Abstract
Despite serious health risks in humans and wild life, the underlying mechanisms that explain the gene-environment effects of chemical toxicants are largely unknown. Polychlorinated biphenyls (PCBs) are one of the most ubiquitous environmental toxicants worldwide, with reported epidemiological evidence for reproductive and neurocognitive anomalies in humans. Here, we show that Aroclor 1254, a mixture of structurally distinct PCBs, causes preterm birth in interleukin (IL)-10-/- mice at a dose that does not show any adverse effects in wild type mice, highlighting the significance of IL-10 as an anti-toxicant cytokine. Aroclor 1254-treated IL-10-/- mice demonstrated increased amniotic fluid, intrauterine growth restriction, and reduced litter size with postnatal neuromotor defects. Further, our results identify aquaporin 1 (AQP1), a potent effector of fluid volume regulation and angiogenic activity, as a novel placental target of PCBs. In vivo or in vitro exposure to Aroclor 1254 coupled with IL-10 deficiency significantly reduced the protein content of AQP1. Reduced uterine AQP1 levels were associated with defective spiral artery transformation. Importantly, recombinant IL-10 reversed PCB-induced in vivo and in vitro effects. These data demonstrate for the first time that the IL-10-AQP1 axis is a novel regulator of PCB-induced in utero effects.
The health consequences of environmental toxicants are likely to have critical effects during in utero fetal development because of the complex signaling cascades, high cellular proliferation rates, and differentiation events. Mammalian reproduction involves a complex but highly choreographed sequence of molecular processes. These processes include interactions between the hormonally stimulated uterus and the developing blastocyst, implantation, placental and fetal development, and parturition (1, 2). Although the hormonal milieu, metabolic changes, and placental microenvironment are programmed in a pregnancy compatible manner, pregnancy presents itself as an immunological and hormonal paradox (3, 4). The role of steroid hormones is well known in uterine receptivity, implantation, local immune modulation, and pregnancy success (5). If not temporally produced and regulated, their dysfunction lead to infertility or pregnancy loss. Man-made chemicals like polychlorinated biphenyls (PCBs)2 act like hormones and interfere with their cognate receptor functions impacting normal biological processes (6, 7). Although the genotoxic effects of PCBs have been investigated intensively and epidemiological studies have highlighted their health risks (6, 7), the mechanisms responsible for reproductive and neurodevelopmental effects still remain enigmatic. The overarching goal of our studies is to identify unknown pathways and targets that impart adverse effects on pregnancy. In this study, we directed our efforts toward establishing an experimental system to evaluate the in utero gene-environment effects of PCBs using wild type mice and their counterparts deficient in pregnancy compatible anti-inflammatory cytokines such as interleukin 10 (IL-10).
IL-10 is a potent anti-inflammatory cytokine that controls inflammatory insult in most organs, particularly at the maternal-fetal interface. IL-10 is produced by gestational tissue and maternal immune cells in the intrauterine microenvironment in humans (8, 9) and in mice (10). We and others have reported that IL-10-/- mice experience preterm birth and resorptions in response to low doses of inflammatory triggers such as lipopolysaccharide (LPS) (11, 12) or poly(I-C) (13). Importantly, the pregnancy outcome in treated IL-10-/- mice can be rescued by giving an exogenous dose of IL-10 (11, 14). We have also demonstrated poor IL-10 production in placental and decidual tissues from preterm labor deliveries and missed abortions (15, 16). These data suggest that an inflammatory environment coupled with genetic stress (IL-10 deficiency) may lead to adverse pregnancy outcomes. In consideration of these observations, we hypothesize that exposure to toxicants such as PCBs mimics the physiological counterpart of inflammation that predisposes to adverse pregnancy outcomes when combined with genetic deficiency in loci crucial for pregnancy success such as IL-10.
PCBs are chlorinated aromatic hydrocarbon compounds consisting of a group of 209 structurally diverse congeners, identified based on the position of chlorine atoms (7). Since the start of their manufacture in the 1920s until their ban in late 1970s, PCBs were globally valued for their noninflammability and high heat and chemical stability and thus were used widely in a multitude of commercial and industrial applications (7, 17). Improper disposal and accidental release of these compounds led to their introduction into the environment, placing them in the list of widespread environmental contaminants. Subsequently, their lipophilicity facilitated their bioaccumulation in the food chain and bio-concentration at successively higher levels (6, 18-21). PCBs have now been detected globally, in different environmental matrices, wild life, food, and humans (6, 18, 20). Convincing evidence exist for their toxicity, both in humans as well as in laboratory animals (7). From epidemiological studies in humans it has been observed that exposure to PCBs causes various reproductive anomalies that include irregular and shorter menstrual cycles, delayed conception, miscarriage, reduced lactating time, low birth weight, preterm birth, small for gestational age infants, and higher incidence of still-births and mortality among children (22-27). PCB congeners may work in an aryl hydrocarbon receptor-dependent or -independent pathway (6, 7, 28). Despite the knowledge that PCBs affect either aryl hydrocarbon receptor or estrogen receptor signaling, there is a paucity of molecular mechanisms underlying the most sensitive developmental effects of PCBs, and thus new pathways and targets need to be identified.
Aroclor 1254 is a mixture of more than one hundred different PCB congeners and may impart cumulative adverse effects on female reproductive health (29, 30). In this study, we show that Aroclor 1254 exposure induces preterm birth in IL-10-/- mice with reduced litter size and birth weight, increased amniotic fluid, and postnatal neurocognitive defects. Importantly, we have identified aquaporin 1 (AQP1) as a novel target of PCB action at the maternal-fetal interface. Our findings for the first time provide direct experimental evidence for a protective role of IL-10 against PCB exposure. These findings may have implications for the understanding and management of environmental toxicant-induced female reproductive anomalies in humans.
EXPERIMENTAL PROCEDURES
Mice and in Vivo Procedures—All of the animal protocols were approved by the Lifespan Institutional Animal Care and Use Committee. The mice were housed and mated in a specific pathogen-free facility under the care of the Central Research Department of Rhode Island Hospital. All of the mating experiments were repeated at least three times with at least three mice/treatment. The day of vaginal plug appearance was designated gestational day (gd) 0. We administered daily intraperitoneal injection of 500 μg of Aroclor 1254 (Sigma-Aldrich)/mouse or an equivalent volume (100 μl) of control vehicle (corn oil) to pregnant C57BL/6 wild type mice or their IL-10-/- counterparts from gd 4 to 12. We allowed one set of mice in all matings to deliver the pups. We recorded the time of birth and the litter size. Newborn pups were observed for loss of righting reflex during the first 21 days of their lives. The pups were placed on a horizontal surface, and the righting reflex was expressed in terms of time required for a pup to turn over its four feet on the ground when placed on its side (33).
We euthanized another set of pregnant mice (n = 6) on gd 13 for molecular and immunological analyses. Uterine horns were examined for placental or fetal pathology. We collected amniotic fluid on gd 13 by using tuberculin syringe and recorded the placental and fetal weights. Uteroplacental tissue was snap frozen for biochemical and gas chromatography analysis and also fixed in formalin for histopathology. For rescue experiments, recombinant mouse IL-10 (R & D Systems) at the dose of 500 ng/mouse or saline was injected twice on gd 5 and 8 along with Aroclor 1254 (500 μg/mouse) as described above. One set of animals was allowed to deliver the pups, and the time required for a pup to turn over (Righting Reflex) was recorded as described earlier. Other set was euthanized on gd 13 for experimental analysis.
Semi-quantitative RT-PCR—Total RNA was isolated from uteroplacental tissue using the RNeasy kit (Qiagen) in accordance with the manufacturer's protocol. Total RNA was used for cDNA synthesis by reverse transcription with SuperScript III first-strand synthesis system for RT-PCR (Invitrogen) in accordance with the manufacturer's instructions. Semi-quantitative RT-PCRs were set up for AQP1, 3, 8, and 9 and β-actin using PTC-100 Peltier thermocycler. The primer sets for AQP1, 3, 8, and 9 and β-actin were as follows: AQP1, sense, 5′-TGC GTT CTG GCC ACC ACT GAC-3′, and antisense, 5′-GAT GTC GTC AGC ATC CAG GTC-3′ (326-bp product); the AQP3, sense, 5′-CTG GAC GCT TTC ACT GTG GGC-3′, and antisense, 5′-ATC TGC TCC TTG TGT TTC ATG-3′ (307-bp product); the AQP8, sense, 5′-CAG CCT TTG CCA TCG TCC AGG-3′, and antisense, 5′-CCT CGA CTT TAG AAT CAG GCG-3′ (355-bp product); the AQP9, sense, 5′-CCT TCT GAG AAG GAC CGA GCC-3′, and antisense, 5′-CTT GAA CCA CTC CAT CCT TCC-3′ (299-bp product); and β-actin, sense, 5′-TTC TTT GCA GCT CCT TGG TTG CCG-3′, and antisense, 5′-TGG ATG GCT ACG TAC ATG GCT GGG-3′ (457-bp product). The optimal RT-PCR conditions were standardized for each product. PCR products were separated by electrophoresis on 2% agarose gels and stained with 0.01% ethidium bromide. The images were recorded using Chemi Doc XRS gel imager.
Cell Lines and Cell Culture—We used immortalized first trimester trophoblast cell line HTR8 with properties of invasive extravillous cytotrophoblasts (48). We obtained human umbilical vein endothelial cells (HUVEC) from Cambrex and cultured in EBM-2 medium (Cambrex, NJ). We maintained all cell lines under standard culture conditions of 5% CO2 at 37 °C. Use of commercially available HUVECs was limited to early and limited passage. The cells were grown to 80% confluence prior to their use in experiments.
Western Blotting—We separated tissue and cellular lysates on 12% SDS-polyacrylamide gels and blotted onto polyvinylidene difluoride membranes and probed with antibodies for AQP1, 3, 8, or 9 (Santa Cruz Biotechnology) and β-actin (Biovision). We used ECL chemiluminescence (Amersham Biosciences) to visualize the bands and recorded them using Konica SRX 101A developer. Densitometric analysis were carried out using Gel-Pro-Analyzer (Media Cybernetics).
Immunohistochemistry—Individual uteroplacental units were obtained on gd 13 and fixed with 10% buffered formalin. Fixed tissue was processed for mounting 5-μm sections on glass slides and immunostaining as described (11). Tissue slides were stained with a primary antibody to AQP1 (Santa Cruz Biotechnology) or control isotype IgG (Santa Cruz Biotechnology). We then acquired the images with a Nikon Eclipse 80i microscope (Nikon) at 10× magnification. A standard procedure was followed for hematoxylin and eosin staining (11). Morphometric analysis of spiral arteries was carried out using the SPOT™ Advanced software (Diagnostic Instruments, Inc.) at 40× magnification (Nikon Eclipse 80i microscope). The average areas of the spiral arteries of at least six spiral arteries/implantation site were calculated from three independent animals/group.
Flow Cytometry—We evaluated the surface expression of AQP1, 3, 8, or 9 on human trophoblast cells in response to Aroclor 1254 treatment by immunostaining and FACS analysis (Becton Dickinson, NJ) as described previously (45). Negative controls were performed by incubating the cells with isotype-matched antibodies.
In Vitro Angiogenesis Assay—We examined the ability of Aroclor 1254 to disrupt the capillary network of endovascular cross-talk between endothelial cells and trophoblasts using a three-dimensional dual cell co-culture model as described previously (45). Briefly, 48-well culture plates were coated with 0.1 ml of Matrigel (BD Biosciences, CA) and allowed to gelatinize at 37 °C for 30 min. Trophoblasts or endothelial cells (2.5 × 104), labeled with cell tracker green 5-chloromethylfluorescein diacetate or cell tracker red 5-(and-6)-(((4-chloromethyl)benzoyl)amino) tetramethylrhodamine (Molecular Probes), respectively, were co-cultured (1:1) in the presence of 10% normal pregnancy serum spiked with different concentrations of Aroclor 1254 (1, 10, and 25 μg/ml). The spontaneous interaction and endothelial cell-directed tube formation by trophoblasts was monitored and recorded after 12-14 h of incubation under standard culture conditions using florescence microscopy (Nikon Eclipse TE2000-E coupled with CCD camera; 4× magnification). Similarly, the single cell tube formation assay was carried out with labeled endothelial cells. Using the MetaVue® software, the phase contrast and red fluorescent images in single cell tube assay or the red and green fluorescent images from dual cell co-cultures were overlaid. The average number of tubes/vacuoles formed was quantified by counting the number of tube-like structures formed by connected capillary bridge in four different fields by two independent investigators. To study the involvement of AQP1 in this cross-talk, prior to the assay, AQP1 expression on trophoblast cells was blocked by using AQP1 blocking antibody (Chemicon). For the rescue of Aroclor 1254-induced disrupted cross-talk, 1 μm estradiol (Sigma-Aldrich) or different doses of recombinant human IL-10 (R & D Systems) were added in the culture medium, and the assay was carried out as described earlier.
Gas Chromatography Analysis—For the extraction and analysis of samples by gas chromatography, a modified EPA method was followed (49). Briefly, the target tissues were extracted using acetone:hexane (1:1) mixture (5 ml × 3) in an ultrasonic bath for 10 min. The combined supernatant was transferred to a 40-ml glass vial. The recovered supernatant was then dried under nitrogen and then transferred to a 4-ml vial using hexane. 1 ml of sulfuric acid was added, and after vigorous shaking supernatant was transferred to another 4-ml vial. The recovered supernatant was dried under nitrogen and transferred to a 2-ml vial using hexane. Hexane was again dried under nitrogen gas, and finally 0.5 ml of toluene was added. The extracts were analyzed on an Agilent Tech HP6890 gas chromatography system equipped with a HP 5 MS column (30m × 0.25 mm inner diameter × 0.25-mm thickness) and a Ni μ-ECD detector. The injector temperature was 280 °C, and the detector temperature was 290 °C. The oven temperature program was 20 °C/min from 60 to 140 °C, 6 °C/min from 140 to 160 °C, 15 °C/min from 160 to 220 °C, and 20 °C/min from 220 to 280 °C hold for 5 min.
Statistics—We performed statistical analysis using Student's t test. When required, comparisons between multiple groups were made by analysis of variance using analysis of variance. A probability level of 0.05 was considered significant.
RESULTS
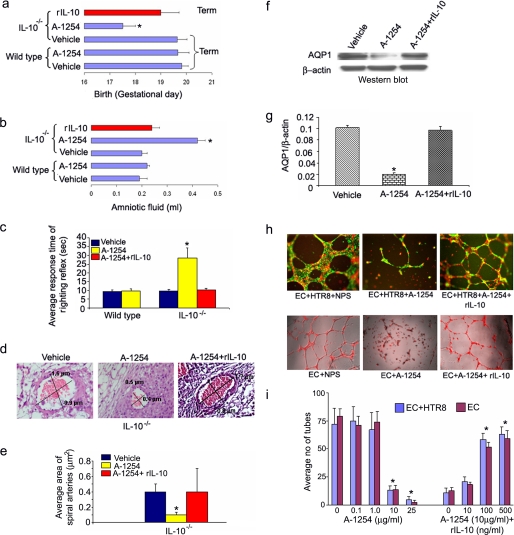
Aroclor 1254 Administration Induces Premature Birth in IL-10-/- Mice—We initially examined the dose-dependent effects of Aroclor 1254 when injected intraperitoneal in wild type or IL-10-/- pregnant mice. Based on pilot experiments, we established that Aroclor 1254 injected on gd 4-10 or 12 at 500 μg/mouse had adverse effects on pregnancy outcome. Although this dose showed no effect in wild type mice, it induced preterm birth in IL-10-/- mice. It is possible that not all PCB congeners in Aroclor 1254 impart adverse pregnancy outcome, thus requiring a seemingly high dose of this PCB mixture for optimal effects. We next performed an expanded set of experiments involving multiple matings using the above described dose of Aroclor 1254 and intraperitoneal injections. As shown in Table 1, Aroclor 1254 induced preterm delivery only in IL-10-/- mice on gd 17 compared with term delivery (gd 20) in vehicle (corn oil)-treated IL-10-/- mice and vehicle or Aroclor 1254-treated wild type animals. Preterm delivery in IL-10-/- mice was accompanied by reduced litter size, placental weight, and fetal weight. Multiple mating experiments resulted in similar pregnancy outcomes. These results demonstrate that IL-10 deficiency is associated with significant adverse in utero effects of PCB.
TABLE 1.
Aroclor 1254 induces preterm birth in C57BL/6 IL-10−/− mice
All of the values are expressed as the means ± S.D.
| Matings | Treatmenta | Preterm birthb | Litter size | Placental weightc | Fetal weightc |
|---|---|---|---|---|---|
| C57BL/6 × C57BL/6 | Vehicle | 0/8d | 10.0 ± 0.9 | 93.5 ± 7.5 | 141.4 ± 16.7 |
| A-1254 | 0/9 | 8.3 ± 1.0 | 90.9 ± 5.3 | 136.7 ± 15.7 | |
| C57BL/6 IL-10−/− × C57BL/6 IL-10−/− | Vehicle | 0/7 | 9.0 ± 1.0 | 92.9 ± 7.7 | 136.2 ± 17.1 |
| A-1254 | 13/13 | 4.1 ± 1.0e | 81.1 ± 6.1e | 119.5 ± 8.9e |
Intraperitoneal injection of 100 μl of vehicle (corn oil) or 500 μg of A-1254/mouse from gd 4 to 12.
Birth on gd 17 ± 0.5.
Weight in mg on gd 13.
The numbers refer to the number of vaginal plug-positive females.
p <0.05 according to Student's t test.
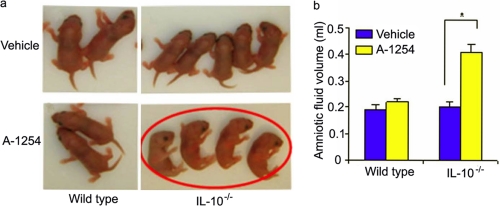
Aroclor 1254 Treatment Causes Defective Righting Reflexes—Because neurocognitive anomalies have been reported in epidemiological studies of children born to PCB-exposed mothers (31, 32), we examined whether newborns from Aroclor 1254-treated IL-10-/- mice suffered from any neurological defects. Righting reflex, a developmental neurologic activity, is a postural reaction that enables an animal to turn over its four feet on ground when placed on its back or side. The righting reflex has been previously used for newborns of rodents (33). We monitored PCB-induced loss of righting reflex in pups born to wild type and IL-10-/- mice. Examination of 7-day-old pups from corn oil or Aroclor 1254-treated wild type and IL-10-/- mice revealed that pups born to Aroclor 1254-treated IL-10-/- mice (n = 13) failed to exhibit righting reflexes in multiple experiments (Fig. 1a). Because pups born to PCB-treated IL-10-/- mice were born prematurely, we repeated these observations on multiple occasions until 21 day of life and observed similar righting reflex defects.
FIGURE 1.
Characterization of postnatal neurocognitive anomaly and amniotic fluid regulation in response to in utero exposure to A-1254. Panel a depicts righting reflex in 7-day-old pups. Pups born to vehicle (corn oil) or A-1254-treated wild type mice and corn oil-treated IL-10-/- mice showed normal righting reflex, whereas pups born to IL-10-/- mice treated with A-1254 showed loss of righting reflex (encircled in red). Panel b shows amniotic fluid volume dysregulation in A-1254-treated IL-10-/- mice. No significant change was observed in amniotic fluid volume in wild type mice. Amniotic fluid was collected on gd 13 from at least six mice from each group. The values are the means ± S.D. A-1254-treated mice showed a significant difference (*, p < 0.05).
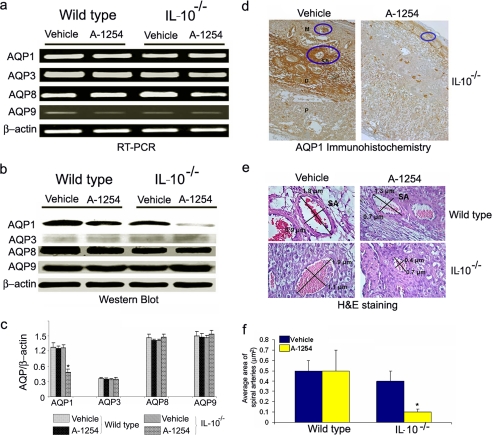
Aquaporin 1 Is a Novel Target of Aroclor 1254 in Uteroplacental Tissue—To our surprise, Aroclor 1254-treated IL-10-/- mice appeared to have increased amniotic fluid volume. In humans, amniotic fluid volume dysregulation and risk factors have been associated with intrauterine infections and inflammation, preterm birth, and postnatal developmental defects (34-36). We quantified amniotic fluid volume in Aroclor 1254-treated animals collected on gd 13 as described under “Experimental Procedures” and found a 2-fold increase in amniotic fluid (Fig. 1b). These data imply that either prematurity or PCB-mediated defects in fluid volume regulation cause amniotic fluid increase in pregnant IL-10-/- mice in response to Aroclor 1254 exposure. Our intriguing observations on preterm delivery and increased amniotic fluid in IL-10-/- mice in response to Aroclor 1254 treatment (Table 1 and Fig. 1b) point to angiogenesis-linked mechanisms. Angiogenesis and fluid volume regulation play critical roles in normal placentation and fetal development. Aquaporins, first described as water channels (37), have been shown to be present in the placenta (38) and to participate in angiogenesis (39) and fluid volume regulation (40, 41). We hypothesized that AQPs could serve as novel targets of PCBs for preterm delivery and increase in amniotic fluid. AQP 1, 3, 8, and 9 have been shown to be expressed in the placental microenvironment (38). To test our hypothesis, we evaluated the expression of these AQPs in uteroplacental tissue obtained on gd 13 by semi-quantitative RT-PCR (Fig. 2a) and immunoblotting (Fig. 2, b and c). We did not detect any differences in the mRNA expression of AQP1, 3, 8, and 9 in tissue from untreated or Aroclor 1254-treated wild type or IL-10-/- mice (Fig. 2a). Surprisingly, we observed a significant reduction in the amounts of AQP1 protein in Aroclor 1254-treated IL-10-/- mice compared with other AQPs (Fig. 2, b and c). It is thus possible that PCBs only affect post-transcriptional or post-translational regulation of AQP1, and further experiments are warranted to decipher such pathways.
FIGURE 2.
A-1254 treatment inhibits the protein expression of AQP1. Panel a shows semi-quantitative RT-PCR for AQP1, 3, 8, and 9 mRNA expressions in uteroplacental tissue from gd 13. A-1254 did not affect expression of any of aquaporins at mRNA level. Panel b shows Western blots for AQP1, 3, 8, and 9. A-1254 treatment reduces AQP1 protein level in IL-10-/- mouse placenta, whereas protein levels of AQP3, 8, and 9 remained unaltered. Panel c shows average densitometric analysis of intensities of AQP1, 3, 8, and 9 protein bands normalized to β-actin from a number of experimental samples. A-1254 significantly reduced AQP1 protein levels in IL-10-/- placental tissue. Panel d shows immunohistochemistry for AQP1 in uteroplacental sections (10× magnification) from IL-10-/- mice. A-1254 treatment reduced AQP1 protein staining and significantly changed the morphology of myometrium compared with tissue from corn oil-treated mice. Mesometrial zone (M), decidua basalis (D), placental zone (P), and transformed spiral arteries (SA) are marked (10× magnification). Panel e shows the morphometry of representative spiral arteries in uteroplacental sections from wild type and IL-10-/- mice stained with hematoxylin and eosin (40× magnification). A-1254 treatment of pregnant IL-10-/- mice resulted in poor remodeling of spiral arteries as indicated by their reduced size as compared with those from corn oil-treated (vehicle) or wild type mice. The diameters of spiral arteries expressed as μm were measured and stamped using SPOT™ Advanced software. One representative data set is shown of three experiments performed. Panel f represents quantitatively the average area of the spiral arteries of at least six spiral arteries per implantation site from three independent animals/group.
We next attempted to verify Aroclor 1254-mediated in vivo effects on AQP1 expression by immunohistochemical analysis of formalin-fixed uteroplacental tissue from IL-10-/- and wild type mice. As shown in Fig. 2d, significant staining for AQP1 was observed in gd 13 uteroplacental tissue from corn oil-treated IL-10-/- mice. The section is marked to identify the mesometrial zone (M), decidua basalis (D), placental zone (P), and transformed spiral arteries (SA). AQP1 was strongly present around transformed spiral arteries and in the decidua basalis region. In contrast, AQP1 staining was poor in uteroplacental tissue from Aroclor 1254-treated IL-10-/- mice, suggesting a pivotal role of this aquaporin in endovascular activity and angiogenesis leading to spiral artery remodeling. No obvious differences between treated and untreated conditions were observed in wild type mice for AQP1 staining (data not shown). Thus, we suggest that AQP1 is a novel target of Aroclor 1254, and its poor levels were associated with defective angiogenic processes at the maternal-fetal interface.
Aroclor 1254 Treatment Disrupts Spiral Artery Remodeling in IL-10-/- Mice—Histological assessment and localization of AQP1 in the uteroplacental tissue suggested adverse effects of Aroclor 1254 on spiral artery transformation. Physiological spiral artery transformation is a critical angiogenic event during placentation and has been reported to be defective in preeclamptic and preterm labor deliveries in humans (42, 43). Morphometric analysis of the hematoxylin and eosin sections of uteroplacental units from multiple animals was carried out to confirm the toxicant-induced effects on spiral artery remodeling. Using SPOT™ Advanced software, the diameters of spiral arteries opening into the myometrium were recorded. As shown in Fig. 2 (e and f), IL-10-/- mice exposed to Aroclor 1254 showed dramatic reduction in diameter and area of the spiral arteries, suggesting that Aroclor 1254 significantly inhibited the transformation of these blood vessels. On the other hand, no significant changes were observed in tissue from wild type mice (Fig. 2f).
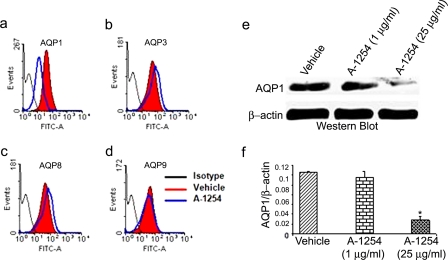
Aroclor 1254 Treatment of Human First Trimester Trophoblast Cells Results in Inhibition of AQP1—As described above, mouse AQP1 is a potent target of PCBs. However, we cannot preclude the possibility that Aroclor 1254-mediated effects on AQP1 were limited to the mouse maternal-fetal interface. To further demonstrate that AQP1 was also a PCB target at the human maternal-fetal interface, we measured protein levels of AQP1, 3, 8, and 9 in Aroclor 1254-treated human HTR8 cells, representing first trimester trophoblasts, by FACS analysis and immunoblotting. Notably, despite similar levels of expression in untreated cells, we detected significant inhibition of AQP1 when analyzed by FACS (Fig. 3a) without affecting AQP3 (Fig. 3b), AQP8 (Fig. 3c) and AQP9 (Fig. 3d). We further confirmed the Aroclor 1254-induced inhibition of AQP1 by Western blotting (Fig. 3e) and densitometry analysis (Fig. 3f). These results in Aroclor 1254-treated human extravillous trophoblast cells support our finding in IL-10-/- mice. Similar results were observed in other first trimester trophoblast cell lines, SWAN 71 and 3A (data not shown).
FIGURE 3.
A-1254-mediated modulation of AQP1 protein levels in human trophoblast HTR8 cells. Representative FACS histograms are shown for AQP1 (panel a), AQP3 (panel b), AQP8 (panel c), and AQP9 (panel d) in response to 24 h of treatment of HTR8 cells with A-1254 (25 μg/ml). Panel e shows a Western blot for AQP1 levels in trophoblast HTR8 cells in response to A-1254. One representative data set is shown of three experiments performed. Panel f shows the average densitometry values of AQP1 normalized to β-actin from three Western blot experiments.
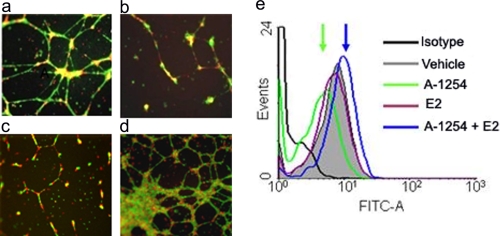
Inhibition of Endovascular Interactions between Trophoblasts and Endothelial Cells by PCB and AQP1 Blocking Antibody—Based on the data presented above, we next aimed to demonstrate that inhibition of AQP1 by Aroclor 1254 treatment of HTR8 cells results in defective endovascular cellular interactions required for optimal spiral artery transformation. We and others have recently developed a three-dimensional culture system on Matrigel that mimics endovascular cross-talk between trophoblast cells and endothelial cells (44, 45). We carried out trophoblast-endothelial cells interactions on Matrigel in the absence or presence of Aroclor 1254, AQP1 blocking antibody, or Aroclor 1254 and estradiol. Doses of Aroclor 1254, AQP1 blocking antibody, and estradiol were determined based on pilot experiments. As shown in Fig. 4a, co-culture of HTR8 cells and HUVECs on Matrigel leads to rapid formation of three-dimensional tube structures. Incubation in the presence of either Aroclor 1254 (Fig. 4b) or AQP1 blocking antibody (Fig. 4c) disrupted tube formation between HTR8 cells and HUVEC cells. This demonstrated that PCB-mediated inhibition of AQP1 or its blocking by an antibody in trophoblast cells elicited detrimental effects on endovascular activity of these cells. On the other hand, estradiol blocked Aroclor 1254 effects and restored tube formation (Fig. 4d). Importantly, the rescue of tube formation by estradiol was due to reversal of Aroclor 1254-induced suppression of AQP1 (Fig. 4e). We also demonstrate that Aroclor 1254 strongly inhibited tube formation in a single cell culture of endothelial cells (Fig. 5h, lower panel), suggesting that PCBs have direct effect on endothelial cells. These effects are not due to poor cell survival of HTR8 or HUVECs because we did not detect any cell death in response to Aroclor 1254 treatment (data not shown).
FIGURE 4.
Effect of A-1254 treatment or AQP1 blocking on endovascular interactions between HTR8 trophoblast cells and HUVECs. HUVECs (labeled with red cell tracker) and HTR8s (labeled with green cell tracker) were cultured overnight on Matrigel in the presence or absence of A-1254. The capillary tube formation was recorded as described under “Experimental Procedures.” Representative figures of HUVEC-directed tube formation by HTR8 cells (4× magnification) are shown. Panel a, in presence of normal pregnancy serum; panel b, trophoblasts pretreated with AQP1 blocking antibody; panel c, in presence of normal pregnancy serum spiked with A-1254 (10 μg/ml); panel d, in presence of normal pregnancy serum spiked with estradiol (E2) (1 μm) and A-1254 (25 μg/ml). Panel e, representative FACS analysis of AQP1 expression in trophoblast HTR8 cells showing estradiol-mediated rescue of AQP1 suppression. One representative data set is shown of three separate experiments performed.
FIGURE 5.
Recombinant IL-10 rescues PCB-induced in vivo and in vitro pregnancy anomalies. A-1254 (500 μg/ml, gd 4-12) or combination with mouse IL-10 (500 ng/mouse, gd 5 and 8) or corn oil (vehicle) was administered to IL-10-/- pregnant mice (n = 3/group). Panel a shows the IL-10-mediated rescue of pregnancy to term in A-1254-treated mice. Panel b shows the IL-10-mediated prevention of amniotic fluid volume increase caused by A-1254 treatment. Panel c shows the average response time of righting reflex. IL-10 treatment significantly reversed A-1254-induced loss of righting reflex. Panel d shows representative spiral arteries in uteroplacental sections from IL-10-/- mice stained with hematoxylin and eosin (40× magnification). Recombinant IL-10 significantly reversed the A-1254-induced poor remodeling of spiral arteries to levels comparable with those from corn oil-treated (vehicle). Panel e represents quantitatively the average area of the spiral arteries of at least six spiral arteries/implantation site from three independent animals/group. Panel f shows the Western blot for AQP1 in placental tissue. IL-10 treatment neutralizes the AQP1 suppression caused by A-1254 treatment. Panel g shows the average densitometry values of placental AQP1 normalized to β-actin from three Western blot experiments. Panel h, upper panel, human IL-10-mediated (100 ng/ml) rescue of A-1254 (10 μg/ml)-induced disruption of angiogenesis on endothelial cell (labeled red)-trophoblast (labeled green) dual cell tube formation assay. Lower panel, IL-10-mediated (100 ng/ml) rescue of A-1254 (10 μg/ml)-induced disruption of angiogenesis on endothelial cell tube formation assay. The endothelial cells were labeled red and overlaid with phase contrast image. All of the images were recorded at 4× magnification. Panel i shows the quantification of tube formation in dual cell and single cell models of angiogenesis with different doses of A-1254 or A-1254 (10 μg/ml) in combination with different doses of IL-10. The average number of tubes/vacuoles formed was quantified from triplicate experiments by counting the number of tube like structures formed by connected capillary bridge in four different fields (4× magnification) by two independent investigators. The values are expressed as the means ± S.D. **, p < 0.05.
IL-10 Functions as Anti-PCB Cytokine—Pregnancy complications observed only in IL-10-/- mice exposed to Aroclor 1254 suggested a protective role for IL-10. However, pregnancy anomalies in these animals could also arise through non-IL-10 pathways. To demonstrate that IL-10 functions as an anti-PCB cytokine, we performed experiments to assess the ability of IL-10 to rescue PCB-mediated in vivo and in vitro effects. As shown in Fig. 5 (a-i), administration of recombinant mouse IL-10 on gd 5 and 8 rescued the phenotypic and molecular anomalies induced by Aroclor 1254. IL-10 treatment rescued preterm birth caused by Aroclor 1254 in IL-10-/- mice (Fig. 5a) and prevented the toxicant-induced increase in amniotic fluid (Fig. 5b). Importantly, administration of recombinant IL-10 significantly reversed the Aroclor-1254-induced loss of righting reflex (Fig. 5c), suggesting a key role played by IL-10 during pregnancy and post-natal neurodevelopment. Moreover, recombinant IL-10 reversed the Aroclor 1254-induced reduction in transformed spiral arteries in IL-10-/- mice (Fig. 5, d and e). At the molecular level, IL-10 co-treatment reversed the Aroclor 1254-induced suppression of AQP1 expression in the placental tissue (Fig. 5, f and g), implicating IL-10 as an anti-PCB cytokine. Moreover, recombinant human IL-10 rescued Aroclor 1254-induced disruption of three-dimensional tube formation involving endothelial cells alone or dual culture of endothelial cells and trophoblasts (Fig. 5h). Quantification of tube formation in response to different doses of Aroclor 1254 or in combination with different doses of recombinant IL-10 is graphically shown in Fig. 5i. Although Aroclor 1254 disrupted in vitro endovascular interactions, co-treatment with recombinant IL-10 significantly rescued Aroclor 1254-mediated disruption in a dose-dependent manner, supporting in vivo protective effects of IL-10 on spiral artery remodeling.
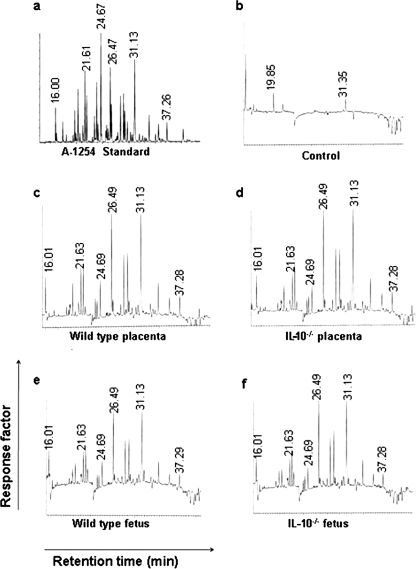
Sensitivity of IL-10-/- Mice to in Utero Anomalies Is Not Associated with Differential Placental Transport of Aroclor 1254—Our in vivo data in IL-10-/- mice are intriguing, but one could argue that IL-10-/- mice were more susceptible to PCBs compared with wild type mice because of excessive placental transport of the toxicant. Thus, we assessed placental and fetal presence of Aroclor 1254 in IL-10-/- and wild type mice. Placenta and fetus from corn oil and Aroclor 1254-treated wild type and IL-10-/- mice were processed for PCB extraction and analysis by gas chromatography. Optimal parameters were established for the Aroclor 1254 mixture and corn oil-treated placenta. The data presented in Fig. 6 support the notion that there was a similar placental and fetal exposure to PCBs in both wild type and IL-10-/- strains. It appears that all congener peaks of Aroclor 1254 were present in all tissues analyzed compared with the total absence of these peaks in tissues from corn oil-treated (control) IL-10-/- mice (Fig. 6) or their wild type counterparts (data not shown). These observations imply that the predisposition in IL-10-/- mice to preterm birth was not due to excessive or congener-specific PCB transport in this strain.
FIGURE 6.
Transplacental transport of A-1254. Frozen uteroplacental tissue from corn oil or A1254-treated pregnant mice was processed for PCB content determination by gas chromatography as described under “Experimental Procedures.” Representative gas chromatograms for standard A-1254 (panel a), tissue from corn oil-treated wild type mice (panel b), tissue from A1254-treated wild type mice (panel c), tissue from IL-10-/- mouse (panel d), wild type fetus (panel e), and IL-10-/- fetus (panel f). Uteroplacental and fetal tissues were collected on gd 13. One representative data set is shown of four experiments performed.
DISCUSSION
The gene-environment origin of early developmental health risks posits that in utero exposures disrupt key developmental pathways. This can involve the angiogenic and hormonal activities at the maternal-fetal interface. Using PCBs as environmental triggers and IL-10-/- mice as the host, we have shown that pregnant mice exposed to a PCB mixture experienced preterm delivery. Exposure was associated with reduced litter size, reduced placental and fetal weight, increased amniotic fluid, and defective righting reflex in newborns. These observations support the epidemiological reports on PCB-induced low birth weight and premature deliveries, postnatal low IQ and behavioral alterations, and high life time risks in humans (7, 26, 27, 32). Importantly, we found that AQP1 is a novel in utero target of PCBs, linking amniotic fluid volume regulation and angiogenesis with toxicant-mediated effects. Our findings on aquaporins, amniotic fluid, and angiogenesis for the first time provide insights into new pathways that can be targeted to counter the toxic developmental effects of PCBs. Our findings are important in consideration of the fact that the environmental levels of PCBs have not declined, and these toxicants remain serious health risks (19, 21). Thus, knowledge of the pathways for toxic effects is important.
We believe that regulation of PCB effects by IL-10 has several possible rationales. First, IL-10 is a potent anti-inflammatory cytokine robustly produced at the maternal-fetal interface in both humans (8, 9) and mice (10). It has been shown to control inflammatory immune cells and cytokines and serves as a temporal regulator of gestational age (9, 11). Its deficiency in mice has been associated with adverse pregnancy outcomes and allows cytotoxic activation of uterine immune cells at very low doses of inflammatory triggers (9). IL-10 is considered a therapeutically important cytokine (46). Because IL-10 proficient wild type mice did not experience any adverse effects of PCB exposure, our results imply that IL-10 functions as an “anti-PCB” cytokine. This hypothesis is strongly supported by the in vivo and in vitro evidence presented here. Administration of recombinant IL-10 reversed phenotypic anomalies induced by Aroclor 1254 such as preterm birth, loss of righting reflex, and increase in amniotic fluid volume. Importantly, a decrease in AQP1 protein expression in the placenta was prevented by IL-10 treatment. Moreover, recombinant IL-10 reversed the PCB-induced decrease in spiral arteries, implying its protective role in at the maternal-fetal interface. These observations are further supported by IL-10-mediated rescue of PCB-induced disruption of tube formation in both single cell and dual cell three-dimensional models of angiogenesis. Given these observations, it will be important to examine the effects of single co-planar and non-co-planar congeners and reversal of their effects by IL-10. Although estradiol was able to rescue the PCB-induced disruption of tube formation and suppression of AQP1 expression in vitro, pregnancy rescue experiments with the administration of estradiol are not feasible. In this context, it is well documented that estrogen receptors are expressed at the maternal-fetal interface. However, for successful pregnancy outcome the levels of estrogen and progesterone are critical. The estrogen levels wane off once implantation takes place with a concomitant increase in progesterone (50). It is thus possible that alterations in these highly choreographed levels of hormones caused by exogeneous administration of estradiol would compromise pregnancy.
The identification of AQP1 as a novel PCB target at the maternal-fetal interface in mice and in human trophoblasts is noteworthy. AQPs were first identified as water channels and are small membrane proteins (37). Although there are 13 known mammalian proteins that increase permeability of not only water but other small molecules such as glycerol and urea, their individual presence is strictly organ-specific (47). The placenta has been shown to express a spectrum of AQPs, particularly AQP1, 3, 8 and 9 (38). Importantly, AQP1 has been shown to regulate angiogenesis (39) and amniotic fluid volume (40, 41, 47). In this regard, our results on Aroclor1254-mediated inhibition of AQP1 are intriguing. Reduced AQP1 levels were associated with defective spiral artery transformation in IL-10-/- mice and an AQP1 blocking antibody disrupted endovascular interactions between human trophoblast cells and endothelial cells in vitro. In addition, Aroclor 1254 treatment of human trophoblasts resulted in AQP1 inhibition and disruption of endovascular interaction with endothelial cells. We thus propose that AQP1 is a critical molecule in programming angiogenesis and spiral artery transformation in the pregnant uterus. In the present study, our data clearly reveal novel targets and pathways for PCB-mediated effects that control highly choreographed events at the maternal-fetal interface and can lead to better understanding of gene-environment biology in general.
Acknowledgments
We thank Kim Boekelheide and James Padbury for thoughtful critique and suggestions for the manuscript. We also thank Marcelo Alexandre and Yongsong Huang for help with PCB analysis and Paula Weston for help with histochemistry.
This work was supported, in whole or in part, by National Institutes of Health Award P20RR018728 through the National Center for Research Resources. This work was also supported in part by Superfund Basic Research Program Award P42ES013660.
Footnotes
The abbreviations used are: PCB, polychlorinated biphenyls; AQP, aquaporins; HUVEC, human umbilical vein endothelial cell(s); A-1254, Aroclor 1254; gd, gestational day(s); IL, interleukin; RT, reverse transcription; FACS, fluorescence-activated cell sorter.
References
- 1.Norwitz, E. R. D., Schust, J., and Fisher, S. J. (2001) N. Engl. J. Med. 345 1400-1408 [DOI] [PubMed] [Google Scholar]
- 2.Moffett-King, A., and Loke, C. (2006) Nat. Rev. Immunol. 6 584-594 [DOI] [PubMed] [Google Scholar]
- 3.Medawar, P. B. (1953) Symp. Soc. Exp. Biol. 7 320-338 [Google Scholar]
- 4.Moffett-King, A. (2002) Nat. Rev. Immunol. 2 656-663 [DOI] [PubMed] [Google Scholar]
- 5.Paria, B. C., Reese, J., Das, S. K., and Dey, S. K. (2002) Science 296 2185-2188 [DOI] [PubMed] [Google Scholar]
- 6.W. H. O. (2003) Polychlorinated Biphenyls: Human Health Aspects, CICADS 55, International Programme for Chemical Safety, World Health Organization, Geneva
- 7.Carpenter, D. O. (2006) Rev. Environ. Health 21 1-23 [DOI] [PubMed] [Google Scholar]
- 8.Trautman, M. S., Collmer, D., Edwin, S. S., White, W., Mitchell, M. D., and Dudley, D. J. (1997) J. Soc. Gynecol. Investig. 4 247-253 [PubMed] [Google Scholar]
- 9.Hanna, N., Hanna, I., Hleb, M., Wagner, E., Dougherty, J., Balkundi, D., Padbury, J., and Sharma, S. (2000) J. Immunol. 164 5721-5728 [DOI] [PubMed] [Google Scholar]
- 10.Krishnan, L., Guilbert, L. J., Russell, A. S., Wegmann, T. G., Mosmann, T. R., and Belosevic, M. (1996) J. Immunol. 156 644-652 [PubMed] [Google Scholar]
- 11.Murphy, S. P., Fast, L. D., Hanna, N. N., and Sharma, S. (2005) J. Immunol. 175 4084-4090 [DOI] [PubMed] [Google Scholar]
- 12.Robertson, S. A., Skinner, R. J., and Care, A. S. (2006) J. Immunol. 177 4888-4896 [DOI] [PubMed] [Google Scholar]
- 13.Kinsky, R., Delage, G., Rosin, N., Thang, M. N., Hoffmann, M., and Chaouat, G. (1990) Am. J. Reprod. Immunol. 23 73-77 [DOI] [PubMed] [Google Scholar]
- 14.Sadowsky, D. W., Novy, M. J., Witkin, S. S., and Gravett, M. G. (2003) Am. J. Obstet. Gynecol. 188 252-263 [DOI] [PubMed] [Google Scholar]
- 15.Plevyak, M., Hanna, N., Mayer, S., Murphy, S., Pinar, H., Fast, L., Ekerfelt, C., Ernerudh, J., Berg, G., Matthiesen, L., and Sharma, S. (2002) Am. J. Reprod. Immunol. 47 242-250 [DOI] [PubMed] [Google Scholar]
- 16.Hanna, N., Bonifacio, L., Weinberger, B., Reddy, P., Murphy, S., Romero, R., and Sharma, S. (2006) Am. J. Reprod. Immunol. 55 19-27 [DOI] [PubMed] [Google Scholar]
- 17.Hensen, L. G., and Robertson, L. W. (2008) PCBs: Human and Environmental Disposition and Toxicology, pp. 1-207, Illinois University Press, Champaign, IL
- 18.Safe, S., Safe, L., and Mullin, M. (1987) Polychlorinated Biphenyls (PCBs): Mammalian and Environmental Toxicology, pp. 1-3, Springer-Verlag, New York
- 19.Ross, P. S., Vos, J. G., Biranbaum, L. S., and Osterhaus, A. D. (2000) Science 289 1878-1879 [DOI] [PubMed] [Google Scholar]
- 20.Rocca, C. L., and Mantovani, A. (2006) Ann. Ist. Super Sanita. 42 410-416 [PubMed] [Google Scholar]
- 21.Weintraub, M., and Birnbaum, L. S. (2008) Environ. Res. 107 412-417 [DOI] [PubMed] [Google Scholar]
- 22.Mendola, P., Buck, G. M., Sever, L. E., and Vena, J. E. (1997) Am. J. Epidemiol. 146 955-960 [DOI] [PubMed] [Google Scholar]
- 23.Gerhard, I., Daniel, B., Link, S., Monga, B., and Runnebaum, B. (1998) Environ. Health Perspect. 106 675-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostyniak, P. J., Stinson, C., Greizerstein, H. B., Vena, J., Buck, G., and Mendola, P. (1999) Environ. Res. 80 S166-S174 [DOI] [PubMed] [Google Scholar]
- 25.Yu, M. L., Guo, Y. L., Hsu, C. C., and Rogan, W. J. (2000) Int. J. Epidemiol. 29 672-677 [DOI] [PubMed] [Google Scholar]
- 26.Baibergenova, A., Kudyakov, R., Zdeb, M., and Carpenter, D. O. (2003) Environ. Health Perspect. 111 1352-1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukimori, K., Tokunaga, S., Shibata, S., Uchi, H., Nakayama, D., Ishimaru, T., Nakano, H., Wake, N., Yoshimura, T., and Furue, M. (2008) Environ. Health Perspect. 116 626-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennig, B., Meerarani, P., Slim, R., Toborek, M., Daugherty, A., Silverstone, A. E., and Robertson, L. W. (2002) Toxicol. Appl. Pharmacol. 181 174-183 [DOI] [PubMed] [Google Scholar]
- 29.Arnold, D. L., Bryce, F., McGuire, P. F., Stapley, R., Tanner, J. R., Wrenshall, E., Mes, J., Fernie, S., Tryphonas, H., and Hayward, S. (1995) Food Chem. Toxicol. 33 457-474 [DOI] [PubMed] [Google Scholar]
- 30.Lee, C. K., Kang, H. S., Kim, J. R., Lee, B. J., Lee, J. T., Kim, J. H., Kim, D. H., Lee, C. H., Ahn, J. H., Lee, C. U., Yu, S. J., and Kang, S. G. (2007) Reprod. Fertil. Dev. 19 539-547 [DOI] [PubMed] [Google Scholar]
- 31.Gray, K. A., Klebanoff, M. A., Brock, J. W., Zhou, H., Darden, R., Needham, L., and Longnecker, M. P. (2005) Am. J. Epidemiol. 162 17-26 [DOI] [PubMed] [Google Scholar]
- 32.Grandjean, P., and Landrigan, P. J. (2006) Lancet, 368 2167-2178 [DOI] [PubMed] [Google Scholar]
- 33.Sugawara, N. Nakai, K., Nakamura, T., Ohba, T., Suzuki, K., Kameo, S., Satoh, C., and Satoh, H. (2006) Arch. Toxicol. 80 286-292 [DOI] [PubMed] [Google Scholar]
- 34.Jacobsson, B., Mattsby-Baltzer, I., Andersch, B., Bokström, H., Holst, R. M., Wennerholm, U. B., and Hagberg, H. (2003) Acta Obstet. Gynecol. Scand. 82 120-128 [DOI] [PubMed] [Google Scholar]
- 35.Harman, C. R. (2008) Semin. Perinatol. 32 288-294 [DOI] [PubMed] [Google Scholar]
- 36.Lee, S. E., Romero, R., Park, I. S., Seong, H. S., Park, C. W., and Yoon, B. H. (2008) J. Matern. Fetal. Neonatal Med. 21 89-94 [DOI] [PubMed] [Google Scholar]
- 37.Agre, P., King, L. S., Yasui, M., Guggino, W. B., Ottersen, O. P., Fujiyoshi, Y., Engel, A., and Nielsen, S. (2002) J. Physiol. 542 3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beall, M. H., Wang, S., Yang, B., Chaudhri, N., Amidi, F., and Ross, M. G. (2007) Placenta 28 421-428 [DOI] [PubMed] [Google Scholar]
- 39.Saadoun, S., Papadopoulos, M. C., Hara-Chikuma, M., and Verkman, A. S. (2005) Nature 434 786-792 [DOI] [PubMed] [Google Scholar]
- 40.Mann, S. E., Ricke, E. A., Torres, E. A., and Taylor, R. N. (2005) Am. J. Obstet. Gynecol. 192 2041-2044 [DOI] [PubMed] [Google Scholar]
- 41.Beall, M. H., van den Wijngaard, J. P., van Gemert, M. J., and Ross, M. G. (2007) Placenta 28 824-832 [DOI] [PubMed] [Google Scholar]
- 42.Kim, Y. M., Bujold, E., Chaiworapongsa, T., Gomez, R., Yoon, B. H., Thaler, H. T., Rotmensch, S., and Romero, R. (2003) Am. J. Obstet. Gynecol. 189 1063-1069 [DOI] [PubMed] [Google Scholar]
- 43.Parham, P. (2004) J. Exp. Med. 200 951-955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aldo, P. B., Krikun, G., Visintin, I., Lockwood, C., Romero, R., and Mor, G. (2007) Am. J. Reprod. Immunol. 58 98-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalkunte, S., Lai, Z., Tewari, N., Chichester, C., Romero, R., Padbury, J., and Sharma, S. (2008) Placenta 29 871-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumont, F. J. (2003) Expert Opin. Ther. Pat. 13 1551-1577 [Google Scholar]
- 47.Liu, H., Zheng, Z., and Wintour, E. M. (2008) Placenta 29 840-847 [DOI] [PubMed] [Google Scholar]
- 48.Graham, C. H., Hawley, T. S., Hawley, R. G., MacDougall, J. R., Kerbel, R. S., Khoo, N., and Lala, P. K. (1993) Exp. Cell Res. 206 204-211 [DOI] [PubMed] [Google Scholar]
- 49.Environmental Protection Agency (1996) Polychloride Biphenyls (PCBs) by Gas Chromatography, Method, 8082A, U. S. EPA, Washington, D. C. 8082A-1-8082A-56
- 50.Dey, S. K. (2006) Nat. Rev. Genetics 7 185-199 [DOI] [PubMed] [Google Scholar]