Abstract
Perturbation of the cytoplasmic protein folding environment by exposure to oxidative stress-inducing As(III)-containing compounds challenges the ubiquitin-proteasome system. Here we report on mass spectrometric analysis of As(III)-induced changes in the proteasome's composition in samples prepared by stable isotope labeling with amino acids in cell culture, using mammalian cells in which TRP32 (thioredoxin-related protein of 32 kDa; also referred to as TXNL1) was identified as a novel subunit of the 26 S proteasome. Quantitative genetic interaction mapping, using the epistatic miniarray profiling approach, identified a functional connection between TRP32 and the proteasome. Deletion of txl1, the Schizosaccharomyces pombe homolog of TRP32, results in a slow growth phenotype when combined with deletion of cut8, a gene required for normal proteasome localization. Deletion analysis in vivo, chemical cross-linking, and manipulation of the ATP concentration in vitro during proteasome immunopurification revealed that the C-terminal domain of mammalian TRP32 binds the 19 S regulatory particle in proximity to the proteasome substrate binding site. Thiol modification with polyethylene glycol-maleimide showed disulfide bond formation at the active site of TRP32 in cells exposed to As(III). Pulse-chase labeling showed that TRP32 is a stable protein whose half-life of >6 h is surprisingly reduced to 1 h upon exposure of cells to As(III). These findings reveal a previously undescribed thiol reductase at the proteasome's regulatory particle.
Exposure to the environmentally prevalent toxin, arsenic (As(III)), challenges the redox defense mechanisms of the cell, causing oxidative stress (1). The accumulation of reactive oxygen species associated with As(III) exposure results in the modification of protein structures and promotes protein misfolding and aggregation, which leads to activation of the heat shock response (2, 3) and the integrated stress response (4) that promote chaperone gene expression and attenuate new protein synthesis, respectively. Although activation of these pathways promotes the reestablishment of proteostasis by affecting the balance between newly synthesized unfolded proteins and chaperones (5), misfolded proteins that have undergone irreversible oxidative modification must be removed from the intracellular environment to prevent chronic intracellular protein misfolding that is often associated with disease.
The primary intracellular protease involved in degrading oxidatively damaged proteins is the 26 S proteasome, a large multisubunit protein complex consisting of the 20 S proteasome and the 19 S regulatory particle (6). Misfolded proteins are targeted to the 26 S proteasome by polyubiquitination, which directs their localization to the 19 S particle. At the 19 S particle, proteasome substrates undergo both deubiquitination and unfolding, which facilitates their translocation into the 20 S core for proteolysis. Through this mechanism of substrate recruitment and unfolding-coupled translocation, the 19 S complex regulates protein accessibility to the 20 S core, preventing premature degradation of functional polypeptides and controlling the proteolytic activity of the proteasomes (7).
Under conditions of oxidative stress, proteasome activity is challenged by the accumulation of oxidatively modified protein structures (e.g. protein cross-links) (6). Similarly, exposure to cellular oxidants, such as H2O2 and As(III), increase the intracellular population of polyubiquitinated proteins targeted for degradation (8, 9). Proteasome subunits themselves can also be targets for oxidative modification, which can reduce activity of the 26 S proteasome by disruption of specific steps in the proteasome pathway (10).
Recent studies have called attention to the importance of proteasome-interacting proteins that bind to the 19 S regulatory particle and alter specific aspects of proteasome function to match changes in protein folding homeostasis (11). In As(III)-treated mammalian cells, the 19 S associating protein AIRAP is up-regulated transcriptionally, and the related protein, AIRAP-L, is recruited to the 19 S particle post-translationally. Together, this accelerates ATP-independent translocation of misfolded proteins into the 20 S core and increases the degradation capacity of the proteasome (12, 13).
Here we report on our efforts to further characterize alterations in proteasome composition induced by oxidative stress by an unbiased comparative proteomic approach directed toward the identification of As(III)-sensitive proteasome-associating proteins. This led to the identification of TRP32 (thioredoxin-related protein of 32 kDa) as a hitherto unrecognized redox-active 19 S proteasome subunit.
EXPERIMENTAL PROCEDURES
Mammalian Cell Culture, Transfection, and Protein Purification—Plasmids expressing HA.TRP32.WT and HA.TRP32.CS were prepared from human cDNA and were generous gifts from Professor Shin Yonehara of Kyoto University. Constructs expressing TRP32-(1-112).HA and HA.TRP32-(112-289) were prepared by amplification of the corresponding domain and ligation in-frame with an HA2 epitope in mammalian expression plasmids. 293T cells were transfected using the calcium phosphate transfection method.
Cells were disrupted in lysis buffer A (20 mm Hepes, pH 7.5, 100 mm NaCl, 5 mm EDTA, 0.5% Triton X-100, 0.1 mm DTT, and protease inhibitors) and clarified at 20,000 × g for 15 min. In Fig. 3A, cells were lysed in TNH buffer (20 mm Hepes, pH 7.5, 100 mm NaCl, 1.5 mm MgCl2, 1 mm EDTA, 0.1 mm EDTA, and proteases inhibitors) with either 5 mm ATP or hexokinase (13.5 μg/ml) plus glucose (20 mm) followed by a 10× dilution into buffer A prior to immunoprecipitation.
FIGURE 3.
TRP32 associates with the 19 S regulatory particle through its C-terminal domain. A, TRP32 immunoblot of proteasomal proteins immunopurified from HEK 293T cell lysates with a monoclonal antibody to the PSMA1 subunit of the 20 S particle. Where indicated, the lysates were supplemented with 5 mm ATP (+ATP) or depleted of their ATP content with glucose-hexokinase (ΔATP). *, an irrelevant band detected by the anti-TRP32 antiserum. PSMD7 and PSMA1 serve as recovery markers of the 20 and 19 S particle. Note the decline in TRP32 and PSMD7 signals in the ATP-depleted sample (lane 3). B, autoradiograph of metabolically labeled endogenous proteasomal proteins immunopurified from HEK 293T cells. Where indicated, the immunopurified material was exposed to the reversible cross-linker DSP (which was subsequently quenched), and the labeled proteins were resolved by reducing SDS-PAGE (which disrupts cross-links to reveal the proteasomal proteins; lanes 1 and 2) or disrupted in SDS under nonreducing conditions (preserving the cross-links) and reimmunopurified with an antiserum to TRP32 (lanes 3 and 4) or an irrelevant antiserum (lanes 5 and 6) and then resolved by reducing SDS-PAGE. Note the presence of DSP-dependent, TRP32-associated radiolabeled bands identical in size to the 19 S particle proteins PSMD1 and PSMD2 (arrows) in the TRP32 immunoprecipitate. C, domain structure and location of the HA epitopes in the TRP32 expression constructs. D, immunoblot of proteasomes purified from HEK 293T cells overexpressing the TRP32 constructs depicted in C.
For isoelectric focusing (IEF)-SDS-PAGE, proteasomes were eluted from monoclonal anti-PSMA1 (mAb 2-18) covalently attached to Protein G-Sepharose with 7 m urea, 4% CHAPS, diffused into a ReadyStrip IPG strip, pH 3-10 (NL) (7 cm; Bio-Rad), and resolved on a Bio-Rad Protean IEF Cell followed by SDS-PAGE.
Glycerol gradient fractionation was performed by layering 500 μl of cellular lysates on a 10-40% glycerol gradient prepared in buffer A and centrifuged in a SW50 rotor for 13.85 h at 32,500 rpm.
Metabolic labeling of 293T and MEF cells was achieved using 20 μCi/ml 35S-TRANSlabel (ICN), for the indicated time (3 h or overnight). Dithiobis(succinimidyl propionate) (DSP) cross-linking was performed on purified proteasomes using 250 μm dithiobis(succinimidyl propionate) (Pierce) following protocols recommended by the manufacturer.
Polyethylene glycol 5000 (PEG(5K)) modification was performed on MEF lysates using 10 mm PEG(5K)-maleimide (Nektar) for 30 min at 37 °C essentially as described (14), except that the modified proteins were purified from free PEG(5K)-maleimide by trichloroacetic acid precipitation before SDS-PAGE and immunoblotting.
Fluorescein-malemide modification was performed by incubation of 100 nm fluorescein-maleimide (Pierce) with 2 μm recombinant TRP32-(1-112) pretreated with 100 μm NEM (Sigma) or 1 mm diamide (Sigma) for 30 min at room temperature. Fluorescence polarization was measured using a Tecan Infinite F500 spectrofluorometer with a 485 ± 20-nm excitation filter and a 535 ± 25-nm emission filter.
SILAC Analysis of Purified Proteasomes—SILAC analysis of proteasomes was performed on 293T cells acclimated to Dulbecco's modified Eagle's medium (-Arg, -Lys) (Invitrogen) with 10% dialyzed fetal calf serum, penicillin/streptomycin, supplemented with either 146 μg/ml Lys plus 84 μg/ml Arg or 146 μg/ml 13C6-Lys plus 84 μg/ml 13C6-Arg. Cells were acclimated through >5 dilutions (1:10) of confluent cells into the appropriate media. Cellular lysates were prepared, and proteasomes were immunopurified and resolved by SDS-PAGE. Excised gel bands were digested with trypsin and processed for mass spectrometric sequencing as described (15). For the phospho-SILAC experiments, the extracted peptides were further purified on a TiO2 tip (1-10-μl NuTip; Glygen). Five μl of each peptide mixture were analyzed using nanoflow LC/ESI-MS/MS, with a NanoLC-2D system (Eksigent) coupled directly to an LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific) equipped with a nano-ESI source (Jamie Hill Instrument Services). Mass spectra were acquired in data-dependent mode, and mascot generic format files were generated from the raw data using DTASuperCharge (version 1.01) and Bioworks (version 3.2; Thermo Fisher Scientific) for data base searching against an IPI human protein sequence data base using the Mascot software (version 2.1.0; Matrix Science). The search parameters included peptide mass tolerance of 10 ppm, MS/MS mass tolerance of 0.8 Da, and variable oxidation of methionines with up to one missed tryptic cleavage allowed. MSQuant software (version 1.4.2a13) was used for the SILAC quantitation. The automatic quantitation results were verified and corrected, manually aided by the same software.
Antibodies—Antisera to AIRAP, eIF2α, glutathione S-transferase, GADD34, and CHOP have been described previously (13). Rabbit antisera directed against the TRP32 C-terminal domain and the TRP32 N-terminal domain were prepared by immunization of rabbits against recombinant His6.TRP32-(112-289) and TRP32-(1-112).His6, respectively. Other antisera used are as follows: anti-PSMA1 (ABR PAI-963 or mAb 2-17 to human PSMA1, a kind gift from Keiji Tanaka (Tokyo Metropolitan Institute of Medical Science)), anti-PSMD7 (ABR PAI-1963), anti-PSMD2 (ABR PAI-964), anti-ubiquitin (Zymed 13-1600), and anti-HSP-70 (Stressgen SPA810).
TRP32i/i Mutant Mice—The mouse embryonic stem cell line XH718, with a splice trap transgene integrated at intron 1 of Txnl1 (BayGenomics) was injected into C57BL/6 blastocysts, and chimeric male mice were derived by conventional methods. The mutant alleles were passed through the germ line, and TRP32+/i offspring of the chimera were intercrossed to produce TRP32i/i and TRP32+/+ progeny, which were recovered as day 13.5 embryos for the production of mouse fibroblasts.
Cellular Growth and Proteasome Activity Were Measured in TRP32+/+ and TRP32i/i MEF cells treated with varying concentrations of As(III), as described (13).
Genetic Manipulation and Phenotypic Analysis of Schizosaccharomyces pombe—E-MAP data on the interactions of txl1 with nonessential S. pombe genes were obtained as previously described (16).
S. pombe h- and their derivatives were employed. The complete YPD and the minimal EMM2 media were used and yeast were grown at 30 °C. To introduce the txl1del::KAN allele into 972 (WT) and Δcut8 yeast strains (a generous gift from Prof. Kojiro Takeda), oligonucleotides flanking the disrupted allele were used to amplify the allele from strains of the S. pombe Genome Deletion Project and inserted into the yeast genome through homologous recombination, resulting in h-Δtxl1::Kan and h- leu1 ura4 Δcut8::ura4+Δtxl1::Kan. Plasmids used for expression of HA.spTxl1.WT were prepared by amplification of the txl1 gene introducing unique restriction sites to the 5′- and 3′-ends for in-frame ligation into the S. pombe expression vector pART. Plasmids expressing HA.TRP32.WT and HA.TRP32.CS were prepared by ligation of the HA.TRP32.WT/CS coding sequence (acquired from mammalian expression vectors described above) into pART. Lysates of S. pombe were prepared by mechanical disruption of S. pombe with glass beads in Thorner buffer (8 m urea, 5% SDS, 40 mm Tris-HCl, pH 7.5, 0.1 mm EDTA, 100 mm DTT) followed by centrifugation at 20,000 × g.
RESULTS
TRP32 Is a Stoichiometric Component of the 19 S Regulatory Particle—A SILAC-based proteomic approach was used to identify arsenic-induced alterations in the subunit composition of the 26 S proteasome (Fig. 1A) (17). Proteasomes immunopurified with a monoclonal antibody to the PSMA1 subunit of the 20 S particle (mAb 2-17) from heterogeneous lysates prepared from As(III)-exposed 13C6-Arg/13C6-Lys-labeled HEK 293T cells (“heavy” sample) and untreated and unlabeled HEK 293T cells (“light” sample) were combined and separated by SDS-PAGE (Fig. 1B). The resulting gel was sliced into four fractions that underwent in-gel tryptic digestion, and the eluted peptides were subjected to LC-MS/MS sequence analysis. The isotopically derived mass differences between peptides purified from the two samples enabled comparison of proteasome composition in control and As(III)-treated cells (Fig. 1, A and C).
FIGURE 1.
SILAC analysis of proteasomes purified from As(III)-treated HEK 293T cells. A, schematic of the experimental procedure for SILAC analysis of proteasomes purified from control (light) and As(III)-exposed (heavy) cells. The quantified data appears in supplemental Table 1. B, Coomassie-stained SDS-polyacrylamide gel of proteasomes immunopurified from untreated (light) and As(III)-exposed (heavy) HEK 293T cells (20S IP). The sample in the lane labeled Mock was immunopurified with an irrelevant antibody. C, LC-MS/MS spectrum of a typical TRP32-derived tryptic peptide (corresponding to amino acids 196-203 (NH2-IFINLPR-COOH)) from the SILAC experiment. The positions of the “light” (12C6-Arg) and “heavy” (13C6-Arg) natural isotopic series are indicated. Note the marked (∼50-fold) reduction in signal from the sample derived from As(III)-treated cells.
All known (canonical, stoichiometric) subunits of the 20 S proteasome and the 19 S regulatory particle were identified by LC-MS/MS sequence analysis, as were many of the known substoichiometric constituents that modulate proteasome activity (supplemental Table 1). This analysis revealed modest As(III)-induced alterations in overall particle composition; e.g. proteasomes immunopurified from As(III)-treated cells had ∼30% fewer 19 S components, suggesting a measure of 26 S instability in cells exposed to As(III).
The most striking alteration identified by the SILAC approach was an As(III)-dependent decrease in the association of a hitherto unrecognized proteasome constituent, identical in sequence to TRP32 (thioredoxin-related protein of 32 kDa; also known as TXNL1 (thioredoxin like-1)) (18, 19). Comparing the relative signals of TRP32 purified from control and As(III)-treated cells revealed that proteasome-associated TRP32 decreased nearly 50-fold after 4 h of exposure to As(III) (Fig. 1C).
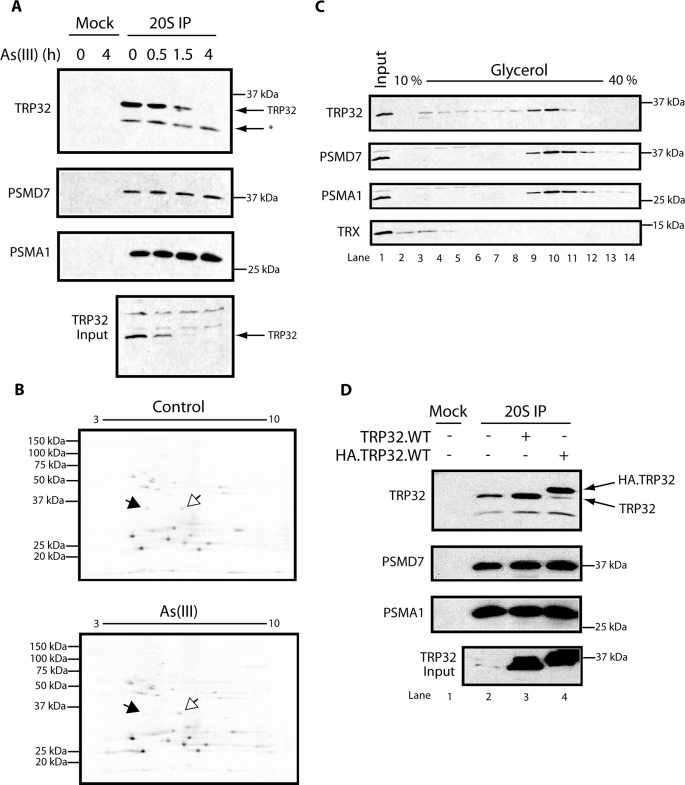
To analyze this suggestion of association with proteasomes, we raised an antiserum to the bacterially expressed C terminus of TRP32 and immunoblotted the constituents of proteasomes immunopurified from untreated HEK 293T cells and cells exposed to As(III). TRP32 was readily detected in proteasomes purified from untreated cells, and its signal decreased to below the level of detection following exposure to As(III) (Fig. 2A). Similarly, TRP32 could also be detected in proteasomes purified by alternative methods (supplemental Fig. 1). Integrity of the proteasomes is revealed by the observation that levels of the 19 S component PSMD7 and the 20 S component PSMA1 recovered in the immunopurification were unaltered by exposure to As(III) (Fig. 2A). The decline in proteasome-associated TRP32 mirrored changes in total cellular level of the protein (Fig. 2A, input). Together, these observations confirm those of the SILAC experiment indicating that TRP32 is an As(III)-sensitive proteasome constituent.
FIGURE 2.
TRP32 associates with proteasomes. A, TRP32 immunoblot of proteasomes immunopurified from HEK 293T cells exposed to 100 μm As(III) for the indicated time. Lanes 1 and 2 (Mock), immunopurified with an irrelevant monoclonal antibody; lanes 3-6, immunopurified with mAb 2-17 to human PSMA1 (a 20 S subunit; 20S IP). The PSMD7 and PSMA1 immunoblots serve as recovery markers for the 19 and 20 S particles, respectively. Bottom (Input), the content of TRP32 in the cell lysate; *, irrelevant bands detected by the anti-TRP32 serum. B, autoradiograph of two-dimensional IEF-SDS-PAGE of proteasomes immunopurified from [35S]Met/Cys-labeled HEK 293T cells exposed to 100 μm As(III) for 2 h or control cells. Filled arrows, TRP32; open arrows, PSMD14. C, immunoblot of MEF lysates fractionated by a 10-40% glycerol gradient. Note the presence of a conspicuous TRP32 peak in fractions that are also enriched in 19 S (PSMD7) and 20 S (PSMA1) constituents and distinct from the light thioredoxin (TRX) peak. D, immunoblot of proteasomes immunopurified from HEK 293T cells overexpressing untagged (TRP32.WT) or N-terminally HA-tagged TRP32 (HA.TRP32.WT). Note the marked discrepancy between the recovery of overexpressed TRP32 in the immunopurified proteasomes (top) and their abundance in the cell lysate (bottom, input).
To further explore TRP32 binding, proteasomes immunopurified from [35S]Met/Cys-labeled HEK 293T cells were analyzed by IEF-SDS-PAGE. TRP32 was readily identified in IEF-SDS-PAGE by its predicted molecular mass and isoelectric point (32 kDa/pI = 4.84) and by the diminution of the signal following As(III) treatment (Fig. 2B, filled arrows). After taking into account the relative Met/Cys composition of TRP32 and the similarly sized 19 S subunit PSMD14 (that serves as a reference; Fig. 2B, open arrows) and assuming the two proteins to have similar half-lives, a comparison of the relative intensity of the two protein spots, suggests that TRP32 occupancy at the proteasome is about ∼0.75-fold that of a canonical 19 S proteasome subunit (PSMD14). Given the relative instability of the TRP32-proteasome complex in vitro (supplemental Fig. 2), these observations suggest nearly stoichiometric incorporation of TRP32 into proteasomes in vivo.
Glycerol gradient fractionation of cell lysates revealed that most of the TRP32 co-migrated with markers of the 26 S proteasome (Fig. 2C). Thioredoxin, by contrast, migrated exclusively in lighter fractions, demonstrating that proteasome association is not a property common to thioredoxin domain-containing proteins (Fig. 2C, bottom). Furthermore, overexpression of untagged TRP32 or the N-terminally HA-tagged HA.TRP32.WT in HEK 293T cells only modestly increased the amount of TRP32 associated with immunopurified proteasomes (Fig. 2D, compare lane 2 with lanes 3 and 4), suggesting saturable and therefore specific binding of TRP32 at the proteasome.
ATP depletion promotes dissociation of the 19 S regulatory particle from the 20 S proteolytic core and provides a criterion to identify proteins that associate with proteasomes through either particle (20, 21). Proteasomes immunopurified from cellular lysates that had been depleted of ATP using glucose and hexokinase contained less TRP32 than those purified with ATP (Fig. 3A, compare lane 3 with lanes 1 and 2), suggesting that TRP32 associates with the proteasome through the 19 S regulatory particle.
Chemical cross-linking was used to identify proteasome subunits in proximity to TRP32. Endogenous proteins of HEK 293T cells were metabolically labeled with [35S]Met/Cys, and proteasomes were immunopurified with a covalently immobilized monoclonal antibody directed to the PSMA1 subunit of 20 S proteasome. The immunopurified proteasomes were exposed in vitro to the reversible cross-linker DSP. After quenching the cross-linker, the sample was boiled in 2% SDS to dissociate the proteasome from the anti-PSMA1 antibody and to disrupt non-covalent interactions. The soluble proteins were diluted to 0.1% SDS, and TRP32 (with cross-linked partners) was immunopurified with antiserum to TRP32. The immunopurified complex was disrupted with 2% SDS under reducing conditions (that reverse the cross-linker), revealing the labeled proteins by SDS-PAGE. The addition of cross-linker to the immunopurified proteasomes invariably resulted in the association of TRP32 with two high molecular weight bands identical in size to PSMD1 and PSMD2 and several lighter bands (Fig. 3B, lane 4). The association of these two bands with TRP32 depended on both the addition of cross-linker (compare lanes 3 and 4) and the immunopurification of TRP32 (lanes 5 and 6) and thus indicates that they represent proteasome subunits proximal to TRP32.
TRP32 consists of two independently folding domains (22, 23) (Fig. 3C). To map the portion of TRP32 that specifies proteasome association, we expressed each domain separately in HEK 293T cells and sought to detect their presence in immunopurified proteasomes by immunoblot using a combination of antisera that recognize the N- and C-terminal portions of the protein. Endogenous TRP32, exogenously expressed HA epitope-tagged full-length TRP32 (HA.TRP32.WT), and its C-terminal fragment (HA.TRP32-(112-289)) were recovered in association with immunopurified proteasomes (Fig. 3D, lanes 5, 6, and 8). However, the N-terminal fragment (TRP32-(1-112).HA), which gave a strong signal in the cell lysate (lane 3), did not form a stable complex with proteasomes (lane 7). Thus, the C-terminal portion of TRP32 is both necessary and sufficient for proteasome binding. Together, the observations presented in Fig. 3 place the thioredoxin domain of TRP32 near the substrate-binding site of the 19 S regulatory particle (24) and suggest that it is free to engage substrates.
TRP32 Is a Proteasome-associated Disulfide Reductase—An established procedure for cysteine modification with maleimide-conjugated PEG(5K) (PEG(5K)-maleimide) was used to probe the redox state of the TRP32 active site in vivo (14). HEK 293T cells expressing HA.TRP32.WT or mutant human TRP32 lacking both active site cysteine residues (HA.TRP32.CS) were exposed to As(III) before lysis in the presence of the cysteine alkylating agent N-ethylmaleimide (NEM), irreversibly modifying reduced cysteines. Disulfides in this blocked sample were then reduced with an excess of DTT followed by protein precipitation with trichloroacetic acid, and the pellet was washed free of DTT and residual NEM. The resolubilized proteins in the pellet were exposed to PEG(5K)-maleimide, which modifies reduced cysteines, leading to a reduced electrophoretic mobility in SDS-PAGE and thus identifying proteins that contained disulfide bonds in vivo (Fig. 4A).
FIGURE 4.
TRP32 is a redox-active protein that undergoes disulfide bond formation in cells exposed to As(III). A, schematic of the procedure for PEG(5K)-maleimide labeling of thiols engaged in disulfide bonds in vivo. B, TRP32 immunoblot of samples prepared from lysates of HEK 293T cells expressing WT or active site mutant (CS) HA-tagged TRP32 that had been exposed to As(III), as indicated. Following trichloroacetic acid precipitation, the proteins in the lysate were reduced with DTT and exposed to PEG(5K)-maleimide, as indicated. Note the conspicuous DTT and PEG(5K)-maleimide-dependent shift in mobility of the wild-type TRP32 (PEG(5K)-HA.TRP32), which is enhanced in the sample from cells exposed to As(III) (lanes 1 and 5). The lower panel shows an immunoblot of the TRP32 in the lysates before trichloroacetic acid precipitation (the input). C, bar graph depicting the fluorescence polarization signal of fluorescein-maleimide following incubation with recombinant TRP32-(1-112) treated, where indicated, with 100 μm NEM, 1 mm diamide, or 1 mm As(III). Polarization was blocked by both NEM and diamide but not As(III). D, immunoblot of endogenous TRP32 and eIF2α (a non-redox-active reference protein) from lysates of cells exposed to As(III) (in the presence of MG132) (Input) and PEG(5K)-maleimide labeled samples prepared from these lysates, as described in A. Note the conspicuous shift in mobility of the endogenous TRP32, but not for the non-redox-active cytosolic protein eIF2α, in the As(III)-treated samples.
HA.TRP32.WT was readily modified by PEG(5K)-maleimide, whereas HA.TRP32.CS was not (Fig. 4B, compare lane 1 with lane 4). Furthermore, the amount of TRP32 protein modified was increased by exposing the transfected cells to As(III) (Fig. 4B, compare lanes 1 and 5). These findings suggest the presence of disulfide bonding at the active site of TRP32, whose extent increases in As(III)-treated cells. The observed modification of TRP32 was dependent on exposure to both DTT and PEG(5K)-maleimide in vitro (Fig. 4B, lanes 2 and 3, respectively), suggesting that the slower migrating band reports on disulfides in TRP32. The observed shift in TRP32 mobility primarily corresponded to that expected of a protein modified by a single PEG(5K). This suggests that the active site of TRP32 might be selectively engaged in intermolecular disulfide bonding. Alternatively, steric hindrance may restrict PEG(5K) modification to only one of the two thiols at the TRP32 active site exposed by reduction of an intramolecular disulfide in TRP32. Finally, our observations may reflect a bias against transfer of heavily PEGylated protein to nitrocellulose membranes (25).
In order to confirm that the As(III)-induced change in PEG(5K) reactivity of TRP32 active site thiols is due to disulfide bond formation and not stable coordination of As(III), the accessibility of active site thiols in recombinant TRP32-(1-112) was explicitly explored. Modification of TRP32-(1-112) by fluorescein-maleimide was followed by an increase in fluorescence polarization upon covalent attachment of fluorescein-maleimide to thiols on the protein (Fig. 4C). Incubation of TRP32-(1-112) with either NEM or diamide prior to labeling blocked fluorescein-maleimide conjugation of TRP32-(1-112), indicating that the increase in polarization reports directly on the accessibility of thiols in TRP32-(1-112) and that these can be blocked by NEM. Importantly, incubation with as much as 1 mm As(III) did not affect the accessibility of the TRP32-(1-112) thiols, demonstrating that the TRP32 thioredoxin domain does not stably associate with As(III) and that the PEG(5K) modification of TRP32 in Fig. 4B reports on the formation of a disulfide bond at the TRP32 active site.
As(III)-dependent disulfide formation was also observed in endogenous TRP32 from MEFs, the process being nearly complete within 30 min (Fig. 4D, top). As(III) also promoted disulfide formation in thioredoxin (data not shown), consistent with a global perturbation in cytosolic redox in As(III)-treated cells that is counteracted by redox-active proteins. As expected, As(III) exposure did not alter the thiol reactivity of the translation initiation factor eIF2α, a reference protein that lacks a redox active site (Fig. 4D, bottom).
The above findings demonstrate that TRP32 is a 19 S-localized disulfide reductase, which probably donates electrons to an oxidatively modified protein. However, we were unable to trap a mixed disulfide between TRP32 (either wild type or the active site TRP32C37S trapping mutant) and a second protein in proteasomes purified from either untreated or As(III)-treated cells (data not shown). Also, although profiling of proteasomes isolated from As(III)-treated cells revealed the unexpected presence of a disulfide in the 11 S cap subunit PSME3, the presence of that disulfide was unaltered by TRP32 activity (supplemental Fig. 3). Thus, the redox substrate(s) of TRP32 remains unknown.
TRP32 Is Rapidly Degraded in As(III)-treated Cells—Exposure of HEK 293T cells to As(III) reduced both the amount of TRP32 that co-immunoprecipitates with proteasomes and the total cellular content of TRP32 (Figs. 1C and 2A). Similar observations were made in MEFs, and the decline in TRP32 protein levels in response to As(III) was reversed by the proteasome inhibitor MG132 (Fig. 5A, compare lanes 1-4 and lanes 5-8). The decline in TRP32 levels is very rapid, preceding the induction of As(III)-induced proteins (of which AIRAP is an example; Fig. 5A, bottom) by several h. Consistent with that time course, the As(III)-mediated decline in TRP32 levels was also observed when protein synthesis was inhibited by cycloheximide, implicating a rapid post-translational mechanism (Fig. 5A, lanes 9-12).
FIGURE 5.
TRP32 is rapidly degraded by the proteasome in As(III)-treated cells. A, immunoblots of TRP32, thioredoxin, eIF2α, and the As(III)-inducible protein AIRAP in lysates of MEFs exposed to 100 μm As(III) in the absence of presence of 10 μm MG132 or 50 μg/ml cycloheximide for the indicated time. B, autoradiogram of TRP32 immunopurified from metabolically labeled MEFs, immediately after the 3-h labeling pulse or following a cold chase. Where indicated, the cells were exposed to 100 μm As(III) with or without 10 μm MG132 during the chase. The TRP32 signal is plotted in C, revealing the As(III)-dependent decrease in the t½ of TRP32 from >6 to 1 h. D, immunoblots of endogenous and HA-tagged active site mutant TRP32 (HA.TRP32.CS) in lysates (lanes 1-3) of HEK 293T cells exposed to 100 μm As(III) and in proteasomes immunopurified from the same cells (lanes 5-7). Immunoblotted PSMD2 and PSMA1 served as recovery controls for the 19 and 20 S particles, respectively. The monoclonal antibody for proteasome immunopurification was omitted from the sample in lane 4 (Mock).
Metabolic labeling and pulse-chase analysis in MEF cells showed that the half-life of endogenous TRP32 exceeded 6 h in untreated cells and was decreased upon As(III) exposure to 1 h. The proteasome inhibitor MG132 blocked As(III)-mediated degradation of TRP32 (Fig. 5B). Together, these observations demonstrate that As(III) treatment induces the proteasome-mediated degradation of TRP32.
As(III) induces both oxidation of the TRP32 thioredoxin active site and proteasome-mediated degradation of the protein. Therefore, the relationship between the redox activity of TRP32 and TRP32 degradation was explored by comparing the fate of the active site mutant HA.TRP32.CS and the endogenous wild-type protein in transfected HEK 293T cells exposed to As(III). Both endogenous TRP32 and the lower mobility HA-tagged mutant dissociated from the proteasome and were depleted from the cell lysate with similar kinetics in As(III)-treated cells (Fig. 5D). This observation indicates that the redox activity of TRP32 and the As(III)-induced degradation of TRP32 are independent of one another.
Functional Role of TRP32—To evaluate the functional role of TRP32 in mammalian cells, we developed TRP32 mutant mice. A mouse embryonic stem cell line with a β-geo gene trapping vector integrated at the TRP32 (Txnl1) locus was obtained from Bay Genomics (line XH718). Sequencing of the targeted allele revealed that vector integration occurred in intron 1, disrupting the gene immediately 5′ of the region encoding the thioredoxin active site. The mutant allele, Trp32i, was transferred through the germ line of chimeric mice to produce Trp32+/i mice, which were intercrossed to produce Trp32+/+ and Trp32i/i embryos, from which MEFs were prepared. Immunoblotting revealed no detectable TRP32 protein in Trp32i/i fibroblasts (Fig. 6A), confirming significant disruption of gene function by the insertion.
FIGURE 6.
Cells derived from mice with an inactivating insertion at the Txnl1 locus (Trp32i) are not hypersensitive to oxidative insults. A, anti-TRP32 immunoblot of lysates of primary MEFs of the indicated Trp32 genotype. Where indicated, the cells were exposed to 100 μm As(III) for 4 h. B, proteasome activity of postmitochondrial supernatants prepared from Trp32+/+ and Trp32i/i MEF following exposure to the indicated concentrations of As(III) for 4 h measured by fluorescence of cleaved succinyl-LLVY-7-amido-4-methylcoumarin. The proteasome inhibitor MG132 (10 μm) was added to lysates as a specificity control (shown are the mean ± S.E. values, n = 3). C, anti-ubiquitin immunoblot of lysates from MEFs of the indicated Trp32 genotype following exposure to As(III). Note similar accumulation of high molecular weight polyubiquitinated proteins in the three genotypes. D, viability of MEF of the indicated Trp32 genotype following exposure to As(III) for 72 h measured by WST-1 absorption. The signal in the untreated sample of each genotype is arbitrarily set at 1 (shown are the mean ± S.E., n = 3). E, immunoblot of the indicated stress markers in lysates of Trp32+/+ and Trp32i/i MEFs exposed to 100 μm As(III). * (bottom), a nonspecific band reactive with the rabbit anti-TRP32 sera. F, immunoblot of the indicated stress markers in lysates of Trp32+/+ and Trp32i/i MEFs exposed to 0.25 μg/ml tunicamycin for the indicated time. TRP32 (bottom) was detected with a murine monoclonal antibody lacking the background reactivity of the rabbit anti-TRP32 serum used in E.
Trp32i/i mice were born at the expected frequency from heterozygous matings and were superficially indistinguishable from wild type. The cytosol of wild-type and Trp32i/i MEFs had a similar ability to digest a model proteasome substrate, succinyl-LLVY-7-amido-4-methylcoumarin, in vitro, and this activity was unaltered by exposure of cells of either genotype to As(III) (Fig. 6B). Exposure to As(III) resulted in a similar time-dependent increase in levels of polyubiquitinated proteins in MEFs of either genotype (Fig. 6C), and there was no evidence for hypersensitivity of Trp32i/i MEFs to cellular oxidants, such as As(III) or t-BuOOH (Fig. 6D and supplemental Fig. 4A). Furthermore, the activation of known As(III)-induced stress signaling pathways was indistinguishable in Trp32i/i and wild-type MEFs, as indicated by the efficient induction of Hsp70, CHOP, GADD34, and AIRAP (Fig. 6E). The potential role of TRP32 in reducing disulfides in the ER-associated degradation pathway was also explored. In Caenorhabditis elegans, RNA interference directed against components of the ER-associated degradation pathway (e.g. SEL-1; Hrd3p in yeast) activate the ER stress reporter hsp-4pr::gfp. Alternatively, RNA interference targeting the C. elegans homolog of TRP32 (trp-32) did not activate hsp4pr::gfp (supplemental Fig. 4B). Similarly, there is no evidence to suggest that Trp32i/i MEF cells are sensitized to low doses of a toxin (tunicamycin) that leads to protein misfolding and enhanced production of ER-associated degradation substrates, as indicated by the indistinguishable induction kinetics of ER stress markers (Fig. 6F). These observations suggest that the activity of TRP32 is not critical to readily assayable cellular functions.
To further explore the potential significance of the TRP32 association with proteasomes, we turned to the fission yeast, S. pombe, that possesses a readily identifiable TRP32 homolog, Txl1 (26). Like the Trp32i/i mice, Δtxl1 yeasts were indistinguishable from the parent wild-type strain in growth rate and morphology (Fig. 7A) and were not observably hypersensitive to As(III) or the cellular oxidant tert-BuOOH (Fig. 7B).
FIGURE 7.
Deletion of the TRP32 S. pombe homolog txl1 inhibits growth of cut8-deleted yeasts. A, growth of WT and Δtxl1 S. pombe in suspension analyzed by measuring the OD600. B, As(III)- and tert-butyl hydrogen peroxide (tBuOOH)-mediated growth inhibition of WT and Δtxl1 S. pombe following a 10-h incubation. C, growth on agarose plates of 10-fold serially diluted WT, Δtxl1, Δcut8, and Δcut8Δtxl1 S. pombe at the indicated temperature. D, growth of Δtxl1 and Δcut8Δtxl1 S. pombe in suspension. E, growth of Δcut8, Δcut8Δtxl1, or the double mutant yeasts rescued with an HA-tagged wild-type S. pombe Txl1 or wild-type or CS mutant HA-tagged human TRP32. F, anti-HA immunoblot of lysates prepared from the yeast strains shown used in E demonstrating the expression of HA.spTxl1.WT, HA.TRP32.WT, and HA.TRP32.CS. The anti-Hsp60 blot served as a loading control.
To further characterize the role of Txl1 in fission yeast, we used the E-MAP approach, which quantitatively reports on genetic interactions, both negative (e.g. synthetic sickness/lethality) and positive (e.g. suppression) (27, 28). Using the Pombe Epistatic Mapper system developed for fission yeast (16), we crossed the Δtxl1 to a set of 2,295 viable deletion strains. The strongest negative interaction was observed with Δcut8, a gene encoding a proteasome-associated protein involved in localizing 26 S proteasomes to the nucleus (29, 30) (Table 1).
TABLE 1.
List of significant negative interactions (synthetic sick) observed after crossing Δtxl1 to a set of 2,295 deletion strains
The list is arranged in descending S-score (or E-MAP score) (28), and a cut-off of −3 was used. Where known, the encoded S. pombe and corresponding S. cerevisiae homologs are listed.
| S. pombe open reading frame | S. pombe protein | S. cerevisiae homolog | E-MAP score |
|---|---|---|---|
| SPAC17C9.13C | Cut8 | STS1 | −10.657017 |
| SPAC589.06C | PHO88 | −10.360266 | |
| SPBC27B12.11C | None | −9.167332 | |
| SPAC3H5.08C | YKL121W,YMR102C | −8.244299 | |
| SPBP8B7.28C | None | −7.264413 | |
| SPCC736.08 | Cbf11 | None | −6.651497 |
| SPAC8C9.11 | YGL220W | −5.66225 | |
| SPBC1734.12C | Alg12 | ALG12 | −5.216294 |
| SPAC8C9.12C | MRS3,MRS4 | −5.090238 | |
| SPBC1734.13 | Atp3 | ATP3 | −4.801525 |
| SPBC337.03 | RTT103 | −4.51371 | |
| SPCC594.06C | VAM7 | −4.089038 | |
| SPAC29B12.04 | Snz1 | SNZ3,SNZ1,SNZ2 | −3.9765 |
| SPBC1778.09 | None | −3.880673 | |
| SPBC337.11 | YIM1 | −3.450755 | |
| SPBC725.05C | NPP1,NPP2 | −3.245368 | |
| SPBC337.04 | Ppk27 | PRR1 | −3.224489 |
| SPBC1773.06C | None | −3.139316 | |
| SPBC12D12.06 | Srb11 | SSN8 | −3.123008 |
The negative interaction between Δcut8 and Δtxl1 was confirmed by recreating the double deletion in a wild-type background (Fig. 7, C and D). The growth defect of the double mutant was rescued in trans by expression of HA-tagged S. pombe Txl1 (HA.spTxl1.WT) and by expression of mammalian HA.TRP32.WT, suggesting that Txl1 and TRP32 perform similar functions in eukaryotic cells (Fig. 7, E and F). Surprisingly, both the wild-type HA.TRP32.WT and the active site mutant HA.TRP32.CS allowed for complete rescue of the synthetic phenotype of the compound deletion, Δcut8 and Δtxl1. This last finding indicates that the positive effect of Txl1 in the growth of Δcut8 yeast does not require its redox active site.
DISCUSSION
Strong biochemical evidence supports the conclusion that TRP32 is bound to the 19 S regulatory particle of the proteasome. Furthermore, it is likely that most of the TRP32 in cells is associated with proteasomes and that a substantial fraction of 26 S proteasomes have TRP32 associated with them. The biochemical findings in mammalian cells are buttressed by genetic evidence of an enfeebling synthetic interaction between deletions of the S. pombe TRP32 homolog, txl1, and cut8, a gene involved in localizing proteasomes to the nuclear envelope. Furthermore probing of the activity of TRP32 demonstrated the formation of an active site disulfide (Fig. 4D), providing evidence for its involvement in disulfide bond reduction in vivo. Together, these observations indicate that in addition to its known activities of deubiquitinating, ubiquitinating, and ATPase-mediated unfolding and translocation of proteins, the 19 S regulatory particle is also endowed with a subunit able to reduce disulfide bonds. The experiments described in this paper provide some clues to the significance of this key discovery.
Under normal circumstances, TRP32 is a relatively long lived protein (t½ > 6 h); it is thus unlikely that its association with the proteasome is that of a degradation substrate in transit. Our chemical cross-linking experiments place proteasome-bound TRP32 in proximity to PSMD2, but other contacts, such as with the identically sized PSMD14 (31), would have been missed in our assay. Nonetheless, both our study and that of Andersen et al. (31) place TRP32 near the substrate-binding site of the 19 S cap. This suggests that TRP32 might be poised to reduce disulfide-containing substrates recruited to the proteasome, perhaps as a mechanism to facilitate their unfolding and degradation. Our finding of enhanced disulfide-bonded TRP32 in cells exposed to As(III) is consistent with that idea, since As(III) promotes oxidative protein misfolding and challenges the cell's protein degradation machinery (32). Furthermore, previous reports implicating TRP32 in preventing cytotoxicity under conditions of metabolic stress (33) suggest a role for the protein in combating aspects of oxidative protein misfolding in the cell.
However, other findings are less supportive of a role for TRP32 in reduction of oxidized, difficult-to-degrade proteasome substrates; neither yeasts nor mammalian cells lacking TRP32 are hypersensitive to As(III), and TRP32 knock-out MEFs cells do not accumulate polyubiquitinated substrates upon exposure to As(III). This contrasts with cells lacking the As(III)-inducible proteasome subunit AIRAP that are hypersensitive to the toxin and accumulate excess polyubiquitinated substrates upon exposure to As(III) (13). Furthermore, we were unable to trap disulfide-bonded intermediates between TRP32 and its putative substrates in cells exposed to As(III), and, although our studies led to the identification of a hitherto unanticipated As(III)-induced disulfide bond in the PSME3 subunit of the 11 S proteasome regulator, manipulations of TRP32 function did not affect that modification (supplemental Fig. 3).
We also considered the possibility that TRP32 reduces the oxidatively damaged active site cysteine of the proteasome-associated deubiquitinating enzyme USP14 or UCH37. However, we were unable to detect a disulfide bonded complex between TRP32 and USP14 by immunoblotting, and direct measurements of total proteasome-associated N-ethylmaleimide-sensitive deubiquitination of ubiquitin-7-amido-4-methylcoumarin in preparations derived from unstressed and As(III)-treated cells failed to implicate TRP32 in the process (data not shown). Finally, a role for TRP32 in enhancing proteasomal capacity to deubiquitinate or degrade oxidized misfolded proteins is difficult to reconcile with the fact that TRP32 itself is degraded under the same conditions that promote accumulation of oxidatively damaged proteins.
An alternative hypothesis ascribes to the TRP32 redox active site the role of a sensor, communicating the redox state of the cytosol to the proteasome. This idea is consistent with the finding that TRP32 engages the proteasome via its C-terminal domain, leaving the thioredoxin active site free to probe the redox environment in the cell. In a fanciful extension of that hypothesis, As(III)-mediated degradation of TRP32 switches proteasome function from a mode favored in unstressed cells containing TRP32 to a mode favored in stressed cells lacking TRP32. The enfeeblement of cut8 mutant yeast by coincidental deletion of txl1 is consistent with a role for TRP32 in maintaining some aspect of proteasome function under basal conditions, as is the ability of the redox mutant TRP32.CS to rescue the growth defect of the null in a cut8 background. In Drosophila, TRP32 mRNA levels are coordinately regulated with other proteasome subunits, increasing in abundance when proteasome function is blocked (34). Finally the notion of a role for TRP32 in enabling proteasome function under basal, nonstressed conditions receives further support from the existence of a plant homolog that has a close counterpart of the presumed proteasome-interacting TRP32 C-terminal domain but lacks the thioredoxin domain altogether (23).
However, the idea that a change in redox state of TRP32 triggers a switch at the proteasome is difficult to reconcile with the finding that redox activity is dispensable for As(III)-mediated degradation of TRP32 in mammalian cells. Thus, both the mechanism and significance of As(III)-mediated degradation of TRP32 remain obscure. Furthermore, although our global analysis of As(III)-induced changes in phosphorylation of proteasome-associated proteins led to the identification of stress-induced phosphorylation of the ADRM1 subunit of the 19 S particle, further scrutiny failed to implicate that phosphorylation event in either TRP32 degradation or in modulation of the known functions of ADRM1 (ubiquitin binding or activation/recruitment of the deubiquitinating enzyme UCH37; supplemental Fig. 5).
In conclusion, our study and the independent, coincidental study by Andersen et al. (31) have uncovered a novel, redoxactive proteasome subunit whose function remains to be determined. To date, our various hypothesis-driven attempts to understand the function of TRP32 have been unproductive, and the absence of a discernable phenotype in mice and MEFs lacking TRP32 limits our ability to generate new hypotheses. Nonetheless, we hope and expect that further progress may be made by the future discovery of proteins that form S-S bonds with TRP32.
Acknowledgments
We thank Bay Genomics for the XH718 clone, Shin Yonehara (Kyoto University) for the human TRP32 expression plasmids and for anti-TRP32 monoclonal antibody used in the early phases of the study, Keiiji Tanaka (Tokyo Metropolitan Institute of Medical Science) for the monoclonal anti-PSMA1 (mAb 2-17), George N. DeMartino (University of Texas Southwestern) for purified 20 S proteasomes, and Robert E. Cohen (The Johns Hopkins University) for informative discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1-ES08681 (to D. R.), F32-ES014775 (to R. L. W.), MC-IRG203123 (to A. S.), P30-NS050276 (to T. A. N.), F32-NS050901 (to C. M. H.), and RO1-GM084279 (to N. J. K.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1-5.
Footnotes
The abbreviations used are: HA, hemagglutinin; DTT, dithiothreitol; IEF, isoelectric focusing; WT, wild type; mAb, monoclonal antibody; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; MEF, mouse embryo fibroblast; PEG(5K), polyethylene glycol 5000; NEM, N-ethylmaleimide; SILAC, stable isotope labeling with amino acids in cell culture; LC, liquid chromatography; ESI, electrospray ionization; MS, mass spectrometry; ER, endoplasmic reticulum; DSP, dithiobis(succinimidyl propionate).
References
- 1.Aposhian, H. V. (1989) Rev. Biochem. Toxicol. 10 265-299 [Google Scholar]
- 2.Johnston, D., Oppermann, H., Jackson, J., and Levinson, W. (1980) J. Biol. Chem. 255 6975-6980 [PubMed] [Google Scholar]
- 3.Levinson, W., Oppermann, H., and Jackson, J. (1980) Biochim. Biophys. Acta 606 170-180 [DOI] [PubMed] [Google Scholar]
- 4.Duncan, R. F., and Hershey, J. W. (1987) Arch. Biochem. Biophys. 256 651-661 [DOI] [PubMed] [Google Scholar]
- 5.Balch, W. E., Morimoto, R. I., Dillin, A., and Kelly, J. W. (2008) Science 319 916-919 [DOI] [PubMed] [Google Scholar]
- 6.Goldberg, A. L. (2003) Nature 426 895-899 [DOI] [PubMed] [Google Scholar]
- 7.Elsasser, S., and Finley, D. (2005) Nat. Cell Biol. 7 742-749 [DOI] [PubMed] [Google Scholar]
- 8.Bond, U., Agell, N., Haas, A. L., Redman, K., and Schlesinger, M. J. (1988) J. Biol. Chem. 263 2384-2388 [PubMed] [Google Scholar]
- 9.Kirkpatrick, D. S., Dale, K. V., Catania, J. M., and Gandolfi, A. J. (2003) Toxicol. Appl. Pharmacol. 186 101-109 [DOI] [PubMed] [Google Scholar]
- 10.Ishii, T., Sakurai, T., Usami, H., and Uchida, K. (2005) Biochemistry (Mosc.) 44 13893-13901 [DOI] [PubMed] [Google Scholar]
- 11.Hanna, J., and Finley, D. (2007) FEBS Lett. 581 2854-2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun, C., Stanhill, A., Yang, Y., Zhang, Y., Haynes, C. M., Xu, C., Neubert, T., Mor, A., Philips, M. R., and Ron, D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105 7094-7099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanhill, A., Haynes, C. M., Zhang, Y., Min, G., Steele, M. C., Kalinina, J., Martinez, E., Pickart, C. M., Kong, X.-P., and Ron, D. (2006) Mol. Cell 23 875-995 [DOI] [PubMed] [Google Scholar]
- 14.Lu, J., and Deutsch, C. (2001) Biochemistry (Mosc.) 40 13288-13301 [DOI] [PubMed] [Google Scholar]
- 15.Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996) Anal. Chem. 68 850-858 [DOI] [PubMed] [Google Scholar]
- 16.Roguev, A., Wiren, M., Weissman, J. S., and Krogan, N. J. (2007) Nat. Methods 4 861-866 [DOI] [PubMed] [Google Scholar]
- 17.Blagoev, B., Kratchmarova, I., Ong, S. E., Nielsen, M., Foster, L. J., and Mann, M. (2003) Nat. Biotechnol. 21 315-318 [DOI] [PubMed] [Google Scholar]
- 18.Lee, K. K., Murakawa, M., Takahashi, S., Tsubuki, S., Kawashima, S., Sakamaki, K., and Yonehara, S. (1998) J. Biol. Chem. 273 19160-19166 [DOI] [PubMed] [Google Scholar]
- 19.Miranda-Vizuete, A., Gustafsson, J. A., and Spyrou, G. (1998) Biochem. Biophys. Res. Commun. 243 284-288 [DOI] [PubMed] [Google Scholar]
- 20.Orino, E., Tanaka, K., Tamura, T., Sone, S., Ogura, T., and Ichihara, A. (1991) FEBS Lett. 284 206-210 [DOI] [PubMed] [Google Scholar]
- 21.Eytan, E., Ganoth, D., Armon, T., and Hershko, A. (1989) Proc. Natl. Acad. Sci. U.S.A. 86 7751-7755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin, J., Chen, X., Zhou, Y., Bartlam, M., Guo, Q., Liu, Y., Sun, Y., Gao, Y., Ye, S., Li, G., Rao, Z., Qiang, B., and Yuan, J. (2002) Eur. J. Biochem. 269 2060-2068 [DOI] [PubMed] [Google Scholar]
- 23.Song, J., Tyler, R. C., Wrobel, R. L., Frederick, R. O., Vojtek, F. C., Jeon, W. B., Lee, M. S., and Markley, J. L. (2005) Protein Sci. 14 1059-1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenzweig, R., Osmulski, P. A., Gaczynska, M., and Glickman, M. H. (2008) Nat. Struct. Mol. Biol. 15 573-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appenzeller-Herzog, C., and Ellgaard, L. (2008) Antioxid. Redox Signal. 10 55-64 [DOI] [PubMed] [Google Scholar]
- 26.Jimenez, A., Mateos, L., Pedrajas, J. R., Miranda-Vizuete, A., and Revuelta, J. L. (2007) Yeast 24 481-490 [DOI] [PubMed] [Google Scholar]
- 27.Schuldiner, M., Collins, S. R., Thompson, N. J., Denic, V., Bhamidipati, A., Punna, T., Ihmels, J., Andrews, B., Boone, C., Greenblatt, J. F., Weissman, J. S., and Krogan, N. J. (2005) Cell 123 507-519 [DOI] [PubMed] [Google Scholar]
- 28.Roguev, A., Bandyopadhyay, S., Zofall, M., Zhang, K., Fischer, T., Collins, S. R., Qu, H., Shales, M., Park, H. O., Hayles, J., Hoe, K. L., Kim, D. U., Ideker, T., Grewal, S. I., Weissman, J. S., and Krogan, N. J. (2008) Science 322 405-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda, K., and Yanagida, M. (2005) Cell 122 393-405 [DOI] [PubMed] [Google Scholar]
- 30.Tatebe, H., and Yanagida, M. (2000) Curr. Biol. 10 1329-1338 [DOI] [PubMed] [Google Scholar]
- 31.Andersen, K., Madsen, L., S, P., Johnsen, A., Semple, C., Hendil, K., and Hartmann-Petersen, R. (2009) J. Biol. Chem. 284 15246-15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng, P. Z., Wang, K. K., Zhang, Q. Y., Huang, Q. H., Du, Y. Z., Zhang, Q. H., Xiao, D. K., Shen, S. H., Imbeaud, S., Eveno, E., Zhao, C. J., Chen, Y. L., Fan, H. Y., Waxman, S., Auffray, C., Jin, G., Chen, S. J., Chen, Z., and Zhang, J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102 7653-7658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimenez, A., Pelto-Huikko, M., Gustafsson, J. A., and Miranda-Vizuete, A. (2006) FEBS Lett. 580 960-967 [DOI] [PubMed] [Google Scholar]
- 34.Lundgren, J., Masson, P., Mirzaei, Z., and Young, P. (2005) Mol. Cell. Biol. 25 4662-4675 [DOI] [PMC free article] [PubMed] [Google Scholar]