural Research Program).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
Footnotes
The abbreviations used are: DAR, dopamine receptor; GRK, G protein-coupled receptor kinase; GPCR, G protein-coupled receptor; DMEM, Dulbecco's modified Eagle's medium; EBSS, Earle's balanced salt solution; PKA, cAMP-dependent protein kinase; GTPγS, guanosine 5′-3-O-(thio)triphosphate; PKC, protein kinase C; PMS, phorbol 12-myristate 13-acetate; DA, dopamine; WT, wild type; BRET, bioluminescence resonance energy transfer; IC3, third intracellular loop.
D. R. Sibley and Y. Namkung, unpublished observations.
References
- 1.Missale, C., Nash, S. R., Robinson, S. W., Jaber, M., and Caron, M. G. (1998) Physiol. Rev. 78 189-225 [DOI] [PubMed] [Google Scholar]
- 2.Sibley, D. R., and Monsma, F. J., Jr. (1992) Trends Pharmacol. Sci. 13 61-69 [DOI] [PubMed] [Google Scholar]
- 3.Neve, K. A., Seamans, J. K., and Trantham-Davidson, H. (2004) J. Recept. Signal. Transduct. Res. 24 165-205 [DOI] [PubMed] [Google Scholar]
- 4.Sibley, D. R., and Neve, K. A. (1997) in The Dopamine Receptors (Neve, K. A., and Neve, R. L., eds), pp. 383-424, Humana Press Inc., Totowa, NJ
- 5.Ferguson, S. S., Barak, L. S., Zhang, J., and Caron, M. G. (1996) Can. J. Physiol. Pharmacol. 74 1095-1110 [DOI] [PubMed] [Google Scholar]
- 6.Gainetdinov, R. R., Premont, R. T., Bohn, L. M., Lefkowitz, R. J., and Caron, M. G. (2004) Annu. Rev. Neurosci. 27 107-144 [DOI] [PubMed] [Google Scholar]
- 7.Hanyaloglu, A. C., and von Zastrow, M. (2008) Annu. Rev. Pharmacol. Toxicol. 48 537-568 [DOI] [PubMed] [Google Scholar]
- 8.Marchese, A., Paing, M. M., Temple, B. R., and Trejo, J. (2008) Annu. Rev. Pharmacol. Toxicol. 48 601-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore, C. A., Milano, S. K., and Benovic, J. L. (2007) Annu. Rev. Physiol. 69 451-482 [DOI] [PubMed] [Google Scholar]
- 10.DeWire, S. M., Ahn, S., Lefkowitz, R. J., and Shenoy, S. K. (2007) Annu. Rev. Physiol. 69 483-510 [DOI] [PubMed] [Google Scholar]
- 11.Seeman, P. (2007) Synapse 61 1013-1018 [DOI] [PubMed] [Google Scholar]
- 12.Kapur, S., and Remington, G. (2001) Biol. Psychiatry 50 873-883 [DOI] [PubMed] [Google Scholar]
- 13.Tandon, R., Keshavan, M. S., and Nasrallah, H. A. (2008) Schizophr. Res. 100 4-19 [DOI] [PubMed] [Google Scholar]
- 14.Namkung, Y., and Sibley, D. R. (2004) J. Biol. Chem. 279 49533-49541 [DOI] [PubMed] [Google Scholar]
- 15.Morris, S. J., Van Ham, I. I., Daigle, M., Robillard, L., Sajedi, N., and Albert, P. R. (2007) Eur. J. Pharmacol. 577 44-53 [DOI] [PubMed] [Google Scholar]
- 16.Ito, K., Haga, T., Lameh, J., and Sadee, W. (1999) Eur. J. Biochem. 260 112-119 [DOI] [PubMed] [Google Scholar]
- 17.Itokawa, M., Toru, M., Ito, K., Tsuga, H., Kameyama, K., Haga, T., Arinami, T., and Hamaguchi, H. (1996) Mol. Pharmacol. 49 560-566 [PubMed] [Google Scholar]
- 18.Iwata, K., Luo, J., Penn, R. B., and Benovic, J. L. (2005) J. Biol. Chem. 280 2197-2204 [DOI] [PubMed] [Google Scholar]
- 19.Kim, K. M., Valenzano, K. J., Robinson, S. R., Yao, W. D., Barak, L. S., and Caron, M. G. (2001) J. Biol. Chem. 276 37409-37414 [DOI] [PubMed] [Google Scholar]
- 20.Macey, T. A., Gurevich, V. V., and Neve, K. A. (2004) Mol. Pharmacol. 66 1635-1642 [DOI] [PubMed] [Google Scholar]
- 21.Vickery, R. G., and von Zastrow, M. (1999) J. Cell Biol. 144 31-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartlett, S. E., Enquist, J., Hopf, F. W., Lee, J. H., Gladher, F., Kharazia, V., Waldhoer, M., Mailliard, W. S., Armstrong, R., Bonci, A., and Whistler, J. L. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 11521-11526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwata, K., Ito, K., Fukuzaki, A., Inaki, K., and Haga, T. (1999) Eur. J. Biochem. 263 596-602 [DOI] [PubMed] [Google Scholar]
- 24.Kabbani, N., Jeromin, A., and Levenson, R. (2004) Cell. Signal. 16 497-503 [DOI] [PubMed] [Google Scholar]
- 25.Cho, D. I., Beom, S., Van Tol, H. H., Caron, M. G., and Kim, K. M. (2006) Biochem. Biophys. Res. Commun. 350 634-640 [DOI] [PubMed] [Google Scholar]
- 26.Loening, A. M., Fenn, T. D., Wu, A. M., and Gambhir, S. S. (2006) Protein Eng. Des. Sel. 19 391-400 [DOI] [PubMed] [Google Scholar]
- 27.Watts, V. J., and Neve, K. A. (1996) Mol. Pharmacol. 50 966-976 [PubMed] [Google Scholar]
- 28.Gardner, B., Hall, D. A., and Strange, P. G. (1996) Br. J. Pharmacol. 118 1544-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Premont, R. T., and Gainetdinov, R. R. (2007) Annu. Rev. Physiol. 69 511-534 [DOI] [PubMed] [Google Scholar]
- 30.Ferguson, S. S. (2001) Pharmacol. Rev. 53 1-24 [PubMed] [Google Scholar]
- 31.Pitcher, J. A., Freedman, N. J., and Lefkowitz, R. J. (1998) Annu. Rev. Biochem. 67 653-692 [DOI] [PubMed] [Google Scholar]
- 32.Watts, V. J., and Neve, K. A. (2005) Pharmacol. Ther. 106 405-421 [DOI] [PubMed] [Google Scholar]
- 33.Gurevich, V. V., and Gurevich, E. V. (2006) Pharmacol. Ther. 110 465-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klewe, I. V., Nielsen, S. M., Tarpo, L., Urizar, E., Dipace, C., Javitch, J. A., Gether, U., Egebjerg, J., and Christensen, K. V. (2008) Neuropharmacology 54 1215-1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rankin, M. L., Marinec, P. S., Cabrera, D. M., Wang, Z., Jose, P. A., and Sibley, D. R. (2006) Mol. Pharmacol. 69 759-769 [DOI] [PubMed] [Google Scholar]
- 36.Gainetdinov, R. R., Bohn, L. M., Sotnikova, T. D., Cyr, M., Laakso, A., Macrae, A. D., Torres, G. E., Kim, K. M., Lefkowitz, R. J., Caron, M. G., and Premont, R. T. (2003) Neuron 38 291-303 [DOI] [PubMed] [Google Scholar]
- 37.Agui, T., Amlaiky, N., Caron, M. G., and Kebabian, J. W. (1988) J. Biochem. (Tokyo) 103 436-441 [DOI] [PubMed] [Google Scholar]
- 38.Barton, A. C., Black, L. E., and Sibley, D. R. (1991) Mol. Pharmacol. 39 650-658 [PubMed] [Google Scholar]
- 39.Bates, M. D., Senogles, S. E., Bunzow, J. R., Liggett, S. B., Civelli, O., and Caron, M. G. (1991) Mol. Pharmacol. 39 55-63 [PubMed] [Google Scholar]
- 40.Zhang, L. J., Lachowicz, J. E., and Sibley, D. R. (1994) Mol. Pharmacol. 45 878-889 [PubMed] [Google Scholar]
- 41.Ferguson, S. S. (2007) Trends Pharmacol. Sci. 28 173-179 [DOI] [PubMed] [Google Scholar]
- 42.Neve, K. A. (2006) Mol. Pharmacol. 69 673-676 [DOI] [PubMed] [Google Scholar]
- 43.Lan, H., Liu, Y., Bell, M. I., Gurevich, V. V., and Neve, K. A. (2009) Mol. Pharmacol. 75 113-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skinbjerg, M., Ariano, M. A., Thorsell, A., Heilig, M., Halldin, C., Innis, R. B., and Sibley, D. R. (2009) Synapse 63 621-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lan, H., Teeter, M. M., Gurevich, V. V., and Neve, K. A. (2009) Mol. Pharmacol. 75 19-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marion, S., Oakley, R. H., Kim, K. M., Caron, M. G., and Barak, L. S. (2006) J. Biol. Chem. 281 2932-2938 [DOI] [PubMed] [Google Scholar]
- 47.Galliera, E., Jala, V. R., Trent, J. O., Bonecchi, R., Signorelli, P., Lefkowitz, R. J., Mantovani, A., Locati, M., and Haribabu, B. (2004) J. Biol. Chem. 279 25590-25597 [DOI] [PubMed] [Google Scholar]
- 48.Mukherjee, S., Gurevich, V. V., Preninger, A., Hamm, H. E., Bader, M. F., Fazleabas, A. T., Birnbaumer, L., and Hunzicker-Dunn, M. (2002) J. Biol. Chem. 277 17916-17927 [DOI] [PubMed] [Google Scholar]
- 49.Shukla, A. K., Violin, J. D., Whalen, E. J., Gesty-Palmer, D., Shenoy, S. K., and Lefkowitz, R. J. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 9988-9993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim, O. J., Ariano, M. A., Namkung, Y., Marinec, P., Kim, E., Han, J., and Sibley, D. R. (2008) J. Neurochem. 106 83-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim, O. J. (2008) J. Recept. Signal. Transduct. Res. 28 453-464 [DOI] [PubMed] [Google Scholar]
- 52.Wang, F., Chen, X., Zhang, X., and Ma, L. (2008) Mol. Endocrinol. 22 1881-1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson, D., Pusch, M., and Whistler, J. L. (2007) J. Biol. Chem. 282 29178-29185 [DOI] [PubMed] [Google Scholar]
- 54.Whistler, J. L., Enquist, J., Marley, A., Fong, J., Gladher, F., Tsuruda, P., Murray, S. R., and Von Zastrow, M. (2002) Science 297 615-620 [DOI] [PubMed] [Google Scholar]
- 55.Hanyaloglu, A. C., McCullagh, E., and von Zastrow, M. (2005) EMBO J. 24 2265-2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.