Abstract
Exposure to bright light can cause visual dysfunction and retinal photoreceptor damage in humans and experimental animals, but the mechanism(s) remain unclear. We investigated whether the retinoid cycle (i.e. the series of biochemical reactions required for vision through continuous generation of 11-cis-retinal and clearance of all-trans-retinal, respectively) might be involved. Previously, we reported that mice lacking two enzymes responsible for clearing all-trans-retinal, namely photoreceptor-specific ABCA4 (ATP-binding cassette transporter 4) and RDH8 (retinol dehydrogenase 8), manifested retinal abnormalities exacerbated by light and associated with accumulation of diretinoid-pyridinium-ethanolamine (A2E), a condensation product of all-trans-retinal and a surrogate marker for toxic retinoids. Now we show that these mice develop an acute, light-induced retinopathy. However, cross-breeding these animals with lecithin:retinol acyltransferase knock-out mice lacking retinoids within the eye produced progeny that did not exhibit such light-induced retinopathy until gavaged with the artificial chromophore, 9-cis-retinal. No significant ocular accumulation of A2E occurred under these conditions. These results indicate that this acute light-induced retinopathy requires the presence of free all-trans-retinal and not, as generally believed, A2E or other retinoid condensation products. Evidence is presented that the mechanism of toxicity may include plasma membrane permeability and mitochondrial poisoning that lead to caspase activation and mitochondria-associated cell death. These findings further understanding of the mechanisms involved in light-induced retinal degeneration.
The retinoid cycle is a fundamental metabolic process in the vertebrate retina responsible for continuous generation of 11-cis-retinal from its all-trans-isomer (1-3). Because 11-cis-retinal is the chromophore of rhodopsin and cone visual pigments (4), disabling mutations in genes encoding proteins of the retinoid cycle can cause a spectrum of retinal diseases affecting sight (3). Moreover, the efficiency of the mammalian visual system and health of photoreceptors and retinal pigment epithelium (RPE)2 decrease significantly with age. Even in the presence of a functional retinoid cycle, A2E, retinal dimer (RALdi), and other toxic all-trans-retinal condensation products (5-7) can accumulate as a consequence of aging (8). Under experimental conditions, these compounds can produce toxic effects on RPE cells (9-11). Patients affected by age-related macular degeneration, Stargardt disease, or other retinal diseases associated with accumulation of surrogate markers, such as A2E, all develop retinal degeneration (12). Thus, elucidating the fundamental causes of these age-dependent changes is of increasing importance. Encouragingly, our understanding of both retinoid metabolism outside the eye and production of 11-cis-retinal unique to the eye has accelerated recently (Scheme 1) (1-3), and genetic mouse models are readily available to study these processes and their potential aberrations in vivo (13). Thus, a central question can be addressed, namely what initiates the death of photoreceptor cells and the underlining RPE?
SCHEME 1.
Retinoid flow and all-trans-retinal clearance in the visual cycle. After diffusion from the RPE, the visual chromophore, 11-cis-retinal, combines with rhodopsin and then is photoisomerized to all-trans-retinal. Most of the all-trans-retinal dissociates from opsin into the cytoplasm, where it is reduced to all-trans-retinol by RDHs, including RDH8. The fraction of all-trans-retinal that dissociates into the disc lumen is transported by ABCA4 into the cytoplasm (23) before it is reduced. All-trans-retinol then is translocated to the RPE, esterified by LRAT, and recycled back to 11-cis-retinal. Mutations of ABCA4 are associated with human macular degeneration, Stargardt disease, and age-related macular degeneration (55, 56).
Several mechanisms associated with retinoid metabolism may contribute to different retinopathies (1). For example, lack of retinoids in LRAT (lecithin:retinol acyltransferase) or chromophore in retinoid isomerase knock-out (Rpe65-/-) mice leads to rapid degeneration of cone photoreceptors and slowly progressive death of rods (14). Such mice do not produce toxic condensation products from all-trans-retinal. Instead, their retinopathies have been attributed to continuous activation of visual phototransduction (15) due to either the basal activity of opsin (16-18) or disordered vectorial transport of cone visual pigments without bound chromophore (19). Paradoxically, an abnormally high flux of retinoids through the retinoid cycle can also lead to retinopathy in other mouse models (20, 21). Animal models featuring anomalies in the retinoid cycle illustrate the importance of chromophore regeneration and provide an approach to elucidating mechanisms involved in human retinal dysfunction and disease.
Recently, we showed that mice carrying a double knock-out of Rdh8 (retinol dehydrogenase 8), one of the main enzymes that reduces all-trans-retinal in rod and cone outer segments (22), and Abca4 (ATP-binding cassette transporter 4), which transports all-trans-retinal from the inside to the outside of disc membranes (23), rapidly accumulate all-trans-retinal condensation products and exhibit accentuated RPE/photoreceptor dystrophy at an early age (24). Although these studies suggest retinoid toxicity, it is still unclear if the elevated levels of retinal and/or its condensation products, such as A2E, are the cause of this retinopathy or merely a nonspecific reflection of impaired retinoid metabolism. Here, we report that spent chromophore, all-trans-retinal, is most likely responsible for photoreceptor degeneration in Rdh8-/-Abca4-/- mice. Toxic effects of all-trans-retinal include caspase activation and mitochondria-associated cell death.
EXPERIMENTAL PROCEDURES
Animals—Lrat-/-Rdh8-/-Abca4-/- triple knock-out mice were generated by cross-breeding Rdh8-/-Abca4-/- mice with Lrat-/- mice, and progeny were genotyped as previously described (24-26). Gnat1-/-Rdh8-/-Abca4-/- triple knock-out mice were generated by cross-breeding Rdh8-/-Abca4-/- mice with Gnat1-/- mice, and progeny also were genotyped as previously described (24, 27). Mice with the Leu residue variant at amino acid position 450 of RPE65 (retinal pigment epithelium-specific protein of 65 kDa) were used for this study. Animals were housed in the animal facility at the School of Medicine, Case Western Reserve University, where they were maintained either under complete darkness or in a 12-h light (∼5 lux)/12-h dark cycle environment. Manipulations were done in the dark under dim red light transmitted through a Kodak No. 1 safe light filter (transmittance >560 nm). All animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committees and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the Association of Research for Vision and Ophthalmology.
Materials and Chemical Syntheses—All-trans-retinoic acid, 13-cis-retinoic acid, 9-cis-retinal, and 9-cis-retinol were purchased from Sigma, and all-trans-retinal was purchased from Toronto Research Chemicals Inc. (Toronto, Canada). 9-cis-Retinyl acetate was prepared from 9-cis-retinol and acetic anhydride by a method previously described (26). A2E was synthesized by condensation of all-trans-retinal with phosphatidylethanolamine, purified by HPLC, and quantified by UV-visible absorption (28-30). RALdi was synthesized based on a modified procedure previously published (31). Specifically, condensation of all-trans-retinal to RALdi was performed in an argon-flushed reaction chamber. 1 mol eq of sodium hydride in mineral oil (60% NaH) was carefully mixed with 1 mol eq of all-trans-retinal delivered in dry tetrahydrofuran. The reaction mixture was stirred at room temperature for 3 h prior to the addition of saturated ammonium chloride and extraction with hexane. The organic layer was washed twice with saturated sodium chloride and dried down in a SpeedVac. The crude product was purified by reverse phase HPLC (Phenomenex C18 column; 250 × 4.60 mm; 5 μm) in an acetonitrile-acetonitrile/isopropyl alcohol (33%/67%, v/v) gradient for 20 min at a flow rate of 1 ml/min. All solvents contained 0.1% formic acid (v/v). The RALdi peak was identified by its characteristic UV-visible spectrum with maxima at 293 and 432 nm. The identity of the compound was further confirmed by LC/MS using a LXQ mass spectrometer (Thermo Scientific, Waltham, MA) equipped with an APCI source under chromatographic conditions described above. To obtain oxidized forms of RALdi, the compound was redissolved in acetonitrile and incubated at room temperature in daylight for 2 h. Formation of oxo-RALdi species was indicated by a shorter retention time on a reverse phase column, a hypochromic shift in the UV-visible absorbance spectra characteristic of losing conjugated double bonds, and a +16, +32 molecular mass shift.
Retinoid and A2E Analyses—All experimental procedures related to extraction, derivatization, and separation of retinoids from dissected mouse eyes were carried out as formerly described (32). A2E was extracted twice with 1 ml of acetonitrile from two eyes processed with a glass/glass homogenizer. After solvent evaporation, residues were dissolved in 150 μl of acetonitrile with 0.1% trifluoroacetic acid (v/v) and filtered through a Teflon syringe filter (National Scientific Co.). Samples (100 μl) were loaded on C18 columns (250 × 4.60 mm; 5 μm) (Phenomenex) and analyzed by normal phase HPLC with gradients of acetonitrile/H2O (80:20) and acetonitrile/H2O (100:0) with 0.1% trifluoroacetic acid (v/v/v) as the mobile phase for 30 min. Quantification of A2E was performed by comparison with known concentrations of pure synthetic A2E (32). Oxidized A2E was detected and quantified with a LXQ mass spectrometer (Thermo Scientific, Waltham, MA) equipped with an APCI source and combined with an 1100 series HPLC (Agilent Technologies, Santa Clara, CA) (28).
Histology and Immunohistochemistry—Histological and immunohistochemical procedures employed were previously reported (33). Briefly, eyecups for histology were fixed in 2% glutaraldehyde, 4% paraformaldehyde and processed for embedding in Epon. Sections for routine histology were cut at 1 μm and stained with toluidine blue. For immunocytochemistry, eyes were immersion-fixed for 2 h with freshly prepared 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4, and processed for optimal cutting temperature (Miles) embedding. Sections cut at 10 μm were viewed through a Zeiss LSM 510 inverted laser scan confocal microscope. TUNEL staining was done according to methods provided by the manufacturer (Chemicon).
ERG—All ERG procedures were performed by well established methods (32). Briefly, mice under a safety light were anesthetized by intraperitoneal injection of 20 μl/g body weight of 6 mg/ml ketamine and 0.44 mg/ml xylazine diluted with 10 mm sodium phosphate, pH 7.2, containing 100 mm NaCl. Pupils were dilated with 1% tropicamide. A contact lens electrode was placed on the eye, and a reference electrode and ground electrode were positioned on the ear and tail, respectively. ERGs were recorded with the universal testing and electrophysiologic system, UTAS E-3000 (LKC Technologies, Inc.).
Single Flash Recording—White light flash stimuli had a range of intensities from -3.7 to 1.6 log cd·s/m2, and flash duration was adjusted according to intensity from 20 μs to 1 ms. 3-5 recordings were made at sufficient intervals (from 10 s to 10 min) between flash stimuli to allow mice to recover.
Light Damage—After dark adaptation for 24 h, one or two mice with pupils dilated by 1% tropicamide were exposed to fluorescent light (10,000 lux) (150-watt spiral lamp; Commercial Electric) for the indicated times in a white bucket (Papersmith, San Marcos, TX) with food and water and then kept in the dark. Quantification of A2E and oxidized A2E in mouse eyes was done at 0 min, 24 h, and 3 days after light illumination, whereas histological analyses of the retina were performed 1 week after such exposure.
Illumination of 9-cis-Retinyl Acetate-gavaged Mice—Mice were gavaged with 2 mg of 9-cis-retinyl acetate and kept for 3 days in the dark. Then they were exposed to intense constant illumination (500 cd·m-2) for 24 min. After 24 h of dark adaptation, this procedure was repeated up to three times.
Viability Assay of Retinoid-laden ARPE19 and HEK293 Cells—Retinoid-laden ARPE19 cells (human RPE cells) and HEK293 cells (human renal epithelial cells) were produced by the methods described in Refs. 34 and 35 with modifications. Cells (3 × 105 cells in 24-well plates) were incubated for 16 h at 37 °C with a 30 μm concentration of the indicated retinoids, followed by 3 days of incubation with 1 ml of Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum alone. Cells then were either exposed or not exposed to blue light (440 nm; X-Cite120 Fluorescence illumination system with 440-nm filters) for 5 min, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were performed after 16 h of additional incubation in the dark. For the MTT assay, 50 μl of MTT solution (2 mg/ml in DMEM) was added to each well. After a 16-h incubation, the medium was aspirated; next, intracellular formazan dye crystals were dissolved with 1 ml of DMSO at room temperature in the dark. The absorbance of formazan at 560 nm was measured using a Cary 50 UV-visible spectrophotometer (Varian).
In Vitro Calcium Monitoring—Fluo-3 AM (Invitrogen) at 2 μm was loaded onto ARPE19 cells cultured in 24-well plates (1 × 105 cells/well) in DMEM with 10% fetal calf serum. After a 30-min incubation, cells were washed three times with DMEM. All-trans-retinal or calcium ionophore A23187 (Sigma) was added to these cells at the indicated concentrations, and the intracellular calcium concentration was monitored with a Leica DMI 6000 B inverted microscope.
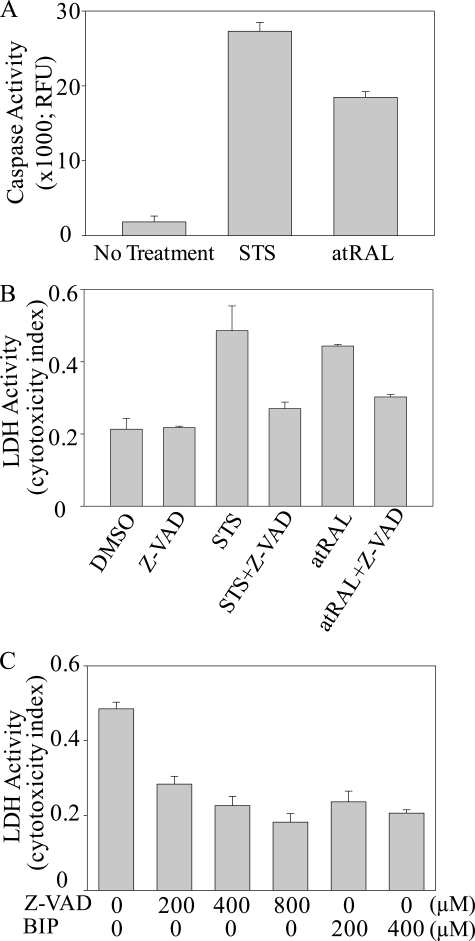
Caspase Activity—Cells in 6-cm dishes (6 × 105 cells/3 ml/dish) were cultured at 37 °C in 10% fetal calf serum in DMEM to which either all-trans-retinal (30 μm) or staurosporin (STS; 100 nm) was added. After a 24-h incubation at 37 °C, both floating and adhering cells were collected for caspase activity determinations, as previously reported (36). Cells were lysed in CHAPS cell lysis buffer (1% CHAPS in 137 mm NaCl, 2.7 mm KCl, 4.3 Na2HPO4, 1.4 mm KH2PO4, pH 7.4), and the soluble fraction was collected after centrifugation at 10,000 rpm for 30 min (Eppendorf microcentrifuge). Caspase activity was detected by cleavage of the fluorescent caspase substrate, DEVD-7-amino-4-trifluoromethylcoumarin, as previously reported (36), after incubation with cell lysates (30 μg of protein) for 30 min at 37 °C.
Lactate Dehydrogenase Activity—Cells were cultured in 24-well plates (6 × 104 cells/300 μl/well), and all-trans-retinal (30 μm) was added to the medium with and without various concentrations of cell death inhibitors (caspase inhibitor, Z-VAD-fluoromethyl ketone) or Bax-inhibiting peptide (BIP), as indicated in the legends to Figs. 7 and 9. To examine the role of caspase and Bax in all-trans-retinal-induced cell death, we preincubated cells with either z-VAD-fluoromethyl ketone or BIP for 3 or 12 h, respectively. Then all-trans-retinal was added, and incubation continued for 16 h before collection of media. Activity of lactate dehydrogenase released from dead cells was measured with a lactate dehydrogenase assay kit from Bio-Vision (Mountain View, CA).
FIGURE 7.
All-trans-retinal causes ARPE19 cell death. A, ARPE19 cells were incubated with the indicated concentrations of all-trans-retinal (atRAL) for 16 h at 37 °C. Dose-dependent cell death was evident at 5, 10, and 30 μm concentrations. B, ARPE19 cells were incubated with 30 μm all-trans-retinal with and without 100 μm Bax inhibitor (BIP) for 16 h at 37 °C and then stained with trypan blue for 5 min. BIP inhibited all-trans-retinal-induced cell death, indicating that all-trans-retinal induces Bax-dependent apoptosis through the intrinsic mitochondrial/endoplasmic reticulum pathway.
FIGURE 9.
All-trans-retinal induces caspase- and Bax-dependent cell apoptosis. A, ARPE19 cells were incubated with 100 nm STS or 30 μm all-trans-retinal (atRAL) for 24 h at 37 °C, and caspase activity was measured by detecting cleavage of the fluorescent caspase substrate, DEVD-7-amino-4-trifluoromethylcoumarin. B, the caspase inhibitor, Z-VAD-fluoromethyl ketone (Z-VAD) (200 μm), was added together with 100 nm STS or 30 μm all-trans-retinal to ARPE19 cells and incubated for 24 h at 37 °C. ARPE19 cell toxicity was assessed by lactate dehydrogenase release. C, caspase inhibitor Z-VAD-fluoromethyl ketone and Bax inhibitor (BIP) were admixed at the indicated concentrations with culture medium containing 30 μm all-trans-retinal. ARPE19 cell cytotoxicity was assessed by lactate dehydrogenase measurements after a 16-h incubation at 37 °C.
Mitochondrial Oxidative Phosphorylation—Cardiac interfibrillar mitochondria were isolated from Sprague-Dawley rats using a protocol reported by Palmer et al. (37) with minor modifications. Cardiac interfibrillar mitochondria were separated by treating minced cardiac muscle with modified Chappel-Perry buffer (50 mm MOPS, pH 7.4, containing 100 mm KCl, 5.0 mm MgSO4, 1.0 mm EGTA, and 1.0 mm ATP) followed by homogenization in a loose then tight fitting Potter-Elvehjem Teflon-glass apparatus. After centrifugation (10 min at 2200 rpm twice and 10 min at 5000 rpm) to isolate the subsarcolemmal mitochondria, the cardiac interfibrillar mitochondria were extracted after treatment with trypsin (5 mg/ml), homogenization, and centrifugation (10 min at 5000 rpm twice). Mitochondrial pellets then were suspended in 50 mm MOPS, pH 7.4, containing 100 mm KCl and 0.5 mm EGTA, and protein analysis was performed using the Lowry assay.
Oxidative phosphorylation of mitochondria was used to assess integrated mitochondrial function. Oxygen consumption of isolated cardiac interfibrillar mitochondria was measured in three separate experiments with a Clark-type oxygen electrode immersed in 50 mm MOPS, pH 7.4, containing 100 mm KCl, 1.0 mm EGTA, 5.0 mm K3PO4, and 1.0 mg/ml bovine serum albumin with 20 mm glutamate as substrate. State 3 respiration was measured after the addition of 180 μm ADP, and state 4 was measured upon ADP depletion. Respiratory control ratios reflecting the coupling of oxidation with phosphorylation were determined as ratios of state 3 respiration/state 4 respiration. Dinitrophenol (0.2 mm) was added to measure uncoupled respiration (38).
RESULTS
Rdh8-/-Abca4-/- mice exposed to a 12-h dark/12-h light (∼5 lux) cycle exhibited progressive severe retinal degeneration (Fig. 1A). The photoreceptor layer thinned to 4-6 rows of disorganized cells in 5-month-old mice and was absent in 12-month-old animals. Retinal degeneration under a normal 12-h dark/12-h light cycle was not obvious by the age of 4-6 weeks in these mice, but the same 4-week-old strain displayed acute severe retinal degeneration after exposure to bright light (Fig. 1B). Rod and cone photoreceptor cell death was observed in Rdh8-/-Abca4-/- mice illuminated at 10,000 lux for 15 min, whereas no degeneration was evident in wild type (WT) mice, even after 60 min of illumination (Fig. 1C). Photoreceptor cell death was detected promptly after light exposure, and a large number of photoreceptor cells were TUNEL-positive at 24 h after 10,000-lux light exposure for 60 min (Fig. 1D). Because rapid retinal degeneration was observed immediately after bright light exposure, we speculated that light-sensitive compounds within the eye might trigger this pathology. One such compound that accumulates in the eyes of Rdh8-/--Abca4-/- mice is A2E (24), and toxicity of oxidized A2E produced by light has been suggested as well (39-41). Therefore, we quantified A2E plus oxidized A2E and found A2E but no oxidized A2E in the eyes of 4-week-old and 3-month-old Rdh8-/-Abca4-/- mice after bright light exposure (Fig. 2, A-D). Moreover, although production of oxidized A2E from A2E had been reported for in vitro systems (6, 10, 11, 39, 42), amounts of A2E did not change after illumination (Fig. 2D), suggesting that neither A2E nor oxidized A2E causes light-induced acute retinal degeneration in Rdh8-/-Abca4-/- mice. No degeneration was observed in WT mice under these experimental conditions, and only mild retinal degeneration was noted in single knock-out Rdh8-/- or Abca4-/- mice (data not shown). The severity of light-induced retinal degeneration due to persisting phototransduction could affect the observed retinal degeneration, so we genetically eliminated rod-specific G protein transducin (Gnat1) by testing Gnat1-/-Rdh8-/-Abca4-/- mice. Gnat1 reportedly modulates retinal sensitivity to light-induced degeneration with 1000-3000-lux illumination (27). However, we observed no difference in retinal histology between Gnat1-/-Rdh8-/--Abca4-/- and Rdh8-/-Abca4-/- mice illuminated with 1000 lux for 2 or 4 weeks, indicating that Gnat1 and, in turn, activation of phototransduction did not alter sensitivity to light in Rdh8-/-Abca4-/- mice (data not shown).
FIGURE 1.
Age-dependent and acute retinal degeneration in Rdh8-/--Abca4-/- mice. A, retinal histology of 5- and 12-month-old Rdh8-/-Abca4-/- mice shows severe progressive degenerative changes compared with 1-month-old mice. Bars, 10 μm. OS, outer segment; IS, inner segment; INL, inner nuclear layer; ONL, outer nuclear layer. B, light exposure (10,000 lux for periods up to 60 min)-induced acute retinal degeneration in 4-week-old Rdh8-/-Abca4-/- mice. DAPI nuclear staining is shown. Mice were placed in the dark for 7 days after light exposure. Bars, 20 μm. C, immunohistochemical staining of retinal rhodopsin (anti-1D4 antibody; red), cone photoreceptors (peanut agglutinin lectin; green), and nuclear layer (DAPI; blue) was performed with 15-min illuminated (10,000 lux) 4-week-old Rdh8-/-Abca4-/- mice (left) and 60-min illuminated (10,000 lux) 4-week-old WT mice (right). Bars, 20 μm. D, TUNEL stain was applied to 4-week-old Rdh8-/-Abca4-/- retinas illuminated with 10,000 lux for 60 min. Eyes were collected at the indicated times after exposure. Bars, 20 μm.
FIGURE 2.
Detection of retinal A2E and oxidized A2E after light exposure by HPLC and LC-MS. Rdh8-/-Abca4-/- mice at 4 weeks and 3 months of age were exposed to light (10,000 lux for 60 min), and then A2E and oxidized A2E were analyzed by HPLC and liquid chromatography-mass spectrometry. Representative ion mass chromatograms at 592.6, corresponding to A2E [M + H]+ (A) with MS/MS pattern (B) and 608.5 and 624.5 for oxidized A2E (C), are shown for eyes of 4-week-old mice examined 24 h after light exposure. D, top, UV-visible spectrum of A2E from the Rdh8-/-Abca4-/- mouse at 0 min after light exposure (10,000 lux for 60 min) at the age of 3 months and a standard. Middle, representative HPLC chromatograms of A2E detected in Rdh8-/-Abca4-/- mice at 0 min after light exposure at the age of 3 months and a standard. Bottom, A2E quantities in eyes of 3-month-old mice assessed by HPLC are shown at 0 min, 24 h, and 3 days after illumination.
Thus, it appeared that another retinoid produced by light might be the trigger of this retinopathy. A likely candidate is the product derived from photoactivated rhodopsin, namely the isomerized chromophore, all-trans-retinal (4). To test if this retinoid might cause this acute light-induced retinopathy, we established Lrat-/--Rdh8-/-Abca4-/- triple knock-out mice (Fig. 3A). These triple knock-out animals lacked ocular retinoids (Fig. 3B), because LRAT is essential for retinoid storage in the eye (25). Gavage of 9-cis-retinyl acetate was effective in generating isorhodopsin in these triple knock-out mice (Fig. 3B) and in restoring visual function, as measured by ERG recordings (Fig. 3C). After triple knock-out and Lrat-/- mice (not shown) were gavaged with 2 mg of 9-cis-retinyl acetate and dark-adapted for 3 days, 9-cis-retinal was quantified at 339.8 ± 51.6 pmol/eye, and all-trans-retinal was undetectable. Thus, these triple knock-out mice can either be deprived or charged with artificial chromophore and thereby provide an excellent model to analyze the role of retinoids in light-induced retinopathy.
FIGURE 3.
The artificial chromophore precursor, 9-cis-retinyl acetate, preserves retinal structure and function in Lrat-/-Rdh8-/-Abca4-/- mice. A, retinal histology of 6-week-old WT, Lrat-/-, and Lrat-/-Rdh8-/-Abca4-/- mice are shown (top). Cone photoreceptor degeneration is evident in the inferior retina of 6-week-old triple knock-out and Lrat-/- mice. Cone photoreceptors and photoreceptor nuclei were stained with peanut agglutinin lectin (green) and DAPI (red), respectively. B, retinoid content of mouse eyes was determined by normal phase HPLC. Lrat-/-Rdh8-/-Abca4-/- mice at the age of 6 weeks were gavaged once with 2 mg of 9-cis-retinyl acetate and kept in the dark for 3 days before analyses. Peaks corresponding to 9-cis-retinal were detected in gavaged Lrat-/-Rdh8-/-Abca4-/- mice. atRE, all-trans-retinylester; 11cRAL, 11-cis-retinal; atRAL, all-trans-retinal; atROL, all-trans-retinol; 9cRAL, 9-cis-retinal; 9cRAc, 9-cis-retinyl acetate. C, scotopic ERGs were recorded from 6-week-old Lrat-/- Rdh8-/-Abca4-/- mice. Representative ERG traces before (right) and after gavage with 2 mg of 9-cis-retinyl acetate (left) are presented. Retinal function was significantly preserved by pretreatment with 9-cis-retinyl acetate.
Young triple knock-out mice exhibited a preserved retinal structure, with the exception that a lack of 11-cis-retinal caused cone photoreceptor cell death in the inferior retina at 6 weeks of age, as reported previously (Fig. 3, A and B) (19). When 9-cis-retinyl acetate-treated triple knockouts were exposed to 10,000 lux light for 12 min or 24 min to bleach visual pigment, 170.3 ± 13.6 and 287.6 ± 20.8 pmol/eye of all-trans-retinal were detected with a concomitant decrease in 9-cis-retinal (Fig. 4A). These results indicate that isorhodopsin can be converted by light into all-trans-retinal that accumulates. Some A2E also became detectable in the eye after three cycles of 9-cis-retinyl acetate gavage followed 3 days later by exposure to bright light. But the amounts were less than in untreated control 6-week-old Rdh8-/-Abca4-/- mice without retinal degeneration (Fig. 4B).
FIGURE 4.
Regeneration of isorhodopsin with 9-cis-retinyl acetate in Lrat-/-Rdh8-/-Abca4-/- mice. A, 6-week-old Lrat-/-Rdh8-/-Abca4-/- mice were gavaged once with 2 mg of 9-cis-retinyl acetate (9cRAc) and kept in the dark for 3 days. Then mice were exposed for 12 or 24 min to bright light (intensity 500 cd·m-2), and amounts of 9-cis-retinal and all-trans-retinal in the eyes were determined by normal phase HPLC. Means ± S.E. are presented (n > 3). B, HPLC was used to quantify amounts of A2E in the eyes of 6-week-old Lrat-/-Rdh8-/-Abca4-/- mice after the indicated numbers of cycles (left and center), each consisting of a 2-mg 9-cis-retinyl acetate gavage followed by 3 days of dark adaptation and then a 24-min exposure to bright light (intensity 500 cd·m-2). A2E levels in untreated control mice (double knockouts; 6-week-old Rdh8-/-Abca4-/- mice) are shown on the right. Means ± S.E. are presented (n > 3).
Next, eyes of 6-week-old triple knock-out mice and Lrat-/- controls gavaged with 9-cis-retinyl acetate and exposed to bright light were examined histologically. Only the triple knock-out mice treated with 9-cis-retinyl acetate showed regional retinal degeneration (Fig. 5A) similar to that seen in light-exposed Rdh8-/-Abca4-/- mice (Fig. 1B). The severity of retinal degeneration was similar to that in triple knock-out mice undergoing either one or three cycles of 9-cis-retinyl acetate gavage and subsequent illumination, but the incidence of retinal degeneration was increased in mice undergoing three cycles of this regimen (66.7% for 1 cycle versus 100% for 3 cycles) (Fig. 5B). No degeneration was detected in triple knock-out mice that were not gavaged with 9-cis-retinyl acetate or in Lrat-/- mice with or without such treatment (Fig. 5A). Together, these results suggest that retinoids produced by light in triple knock-out mice and, most likely, in Rdh8-/-Abca4-/- mice as well can cause light-induced acute retinal degeneration. Moreover, the obvious agent triggering this process appears to be all-trans-retinal.
FIGURE 5.
Induction of light-induced acute retinal degeneration in 9-cis-retinyl acetate-gavaged Lrat-/-Rdh8-/-Abca4-/- mice. A, 6-week-old Lrat-/-Rdh8-/-Abca4-/- and Lrat-/- mice were gavaged once with 2 mg of 9-cis-retinyl acetate (9cRAc) and kept in the dark for 3 days. Then mice were illuminated with light at an intensity of 10,000 lux. After 7 days of dark adaptation, retinal morphology was viewed after DAPI staining of cryosections. Representative images are shown (n > 5). B, incidence of light-induced retinal degeneration (numbers of mice with retinal degeneration/total numbers of tested animals) is shown for Lrat-/-Rdh8-/- Abca4-/- mice receiving 1 or 3 cycles of gavage with 2 mg of 9-cis-retinyl acetate followed by 3 days of dark adaptation and then a 24-min exposure to bright light (intensity 500 cd·m-2).
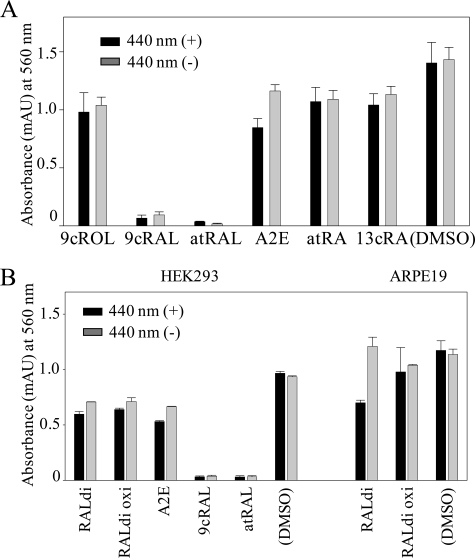
Experimental cell lines were used to assess possible mechanisms of toxicity induced by all-trans-retinal and other retinoids formed from this precursor. First, human ARPE19 cells were treated with candidate retinoids produced by bleaching the retina in vivo. Surprisingly, 9-cis-retinal and all-trans-retinal evidenced strong cytotoxicity either without or after blue light exposure, whereas other compounds were less toxic (Fig. 6A). Blue light was tested, because it can enhance the production of oxidative retinoid products. The same phenomenon was observed in HEK293 cells derived from human kidney (Fig. 6B). Dose-dependent cell death was demonstrated in ARPE19 cells incubated for 16 h at 37 °C with up to 30 μm all-trans-retinal (Fig. 7A). These results that free reactive retinal aldehydes can cause cell death in various cell lines. Similarly, accumulated all-trans-retinal resulting from its delayed clearance in Rdh8-/-Abca4-/- mice can also be a cause of light-induced acute retinal degeneration.
FIGURE 6.
Viability of retinoid-laden ARPE19 and HEK293 cells. A, retinoid-laden ARPE19 cells were produced by the published methods (34, 35) with modifications. Cells were incubated for 16 h at 37 °C with a 30 μm concentration of the indicated retinoids followed by 3 days of incubation with medium alone. Cells then were either exposed or not exposed to blue light (440 nm; X-Cite120 Fluorescence illumination system with 440-nm filters) for 5 min, and MTT assays were performed after 16 h of additional incubation in the dark. Some blue light-dependent cell death was observed with A2E-loaded cells, whereas nearly all cells loaded with either 9-cis-retinal (9cRAL) or all-trans-retinal (atRAL) died whether or not they were exposed to light. Means ± S.E. are shown (n > 3). B, A2E, all-trans-retinal dimer (RALdi), and oxidized RALdi-laden HEK293 cells (human renal epithelial cells) and ARPE19 cells (human RPE cells) were produced by a 16-h incubation with a 30 μm concentration of the indicated retinoids followed by a 3-day incubation with fresh medium. 9-cis-Retinal and all-trans-retinal were added to the medium just before cells were either exposed or not exposed to blue light (440 nm) for 5 min and then analyzed by MTT assays. Strong cytotoxicity was evidenced by 9-cis-retinal- and all-trans-retinal-laden HEK293 cells independent of light exposure (left). Blue light-dependent cell death was noted in all-trans-retinal dimer-loaded ARPE19 cells (right). Means ± S.E. are shown (n > 5).
Because strong toxicity of all-trans-retinal was observed in ARPE19 cells (Fig. 6) and because free aldehyde changes membrane stability and may promote calcium influx associated with cell apoptosis (43, 44), in vitro calcium concentration was monitored by using the cell-permeable dye, Fluo-3 AM. All-trans-retinal and calcium ionophore A23187 increased the fluorescent intensity of cells loaded with Fluo-3, indicating an increased intracellular calcium concentration, whereas all-trans-retinol, rapidly produced from all-trans-retinal in the eye, had no significant effect (Fig. 8A). This increase in fluorescence elevation occurred rapidly (Fig. 8B) and in a dose-dependent manner (Fig. S1).
FIGURE 8.
All-trans-retinal rapidly increases intracellular calcium. A, ARPE19 cells were incubated with 2 μm cell-permeable Fluo-3 for 30 min at 37 °C and then washed three times with medium. All-trans-retinal (atRAL) (30 μm), all-trans-retinol (atROL) (30 μm), calcium ionophore A23187 (2 μm), and DMSO were added to cell cultures and incubated for 15 min at 37 °C. Fluorescent intensity of cells after three medium washes was detected with a Leica inverted microscope. B, Fluo-3-loaded ARPE19 cells were incubated with all-trans-retinal (30 μm) for the indicated durations at 37 °C. Fluorescent intensity of cells after three medium washes was detected by a Leica inverted microscope.
Next, we measured caspase activity as a surrogate marker for an apoptotic mechanism of all-trans-retinal-induced cell death. All-trans-retinal at 30 μm, a concentration that equates to that after <0.5% of rhodopsin is bleached, induced caspase activity at a level similar to that shown by STS (Fig. 9A), a well known apoptosis inducer (45). Moreover, cell death induced by all-trans-retinal and STS was completely inhibited by Z-VAD-fluoromethyl ketone, a caspase inhibitor (Figs. 9, B and C). Because Bax is a proapoptotic member of the Bcl-2 family proteins central to mitochondria-dependent apoptosis (46), we then tested the effect on cell viability of BIP, a Bax inhibitor composed of five amino acids designed from the Bax-binding domain of the Bax suppressor, Ku70 (47, 48). BIP completely inhibited all-trans-retinal-induced cell death (Figs. 7B and 9C), indicating that all-trans-retinal induces Bax-dependent apoptosis involving the intrinsic mitochondria/ER pathway.
Oxidative phosphorylation also was assessed to examine the effects of all-trans-retinal and A2E on mitochondria. Mitochondria were isolated from rat heart, and glutamate was used as the oxidizable substrate. Glutamate oxidation reflects a combination of mitochondrial functions, including glutamate uptake, glutamate dehydrogenase activity, electron transport, and ATP biosynthesis. The addition of all-trans-retinal decreased state 3 respiratory rates, respiratory control ratios (state 3/state 4), and dinitrophenol-induced oxidation in a dose-dependent manner, whereas A2E did not affect these parameters even at a 600 μm concentration (Table 1). In addition, all-trans-retinal uncoupled oxidative phosphorylation at concentrations of >50 μm. These data indicate that all-trans-retinal both inhibits mitochondrial oxidation and, above a certain concentration, uncouples oxidative phosphorylation. Of interest, A2E neither inhibited oxidation nor uncoupled oxidative phosphorylation.
TABLE 1.
Oxidative properties of mitochondria isolated from the hearts of Sprague Dawley rats
Assays were carried out as described under “Experimental Procedures.” Glutamate was used as substrate. RCR, respiratory control ratio (state 3/state 4); ADP/O, ADP to atomic oxygen phosphorylation ratio (54); DNP, dinitrophenol. Respiratory rates are expressed as nanoatoms of oxygen/min/mg of mitochondrial protein. n = 3 (mean ± S.E. of measurement).
| State 3 | State 4 | RCR | ADP/O | DNP | |
|---|---|---|---|---|---|
| All-trans-retinal | |||||
| 0 μm | 225.0 ± 31.6 | 28.5 ± 4.0 | 8.2 ± 1.5 | 3.1 ± 0.1 | 284.9 ± 49.8 |
| 12 μm | 196.3 ± 21.0 | 29.0 ± 3.6 | 7.1 ± 1.5 | 3.5 ± 0.5 | 256.4 ± 32.5 |
| 25 μm | 187.3 ± 19.4 | 33.0 ± 3.0 | 5.7 ± 0.2 | 3.4 ± 0.1 | 222.0 ± 23.4 |
| 50 μm | 178.3 ± 14.2a | 36.3 ± 4.9 | 5.5 ± 1.4 | 3.1 ± 0.02 | 201.0 ± 26.7a |
| 75 μm | 153.0 ± 13.4a | 87.0 ± 3.0a | 1.8 ± 0.1a | 2.9 ± 0.2 | 30.0 ± 6.0a |
| 100 μm | 45.0 ± 10.4a | 45.0 ± 10.4 | 1 ± 0a | 0 ± 0a | 5.5 ± 0.1a |
| A2E | |||||
| 120 μm | 228.0 ± 21.6 | 40.7 ± 4.3 | 5.7 ± 0.5 | 3.1 ± 0.1 | 290.9 ± 30.3 |
| 240 μm | 225.0 ± 27.5 | 37.7 ± 3.8 | 6.0 ± 0.1 | 2.9 ± 0.1 | 296.9 ± 31.5 |
| 600 μm | 213.0 ± 19.7 | 39.0 ± 3.0 | 5.5 ± 0.1 | 3.0 ± 0.1 | 272.9 ± 21.5 |
p < 0.05.
DISCUSSION
Although a large body of evidence supports a correlation between toxic retinoids and various retinopathies, there is no direct link between them. As noted previously, light exacerbated retinopathy in Rdh8-/-Abca4-/- double knock-out mice that manifested retinal abnormalities, including A2E/RALdi/lipofuscin accumulation, RPE/photoreceptor atrophy, and other abnormalities (24). Herein we describe an acute, light-dependent retinopathy in these mice, and a set of results with triple knock-out engineered mice that clearly demonstrate that acute retinopathy in these animals is induced by free all-trans-retinal and not, as generally believed, by A2E or other aldehyde condensation products. At a rhodopsin concentration close to 5 mm (49), even <0.5% bleach would generate all-trans-retinal at concentrations exceeding those used in our experiments if this retinoid is not properly cleared. Finally, we show that one possible mechanism of toxicity involves plasma membrane permeability and mitochondrial poisoning that lead to caspase activation and mitochondria-associated cell death.
Rapid Retinal Degeneration Induced by Light in Rdh8-/--Abca4-/- Mice—Mice lacking either ABCA4 or RDH8 alone failed to show much retinal pathology despite accumulation of A2E (Abca4-/- mice) (50) or delayed clearance of all-trans-retinal (Rdh8-/- mice) (32). The present study demonstrates that delayed clearance of all-trans-retinal due to lack of both ABCA4 and RDH8 causes two types of retinal degeneration (i.e. chronic degeneration and bright light-induced acute retinal degeneration) (Fig. 1, A and B). As for acute retinal degeneration, light exposure for even 5 min at 10,000 lux initiated photoreceptor cell death, as indicated by apoptotic photoreceptors (Fig. 1D). These observations show that vitamin A-related products formed upon exposure to light are strongly associated with light-induced acute retinal degeneration in Rdh8-/--Abca4-/- mice. A2E, believed to be toxic to the RPE, was the first causative candidate considered, but formation of A2E takes days because it involves rod outer segment phagocytosis by the RPE. Oxidized A2E, a second candidate, should be produced quickly by light, but no oxidized A2E was detected in Rdh8-/-Abca4-/- mice, either before or after light exposure (Fig. 2). Therefore, A2E may not be involved in the pathogenesis of the rapid retinal degeneration induced by light in these animals.
Light-induced Acute Retinal Degeneration in Lrat-/--Rdh8-/-Abca4-/- Mice—Lrat-/- mice have no retinoids in their eyes (25), because LRAT is an important enzyme involved in retinoid storage in the eye. Therefore, we generated Lrat-/- Rdh8-/-Abca4-/- mice to investigate if retinoids in the retinoid cycle cause retinal degeneration in Rdh8-/-Abca4-/- mice. These triple knock-out mice did not have retinoids in the eye, but they did produce isorhodopsin after gastric gavage with 9-cis-retinyl acetate (Fig. 3B). Moreover, the isorhodopsin was biologically active, because visual function was detected by ERG recordings, and light illumination induced all-trans-retinal formation (Figs. 3C and 4). These data indicate that 9-cis-retinyl acetate-gavaged Lrat-/-Rdh8-/-Abca4-/- mice should have a visual physiology similar to that of Rdh8-/-Abca4-/- mice. Therefore, an excellent way of testing toxicity of retinoids in Rdh8-/-Abca4-/- mice would be to compare their effects in Lrat-/-Rdh8-/-Abca4-/- mice either exposed or not exposed to bright light after gavage with/without 9-cis-retinal.
Light-induced acute retinal degeneration was observed in Lrat-/-Rdh8-/-Abca4-/- mice only after they were gavaged with 9-cis-retinyl acetate. Two observations implicate all-trans- retinal as a cause of this acute degeneration; namely Rdh8-/--Abca4-/- mice evidence a delay in all-trans-retinal clearance (24), and free retinals are strongly toxic to cultured cells (Fig. 6). All-trans-retinal caused the death of cultured cells in a time- and dose-dependent manner (Figs. 6 and 7).3 Concentrations of all-trans-retinal needed to produce this effect after a 16-h incubation at 37 °C ranged from 5 to 30 μm. Although apparently high, these concentrations represent only a minor fraction of the concentration of free all-trans-retinal released from mouse opsin in vivo.
Surprisingly, just a single exposure to all-trans-retinal (287.6 ± 20.8 pmol/eye) induced photoreceptor death in Lrat-/-Rdh8-/-Abca4-/- mice (Fig. 5). Additionally, light-induced retinal changes in Rdh8-/- mice were of a severity similar to those noted in Lrat-/-Rdh8-/-Abca4-/- mice gavaged with a single dose of 9-cis-retinal (24). Although rates of all-trans-retinal clearance were equally delayed in Rdh8-/- and Rdh8-/-Abca4-/- mice (24), transport of all-trans-retinal from inner to outer disc membranes proceeds normally in Rdh8-/- mice because of the presence of ABCA4. A more prolonged presence of all-trans-retinal in the discs may account for the enhanced photoreceptor death seen in Rdh8-/-Abca4-/- mice. Thus, it is relevant that Rdh8-/-Abca4-/- mice exhibited much more severe retinal degeneration after exposure to bright light for 60 min.
Mitochondrial Poisoning by All-trans-retinal—Mitochondrial impairment has been suggested as an important mechanism for neurodegeneration (51). Related to our study, previous work showed that carotenoid cleavage products, such as highly reactive aldehydes and epoxides, cause oxidative stress by impairing mitochondrial function (52). Evidence is presented here that one mechanism of cell death induced by all-trans-retinal involves an intrinsic apoptotic pathway associated with mitochondrial impairment due to a rapid increase of intracellular calcium concentration (Figs. 8 and 9). In addition to acute photoreceptor degeneration caused by intense exposure to all-trans-retinal, prolonged exposure to low concentrations may lead to chronic photoreceptor and RPE cell death. Interestingly, although mitochondrial impairment was caused by all-trans-retinal, no damage was detected in purified rat heart mitochondria exposed to A2E (Table 1). Loss of the all-trans-RDHs, RDH8 and RDH12, facilitated conversion of all-trans-retinal to all-trans-retinol in the RPE (28), suggesting that all-trans-retinal diffuses into the RPE after excessive production in photoreceptors. All-trans-retinal may also impair the RPE in Rdh8-/-Abca4-/- mice, contributing to acute and chronic retinal dystrophy.
Are A2E and Other All-trans-retinal Condensation Products Instigators or Just Surrogates of Retinal Degeneration?—A2E accumulation has been detected in Rdh8-/-Abca4-/- and other mice with delayed all-trans-retinal clearance (20, 24, 28, 32, 50), and toxicity of A2E/oxidized A2E and ensuing immunoreactivity has been reported as well (10, 11, 39, 53). However, oxidized A2E was not detected in 4-week- and 3-month-old Rdh8-/-Abca4-/- mice, either before or after light exposure (Fig. 2). In control experiments, we have found light-induced production of oxidized A2E at 365, 405, and 440 nm, as reported previously. Therefore, production of toxic oxidized A2E byproducts may be largely reduced in vivo. Analyses of untreated Rdh8-/-Abca4-/- mice and Lrat-/-Rdh8-/--Abca4-/- mice gavaged with 9-cis-retinyl acetate indicate that all-trans-retinal is a cause of retinal degeneration, so A2E may be either just a surrogate for or a contributor to retinal degeneration induced by all-trans-retinal. Several studies showed that A2E is toxic to RPE cells in culture and that blue light increases this toxicity (11, 39, 53). However, this study strongly indicates that these products are less toxic than all-trans-retinal (Fig. 6 and Table 1). Facilitating transport of all-trans-retinal from inner to outer disc membranes, inhibition of all-trans-retinal production, and removal of excess all-trans-retinal from the retina all constitute possible therapies for Stargardt disease and age-related macular degeneration. This work also suggests that A2E production may lower all-trans-retinal toxicity. Although A2E formation could be a surrogate marker for aberrations in all-trans-retinal clearance, it may also represent a photoreceptor detoxification product and not the primary toxin previously supposed.
Supplementary Material
Acknowledgments
We thank Dr. J. Lem for Gnat1-/- mice; Drs. L. T. Webster and J. von Lintig (Case Western Reserve University) for comments on the manuscript; and Drs. K. Lodowski, S. Shiose, S. Howell, S. Roos, M. Matosky, M. Singer, and H. Matsuyama (Case Western Reserve University) for technical support and advice about some of the procedures employed.
This work was supported, in whole or in part, by National Institutes of Health Grants K08EY019031, EY09339, P30 EY11373, and EY08123.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: RPE, retinal pigment epithelium; A2E, diretinoid-pyridinium-ethanolamine; RALdi, all-trans-retinal dimer; WT, wild type; HPLC, high pressure liquid chromatography; DMEM, Dulbecco's modified Eagle's medium; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; STS, staurosporin; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; Z, benzyloxycarbonyl; BIP, Bax-inhibiting peptide; MOPS, 4-morpholinepropanesulfonic acid; DAPI, 4′,6-diamidino-2-phenylindole; cd, candela; ERG, electroretinogram; TUNEL, terminal deoxynucleotidyltransferase biotin-dUTP nick end labeling.
A. Maeda, T. Maeda, M. Golczak, S. Chou, A. Desai, C. L. Hoppel, S. Matsuyama, and K. Palczewski, unpublished results.
References
- 1.Travis, G. H., Golczak, M., Moise, A. R., and Palczewski, K. (2007) Annu. Rev. Pharmacol. Toxicol. 47 469-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBee, J. K., Palczewski, K., Baehr, W., and Pepperberg, D. R. (2001) Prog Retin Eye Res 20 469-529 [DOI] [PubMed] [Google Scholar]
- 3.Thompson, D. A., and Gal, A. (2003) Prog. Retin. Eye Res. 22 683-703 [DOI] [PubMed] [Google Scholar]
- 4.Palczewski, K. (2006) Annu. Rev. Biochem. 75 743-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mata, N. L., Weng, J., and Travis, G. H. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 7154-7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim, S. R., Jang, Y. P., Jockusch, S., Fishkin, N. E., Turro, N. J., and Sparrow, J. R. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 19273-19278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishkin, N. E., Sparrow, J. R., Allikmets, R., and Nakanishi, K. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 7091-7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yannuzzi, L. A., Ober, M. D., Slakter, J. S., Spaide, R. F., Fisher, Y. L., Flower, R. W., and Rosen, R. (2004) Am. J. Ophthalmol. 137 511-524 [DOI] [PubMed] [Google Scholar]
- 9.Finnemann, S. C., Leung, L. W., and Rodriguez-Boulan, E. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 3842-3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, S. R., Nakanishi, K., Itagaki, Y., and Sparrow, J. R. (2006) Exp. Eye Res. 82 828-839 [DOI] [PubMed] [Google Scholar]
- 11.Kim, S. R., Jockusch, S., Itagaki, Y., Turro, N. J., and Sparrow, J. R. (2008) Exp. Eye Res. 86 975-982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allikmets, R. (2000) Am. J. Hum. Genet. 67 487-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baehr, W., Wu, S. M., Bird, A. C., and Palczewski, K. (2003) Vision Res. 43 2957-2958 [DOI] [PubMed] [Google Scholar]
- 14.Rohrer, B., Lohr, H. R., Humphries, P., Redmond, T. M., Seeliger, M. W., and Crouch, R. K. (2005) Invest. Ophthalmol. Vis. Sci. 46 3876-3882 [DOI] [PubMed] [Google Scholar]
- 15.Fan, J., Woodruff, M. L., Cilluffo, M. C., Crouch, R. K., and Fain, G. L. (2005) J. Physiol. 568 83-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann, K. P., Pulvermuller, A., Buczylko, J., Van Hooser, P., and Palczewski, K. (1992) J. Biol. Chem. 267 15701-15706 [PubMed] [Google Scholar]
- 17.Palczewski, K., Jager, S., Buczylko, J., Crouch, R. K., Bredberg, D. L., Hofmann, K. P., Asson-Batres, M. A., and Saari, J. C. (1994) Biochemistry 33 13741-13750 [DOI] [PubMed] [Google Scholar]
- 18.Jager, S., Palczewski, K., and Hofmann, K. P. (1996) Biochemistry 35 2901-2908 [DOI] [PubMed] [Google Scholar]
- 19.Zhang, H., Fan, J., Li, S., Karan, S., Rohrer, B., Palczewski, K., Frederick, J. M., Crouch, R. K., and Baehr, W. (2008) J. Neurosci. 28 4008-4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda, A., Maeda, T., Imanishi, Y., Sun, W., Jastrzebska, B., Hatala, D. A., Winkens, H. J., Hofmann, K. P., Janssen, J. J., Baehr, W., Driessen, C. A., and Palczewski, K. (2006) J. Biol. Chem. 281 37697-37704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenzel, A., Reme, C. E., Williams, T. P., Hafezi, F., and Grimm, C. (2001) J. Neurosci. 21 53-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rattner, A., Smallwood, P. M., and Nathans, J. (2000) J. Biol. Chem. 275 11034-11043 [DOI] [PubMed] [Google Scholar]
- 23.Molday, R. S. (2007) J. Bioenerg. Biomembr. 39 507-517 [DOI] [PubMed] [Google Scholar]
- 24.Maeda, A., Maeda, T., Golczak, M., and Palczewski, K. (2008) J. Biol. Chem. 283 26684-26693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batten, M. L., Imanishi, Y., Maeda, T., Tu, D. C., Moise, A. R., Bronson, D., Possin, D., Van Gelder, R. N., Baehr, W., and Palczewski, K. (2004) J. Biol. Chem. 279 10422-10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batten, M. L., Imanishi, Y., Tu, D. C., Doan, T., Zhu, L., Pang, J., Glushakova, L., Moise, A. R., Baehr, W., Van Gelder, R. N., Hauswirth, W. W., Rieke, F., and Palczewski, K. (2005) PLoS Med. 2 e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao, W., Wenzel, A., Obin, M. S., Chen, C. K., Brill, E., Krasnoperova, N. V., Eversole-Cire, P., Kleyner, Y., Taylor, A., Simon, M. I., Grimm, C., Reme, C. E., and Lem, J. (2002) Nat. Genet. 32 254-260 [DOI] [PubMed] [Google Scholar]
- 28.Maeda, A., Maeda, T., Sun, W., Zhang, H., Baehr, W., and Palczewski, K. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 19565-19570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golczak, M., Kuksa, V., Maeda, T., Moise, A. R., and Palczewski, K. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 8162-8167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golczak, M., Imanishi, Y., Kuksa, V., Maeda, T., Kubota, R., and Palczewski, K. (2005) J. Biol. Chem. 280 42263-42273 [DOI] [PubMed] [Google Scholar]
- 31.Verdegem, P. J. E., Monnee, M. C. F., Mulder, P. P. J., and Lugtenburg, J. (1997) Tetrahedron Lett. 38 5355-5358 [Google Scholar]
- 32.Maeda, A., Maeda, T., Imanishi, Y., Kuksa, V., Alekseev, A., Bronson, J. D., Zhang, H., Zhu, L., Sun, W., Saperstein, D. A., Rieke, F., Baehr, W., and Palczewski, K. (2005) J. Biol. Chem. 280 18822-18832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda, T., Lem, J., Palczewski, K., and Haeseleer, F. (2005) Invest. Ophthalmol. Vis. Sci. 46 4320-4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparrow, J. R., Parish, C. A., Hashimoto, M., and Nakanishi, K. (1999) Invest. Ophthalmol. Vis. Sci. 40 2988-2995 [PubMed] [Google Scholar]
- 35.Sparrow, J. R., Nakanishi, K., and Parish, C. A. (2000) Invest. Ophthalmol. Vis. Sci. 41 1981-1989 [PubMed] [Google Scholar]
- 36.Deveraux, Q. L., Takahashi, R., Salvesen, G. S., and Reed, J. C. (1997) Nature 388 300-304 [DOI] [PubMed] [Google Scholar]
- 37.Palmer, J. W., Tandler, B., and Hoppel, C. L. (1977) J. Biol. Chem. 252 8731-8739 [PubMed] [Google Scholar]
- 38.Hoppel, C. L., and Tomec, R. J. (1972) J. Biol. Chem. 247 832-841 [PubMed] [Google Scholar]
- 39.Sparrow, J. R., Zhou, J., Ben-Shabat, S., Vollmer, H., Itagaki, Y., and Nakanishi, K. (2002) Invest. Ophthalmol. Vis. Sci. 43 1222-1227 [PubMed] [Google Scholar]
- 40.Ben-Shabat, S., Parish, C. A., Vollmer, H. R., Itagaki, Y., Fishkin, N., Nakanishi, K., and Sparrow, J. R. (2002) J. Biol. Chem. 277 7183-7190 [DOI] [PubMed] [Google Scholar]
- 41.Ben-Shabat, S., Itagaki, Y., Jockusch, S., Sparrow, J. R., Turro, N. J., and Nakanishi, K. (2002) Angew Chem. Int. Ed. Engl. 41 814-817 [DOI] [PubMed] [Google Scholar]
- 42.Kim, S. R., He, J., Yanase, E., Jang, Y. P., Berova, N., Sparrow, J. R., and Nakanishi, K. (2007) Biochemistry 46 10122-10129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruman, I. I., and Mattson, M. P. (1999) J. Neurochem. 72 529-540 [DOI] [PubMed] [Google Scholar]
- 44.Holownia, A., Ledig, M., Braszko, J. J., and Menez, J. F. (1999) Brain Res. 833 202-208 [DOI] [PubMed] [Google Scholar]
- 45.Weil, M., Jacobson, M. D., Coles, H. S., Davies, T. J., Gardner, R. L., Raff, K. D., and Raff, M. C. (1996) J. Cell Biol. 133 1053-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tawa, P., Tam, J., Cassady, R., Nicholson, D. W., and Xanthoudakis, S. (2001) Cell Death Differ. 8 30-37 [DOI] [PubMed] [Google Scholar]
- 47.Gomez, J. A., Gama, V., Yoshida, T., Sun, W., Hayes, P., Leskov, K., Boothman, D., and Matsuyama, S. (2007) Biochem. Soc. Trans. 35 797-801 [DOI] [PubMed] [Google Scholar]
- 48.Li, Y., Yokota, T., Gama, V., Yoshida, T., Gomez, J. A., Ishikawa, K., Sasaguri, H., Cohen, H. Y., Sinclair, D. A., Mizusawa, H., and Matsuyama, S. (2007) Cell Death Differ. 14 2058-2067 [DOI] [PubMed] [Google Scholar]
- 49.Nickell, S., Park, P. S., Baumeister, W., and Palczewski, K. (2007) J. Cell Biol. 177 917-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weng, J., Mata, N. L., Azarian, S. M., Tzekov, R. T., Birch, D. G., and Travis, G. H. (1999) Cell 98 13-23 [DOI] [PubMed] [Google Scholar]
- 51.Fukui, H., and Moraes, C. T. (2008) Trends Neurosci. 31 251-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siems, W., Sommerburg, O., Schild, L., Augustin, W., Langhans, C. D., and Wiswedel, I. (2002) FASEB J. 16 1289-1291 [DOI] [PubMed] [Google Scholar]
- 53.Zhou, J., Jang, Y. P., Kim, S. R., and Sparrow, J. R. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 16182-16187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Estabrook, R. W. (1967) Methods Enzymol. 10 41-47 [Google Scholar]
- 55.Allikmets, R., Shroyer, N. F., Singh, N., Seddon, J. M., Lewis, R. A., Bernstein, P. S., Peiffer, A., Zabriskie, N. A., Li, Y., Hutchinson, A., Dean, M., Lupski, J. R., and Leppert, M. (1997) Science 277 1805-1807 [DOI] [PubMed] [Google Scholar]
- 56.Allikmets, R., Singh, N., Sun, H., Shroyer, N. F., Hutchinson, A., Chidambaram, A., Gerrard, B., Baird, L., Stauffer, D., Peiffer, A., Rattner, A., Smallwood, P., Li, Y., Anderson, K. L., Lewis, R. A., Nathans, J., Leppert, M., Dean, M., and Lupski, J. R. (1997) Nat. Genet. 15 236-246 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.