Abstract
Estrogen (E2) signaling is conveyed by the transcription factors estrogen receptor (ER) α and β. ERs modulate the expression of genes involved in cellular proliferation, motility, and death. The regulation of transcription by E2-ERα through binding to estrogen-responsive elements (EREs) in DNA constitutes the ERE-dependent signaling pathway. E2-ERα also modulates gene expression by interacting with transregulators bound to cognate DNA-regulatory elements, and this regulation is referred to as the ERE-independent signaling pathway. The relative importance of the ERE-independent pathway in E2-ERα signaling is unclear. To address this issue, we engineered an ERE-binding defective ERα mutant (ERαEBD) by changing residues in an α-helix of the protein involved in DNA binding to render the receptor functional only through the ERE-independent signaling pathway. Using recombinant adenovirus-infected ER-negative MDA-MB-231 cells derived from a breast adenocarcinoma, we found that E2-ERαEBD modulated the expression of a subset of ERα-responsive genes identified by microarrays and verified by quantitative PCR. However, E2-ERαEBD did not affect cell cycle progression, cellular growth, death, or motility in contrast to E2-ERα.ERαEBD in the presence of E2 was also ineffective in inducing phenotypic alterations in ER-negative U-2OS cells derived from an osteosarcoma. E2-ERα, on the other hand, effectively repressed growth in this cell line. Our findings suggest that genomic responses from the ERE-dependent signaling pathway are required for E2-ERα to induce alterations in cellular responses.
17β-Estradiol (E2),5 as the main circulating estrogen hormone, plays critical roles in the physiology and pathophysiology of many tissues (1, 2). The effects of E2 are primarily mediated by estrogen receptor (ER) α and β (1, 2). ERs display functionally distinct structural features. The amino terminus of ERα contains a ligand-independent transactivation function. The central region is the DNA binding domain (DBD). The flexible hinge domain contains a nuclear localization signal and links the DBD domain to the multifunctional carboxyl-terminal ligand binding (LBD) domain. The LBD is involved in ligand binding, dimerization, and ligand-dependent transactivation function.
Following synthesis, ERα dimerizes and translocates to the nucleus independent of E2 (3). Fractions of the ERα population also partition to the perimembrane, cytoplasm, and mitochondria (4). The binding of E2 to ERα leads to a major structural reorganization of the LBD that converts the inactive ERα to the functionally active form by generating surfaces that support protein-protein interactions (5). The integration of E2-ERα signaling generated from various cellular locations is thought to be critical for the regulation of responsive gene expression involved in cellular proliferation, differentiation, motility, and death (4, 6).
One of the primary nuclear E2-ERα signaling events involves the interaction of E2-ERα with specific DNA sequences, known as estrogen-responsive elements (EREs) (7), of estrogen-responsive genes and subsequent regulation of transcription. This signaling route is referred to as the ERE-dependent signaling pathway (2, 8). E2-ERα also regulates gene transcription by interacting with a transcription factor, for example SP1 (stimulatory protein 1) and AP1 (activator protein 1), bound to cognate DNA-responsive elements on the regulatory regions of responsive genes. This nuclear E2-ER signaling is called the ERE-independent signaling pathway (2, 8).
However, the importance of the ERE-independent pathway in E2-ERα signaling in physiology and its contribution to pathophysiology remain unclear. Studies showed that changing Glu and Gly residues to Ala in the DNA-binding helix of the DBD of mouse (9) and human (10) ERα generates a mutant receptor capable of mediating E2 signaling through the ERE-independent pathway. Analogous mutations in the DBD of the human ERβ (11, 12) also render the receptor functional only in the ERE-independent signaling pathway. Studies with a knock-in mouse model provide compelling support for the importance of the ERE-independent pathway in the regulation of various tissue functions, albeit in a tissue-specific manner (13-15). In an attempt to correlate genomic responses from the ERE-independent signaling pathway to alterations in cellular phenotypes, we found that changing Glu and Gly residues at positions 203 and 204 to Ala in the DNA-binding helix of human ERα DBD (ERα203/4) reduces but does not prevent functional features of the parent ERα in the ERE-dependent signaling pathway. Based on these observations, we introduced additional mutations in the DBD of ERα203/4. The replacement of Arg at position 211 with Glu in ERα203/4 generated an ERE-binding defective ERα mutant (ERα203/4/11 or ERαEBD) that was functional exclusively at the ERE-independent signaling pathway. We then assessed the effects of ERαEBD on cellular responses to E2 in adenovirus-infected ER-negative MDA-MB-231 cells derived from breast adenocarcinoma, in which exogenously introduced ERα was shown to induce cellular responses (16-18). Our results reveal that ERαEBD mediated gene expression identified by a global gene expression profiling approach and verified by qPCR was insufficient to alter the proliferation, death, or motility of cells in contrast to E2-ERα. E2-ERαEBD was also ineffective in altering the phenotypic characteristics of ER-negative osteosarcoma-derived U-2OS cells used as an estrogen target tissue model wherein E2-ER signaling induces genomic and cellular changes (19-21). Our results suggest that genomic responses from ERE-independent signaling pathways can be dissociated from the induction of phenotypic alterations. These results further imply that the ERE-dependent pathway is a required signaling route for E2-ERα to induce cellular responses.
EXPERIMENTAL PROCEDURES
Generation of DNA Binding Defective ERα Mutants—The human ERα cDNA encoding the 595-amino acid long ERα was described previously (22, 23). This ERα cDNA also contains sequences that encode an amino-terminal FLAG epitope (23). For the engineering of an ERE-binding defective ERα mutant, we utilized an overlapping PCR with the ERα cDNA as the template. The ERα203/4 mutant was generated using primers that contain sequences for amino acid substitutions replacing glutamic acid and glycine at positions 203 and 204, respectively, with alanine residues in the first zinc finger of the DBD. In the generation of the ERα203/4/11, ERα203/4 was used as the PCR template using primers with sequences that replace arginine at position 211 with glutamic acid. Restriction and DNA-modifying enzymes were obtained from New England Biolabs (Beverly, MA) and Invitrogen.
Cell Culture—Culturing of Chinese hamster ovary, HeLa, and MDA-MB-231 cells was described previously (22, 23). U-2OS cells derived from osteosarcoma were purchased from the ATCC (Manassas, VA). U-2OS cells were grown in McCoy's 5α medium (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; Invitrogen). C4 and C4-12 cells were grown in Eagle's modified essential medium without phenol red containing 5% CD-FBS (Hyclone). In all experiments, medium was changed every 3rd day.
Transient Transfections—Transient transfections for simulated ERE-dependent and ERE-independent pathways were accomplished as described previously (23-25). Transfected MDA-MB-231 cells were treated without or with 10-9 m 17β-estradiol (E2) and/or 10-7 m Imperial Chemical Industries 182,780 (ICI, Tocris Inc., Ballwin, MO) for 24 or 40 h to assess the effects of ligands on ER-mediated transcriptional responses from the ERE-dependent or ERE-independent signaling, respectively.
Generation of Recombinant Adenoviruses—Recombinant adenovirus bearing none or an ERα cDNA was produced by using the AdEasy-XL adenoviral system (Stratagene, La Jolla, CA) as described previously (12, 26). The purified viruses were titered using an Adeno-X rapid titer kit (BD Biosciences) to determine the multiplicity of infection (m.o.i.).
Immunocytochemistry (ICC), Western Blot (WB), and Electrophoretic Mobility Shift Assay (EMSA)—Transfected or infected cells in a time-dependent manner were processed for ICC, WB, and EMSA as described previously (22, 23, 27). For WB, proteins were probed with the horseradish peroxidase-conjugated monoclonal FLAG antibody (M2-horseradish peroxidase; Sigma) using the ECL-Plus Western blotting kit (GE Healthcare). For EMSA, we used FLAG M2 antibody (Sigma). Images from WB and EMSA were analyzed and quantified by ImageQuant version 1.2 software (GE Healthcare). For ICC, an ERα-specific antibody (HC-20) (Santa Cruz Biotechnology, Santa Cruz, CA) was used to probe ER proteins and a fluorescein-conjugated secondary antibody (Santa Cruz Biotechnology) for visualization.
In Situ E2 Binding Assay—To assess the synthesis and functionality of ERα species in transfected or infected cells, we used the in situ E2 binding assay as described previously (12, 26). In brief, transiently transfected or infected cells were incubated with 10-7 m [2,4,6,7,16,17-3H]17β-estradiol (118 Ci/mmol; PerkinElmer Life Sciences) in the absence or presence of 10-6 m ICI for 1 h. Cells were then washed extensively with phosphate-buffered saline and collected, and radioactivity was measured in a scintillation counter.
Chromatin Immunoprecipitation Assay (ChIP)—ChIP assays in transiently transfected MDA-MB-231 cells were performed using FLAG-M2 antibody-conjugated agarose beads (Sigma) as described previously (12, 26). The generation of a 366-bp PCR fragment indicates the specificity of PCRs.
Endogenous Gene Expression—MDA-MB-231 cells (100,000 cells/well), plated in 6-well tissue culture plates in phenol red-free Dulbecco's modified Eagle's medium containing 10% CD-FBS for 24 h, were infected with recombinant adenoviruses in the absence of ligands for 48 h to allow the synthesis of receptor proteins to reach maximum and comparable levels. Cells were then treated without or with 10-9 m E2 for 6 or 24 h to assess the effects of ERs on the expression of endogenous genes. At termination, cells were collected and subjected to total RNA extraction using the RNeasy mini kit (Qiagen, Valencia, CA). For quantitative PCR (qPCR), we used custom TaqMan low density arrays with proprietary primer and probe sequences (Applied Biosystems, Foster City, CA) as we recently described (12). All qPCRs were carried out at the Functional Genomic Center of the University of Rochester, Rochester, NY. The expression of the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene was used as control. The real time reverse transcription-PCR amplifications were accomplished using an ABI Prism 7900HT sequence detection system with a TaqMan low density array upgrade (Applied Biosystems). Relative quantification analysis was performed using the comparative CT method (28).
Cellular Proliferation—MDA-MB-231 cells (5,000 cells/well) plated in 24-well tissue culture plates in phenol red-free Dulbecco's modified Eagle's medium containing 10% CD-FBS for 24 h were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 and/or 10-7 m ICI for different durations of time. Cells were collected and counted using a hemocytometer (Hausser Scientific, Horsham, PA) and/or an automated cell counter (Nexcelom Biosciences, Lawrence, MA) or subjected to MTT assay as described previously (12).
For the effects of ERs on U-2OS cell proliferation, cells (2,500 cells/well) were plated in 24-well tissue culture plates, precoated with poly-d-lysine hydrobromide (Sigma), in McCoy's α-medium containing 10% FBS for 24 h. Cells were subsequently incubated with fresh McCoy's α-medium containing 10% CD-FBS for an additional 24 h. Cells were then infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 and/or 10-7 m ICI for different durations of time. We used Ad5-ERα at 40 m.o.i. At this m.o.i., the recombinant adenovirus synthesizes a concentration of ERα that requires E2 for function. Ad5-ERαEBD was used at 50 m.o.i., which produced comparable levels of receptor proteins to that of ERα. In all infections, the total m.o.i. was adjusted to 50 m.o.i. by the parent virus Ad5. At the termination of an experiment, cells were subjected to proliferation assays.
Cell Cycle Analysis—MDA-MB-231 cells (50,000 cells/well) in 6-well tissue culture plates were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 and/or 10-7 m ICI for different durations. Similarly, U-2OS cells (50,000 cells/well) in poly-d-lysine hydrobromide (Sigma)-coated 6-well tissue culture plates were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 and/or 10-7 m ICI for various durations. At the termination of an experiment, collected cells were processed for and subjected to a fluorescence-activated cell sorting (FACS) using EPICS Elite (Coulter Corp., Miami, FL) as described previously (12).
Annexin V and TUNEL Assays—The Vybrant apoptosis assay kit (Invitrogen) was used to study mid-stages of apoptosis as described previously (12). In brief, cells (100,000 cells/well) were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 for different lengths of time. Cells were collected and subjected to annexin V assay according to the instructions of the manufacturer prior to FACS analysis.
For TUNEL assay, cells (25,000 cells/well) plated in poly-d-lysine hydrobromide-coated 48-well tissue culture plates in phenol red-free Dulbecco's modified Eagle's medium containing 10% CD-FBS were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 for different lengths of time. Cells were then subjected to a terminal dUTP nick-end labeling (TUNEL) assay utilizing the DeadEnd Flurometric TUNEL System (Promega) according to the manufacturer's protocol. 4′, 6-Diamidino-2-phenylindole (Vector Laboratories) was used to stain cell nuclei. Stained cells were imaged.
Wound-healing Assay—MDA-MB-231 cells (200,000 cells/well in 12-well tissue culture plates) were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 for 48 h, a duration that allowed the cells to reach near-confluence. A wound was generated with a 1-ml pipette tip. The closure of wound was then imaged every 24 h. Because of the irregular shape of the edges of a wound, five randomly selected cross-edges were used to obtain a mean gap measure for wound healing.
Invasion Assay—Matrigel invasion chambers (BD Biosciences) were used for the invasion assay. MDA-MB-231 cells (100,000 cells/well) in 6-well tissue culture plates were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 for 48 h. Cells were then collected, and equal numbers of cells from each treatment group were seeded into invasion chambers as described previously (12). Cells on the bottom of the chamber membrane as the invading cell population were stained with the Diff-Quik Stain Set (Dade Behring, Newark, DE), dried, and mounted onto a glass slide. Images were captured, and stained cells were counted from images.
Microarray Analysis—To examine the effects of E2 on endogenous gene expression mediated by ERs, MDA-MB-231 cells were infected with recombinant adenoviruses in the absence of E2 for 48 h. Cells were then treated with 10-9 m E2 for 6 h. At the termination of an experiment, total RNA was extracted using RNeasy mini kit (Qiagen) and processed for microarray analysis, which was carried out at the Functional Genomic Center of the University of Rochester, as described previously (12). Affymetrix HG-U133 Plus 2.0 arrays were used as the microarray platform. Arrays were scanned with the GeneChip Scanner 3000 7G. GeneChip operating software (Affymetrix) was used for initial processing of the scanner data, including generation of cel files. Array normalization for the Affymetrix signal method (GeneChip operating software) involves multiplying raw signals by a scaling factor such that the trimmed mean (excluding highest and lowest 2%) of all expression scores is 500 arbitrary units for every array.
Experimental sets for microarrays were replicated six independent times executed on different days. To increase accuracy of gene identification and reduce false discovery rate (29), we used reorganized and updated probe sets (30-32) that were based on the up-to-date genome, cDNA/EST clustering, and single nucleotide polymorphism information through web-based custom GeneChip library files (Chip definition files) in data analysis. Following UniGene transformation, data sets were subjected to N-statistic test (33) in conjunction with the step-down Westfall-Young procedure (34) controlling the family-wise error rate at a level of 0.05, which we reported here. Minimum information about a microarray experiment-compliant microarray data for the six independent replicate studies can be found at the Gene Expression Omnibus data base with an accession number GSE9761.
Statistical Analysis—Results presented as the mean ± S.E. of at least three independent experiments were subjected to Student's t test for comparison of the means between two groups wherein p < 0.05 was considered significant.
RESULTS
Generation of a Mutant Human ERα for the Selective Regulation of the ERE-independent DNA-dependent Signaling Pathway—The initial step in the ERE-dependent signaling pathway involves the interaction of the E2-ERα complex with EREs, which are permutations of a core palindromic sequence, 5′-GGTCAnnnTGACC-3′ (7). The recognition of an ERE is mediated by two zinc-binding motifs in each DBD monomer of the ERα dimer that fold to form a single functional unit (35, 36). Studies indicated that distinct residues in the DNA recognition helix of the first zinc finger of the human ERα DBD, particularly Glu203, Gly204, and Ala206 residues, are critical for DNA sequence discrimination that signifies the cognate response element (37). Moreover, mutagenesis studies using mouse ERα (9), human ERα (10), or human ERβ (11, 12) showed that Glu and Gly residues are also important for binding to ERE, as changing these amino acids to Ala residues is sufficient to hinder the transregulatory capacity of the receptor from the ERE signaling pathway.
To begin to address the importance of the ERE-independent pathway in physiology and pathophysiology of E2-ERα signaling, we also engineered a human ERα mutant by changing Glu203 and Gly204 to Ala residues (ERα203/4). We found in preliminary studies that ERα203/4 retains, albeit at reduced levels, biochemical and functional features of the parent ERα in systems emulating the ERE-dependent signaling pathway (see also Figs. 1 and 2).
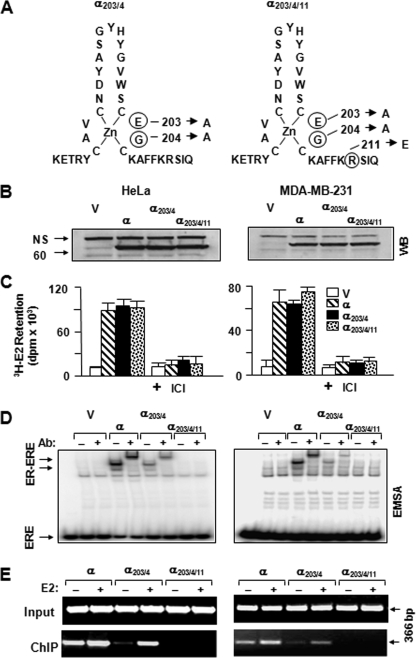
FIGURE 1.
Generation of ERE-binding defective ERα variants. A, ERα203/4 was engineered by changing glutamic acid and glycine at positions 203 and 204 of the DNA-binding helix of the ERα to alanine residues, whereas ERα203/4/11 contains an additional change that replaces arginine at position 211 with glutamic acid. B, synthesis of ERα species. HeLa (left panels) or MDA-MB-231 cells (right panels) were transiently transfected with an expression vector bearing none (V) or an ERα cDNA. Cell extracts (10 μg) were subjected to WB using a horseradish peroxidase-conjugated monoclonal FLAG antibody. Molecular mass in kDa is indicated. NS, nonspecific. C, in situ E2 binding assay. Twenty four hours after transient transfections with an expression vector bearing none (V) or an ERα cDNA, HeLa or MDA-MB-231 cells were incubated in medium containing 10-7 m of [3H]E2 for 1 h. Medium containing the radioactive [3H]E2 was then removed. Cells were extensively washed with fresh medium and dislodged. Radioactivity retained in cells was quantified by scintillation counting. The specific retention of [3H]E2 was assessed by the co-incubation of cells with 10-6 m ICI. The graph represents the mean ± S.E. of three independent experiments performed in duplicate. D, cell extracts (10 μg) of transfected cells were also subjected to EMSA without (-) or with (+) a FLAG antibody (Ab). ERE specifies unbound and ER-ERE denotes ER-bound radiolabeled ERE. A representative result from three independent experiments of WB or EMSA is shown. E, ChIP assay. HeLa or MDA-MB-231 cells were transiently transfected with an expression vector bearing an ERα cDNA together with a reporter plasmid bearing the simple TATA-box promoter with one ERE. Cells were treated without (-) or with (+)10-7 m E2 for 1 h prior to ChIP using FLAG antibody-conjugated agarose beads. Sizes of DNA fragments in base pair are indicated.
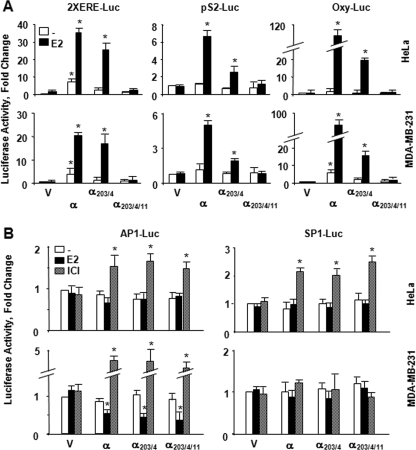
FIGURE 2.
Transcriptional responses to ERα proteins from reporter systems emulating the ERE-dependent and ERE-independent signaling pathways. A, HeLa or MDA-MB-231 cells were transiently transfected with an expression vector bearing none (V) or an ERα cDNA together with a reporter vector bearing two consensus ERE sequences in tandem located at the upstream of the simple TATA box promoter (2XERE-Luc), the proximal promoter from the trefoil factor 1, pS2 (pS2-Luc), or oxytocin (Oxy-Luc) gene in the absence or presence of 10-9 m E2 for 24 h. Simulating the ERE-dependent signaling pathway, these promoters drive the expression of the Firefly luciferase cDNA as the reporter enzyme. Cells were also co-transfected with a reporter vector bearing the Renilla luciferase cDNA for transfection efficiency. Normalized luciferase values are represented as fold changes compared with the luciferase values obtained with control vector in the absence of E2, which was set to 1. Shown are the means ± S.E. of three independent experiments performed in duplicate. B, effects of ligands on transregulatory activities of ERs in simulated ERE-independent signaling pathways. HeLa or MDA-MB-231 cells were transiently transfected with an expression vector bearing none (V) or an ERα cDNA together with reporter vector bearing an AP1 (AP1-Luc) or SP1 (SP1-Luc) promoter driving the expression of the firefly luciferase enzyme cDNA as the reporter. Cells were also co-transfected with a reporter vector bearing the Renilla luciferase cDNA for transfection efficiency. Cells were then treated with fresh medium without or with 10-9 m E2 or 10-7 m ICI for 40 h. Normalized luciferase values are represented as fold change compared with the luciferase values obtained without ligand, which was set to 1. Results are the means ± S.E. of three independent experiments performed in triplicate. Asterisk indicates significant difference.
Recent structural studies uncovered a further complexity in the binding of the human ERα to an ERE (38). It appears that the nature of charge and steric constraint of residues in the DNA recognition helix of ERα contributes to the recognition of and the extent of binding to ERE (38). Based on these findings and our preliminary observations, we introduced single or multiple amino acid changes in the DNA recognition helix of ERα203/4 to generate an ER variant that lacks the ability to interact with ERE. Of mutant receptors, the replacement of positively charged Arg211, which is a conserved residue among the nuclear hormone receptors and is critical for the interaction with bases in a cognate response element (35, 38), with negatively charged Glu residue in ERα203/4 was sufficient to generate an ERα mutant (ERα203/4/11) (Fig. 1A) functional only at the ERE-independent signaling pathway (Figs. 1 and 2).
The initial biochemical characterizations of ERα203/4 and ERα203/4/11 in comparison with the parent ERα were carried out in transiently transfected ER-negative cell models. Cellular extracts from HeLa or MDA-MB-231 cells transfected with expression vector (V) bearing none or an ERα cDNA were subjected to WB analysis using a FLAG antibody directed to the FLAG epitope present at the amino terminus of each receptor (Fig. 1B). Quantitative analysis of results revealed that ERs are synthesized at comparable levels. Moreover, the incubation of transiently transfected cells with 10-7 m [3H]E2 showed that the radiolabeled E2 is similarly retained in cells synthesizing an ERα protein (Fig. 1C). The co-incubation of cells with the E2 antagonist Imperial Chemical Industries 182,780 (ICI) at 10-6 m concentration effectively prevented the retention of [3H]E2 in cells. These results indicate that [3H]E2 binds specifically to ERs. Comparable radiolabeled E2 retention in transfected cells also affirms similar levels of synthesis of functional receptor proteins.
To assess the ER-ERE interaction in vitro, we employed EMSA using extracts from transiently transfected cells. Results showed that although ERα and ERα203/4, albeit at a lesser efficiency, interacted in vitro with ERE, ERα203/4/11, as the parent V, showed no binding (Fig. 1D). We also used ChIP to assess ER-ERE interactions in situ. Cells were transiently transfected with a reporter vector bearing one ERE placed upstream of the TATA box promoter that drives the expression of the Firefly luciferase cDNA together with the expression vector encoding none (V) or an ERα cDNA. We found that the unliganded ERα and ERα203/4 interacted in situ with ERE which was further augmented with E2 (Fig. 1E). On the other hand, ERα203/4/11, as the parent vector (data not shown), showed no interaction with ERE in the absence or presence of E2. Thus, it appears that ERα203/4/11 completely lacks the ability of interacting with ERE in vitro and in situ in contrast to ERα203/4.
These results also predict that ERα203/4/11 is nonfunctional in regulating transcription from the ERE-dependent signaling pathway in heterologous expression systems. To examine this prediction, an expression vector bearing an ERα cDNA was transiently transfected into HeLa or MDA-MB-231 cells lines. Cells were also co-transfected with a vector bearing the Firefly luciferase cDNA as the reporter enzyme and a reporter vector expressing the Renilla luciferase cDNA for transfection efficiency. We used the simple TATA box promoter bearing two EREs in tandem (2XERE-Luc) as the reporter vector (23). In addition, a reporter vector bearing the proximal promoter region derived from the trefoil factor 1, TFF1, pS2 (pS2-Luc), or the oxytocin, OXY (Oxy-Luc), gene that contains a variant ERE sequence was used to emulate the ERE-dependent signaling pathway (12, 23, 24). Normalized activity from each reporter construct was compared with the parent expression V in the absence of E2, with the latter value set to 1. We found that ERα increased the reporter enzyme activity in response to a physiological concentration (10-9 m) of E2 from all promoters in transfected cells. Although the extent of activation varied with the nature of promoter, ERα203/4 also augmented the reporter enzyme activity in the presence of E2. On the other hand, ERα203/4/11 had no effect on luciferase activity from the promoter constructs in transfected cells whether or not cells were treated with E2 (Fig. 2A). The transcriptional responses are receptor-specific because the treatment of cells with 10-7 m ICI had no effect on responses mediated by ERs, whereas the compound effectively blocked E2 effects on reporter enzyme activity mediated by both ERα and ERα203/4 (data not shown).
We observed similar results in transiently transfected ER-negative Chinese hamster ovary and U-2OS cell lines as well as C4 and C412 cells, subclones of ERα-positive MCF-7 cells wherein ERα gene expression is epigenetically silenced (39). We found that ERα and ERα203/4, but not ERα203/4/11, in response to E2 induced reporter enzyme activities from heterologous promoters (supplemental Fig. 1).
The functional interaction of ERs with members of the AP1 protein family bound to an AP1 element provides the basis for the transcriptional regulation of a responsive gene in a ligand- and cell type-dependent manner (40). Similarly, the interaction of ER with SP1 bound to GC boxes is critical for the ligand-mediated regulation of the reporter enzyme activity (41). If transregulatory function of ERα203/4/11 in the ERE-independent signaling pathway is indeed conserved, the ERE-binding defective receptor is expected to simulate the effects of ERα on reporter enzyme activity in response to ER ligands. An expression vector bearing an ERα cDNA was co-transfected into cells with a reporter plasmid that derives the expression of the Firefly luciferase cDNA. We used an AP1-Luc reporter plasmid that bears a fragment of the proximal promoter of the MMP1 gene with single AP1-response element (24, 25, 42) or an SP1-Luc reporter vector derived from the proximal promoter of the RARA gene that contains two GC-boxes (24, 25, 43). Cells were also transfected with the Renilla luciferase reporter plasmid to normalize transfection efficiency. Normalized luciferase activity induced by an ERα expression vector was compared with the parent V in the absence of ligand, with the latter value set to 1. Results showed that although the treatment of cells with 10-9 m E2 had minimal effects on reporter enzyme activity from the AP1 or the SP1 promoter construct in HeLa cells, 10-7 m ICI similarly mediated transcriptional responses to ERs from both promoter constructs (Fig. 2B). On the other hand, E2 repressed and ICI augmented the transcription from the AP1 promoter mediated by ERs in MDA-MB-231 cells. E2 or ICI had no significant effect on reporter enzyme activity from the SP1 promoter construct in MDA-MB-231 cells. These findings collectively show that whereas the transregulatory functions of ERs are conserved in the simulated ERE-independent signaling pathway, only ERα203/4/11 is nonfunctional in systems emulating the ERE-dependent signaling pathway.
Dissection of the Nuclear E2-ERα Signaling Pathways—To address the role of the ERE-independent signaling pathway in cellular responses to E2-ERα, we wanted to further ensure that ERα203/4/11 does not alter the expression of endogenous genes regulated through the ERE-dependent signaling pathway. We used MDA-MB-231 cells as a model in which exogenously expressed ERs are shown to regulate gene expression and alter phenotypic characteristics (12, 16-18). We also used recombinant adenoviruses for an efficient gene delivery (12, 18, 26).
In preliminary studies using various concentrations of the recombinant adenovirus expressing ERα cDNA (Ad5-ERα), we found that MDA-MB-231 cells infected with Ad5-ERα at 50 m.o.i. synthesize a concentration of ERα that requires E2 for function (data not shown). The recombinant adenovirus expressing ERα203/4 and ERα203/4/11 cDNA were used at 100 and 150 m.o.i., respectively. At these multiplicities of infection, the mutants produced comparable levels of receptor proteins to that of the parent ERα (see also Fig. 3B). In all experiments the total m.o.i. was adjusted to 150 by supplementing with the parent adenovirus (Ad5).
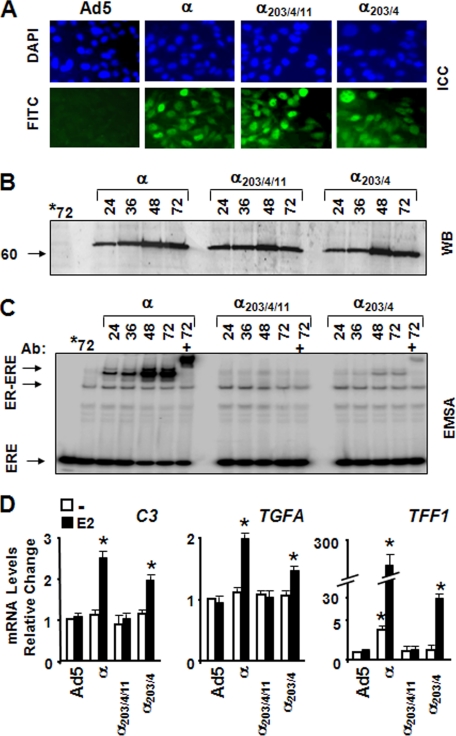
FIGURE 3.
Functional ER synthesis in infected MDA-MB-231 cells. A, cells were infected with the parent recombinant adenovirus (Ad5) at m.o.i. 150, a recombinant adenovirus bearing cDNA for ERα (α) at 50 m.o.i., ERα203/4/11 (α203/4/11) at 150 m.o.i., or ERα203/4 (α203/4) at 100 m.o.i. In all infections, the total m.o.i. was adjusted to 150 by supplementing with the parent Ad5. Intracellular localization of receptor proteins was examined by ICC. Infected cells as a function of time (shown at 48 h post-infection) were probed with an ERα-specific antibody (HC-20) followed by a fluorescein-conjugated secondary antibody (FITC). 4′, 6-Diamidino-2-phenylindole (DAPI) was used to stain nuclei. B, cell extracts (10 μg) of infected cells at indicated times were subjected to WB using the horseradish peroxidase-conjugated monoclonal FLAG antibody. Molecular mass in kDa is indicated. *72 denotes extracts of the parent adenovirus-infected cells at 72 h. C, cell extracts (10 μg) of infected cells for the indicated times were subjected EMSA in the absence (-) or presence (+) of a FLAG antibody (Ab). *72 indicates extracts of the parent adenovirus-infected cells at 72 h. ERE indicates the unbound radiolabeled ERE, and ER-ERE denotes the radiolabeled ERE-bound ERs. D, effects of ERs on the expression of the C3, TGFA, and TFF1 genes. At 48 h post-infection, cells were treated without (-) or with 10-9 m E2 (E2) for 24 h. Isolated total RNA was processed for and subjected to qPCR. The expression of the genes was compared with that observed in cells infected with Ad5 in the absence of E2, which is set to 1. All experiments are repeated three independent times. Asterisk indicates significant change.
The synthesis and function of ER proteins in infected MDA-MB-231 cells were assessed by ICC, WB, and EMSA in a time-dependent manner. ICC using a receptor specific antibody (HC-20) revealed that nearly all cells at 48 h post-infection showed nuclear staining for ERα proteins (Fig. 3A). This coincided with the level of ER synthesis as assessed by WB using a FLAG antibody, which showed that receptor proteins were synthesized comparably with levels reaching to maximum at 48 h post-infection (Fig. 3B). EMSA further demonstrated that ERα and ERα203/4, but not ERα203/4/11, interacted with ERE as a function of time (Fig. 3C) despite the fact that cells synthesizing ERα203/4/11 retained [3H]E2 comparably to those synthesizing ERα or ERα203/4 as assessed by the in situ E2 binding assay (data not shown).
To verify that ERα203/4/11 does not induce the expression of genes regulated by the ERE-dependent signaling pathway, MDA-MB-231 cells infected with recombinant adenoviruses for 48 h were treated without or with 10-9 m E2 for 24 h. Total RNA was isolated and processed for qPCR to determine alterations in the expression of the complement component 3 (C3), the transforming growth factor-α (TGFA), and the TFF1 genes. Like TFF1 (44), and TGFA (45), E2 responsiveness of C3 is mediated by variant ERE sequences within the proximal promoter of the gene (46). We found that ERα and ERα203/4, but not ERα203/4/11, increased mRNA levels of the C3, TGFA, and TFF1 genes (Fig. 3D). Importantly, ERα203/4 significantly affected the growth of the infected cells in response to E2, although with an efficiency lower than E2-ERα (supplemental Fig. 2).
Confirming the results obtained with transient transfections, these findings further indicate that ERα203/4/11 lacks the transregulatory function in the ERE-dependent signaling pathway. Based on these observations, we selected ERα203/4/11 as the ERE-binding defective ERα mutant, ERαEBD, to discriminatorily regulate the ERE-independent signaling pathway.
To ensure that ERαEBD regulates the expression of endogenous genes as an indication of functionality, we used a global gene expression profiling approach. We infected MDA-MB-231 cells with recombinant adenoviruses in the absence of E2 for 48 h. Cells were subsequently treated with 10-9 m E2 for 6 h, a duration that is anticipated to induce changes in the transcription of immediate/early estrogen-responsive genes (47, 48).
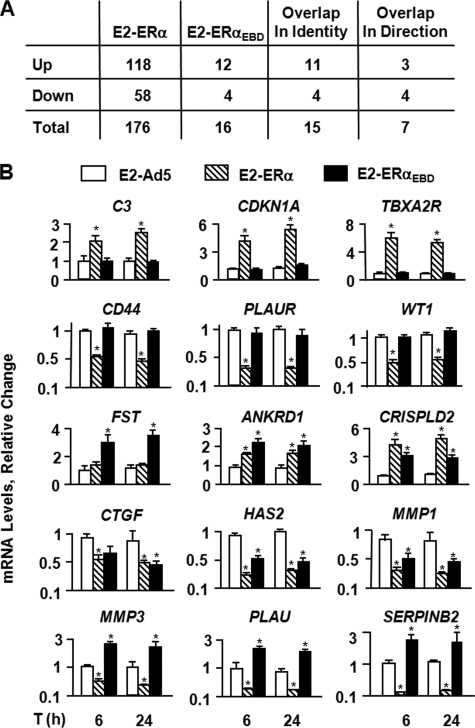
Gene expression profiling, summarized in Fig. 4A, revealed that E2-ERs significantly altered the expression of a number of genes involved in signal transduction, cellular proliferation, apoptosis, and motility. E2-ERα significantly regulated the expression of 176 genes (supplemental Table 1). These include the C3, CTSD (cathepsin D), CDKNA1 (cyclin-dependent kinase inhibitor, WAF1, p21, CIP1), IL18 (interleukin 18), PLAT (plasminogen activator, tissue), PLAU (plasminogen activator, urokinase), PLAUR (plasminogen activator, urokinase receptor), TGFA, and TGFB2 (transforming growth factor β2) genes that are shown to be responsive to E2 (46, 49-54). E2-ERαEBD significantly modified the expression of 16 genes (supplemental Table 2), 15 of which were also altered by E2-ERα.
FIGURE 4.
Effects of ERs on endogenous gene expression. A, summary of E2-ER-responsive genes identified with microarrays. MDA-MB-231 cells were infected with recombinant adenoviruses in the absence of E2 for 48 h. Cells were then treated with 10-9 m E2 for 6 h. Total RNA was subjected to microarrays. Results are the mean of six independent determinations. B, verification by qPCR of a subset of the genes identified by microarrays. MDA-MB-231 cells were infected with recombinant adenoviruses for 48 h. Cells were then treated without (data not shown) or with of 10-9 m E2 for 6 and 24 h. Total RNA from infected cells was processed for and subjected to qPCR. ERα in response to E2 augmented the expression of the C3, CDKNA1, and TBXR2R genes, whereas E2-ERα repressed the CD44, PLAUR, and WT1 gene expressions. The transcription of the FST gene was regulated only by E2-ERαEBD. On the other hand, both E2-ERα and E2-ERαEBD altered the ANKRD1, CRISPLD2, CTGF, HAS2, MMP1, MMP3, and SERPINB2 gene expressions. Results, which are the means ± S.E. of three independent determinations, depict relative change in mRNA levels compared with those observed in cells infected with the parent Ad5 in the absence of E2, which is set to 1. Asterisk indicates significant change.
The expression of the identified genes was further verified by qPCR. Infected MDA-MB-231 cells, which were maintained in the absence of E2 for 48 h, were treated without or with 10-9 m E2 for 6 and 24 h. Total RNA was processed for and subjected to qPCR. Results revealed that the expression of all E2-ERαEBD-responsive genes identified by microarrays was confirmable by qPCR, as was the expression of subset of genes mediated by E2-ERα. For example, ERα in the presence of E2 significantly induced the expression of the C3, CDKN1A, and TBXA2R (thromboxane A2 receptor) genes, while repressing the CD44 (CD44 antigen), PLAUR (plasminogen activator and urokinase receptor), and WT1 (Wilms tumor 1) gene expression. On the other hand, as observed with microarrays, only E2-ERαEBD significantly decreased the expression of the FST (follistatin) gene. E2-ERα and E2-ERαEBD augmented the transcription of the ANKRD1 (ankyrin repeat domain 1) and CRISPLD2 (cysteine-rich secretory protein LCCL domain containing 2) genes, although both receptors in the presence of E2 significantly attenuated the expression of the CTGF (connective tissue growth factor), HAS2 (hyaluronan synthase 2), and MMP1 (matrix metallopeptidase 1, collagenase) genes. Curiously, E2-ERα effectively suppressed the transcription of the MMP3 (matrix metallopeptidase 3, stromelysin 1, and progelatinase), PLAU (plasminogen activator, urokinase), and SERPINB2 (Serpin peptidase inhibitor, clade B, ovalbumin, member 2) genes, whereas E2-ERαEBD significantly enhanced the expression of these genes. Thus, E2-ERαEBD modulates the expression of a subset of endogenous genes regulated by E2-ERα in infected MDA-MB-231 cells.
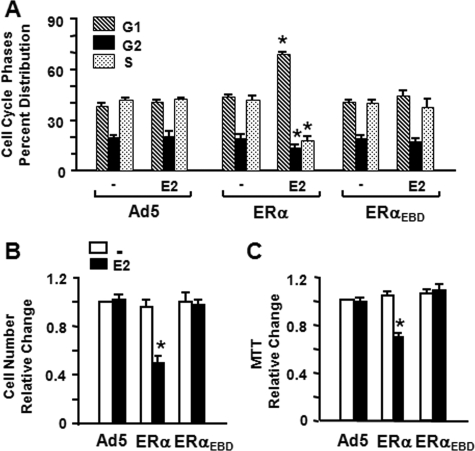
Effect of ERαEBD on Cellular Proliferation—To assess whether ERαEBD-mediated genes are involved in cellular responses, we initially examined the effect of E2-ERαEBD on the distribution of cell cycle phases. MDA-MB-231 cells were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 and maintained for different durations of time. Kinetic analysis of cell cycle histograms generated by FACS revealed that ERαEBD, whether or not cells were treated with 10-9 m E2, does not alter the phase distribution of the infected MDA-MB-231 cells compared with those infected with the parent adenovirus (Ad5) at any time point tested, shown at 48 h post-infection (Fig. 5A and supplemental Fig. 3A). In contrast, ERα in response to E2 increased the number of cells accumulated in G1 phase and decreased the cell number in S and G2 phases. These results indicate that ERαEBD does not affect the distribution of cell cycle phases. ICI alone at 10-7 m concentration had no effect on cell cycle kinetics, but the compound effectively prevented E2-ERα-induced alterations in cell cycle phases (data not shown).
FIGURE 5.
Effects of ERα proteins on cell cycle distribution and the proliferation of infected MDA-MB-231 cells. A, to examine the effects of ERs on cell cycle distribution, MDA-MB-231 cells were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 for 48 h. Cells were then collected, processed for, and subjected to FACS. Results depicted as the percent of cells in G1, G2, and S phases are the means ± S.E. of three independent experiments. B and C, infected cells were maintained in the absence (-) or presence of 10-9 m E2 (E2) for various times. At day 6 of post-infection, cells were subjected to cell counting (B) or MTT assay (C). The graphs represent the mean ± S.E. of three independent experiments performed in duplicate. Asterisk indicates significant change.
Moreover, ERαEBD had no effect on cellular growth. MDA-MB-231 cells infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 were subjected to cell counting (Fig. 5B) and MTT assay (Fig. 5C) as a function of time. Results, shown at day 6 of post-infection, indicated that ERs had no effect on cellular proliferation in the absence of E2 at any time point examined. On the other hand, the treatment of cells synthesizing ERα, but not ERαEBD, with E2 effectively suppressed cellular growth. The treatment of infected cells with ICI alone had no effect on cellular proliferation, whereas it blocked the repression of cellular growth mediated by only ERα in the presence of E2 (data not shown).
Thus, it appears that despite the ability of ERαEBD to regulate gene transcription in response to a physiological level of E2, ERαEBD is ineffective in altering cellular proliferation. Our results suggest that the ERE-independent signaling pathway plays a minimal role in cellular proliferation, and also imply that genomic responses to E2-ERα signaling from the ERE-independent pathway can be dissociated from cellular growth, as we recently suggested for E2-ERβ (12).
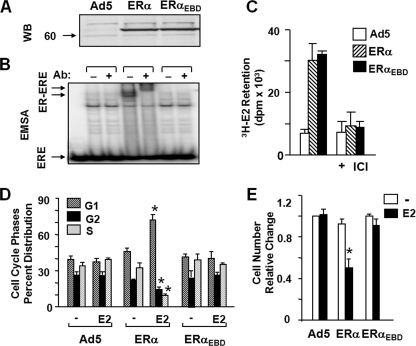
To ensure that observed effects are not cell type-dependent, we also used ER-negative osteosarcoma-derived U-2OS cells, into which the introduction of ERs was shown to induce cellular responses (19-21). As with MDA-MB-231 cells, concentrations of recombinant adenoviruses used to infect U-2OS cells were based on preliminary studies (data not shown). Infection of U-2OS cells with Ad5-ERα and Ad5-ERαEBD at 40 and 50 m.o.i., respectively, produced comparable levels of receptor proteins as assessed by WB (Fig. 6A) and the in situ E2 binding assay (Fig. 6C). As expected, ERα but not ERαDBD interacted with ERE evaluated by EMSA (Fig. 6B).
FIGURE 6.
Effects of ERα and ERαEBD on proliferation and cycle distribution of U-2OS cells. A, synthesis of ERs. Cells were infected for 48 h with the parent recombinant adenovirus (Ad5) at m.o.i. 50, a recombinant adenovirus bearing cDNA for ERα at 40 m.o.i. or ERαEBD at 50 m.o.i. The total m.o.i. was adjusted to 50 by supplementing with parent Ad5. Cell extracts (10 μg) of infected cells were subjected to WB using the horseradish peroxidase-conjugated monoclonal FLAG antibody. Molecular mass in kDa is indicated. B, cell extracts (10 μg) were also subjected EMSA in the absence (-) or presence (+) of a FLAG antibody (Ab). ERE indicates the unbound and ER-ERE depicts the ER-bound radiolabeled ERE. C, assessing the ability of an ERα to binding to E2 in situ. Cells infected with recombinant adenoviruses for 48 h in the absence of a ligand were incubated in medium containing 10-7 m of [3H]E2 for 1 h. Medium containing the radioactive [3H]E2 was removed. Cells were then extensively washed with fresh medium and dislodged. Radioactivity retained in cells was quantified by scintillation counting. The specific retention of [3H]E2 was assessed by the co-incubation of cells with 10-6 m ICI. The graph represents the means ± S.E. of three independent experiments performed in duplicate. D, infected cells with recombinant adenoviruses in the absence (-) or presence (E2) of 10-9 m E2 for 48 h were subjected to FACS. Results, presented as the means ± S.E. of three independent experiments, are the depiction of a cell population in G1, G2, and S phases. E, cells were infected with recombinant adenoviruses bearing none (Ad5) or an ERα cDNA. Cells were maintained in the absence (-) or presence of 10-9 m E2 (E2) for 6 days. At the termination, cells were counted. The means ± S.E., which are three independent experiments performed in duplicate, indicate relative change in cell number compared with those observed in cells infected with the parent Ad5 in the absence of E2, which is set to 1. Asterisk indicates significant change.
In assessing the cellular growth, we found that ERαEBD had no effect on cellular proliferation in the absence or presence of 10-9 m E2. In contrast, E2 effectively repressed the progression through cycle phases (Fig. 6D) and the growth of the infected U-2OS cells synthesizing ERα (Fig. 6E). Although 10-7 m ICI alone did not affect cell growth, the compound prevented the effects of ERα on cellular proliferation in response to E2 (data not shown).
These results collectively indicate that ERαEBD does not alter cellular growth in response to ligands. Thus, it appears that the ERE-dependent pathway is required in the mediation of the effects of E2-ERα signaling on cellular proliferation.
Effects of ERα on Cellular Death—Cellular growth also involves programmed cell death, or apoptosis, which is a complex and multistep process (55). Because E2-ERαEBD failed to affect cellular proliferation, we anticipated that the ERE-independent signaling pathway would contribute minimally to events leading to cell death. To address this issue, we utilized annexin V and TUNEL assays, which assess mid- and late-stages of apoptosis, respectively.
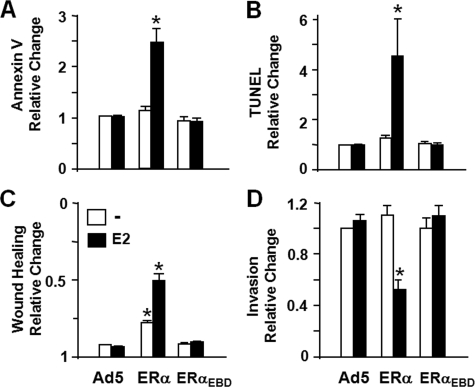
MDA-MB-231 cells were infected with recombinant adenoviruses in the absence or presence of E2 for various durations of time. Cells were then subjected to annexin V staining to examine the integrity of cellular membrane as a marker for the middle stages of apoptosis (56). Results revealed that ERαEBD in the absence or presence of 10-9 m E2 had no effect on the cell population stained with annexin V compared with cells infected with the parent adenovirus (Ad5) at any time point tested. ERα, on the other hand, increased the number of stained cells as a function of time with a significant change occurring at 96 h post-infection only in the presence of E2 (Fig. 7A and supplemental Fig. 3B).
FIGURE 7.
Effects of ERs on death and motility of MDA-MB-231 cells. A and B, infected MDA-MB-231 cells in the absence or presence of 10-9 m E2 were subjected to an annexin V assay using FACS (A) or a TUNEL assay at 96 h post-infection(B). Results, which are the mean ± S.E. of three independent experiments, are summarized as the relative change in the number cells bound to annexin V or as the number cells that incorporated fluorescein isothiocyanate-conjugated dUTP into the fragmented DNA (TUNEL) obtained in comparison with the cells infected with the parent Ad5 in the absence of ligand, which is set to 1. C, wound healing assay. Infected cells were incubated in the absence or presence of 10-9 m E2 for 48 h to allow cells to reach near 100% confluence. A wound was then created, and images were captured at 0 h and at every 24 h thereafter. Results, which are the mean ± S.E. of three independent experiments performed in duplicate, are summarized as the wound closure at 96 h relative to 0 h. D, invasion assay. Cells were infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 for 48 h. Cells were collected and counted. The same number of cells from each experimental group was then seeded on the upper section of the invasion chamber. After 24 h of incubation, cells on the bottom of the chamber membrane as an indication of invasiveness were stained and counted. Results, which represent the mean ± S.E. of three independent experiments performed in duplicate, are relative changes compared with cells infected with the parent adenovirus in the absence of E2, which is set to 1. Asterisk indicates significant change.
To independently corroborate our results, we also used a TUNEL assay, which catalytically incorporates fluorescein isothiocyanate-conjugated nucleotides into the fragmented genomic DNA as one of the last stage characteristics of apoptosis (56). Results, depicted as the relative change in the number of TUNEL-stained cells infected with ERα or ERαEBD compared with cells infected with the parent adenovirus (Ad5) (Fig. 7B, and supplemental Fig. 3C), showed that only E2-ERα induced significant genomic DNA fragmentation at 96 h after infection, whereas ERαEBD in the absence or presence of E2 had no discernible effect on apoptosis at 96 h, or at any time point tested. Similarly, the treatment with E2 of infected U-2OS synthesizing ERα but not ERαEBD significantly affected the cellular death (data not shown).
These results indicate that repression of cellular proliferation by E2-ERα, but not E2-ERαEBD, includes apoptotic events. Our results suggest that the ERE-independent signaling pathway alone does not contribute to the induction of cell death.
Effects of ERα and ERαEBD on Cellular Motility—The ability of cancer cells to invade the extracellular matrix is an indication of malignant growth state. Studies showed that breast cancer cells synthesizing ERs endogenously or exogenously are less motile and invasive than ER-negative cells (16).
To examine whether the ERE-independent pathway participates in the anti-motogenic effect of E2-ERα signaling, we used wound healing and invasion assays. MDA-MB-231 cells infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 were grown for 48 h to allow cells to reach near-confluence. We then created a wound and assessed the rate of wound closure as a function of time. We found that the unliganded ERα delayed the wound healing, which was further delayed by the presence of 10-9 m E2. On the other hand, ERαEBD did not affect gap closure whether or not cells were treated with E2 (Fig. 7C and supplemental Fig. 3D).
An invasion assay that assesses the capacity of cells to migrate through a reconstituted basement membrane further verified our findings. Equal number of MDA-MB-231 cells infected with recombinant adenoviruses in the absence or presence of 10-9 m E2 for 48 h were seeded on the reconstituted basement membrane of invasion chambers without or with E2. After 24 h, cells on the bottom of the chamber membrane were stained and imaged (supplemental Fig. 3E). Quantitative analysis of images indicated that ERαEBD had no effect on cellular invasiveness, whereas ERα in the presence of E2 reduced it (Fig. 7D). Thus, it appears that the ERE-independent pathway mediated by E2-ERα signaling participates minimally in cellular motility as well.
DISCUSSION
Our studies aimed at the dissection of nuclear E2-ERα signaling in the induction of cellular responses using an ERα mutant defective in ERE binding (ERαEBD) suggest that the expression of genes mediated by the nuclear ERE-independent signaling pathway are not sufficient to alter phenotypic characteristics of model cells. Our findings imply that the ERE-dependent pathway is a required signaling route for E2-ERα to induce responses at the cellular level.
Mutagenesis studies using mouse (9) and human (57) ERα suggested that changing Glu203 and Gly204 to Ala prevents binding to ERE and consequently blocks transcription from the ERE-dependent signaling pathway. Consistent with this, we (12) and others (11) showed that analogous changes in the P-box of the first zinc finger motif of the DBD of human ERβ, namely Glu and Gly at positions 167 and 168, respectively, also allow the receptor to convey E2 signaling exclusively through the ERE-independent pathway. On the other hand, our findings here suggest that changing Glu203 and Gly204 to Ala residues significantly attenuates but does not abolish the ability of ERα203/4 to interact in vitro and in situ with ERE and to induce transcriptional responses from heterologous reporter systems or endogenous genes. ERs share a near identical amino acid identity in their zinc finger motifs, which is responsible for binding of the receptors to the same spectrum of response elements (27). The binding affinity of ERβ for an ERE is, however, 2-fold lower than that of ERα (27). The amino terminus of the hinge domain of ERα stabilizes ERα-ERE interactions (58) and diverges significantly from the amino acid sequence of the hinge domain of ERβ (59). In addition, the amino-terminal region of ERβ, but not that of ERα, impairs the ability of the receptor to interact with ERE (26). These dissimilar structural features of ERs could be responsible for differences in the affinities of ER subtypes to bind to an ERE and could also underlie differences between the mutant ER subtypes to interact with and to regulate transcription from ERE sequences. Although differences in species of origin of ERα is withstanding (9), the reconciliation of disparate findings resulting from the use of identical mutations in the DBD of ERα and of similar heterologous expression systems, endogenous genes, and cell models (9, 57) is difficult. We could not assess the effects of ERα203/4 on phenotypic characteristics of C4 or C4-12 cells because of adenovirus-induced cytotoxicity. However, we observed in transiently transfected C4 or C4-12 cells that ERα203/4 in the presence of E2 regulated the reporter enzyme activity from the simulated ERE-dependent signaling pathway in a manner similar to those observed in other cell lines we used here (supplemental Fig. 1) in contrast to the findings of DeNardo et al. (57). We nevertheless found that ERα203/4 alters the growth of MDA-MB-231 cells (supplemental Fig. 2) as DeNardo et al. (57) observed in C4-12 cells. Because ERα203/4 in the presence of E2 regulated ERE bearing genes and altered cellular growth, we inferred that the genomic activity of ERα203/4 from the ERE-dependent signaling pathway could contribute to the E2-mediated modifications in cellular proliferation.
Previous studies indicated that a network of protein-DNA hydrogen bonds confers the binding specificity and stability of ERα to DNA. For the palindromic ERE, the network involves residues Glu203, Lys207, Lys210, and Arg211 (60). Although the recognition of a non-palindromic ERE is achieved by a rearrangement of side chains of various residues, particularly Lys207 and Lys210, the interactions of Glu203 and Arg211 with DNA remains unaltered (60). Based on these findings, we introduced further amino acid replacements in the DNA recognition helix of ERα203/4. Of the mutant receptors, the replacement of positively charged Arg211, which is a conserved residue among nuclear hormone receptors, with negatively charged Glu residue in ERα203/4 was sufficient to abolish in vitro and in situ ability of ERα203/4/11 (ERαEBD) to interact with and to modulate transcription from ERE while retaining the functionality at simulated ERE-independent signaling pathways in various cell lines.
Our global gene expression profiling and qPCR studies revealed that E2-ERαEBD modulates the expression of a subset of genes regulated by E2-ERα. Genes responsive to E2-ERαEBD are involved in various cellular functions, including signal transduction, cellular proliferation, apoptosis, and motility. For instance, the plasminogen activator system (PAS) is an enzymatic cascade involved in fibrinolysis and extracellular matrix (ECM) turnover (61). The central components of PAS include the urokinase-plasminogen activator (uPA, encoded by the PLAU gene), tissue plasminogen activator (expressed by the PLAT gene), the urokinase plasminogen activator receptor (uPAR), plasminogen, and plasminogen activator inhibitors, including PAI-2 encoded by the SERPINB2 gene. uPA triggers the generation of plasmin from plasminogen leading to the degradation of some components of ECM, whereas tissue plasminogen activator controls the breakdown of fibrin. Studies showed that the activity of PAS is increased in ER-positive cell models (62) and that E2 regulates the expression of the PLAT and PLAU genes (53) as we observed here. Although the nature of the regulatory elements critical for the responsiveness to E2 signaling remains to be explored, the expression of the PLAT, PLAU, and SERPINB2 genes by various cytokines and growth factors involves SP1 and AP1 transcription factors (63, 64) that interact with ERs.
Hyaluronan (HA) is a major glycosaminoglycan present in the ECM and is involved in the maintenance of structural integrity of tissues (65). HA is synthesized by the transmembrane glycosyltransferases that include the hyaluronan synthase 2 encoded by the HAS2 gene (65). Preferential and high expression of HAS2 correlate with the invasiveness of breast cancer cells (65). Studies indicate that the basal expression of HAS2 is primarily controlled by SP1 (66), whereas STAT and NFκB mediate the gene responsiveness to endocrine and paracrine signaling (67). Interaction of ERα with SP1, STAT, and/or NFκB could therefore modulate the HAS2 gene expression.
We observed that only ERαEBD, as the ERE-binding defective ERβ (12), regulated the expression of the follistatin (FST) gene. FST acts as an inhibitor of activins, which are the members of the transforming growth factor-β superfamily (68). E2 is shown to repress the FST transcription in the rodent uterus (69), while increasing it in avian granulose cells (70) and in ER-positive MCF-7 cells derived from a human breast adenocarcinoma (71). Our studies here and previously (12) were based on ER concentrations that are primarily dependent upon E2 for function to assess the importance of the ERE-independent pathway in the induction of cellular responses. This consideration was based on observations that augmented synthesis of ERα aberrantly influences target gene specificity (72) and expression pattern (20, 72). Although ERs were synthesized similarly (Fig. 3), it was possible that ERαEBD in the absence of ERE binding generated an excess functional pool that selectively regulated the expression of the FST gene. Indeed, ERαEBD at a decreased level of synthesis lost its ability to induce the transcription of FST without significantly altering the expression pattern of the HAS2 gene or affecting the TGFA gene expression (supplemental Fig. 4). Conversely, an increased level of ERα induced the transcription of the FST gene in response to E2. Moreover, ERα at this level of synthesis gained the ability to modulate the transcription of the HAS2 and TGFA genes in the absence of E2, which was further augmented with E2.
We also found that the transcription of some of the ERαEBD-mediated genes showed opposite directions compared with those regulated by ERα. For example, ERαEBD in response to E2 enhanced the expression of the MMP3, PLAU, and SERPINB2 genes, whereas E2-ERα decreased the transcription of these genes. Studies suggest that the nature of point mutations within the DBD of ERβ alters the pharmacology of ligand to induce transcription from heterologous reporter systems emulating the ERE-independent signaling pathway in a promoter-dependent manner (11). Distinct conformational features of the DBD of ERαEBD compared with ERα may reverse the effects of E2 to modulate the expression of some genes counteracting their cumulative effects on cellular phenotype. We recently showed that an ERE-binding defective ERβ mediates the expression of genes (12), the majority of which were also regulated by ERαEBD, with magnitudes and directions reflective of the parent ERβ. However, the ERE-binding defective ERβ, as we observed here for ERαEBD, in response to E2 was ineffective in altering cellular proliferation, motility, and death in contrast to E2-ERβ (12).
The ineffectiveness of ERαEBD to induce phenotypic changes rather suggests that the expression of genes through the ERE-dependent signaling pathway is necessary for ERα to elicit cellular responses. In addition to the expression of the PLAU, PLAT, and SERPINB2 genes that were responsive to ERαEBD, ERα also modulated the transcription of the PLAUR gene that encodes the uPA receptor, uPAR. uPAR is a glycolipid-anchored membrane receptor and plays an essential role in PAS (73). It appears that the binding of uPA to uPAR converts the inactive pro-uPA to active uPA, which then proteolytically activates plasminogen to form plasmin. Plasmin subsequently degrades the component of the ECM directly and indirectly through the activation of pro-matrix metallopeptidases (73). uPA-uPAR interactions are also involved in intracellular signaling that results in the alteration of cellular proliferation, adhesion, and migration (73). Thus, a coordinated regulation of genes involved in a signaling cascade through both the ERE-dependent and ERE-independent pathways and subsequent integrated effects of their protein products may be required for the manifestation of phenotypic alterations in response to E2-ER signaling. Similarly, the interaction of HA mediated by the HAS2 activity with its surface receptor CD44 signals for the proliferation, adhesion, and migration of tumor cells (65). Although E2-ERα regulated the expression of both CD44 and HAS2, the transcription of only the HAS2 gene was responsive to E2-ERαEBD, which had no effect on cellular phenotype.
Our conclusion that the ERE-dependent signaling pathway is a required route for E2-ER to induce cellular responses in model cells also implies that targeted regulation of the ERE-driven gene expression could provide a novel approach in the treatment of breast cancer independent of ER status and ligands. This prediction is also consistent with approaches that prevent ER-ERE interactions as promising new strategies to combat ER-positive breast cancer. Short sequences of DNA containing a response element for a transcription factor have been used as “decoys” to bind the cognate transcription factor in situ or in vivo. Binding of transcription factor to decoy DNA sequesters the transcription factor away from endogenous binding sites, thereby rendering it ineffective to regulate target gene expression in a variety of systems (74). Synthetic palindromic ERE as the decoy in transfected ER-positive breast cancer cell models was shown to prevent the growth of cells in response to E2 (75). Similarly, the prevention of ER-ERE interaction by ER-specific electrophilic agents that preferentially disrupt zinc fingers of ERα (76-78) effectively suppresses E2-mediated growth of ER-positive breast cancer cell models in situ and in vivo.
In summary, our results show that genomic responses mediated by the ERE-binding defective ERα mutant in response to E2 are insufficient to alter the phenotypic characteristics of model cell lines. We conclude that the ERE-dependent signaling pathway is necessary for E2-ERα to regulate phenotypic features of model cells.
Supplementary Material
Acknowledgments
We thank Dr. Joseph E. Wedekind for the extensive discussion and guidance in the generation of the ERE-binding defective ERα mutants and critical reading of the manuscript. We extend our gratitude to Drs. Mark Plessinger and Russell Hilf for critical reading of the manuscript. We also thank Dr. Stephen Welle, Andrew Cardillo, and Michelle Zanche of the Functional Genomic Center at the University of Rochester for the guidance, assistance, and execution of GeneArrays and qPCRs. We express our gratitude to Dr. Peter Keng and Michael Strong of the Cell Separation and Flow Cytometry Facility at the University of Rochester for the supervision and assistance for FACS studies. We thank Christine Brower of the Biostatistics and Computational Biology Department of the University of Rochester for the assistance in microarray analysis.
This work was supported, in whole or in part, by National Institutes of Health Grant CA113682 from the NCI (to M. M.). This work was also supported by grants from the Susan G. Komen Foundation (to M. M.) and the University of Rochester Clinical and Translational Science Institute (to M. M.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1-4 and Tables 1 and 2.
Footnotes
The abbreviations used are: E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen-responsive element; LBD, ligand binding domain; DBD, DNA binding domain; TUNEL, terminal dUTP nick-end labeling; PAS, plasminogen activator system; FACS, fluorescence-activated cell sorter; ECM, extracellular matrix; uPA, urokinase-plasminogen activator; uPAR, uPA receptor; HA, hyaluronan; m.o.i., multiplicity of infection; CD-FBS, charcoal-dextran treated-fetal bovine serum; qPCR, quantitative PCR; EMSA, electrophoretic mobility shift assay; ICC, immunocytochemistry; WB, Western blot; ChIP, chromatin immunoprecipitation assay; V, vector; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
References
- 1.Deroo, B. J., and Korach, K. S. (2006) J. Clin. Investig. 116 561-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang, J., Li, X., Hilf, R., Bambara, R. A., and Muyan, M. (2005) Curr. Drug. Targets Immune Endocr. Metabol. Disord. 5 379-396 [DOI] [PubMed] [Google Scholar]
- 3.Bai, Y., and Giguére, V. (2003) Mol. Endocrinol. 17 589-599 [DOI] [PubMed] [Google Scholar]
- 4.Hammes, S. R., and Levin, E. R. (2007) Endocr. Rev. 28 726-741 [DOI] [PubMed] [Google Scholar]
- 5.Brzozowski, A. M., Pike, A. C., Dauter, Z., Hubbard, R. E., Bonn, T., Engström, O., Ohman, L., Greene, G. L., Gustafsson, J. A., and Carlquist, M. (1997) Nature 389 753-758 [DOI] [PubMed] [Google Scholar]
- 6.Björnström, L., and Sjöberg, M. (2005) Mol. Endocrinol. 19 833-842 [DOI] [PubMed] [Google Scholar]
- 7.Klinge, C. M. (2001) Nucleic Acids Res. 29 2905-2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall, J. M., Couse, J. F., and Korach, K. S. (2001) J. Biol. Chem. 276 36869-36872 [DOI] [PubMed] [Google Scholar]
- 9.Jakacka, M., Ito, M., Weiss, J., Chien, P. Y., Gehm, B. D., and Jameson, J. L. (2001) J. Biol. Chem. 276 13615-13621 [DOI] [PubMed] [Google Scholar]
- 10.DeNardo, D. G., Kim, H. T., Hilsenbeck, S., Cuba, V., Tsimelzon, A., and Brown, P. H. (2005) Mol. Endocrinol. 19 362-378 [DOI] [PubMed] [Google Scholar]
- 11.Björnström, L., and Sjöberg, M. (2002) J. Biol. Chem. 277 48479-48483 [DOI] [PubMed] [Google Scholar]
- 12.Li, X., Nott, S. L., Huang, Y., Hilf, R., Bambara, R. A., Qiu, X., Yakovlev, A., Welle, S., and Muyan, M. (2008) J. Mol. Endocrinol. 40 211-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakacka, M., Ito, M., Martinson, F., Ishikawa, T., Lee, E. J., and Jameson, J. L. (2002) Mol. Endocrinol. 16 2188-2201 [DOI] [PubMed] [Google Scholar]
- 14.Christian, C. A., Glidewell-Kenney, C., Jameson, J. L., and Moenter, S. M. (2008) Endocrinology 149 5328-5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glidewell-Kenney, C., Weiss, J., Hurley, L. A., Levine, J. E., and Jameson, J. L. (2008) Endocrinology 149 4168-4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, M., Derocq, D., Freiss, G., and Rochefort, H. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 11538-11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, S. Y., and Jordan, V. C. (1992) J. Natl. Cancer Inst. 84 580-591 [DOI] [PubMed] [Google Scholar]
- 18.Lazennec, G., and Katzenellenbogen, B. S. (1999) Mol. Cell. Endocrinol. 149 93-105 [DOI] [PubMed] [Google Scholar]
- 19.Monroe, D. G., Getz, B. J., Johnsen, S. A., Riggs, B. L., Khosla, S., and Spelsberg, T. C. (2003) J. Cell. Biochem. 90 315-326 [DOI] [PubMed] [Google Scholar]
- 20.Kian Tee, M., Rogatsky, I., Tzagarakis-Foster, C., Cvoro, A., An, J., Christy, R. J., Yamamoto, K. R., and Leitman, D. C. (2004) Mol. Biol. Cell 15 1262-1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stossi, F., Barnett, D. H., Frasor, J., Komm, B., Lyttle, C. R., and Katzenellenbogen, B. S. (2004) Endocrinology 145 3473-3486 [DOI] [PubMed] [Google Scholar]
- 22.Muyan, M., Yi, P., Sathya, G., Willmert, L. J., Driscoll, M. D., Hilf, R., and Bambara, R. A. (2001) Mol. Cell. Endocrinol. 182 249-263 [DOI] [PubMed] [Google Scholar]
- 23.Yi, P., Bhagat, S., Hilf, R., Bambara, R. A., and Muyan, M. (2002) Mol. Endocrinol. 16 1810-1827 [DOI] [PubMed] [Google Scholar]
- 24.Huang, J., Li, X., Yi, P., Hilf, R., Bambara, R. A., and Muyan, M. (2004) Mol. Cell. Endocrinol. 218 65-78 [DOI] [PubMed] [Google Scholar]
- 25.Li, X., Huang, J., Yi, P., Bambara, R. A., Hilf, R., and Muyan, M. (2004) Mol. Cell. Biol. 24 7681-7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang, J., Li, X., Maguire, C. A., Hilf, R., Bambara, R. A., and Muyan, M. (2005) Mol. Endocrinol. 19 2696-2712 [DOI] [PubMed] [Google Scholar]
- 27.Yi, P., Driscoll, M. D., Huang, J., Bhagat, S., Hilf, R., Bambara, R. A., and Muyan, M. (2002) Mol. Endocrinol. 16 674-693 [DOI] [PubMed] [Google Scholar]
- 28.Livak, K. J., and Schmittgen, T. D. (2001) Methods 25 402-408 [DOI] [PubMed] [Google Scholar]
- 29.Dai, M., Wang, P., Jakupovic, E., Watson, S. J., and Meng, F. (2007) Bioinformatics 23 2185-2187 [DOI] [PubMed] [Google Scholar]
- 30.Gautier, L., Møller, M., Friis-Hansen, L., and Knudsen, S. (2004) BMC Bioinformatics 5 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai, M., Wang, P., Boyd, A. D., Kostov, G., Athey, B., Jones, E. G., Bunney, W. E., Myers, R. M., Speed, T. P., Akil, H., Watson, S. J., and Meng, F. (2005) Nucleic Acids Res. 33 e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harbig, J., Sprinkle, R., and Enkemann, S. A. (2005) Nucleic Acids Res. 33 e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klebanov, L., Gordon, A., Xiao, Y., Land, H., and Yakovlev, A. (2006) Comput. Statist. Data Anal. 50 3619-3628 [Google Scholar]
- 34.Westfall, P. H., and Young, S. (1993) Resampling-based Multiple Testing, pp. 36-75, Wiley Interscience, New York
- 35.Schwabe, J. W., Chapman, L., Finch, J. T., and Rhodes, D. (1993) Cell 75 567-578 [DOI] [PubMed] [Google Scholar]
- 36.Luisi, B. F., Schwabe, J. W., and Freedman, L. P. (1994) Vitam. Horm. 49 1-47 [DOI] [PubMed] [Google Scholar]
- 37.Mader, S., Kumar, V., de Verneuil, H., and Chambon, P. (1989) Nature 338 271-274 [DOI] [PubMed] [Google Scholar]
- 38.Nguyen, D., Bail, M., Pesant, G., Dupont, V. N., Rouault, E., Deschênes, J., Rocha, W., Melançon, G., Steinberg, S. V., and Mader, S. (2007) Nucleic Acids Res. 35 3465-3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oesterreich, S., Zhang, P., Guler, R. L., Sun, X., Curran, E. M., Welshons, W. V., Osborne, C. K., and Lee, A. V. (2001) Cancer Res. 61 5771-5777 [PubMed] [Google Scholar]
- 40.Kushner, P. J., Agard, D. A., Greene, G. L., Scanlan, T. S., Shiau, A. K., Uht, R. M., and Webb, P. (2000) J. Steroid Biochem. Mol. Biol. 74 311-317 [DOI] [PubMed] [Google Scholar]
- 41.Safe, S. (2001) Vitam. Horm. 62 231-252 [DOI] [PubMed] [Google Scholar]
- 42.Webb, P., Lopez, G. N., Uht, R. M., and Kushner, P. J. (1995) Mol. Endocrinol. 9 443-456 [DOI] [PubMed] [Google Scholar]
- 43.Sun, G., Porter, W., and Safe, S. (1998) Mol. Endocrinol. 12 882-890 [DOI] [PubMed] [Google Scholar]
- 44.Berry, M., Nunez, A. M., and Chambon, P. (1989) Proc. Natl. Acad. Sci. U. S. A. 86 1218-1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Ashry, D., Chrysogelos, S. A., Lippman, M. E., and Kern, F. G. (1996) J. Steroid Biochem. Mol. Biol. 59 261-269 [DOI] [PubMed] [Google Scholar]
- 46.Fan, J. D., Wagner, B. L., and McDonnell, D. P. (1996) Mol. Endocrinol. 10 1605-1616 [DOI] [PubMed] [Google Scholar]
- 47.Soulez, M., and Parker, M. G. (2001) J. Mol. Endocrinol. 27 259-274 [DOI] [PubMed] [Google Scholar]
- 48.Frasor, J., Danes, J. M., Komm, B., Chang, K. C., Lyttle, C. R., and Katzenellenbogen, B. S. (2003) Endocrinology 144 4562-4574 [DOI] [PubMed] [Google Scholar]
- 49.Augereau, P., Miralles, F., Cavaillès, V., Gaudelet, C., Parker, M., and Rochefort, H. (1994) Mol. Endocrinol. 8 693-703 [DOI] [PubMed] [Google Scholar]
- 50.Margueron, R., Licznar, A., Lazennec, G., Vignon, F., and Cavaillès, V. (2003) J. Endocrinol. 179 41-53 [DOI] [PubMed] [Google Scholar]
- 51.Murakami, Y., Otsuki, M., Kusumoto, K., Takeuchi, S., and Takahashi, S. (2005) J. Reprod. Dev. 51 639-647 [DOI] [PubMed] [Google Scholar]
- 52.Bates, S. E., Davidson, N. E., Valverius, E. M., Freter, C. E., Dickson, R. B., Tam, J. P., Kudlow, J. E., Lippman, M. E., and Salomon, D. S. (1988) Mol. Endocrinol. 2 543-555 [DOI] [PubMed] [Google Scholar]
- 53.Levenson, A. S., Kwaan, H. C., Svoboda, K. M., Weiss, I. M., Sakurai, S., and Jordan, V. C. (1998) Br. J. Cancer 78 88-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi, T., Eitzman, B., Bossert, N. L., Walmer, D., Sparrow, K., Flanders, K. C., McLachlan, J., and Nelson, K. G. (1994) Cell Growth & Differ. 5 919-935 [PubMed] [Google Scholar]
- 55.King, K. L., and Cidlowski, J. A. (1998) Annu. Rev. Physiol. 60 601-617 [DOI] [PubMed] [Google Scholar]
- 56.Gorczyca, W., Melamed, M. R., and Darzynkiewicz, Z. (1998) Methods Mol. Biol. 91 217-238 [DOI] [PubMed] [Google Scholar]
- 57.DeNardo, D. G., Cuba, V. L., Kim, H., Wu, K., Lee, A. V., and Brown, P. H. (2007) Mol. Cell. Endocrinol. 277 13-25 [DOI] [PubMed] [Google Scholar]
- 58.Mader, S., Chambon, P., and White, J. H. (1993) Nucleic Acids Res. 21 1125-1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosselman, S., Polman, J., and Dijkema, R. (1996) FEBS Lett. 392 49-53 [DOI] [PubMed] [Google Scholar]
- 60.Schwabe, J. W., Chapman, L., and Rhodes, D. (1995) Structure 3 201-213 [DOI] [PubMed] [Google Scholar]
- 61.Irigoyen, J. P., Muñoz-Cánoves, P., Montero, L., Koziczak, M., and Nagamine, Y. (1999) Cell. Mol. Life Sci. 56 104-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butler, W. B., Kirkland, W. L., and Jorgensen, T. L. (1979) Biochem. Biophys. Res. Commun. 90 1328-1334 [DOI] [PubMed] [Google Scholar]
- 63.Cousin, E., Medcalf, R. L., Bergonzelli, G. E., and Kruithof, E. K. (1991) Nucleic Acids Res. 19 3881-3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costa, M., Shen, Y., Maurer, F., and Medcalf, R. L. (1998) Eur. J. Biochem. 258 123-131 [DOI] [PubMed] [Google Scholar]
- 65.Götte, M., and Yip, G. W. (2006) Cancer Res. 66 10233-10237 [DOI] [PubMed] [Google Scholar]
- 66.Monslow, J., Williams, J. D., Fraser, D. J., Michael, D. R., Foka, P., Kift-Morgan, A. P., Luo, D. D., Fielding, C. A., Craig, K. J., Topley, N., Jones, S. A., Ramji, D. P., and Bowen, T. (2006) J. Biol. Chem. 281 18043-18050 [DOI] [PubMed] [Google Scholar]
- 67.Saavalainen, K., Pasonen-Seppänen, S., Dunlop, T. W., Tammi, R., Tammi, M. I., and Carlberg, C. (2005) J. Biol. Chem. 280 14636-14644 [DOI] [PubMed] [Google Scholar]
- 68.Renier, M. A., Vereecken, A., and Buytaert, P. (1998) Eur. J. Contracept. Reprod. Health Care 3 129-135 [DOI] [PubMed] [Google Scholar]
- 69.Ferreira, M. C., Cavallo, I. K., Florio, P., Petraglia, F., and Reis, F. M. (2008) J. Mol. Histol. 39 535-541 [DOI] [PubMed] [Google Scholar]
- 70.Davis, A. J., Brooks, C. F., and Johnson, P. A. (2000) Gen. Comp. Endocrinol. 119 308-316 [DOI] [PubMed] [Google Scholar]
- 71.Chang, E. C., Frasor, J., Komm, B., and Katzenellenbogen, B. S. (2006) Endocrinology 147 4831-4842 [DOI] [PubMed] [Google Scholar]
- 72.Fowler, A. M., Solodin, N., Preisler-Mashek, M. T., Zhang, P., Lee, A. V., and Alarid, E. T. (2004) FASEB J. 18 81-93 [DOI] [PubMed] [Google Scholar]
- 73.Laufs, S., Schumacher, J., and Allgayer, H. (2006) Cell Cycle 5 1760-1771 [DOI] [PubMed] [Google Scholar]
- 74.Dzau, V. J. (2002) Circ. Res. 90 1234-1236 [DOI] [PubMed] [Google Scholar]
- 75.Wang, L. H., Yang, X. Y., Zhang, X., Mihalic, K., Xiao, W., and Farrar, W. L. (2003) Cancer Res. 63 2046-2051 [PubMed] [Google Scholar]
- 76.Wang, L. H., Yang, X. Y., Zhang, X., Mihalic, K., Fan, Y. X., Xiao, W., Howard, O. M., Appella, E., Maynard, A. T., and Farrar, W. L. (2004) Nat. Med. 10 40-47 [DOI] [PubMed] [Google Scholar]
- 77.Wang, L. H., Yang, X. Y., Zhang, X., An, P., Kim, H. J., Huang, J., Clarke, R., Osborne, C. K., Inman, J. K., Appella, E., and Farrar, W. L. (2006) Cancer Cell 10 487-499 [DOI] [PubMed] [Google Scholar]
- 78.Mao, C., Patterson, N. M., Cherian, M. T., Aninye, I. O., Zhang, C., Montoya, J. B., Cheng, J., Putt, K. S., Hergenrother, P. J., Wilson, E. M., Nardulli, A. M., Nordeen, S. K., and Shapiro, D. J. (2008) J. Biol. Chem. 283 12819-12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.