Summary
The neural crest generates multiple cell types during embryogenesis but the mechanisms regulating neural crest cell diversification are incompletely understood. Previous studies using mutant zebrafish indicated that foxd3 and tfap2a function early and differentially in the development of neural crest sublineages. Here, we show that the simultaneous loss of foxd3 and tfap2a function in zebrafish foxd3zdf10;tfap2alow double mutant embryos globally prevents the specification of developmentally distinct neural crest sublineages. By contrast, neural crest induction occurs independently of foxd3 and tfap2a function. We show that the failure of neural crest cell diversification in double mutants is accompanied by the absence of neural crest sox10 and sox9a/b gene expression, and that forced expression of sox10 and sox9a/b differentially rescues neural crest sublineage specification and derivative differentiation. These results demonstrate the functional necessity for foxd3 and tfap2a for neural crest sublineage specification and that this requirement is mediated by the synergistic regulation of the expression of SoxE family genes. Our results identify a genetic regulatory pathway functionally discrete from the process of neural crest induction that is required for the initiation of neural crest cell diversification during embryonic development.
Keywords: Neural crest, Cell fate specification, foxd3, tfap2a, SoxE, Zebrafish
INTRODUCTION
The neural crest (NC) is an ectoderm-derived embryonic cell population induced at the neural plate border during gastrulation. NC cells subsequently delaminate from the neuroepithelium, migrate and differentiate into a wide variety of derivatives, including peripheral neurons, chromatophores and major elements of the craniofacial skeleton (LeDouarin and Kalcheim, 1999). A number of genes have been identified that are necessary for the development of, and/or that are diagnostic of, different NC sublineages. For example, mitfa is essential for melanophore development and is expressed by melanophore precursors well before overt differentiation (Hodgkinson et al., 1993; Hodgkinson et al., 1998; Mochii et al., 1998; Lister et al., 1999; Levy et al., 2006). Genes with analogous functions or expression patterns have been identified for other NC sublineages as well. However, lineage analyses have indicated that the initial specification of distinct sublineages may considerably precede the initial expression of identified genes that are expressed in a sublineage-specific manner (Bronner-Fraser and Fraser, 1988; Frank and Sanes, 1991; Raible and Eisen, 1994; Schilling and Kimmel, 1994; Henion and Weston, 1997). These observations suggest that an earlier functioning regulatory network initiates neural crest cell (NCC) diversification, resulting in sublineage-specific gene expression and function.
A number of genes that are expressed by NC progenitors upon induction of the neural plate border have been implicated in the early development of the NC and are candidates for mediating the initial specification of NC sublineages (Gammill and Bronner-Fraser, 2003). This group includes the transcription factors foxd3, tfap2a, sox10 and sox9. Phenotypical analysis of zebrafish presumptive loss-of-function mutants indicates that foxd3, tfap2a, sox10 and the sox9 co-orthologs sox9a and sox9b are required for the development of both distinct and overlapping NC subpopulations (Dutton et al., 2001; Knight et al., 2003; Barrallo-Gimeno et al., 2004; Yan et al., 2005; Carney et al., 2006; Montero-Balaguer et al., 2006; Stewart et al., 2006), although the genetic interactions among them are incompletely understood. In addition, a large number of studies that primarily used frog, chick and rodent animal models have documented important functions for this same set of transcription factors in NC development (see Gammill and Bronner-Fraser, 2003; Kos et al., 2001; Cheung et al., 2005; Sakai et al., 2006). Although many of these studies indicate crucial roles in NC development for foxd3, tfap2a and SoxE family genes, inconsistent or conflicting results have been obtained. For example, both gain-of-function and loss-of-function manipulations of foxd3 expression resulted in the upregulation of NC marker gene expression (Dottori et al., 2001; Kos et al., 2001; Pohl and Knochel, 2001; Sasai et al., 2001). Although some inconsistencies can probably be attributed to differences in experimental paradigms or even species, the lack of consensus on the function(s) of individual transcription factors in the regulation of NC development has hampered efforts to address how these factors interact (cf. Cheung et al., 2005; Sakai et al., 2006). In addition, genetic efforts to this end in mouse have been complicated until recently, and with exceptions (Herbarth et al., 1998; Southard-Smith et al., 1998; Kapur, 1999), by severe early pleiotropic phenotypes or haploinsufficiency issues in knockout models (Schorle et al., 1996; Zhang et al., 1996; Morriss-Kay, 1996; Bi et al., 2001; Hanna et al., 2002; Mori-Akiyama et al., 2003; Sock et al., 2003). Therefore, to investigate potential functional genetic interactions between transcriptional regulators of early NC development, and their molecular and cellular consequences in the process of NCC diversification, we have used a genetic approach in zebrafish.
Using the presumptive null foxd3zdf10 and tfap2alow mutant alleles (Knight et al., 2003; Stewart et al., 2006), we have analyzed NC development in zebrafish foxd3zdf10;tfap2alow double mutant embryos and in tfap2a morpholino (MO)-injected foxd3zdf10 embryos. We found that the synergistic functions of foxd3 and tfap2a are required for the specification and differentiation of all major NC sublineages. The absence of NC sublineage specification was preceded by the absence of NC SoxE family gene expression in double mutants. Forced expression of SoxE family genes in tfap2a-depleted foxd3zdf10 embryos differentially rescued the specification of all major NC sublineages. Our results define a minimal genetic transcriptional network required for the initiation of NCC diversification.
MATERIALS AND METHODS
Whole-mount in situ hybridization and immunohistochemistry
In situ hybridizations were performed as previously described (Thisse et al., 1993), with minor modifications. Immunohistochemistry was also performed as previously described (An et al., 2002).
Morpholino and expression vectors
A previously described (O'Brien et al., 2004), ap2E2I2 morpholino, which specifically targets splicing of tfap2a, was used to phenocopy tfap2a mutant phenotypes in both wild-type and foxd3zdf10 mutant backgrounds. The p53 morpholino was the gift of Dr A. T. Look (Dana-Farber Cancer Institute, Boston, MA, USA). To express SoxE genes, we used sox9a-pSP64T and sox9b-pSP64T (Yan et al., 2005) kindly provided by Dr John Postlethwait (University of Oregon, Eugene, OR, USA). We tested a wide range of sox9a/b mRNA concentrations based on Yan et al. (Yan et al., 2005), including concentrations of single sox9 mRNAs that were greater than the concentrations of the combined mRNAs in double injections. sox10-pCSHSP (Elworthy et al., 2003) was a gift from Dr Robert Kelsh (University of Bath, Bath, UK). Heat shock was induced by transferring embryos from 28.5°C to 37°C for 1 hour.
Cartilage staining
Alcian Blue staining was used to detect cartilage, as previously described (Kimmel et al., 1998).
Genotyping
Single and double mutant embryos were genotyped using foxd3zdf10 and tfap2alow allele-specific PCR or sequencing as previously described (Knight et al., 2003; Stewart et al., 2006).
Zebrafish
All zebrafish were maintained in the Ohio State University Zebrafish Facility, raised at 28.5°C, and staged by published criteria (Kimmel et al., 1995). The generation of foxd3zdf10 and tfap2alow mutants has been described previously (Knight et al., 2003; Stewart et al., 2006). The specific nucleotide mutations of both mutants strongly suggest that both represent loss-of-function alleles.
RESULTS
Phenotypical analysis of zebrafish presumptive loss-of-function mutants indicates that foxd3 and tfap2a are required for the development of both distinct and overlapping NC subpopulations (Knight et al., 2003; Barrallo-Gimeno et al., 2004; Montero-Balaguer et al., 2006; Stewart et al., 2006). However, because the induction of tfap2a expression is normal in foxd3zdf10 mutants and foxd3 expression is only defective in a small number of hindbrain NCCs in tfap2low mutants, the reciprocal downregulation of one another's expression in mutant embryos is unlikely to directly contribute to the major phenotypical defects. Consistent with this assertion, we found that misexpression of tfap2a mRNA in foxd3zdf10 mutants failed to rescue any of the NC derivative phenotypes of mutant embryos (n=165; data not shown). Thus, although foxd3 and tfap2a might each directly regulate aspects of NCC diversification, major functions of both transcription factors in NC development are likely to be mediated by other genes. Therefore, to investigate potential functional genetic interactions between foxd3 and tfap2a in the process of NCC diversification, we analyzed NC development in foxd3zdf10;tfap2alow double mutant embryos. Parallel experiments using tfap2a morpholino (MO)-injected foxd3zdf10 embryos efficiently (90%; n>1000) produced identical results (see Figs S1-S3 in the supplementary material).
Global absence of differentiated NC-derived cells in foxd3zdf10;tfap2alow double mutant embryos
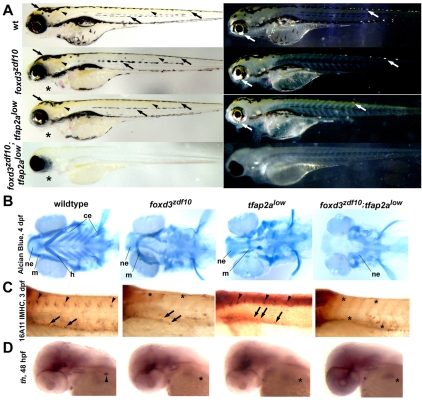
In both foxd3zdf10 and tfap2a single mutants, NC-derived chromatophore development, after a brief developmental delay, occurs normally (Fig. 1A) suggesting that both of these genes are ultimately dispensable for chromatophore development in zebrafish (Knight et al., 2003; Barrallo-Gimeno et al., 2004; Montero-Balaguer et al., 2006; Stewart et al., 2006). Unexpectedly, foxd3zdf10;tfap2alow double mutants are completely devoid of NC-derived chromatophores, with the exception of occasional xanthophores over the head (Fig. 1A). The normal development of the pigmented retinal epithelium in double mutants demonstrates NC specificity of the genetic requirement for foxd3 and tfap2a function among pigmented cells. This result indicates that a synergistic genetic interaction between foxd3 and tfap2a is required for NC-derived chromatophore development.
Fig. 1.
foxd3zdf10;tfap2alow double mutant embryos lack NC derivatives. (A) Live larvae at 4 days post-fertilization (dpf), lateral views with either transmitted (left) or reflected (right) light. Wild-type zebrafish exhibit three NC-derived pigment cell types: black melanophores (left, arrows), yellow xanthophores (left, arrowheads) and iridescent iridophores (right, white arrows). foxd3zdf10 and tfap2alow single mutants develop essentially normal pigment patterns by 4 dpf. Jaw structures protrude ventrally in both foxd3zdf10 and tfap2alow single mutants (asterisks). foxd3zdf10;tfap2alow double mutants completely lack NC-derived pigment cells except for occasional head xanthophores. Jaw structures appear to be missing (asterisk). (B) Craniofacial cartilage development revealed by Alcian Blue staining at 4 dpf, ventral views with anterior to the left. The wild-type larval head skeleton of zebrafish consists of the dorsal neurocranium (ne), as well as upper (mandibular, m) and lower (hyoid, h, and ceratobranchial, ce) jaw structures. In both foxd3zdf10 and tfap2alow single mutants, mandibular and hyoid structures are disorganized and the ceratobranchial elements are absent, whereas the neurocranium remains intact. Double mutants lack upper and lower jaw structures, and all but the most posterior portion of the neurocranium. (C) Immunostaining with 16A11 monoclonal (anti-Hu) antibody at 3 dpf, lateral views, anterior to the left. DRG neurons are found in each trunk segment of wild-type embryos (arrowheads) and enteric neurons populate the gut tube (arrows). DRG neurons are absent in foxd3zdf10 single mutants (asterisks) and present in reduced numbers in tfap2alow single mutants (arrowheads), whereas enteric neurons are reduced in number in both backgrounds (arrows). DRG and enteric neurons are absent in double mutant embryos (asterisks). (D) tyrosine hydroxylase (th) expression in sympathetic neurons at 48 hpf in wild-type embryos (arrowhead). Sympathetic neurons are absent in foxd3zdf10 and tfap2alow single mutants, as well as in double mutants (asterisks).
Loss of either foxd3 or tfap2a function results in similar but non-identical craniofacial defects involving the reduction and disorganization of upper and lower jaw elements, with more dorsal structures of the neurocranium remaining intact (Knight et al., 2003; Barrallo-Gimeno et al., 2004; Montero-Balaguer et al., 2006; Stewart et al., 2006). By contrast, Alcian Blue staining of foxd3zdf10;tfap2alow double mutant embryos revealed the complete absence of all upper and lower jaw structures, and of all but the most posterior portion of the neurocranium (Fig. 1B). The development of pharyngeal mesoderm and endoderm was normal in double mutants (Fig. 2I,J). As was the case for chromatophores, the craniofacial phenotype of double mutants was more severe than would be predicted by an additive effect of the mutations, which further suggests a parallel synergistic interaction between foxd3 and tfap2a that regulates the development of progenitors of the NC-derived craniofacial skeleton.
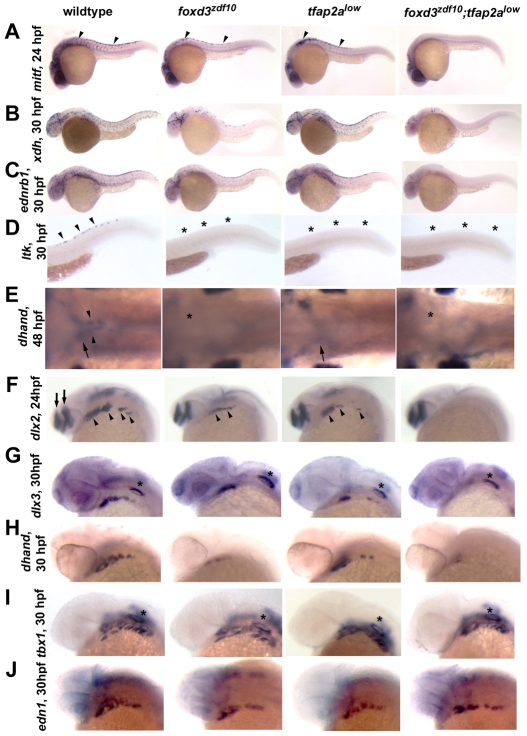
Fig. 2.
Failure of NC sublineage specification in foxd3zdf10;tfap2alow double mutants. (A-D) Lateral views with anterior to the left. (A) mitf expression at 24 hpf (arrowheads). foxd3zdf10;tfap2alow double mutant embryos completely lack NC-derived melanoblast mitf expression. (B) xanthine dehydrogenase (xdh), diagnostic of xanthophore precursors, at 30 hpf. (C) endothelin receptor B (ednrb1), presumed to be expressed by all chromatophore precursors, at 30 hpf and (D) leukocyte tyrosine kinase (ltk), reported to be expressed by iridophore precursors, at 30 hpf. (E) Dorsal views of dhand expression, anterior to left, ventral of the caudal hindbrain, at 48 hpf. Sympathetic neuron precursors are labeled in wild-type embryos (arrowheads), as are the more ventral and laterally localized enteric neuron precursors (arrows). In the majority of foxd3zdf10 embryos, both sympathetic and enteric neuron progenitors are absent (asterisk) (Stewart et al., 2006). In tfap2alow mutants, sympathetic neuron precursors are absent whereas a small number of enteric precursors are present (arrow) (Knight et al., 2003). In foxd3zdf10;tfap2alow double mutants, both sympathetic and enteric neuron precursors are absent (asterisk). (F-J) Lateral (F-I) and dorsolateral views (J), anterior to the left. (F) dlx2 expression at 24 hpf. dlx2 expression (arrowheads) is absent in the branchial arches of double mutant embryos, but is retained in the forebrain (arrows). (G) dlx3 expression at 30 hpf; (H) dhand expression at 30 hpf. NC expression of both genes is absent in double mutant embryos with dlx3 expression maintained in non-NC otic vesicle (asterisks). (I) tbx1 expression in the branchial arches (asterisk) at 30 hpf is diagnostic of the endodermal component of the arches. tbx1 expression is normal in all experimental embryos. (J) endothelin 1 (edn1) expression in the mesodermal component of the branchial arches at 30 hpf. Branchial mesoderm appears to develop normally in foxd3zdf10 and tfap2alow single mutants, as well as in foxd3zdf10;tfap2alow double mutant embryos.
We also examined the development of NC-derived peripheral neurons of the dorsal root ganglia (DRG) and enteric nervous system by using a neuron-specific antibody, and the development of autonomic sympathetic neurons, identified by expression of tyrosine hydroxylase (th). DRG neurons are absent in foxd3zdf10 mutant embryos and are slightly reduced in number in tfap2a mutant embryos, enteric neurons are reduced in homozygous mutants for either gene and sympathetic neurons are absent in both foxd3zdf10 and tfap2a single mutants (Fig. 1C,D) (Knight et al., 2003; Barrallo-Gimeno et al., 2004; Montero-Balaguer et al., 2006; Stewart et al., 2006). All three neuronal populations are entirely absent in foxd3zdf10;tfap2alow double mutants at 3 days post-fertilization (dpf) (Fig. 1C,D). Taken together, our analysis of differentiated NC-derived cells indicates a specific, complete failure of the development of major NC derivatives in foxd3zdf10;tfap2alow double mutants. That the NC phenotype of foxd3zdf10;tfap2alow double mutants is more severe than that of either single mutant indicates that foxd3 and tfap2a regulate NC development in parallel.
The specification of NC sublineages fails to occur in foxd3zdf10;tfap2alow double mutant embryos
Analysis of the expression of genes required for and/or diagnostic of the specification of major NC sublineages prior to the overt differentiation of derivatives in foxd3zdf10;tfap2alow double mutant embryos revealed a global failure in the initiation of NCC diversification (Fig. 2, see also Fig. S2 in the supplementary material; data not shown). For example, chromatophore precursor expression of mitfa (Lister et al., 1999), xdh (Budi et al., 2008), ltk (Lopes et al., 2008) and ednrb1 (Parichy et al., 2000) (Fig. 2A-D), pharyngeal arch progenitor expression of dlx2 and dlx3 (Akimenko et al., 1994) (Fig. 2F,G), and dhand (Yelon et al., 2000) (Fig. 2H), and sympathetic and enteric neuron progenitor expression of dhand (Lucas et al., 2006) (Fig. 2E) and zash1a (Allende and Weinberg, 1994; Lucas et al., 2006) (data not shown) fail to occur. By contrast, the development of mesendodermal components of the early craniofacial skeleton appears to occur normally in double mutants (Fig. 2I,J). Taken together, the absence of NC derivatives in double mutants appears to be a consequence of the failure of NC sublineage specification mediated directly or indirectly by the functions of foxd3 and tfap2a during early stages of NC development.
foxd3 and tfap2a are synergistically required for NC SoxE family gene expression
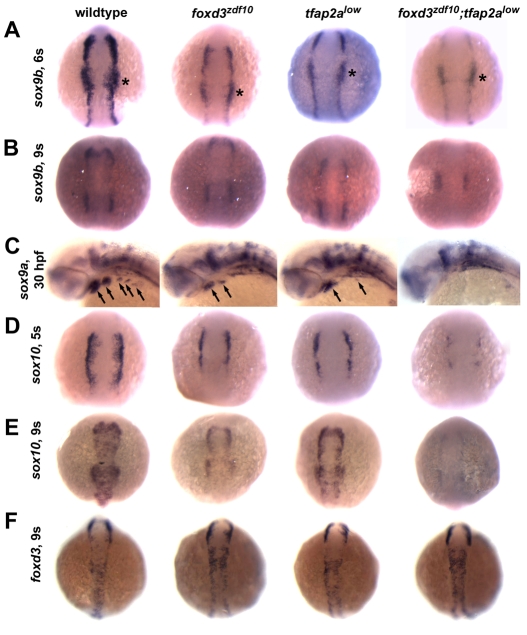
Because members of the SoxE family of genes in zebrafish have been implicated in the specification and development of some NCC sublineages that generate all chromatophore types, DRG, sympathetic and enteric neurons (sox10) (Dutton et al., 2001; Elworthy et al., 2005; Carney et al., 2006), and craniofacial skeleton and melanophores and iridophores (sox9a and sox9b) (Yan et al., 2005), we examined the expression of these genes in foxd3zdf10;tfap2alow double mutant embryos. In both foxd3zdf10 and tfap2alow mutants, NC expression of sox9a, sox9b and sox10 was reduced but still readily detectable by in situ hybridization in the majority of the NCC population (Fig. 3A-E) (Knight et al., 2003; Barrallo-Gimeno et al., 2004; Montero-Balaguer et al., 2006; Stewart et al., 2006). By stark contrast, NC expression of sox9b and sox10 was induced at strikingly reduced levels (Fig. 3A,D; see also Fig. S3 in the supplementary material), was then rapidly and completely extinguished (Fig. 3B,E) in foxd3zdf10;tfap2alow double mutants, and remained undetectable at all axial levels through 27 hours post-fertilization (hpf), the latest stage examined (not shown). NC sox9a expression was absent at all stages in double mutants (Fig. 3C; data not shown; see Fig. S3 in the supplementary material). Failure in the expression of these SoxE genes in foxd3zdf10;tfap2alow double mutant embryos was NC specific, as the induction and maintenance of expression in non-NC-derived tissues was similar to that in wild-type embryos (Fig. 3). Lastly, we also examined the expression of another transcription factor, snai1b, that is normally expressed by premigratory NC during early somitogenesis (Thisse et al., 1993). Consistent with previous findings (Knight et al., 2003; Barrallo-Gimeno et al., 2004; Stewart et al., 2006), we found that NC snai1b expression in tfap2alow mutants was indistinguishable from that in wild-type embryos, whereas NC expression was significantly reduced but still detectable in foxd3zdf10 mutants. NC snai1b expression in foxd3zdf10;tfap2alow double mutants was qualitatively indistinguishable from that in foxd3zdf10 single mutants (data not shown) (see Stewart et al., 2006).
Fig. 3.
Defective expression of NC SoxE family genes in foxd3zdf10;tfap2alow double mutant embryos. (A,B,D-F) Dorsal views with anterior to the top of 6, 5 and 9 somite stage (s) embryos. (C) Lateral views with anterior to the left of 30 hpf embryos. (A) NC sox9b expression is severely depleted in foxd3zdf10;tfap2alow double mutants at the 6 somite stage, but is retained in the non-NC-derived otic placode (asterisks). (B) NC sox9b expression remains reduced in both single mutants, whereas NC expression is undetectable in double mutants by the 9 somite stage. (C) NC sox9a expression in the branchial arches (arrows) is absent in double mutant embryos. (D) There are slight reductions in NC sox10 expression in foxd3zdf10 and tfap2alow single mutants at the 5 somite stage, whereas in double mutant embryos NC sox10 expression is much more reduced. (E) By the 9 somite stage, NC sox10 expression is undetectable in double mutants. (F) In contrast to SoxE gene expression, NC foxd3 expression is maintained in foxd3zdf10;tfap2alow double mutant embryos, demonstrating that NC induction occurs.
NC induction is independent of foxd3 and tfap2a function
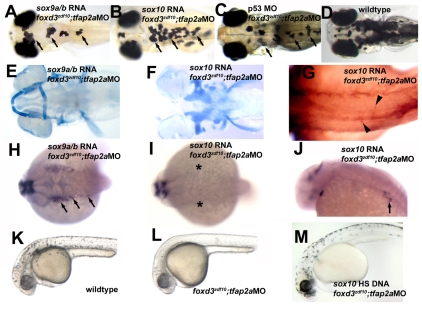
Importantly, NCCs are present at stages when SoxE family genes are expressed in wild-type embryos based on the normal pattern of foxd3 expression in foxd3zdf10;tfap2alow double mutants (Fig. 3F; data not shown), indicating that NC induction is qualitatively normal, although these NCCs are molecularly abnormal. In addition, the level of NCC death over time (6 somites-24 hpf), assessed qualitatively by TUNEL and Acridine Orange staining, in foxd3zdf10;tfap2alow double mutants was modest and not more pronounced than the levels observed in tfap2alow or foxd3zdf10 single mutants nor than the theoretical sum of both (data not shown). Nevertheless, NCC death might play a role in the development of aspects of the single as well as the double mutant phenotypes. Consistent with this possibility, we found that p53 morpholino injection into tfap2a-depleted foxd3zdf10 embryos resulted in limited and variable melanophore and xanthophore rescue in 100% of mutant morphant embryos (n=50), and iridophore rescue in 30% of these embryos when examined at 4 dpf and 7 dpf (Fig. 4C). Qualitatively, chromatophore rescue by p53 morpholino was markedly less robust than that by sox10 misexpression (not shown, see below). No rescue of craniofacial cartilage development was found based on Alcian Blue staining (data not shown). However, we also found that activation of sox10 cDNA expression driven by a heat-shock promoter (Halloran et al., 2000) at 24 hpf in tfap2a MO-injected foxd3zdf10 mutant embryos resulted in robust melanophore rescue (50%, n=17; Fig. 4K-M). Because sox10 function is not necessary for NC induction in zebrafish (not shown) (Kelsh and Eisen, 2000) and has a demonstrable NCC survival function only after 35 hpf (Dutton et al., 2001), these results indicate the persistence of an NCC population in tfap2a-depleted foxd3zdf10 embryos at least as late as 24 hpf. Interestingly, the persistence and/or sox10 responsiveness of these cells is limited, as chromatophore rescue was not observed when sox10 expression was induced at 48 hpf (not shown). Taken together, these results strongly suggest that p53-mediated NCC death is unlikely to fully account for the observed defects in NC sublineage specification and development in double mutants. Finally, the induction and patterning of the neural plate border occurs normally in double mutants, as does the development of Rohon-Beard sensory neurons, identified by huC (elevl3 - Zebrafish Information Network) and isl1 expression (see Fig. S4 in the supplementary material; data not shown). Thus, foxd3 and tfap2a are dispensable for NC induction but are genetically required for NCCs to express SoxE genes and diversify.
Fig. 4.
Misexpression of SoxE genes differentially rescues NC sublineage specification and differentiation in foxd3zdf10;tfap2aMO embryos. (A,E,H) sox9a and sox9b (sox9a/b) RNA co-injection with tfap2a morpholino (tfap2aMO) into foxd3zdf10 mutant embryos (foxd3zdf10-tfap2aMO). (A) Live 4 dpf larva, dorsal view, anterior to the left. Melanophore and xanthophore development is rescued in foxd3zdf10-tfap2aMO embryos co-injected with sox9a/b RNA (arrows; compare with wild-type embryo, D). (E) Alcian Blue staining of a 4 dpf larva, vental views with anterior to the left. Misexpression of sox9a/b in foxd3zdf10-tfap2aMO embryos results in rescue of the neurocranium, as well as of the mandibular and hyoid jaw structures. (H) dlx2 expression at 24 hpf, dorsal views, anterior to the left. Specification of NC craniofacial progenitors occurs in foxd3zdf10-tfap2aMO embryos co-injected with sox9a/b RNA, based on dlx2 expression (arrows). (B,F,G,I,J) foxd3zdf10-tfap2aMO embryos co-injected with sox10 RNA. (B) Live 4 dpf larva, dorsal view, anterior to the left. Consistent with a role in development of non-ectomesenchymal NC derivatives, sox10 misexpression rescues melanophore and xanthophore development in foxd3zdf10-tfap2aMO embryos (arrows; compare with wild-type embryo, D). (F) Alcian Blue staining of 4 dpf larva, ventral view, anterior to the left. sox10 misexpression does not rescue cranial cartilage structures in foxd3zdf10-tfap2aMO embryos. (G) 16A11 immunoreactivity, 3 dpf, dorsal view. Dorsal root ganglion neuron development is rescued by sox10 misexpression (arrowheads). (I) dlx2 expression at 24 hpf, dorsal view with anterior to the left. sox10 RNA injection also does not rescue NC dlx2 expression in foxd3zdf10-tfap2aMO embryos (asterisks), indicating that sox10 function cannot rescue the specification of NC precursors for craniofacial cartilages. (J) th expression at 48 hpf, lateral view, anterior to the left. sox10 misexpression rescues sympathetic neuron development (arrow). (C) Live 4 dpf larva, dorsal view, anterior to the left. Morpholino-mediated knockdown of p53 in foxd3zdf10-tfap2aMO embryos rescues melanophore, xanthophore (arrows; compare with wild-type embryo, D) and iridophore (not visible) development. (K-M) sox10-responsive NCCs persist in foxd3zdf10-tfap2aMO embryos. 33 hpf embryos, lateral view, anterior to left. (K) Wild-type embryo with abundant melanophores. (L) In foxd3zdf10-tfap2aMO embryos injected with a heat shock-inducible sox10 construct but not exposed to heat shock, melanophores fail to develop. (M) In foxd3zdf10-tfap2aMO embryos injected with a heat shock-inducible sox10 construct and heat shocked at 24 hpf, robust melanogenesis occurs.
Coordinate regulation of SoxE family gene expression by foxd3 and tfap2a initiates NCC diversification
To test whether the absence of NC SoxE family gene expression in foxd3zdf10;tfap2alow double mutant embryos can functionally account for the failure of NC sublineage specification and/or the differentiation of NC derivatives, we misexpressed SoxE family genes using mRNA constructs in tfap2a MO-injected foxd3zdf10 mutants. Again, these mutant morphants precisely and efficiently phenocopy double mutants, including NC SoxE family gene induction deficiencies (see Figs S1-S3 in the supplementary material). Misexpression of sox10 mRNA resulted in the efficient rescue of melanophores (50%, n=69), xanthophores (50%, n=69), and DRG and sympathetic neurons (50%, n=38), but had no effect on the failure of craniofacial NC development (0%, n=75; Fig. 4B,F,G,I,J). By contrast, misexpression of combined sox9a and sox9b mRNAs globally rescued the sublineage specification (76%, n=28) and differentiation of craniofacial cartilages (68%, n=44), as well as melanophores and xanthophores (76%, n=29; Fig. 4A,E,H). No phenotype rescue was detected after misexpression of either sox9a (n=44) or sox9b (n=33) alone (data not shown), even when concentrations of single species were in excess of that of the concentration of the combined mRNAs that resulted in phenotype rescue (see Materials and methods). These results indicate that the foxd3;tfap2a-dependent initiation of NCC diversification is mediated in part by SoxE family genes, which in turn differentially specify subpopulations of all of the major NC sublineages.
DISCUSSION
We have shown that foxd3zdf10;tfap2alow double mutant embryos completely lack differentiated NC-derived cells. Furthermore, our results indicate that foxd3 and tfap2a are required in parallel for the initiation of NCC diversification in zebrafish embryos, as demonstrated by the absence of NC expression of genes required for and/or diagnostic of specified NC sublineages. This phenotype is preceded or accompanied by the complete loss of NC expression of the SoxE family genes sox10, sox9b and sox9a. We show that restoration of NC expression of sox10 and both sox9a and sox9b differentially rescues NCC diversification. Together, based on the results of our genetic manipulations, we conclude that the requirement for foxd3 and tfap2a function for the initiation of NCC diversification is mediated to a significant extent through the synergistic regulation of SoxE family gene expression. We also show that p53-mediated NCC death also plays at least a limited role in the double mutant phenotype, although whether this results directly or indirectly from the absence of foxd3, tfap2a or SoxE function, or a combination thereof, is unclear. Our results also provide further evidence for the decoupling of the genetic regulation of the processes of NC induction and NCC diversification, as the generation of the premigratory NC population is largely normal and significant numbers of NCCs persist for an extended period of embryogenesis in foxd3zdf10;tfap2alow double mutants. Our results suggest that in foxd3zdf10;tfap2alow double mutants substantial numbers of NCCs persist in an unspecified state and do not appear to adopt an alternative ectodermal fate.
Our results demonstrate that the coordinated regulation of the NC expression of SoxE family genes by foxd3 and tfap2a underlies the specification of multiple NC sublineages, although the rescue experiments we performed do not address whether all cells within a given sublineage are specified by SoxE genes. For example, the fact that in zebrafish sox10 null mutants subsets of DRG, sympathetic and enteric neurons develop successfully (Kelsh and Eisen, 2000; Dutton et al., 2001) indicates that additional regulators of sublineage specification, dependent upon foxd3 and tfap2a function, are required for the specification of these sublineages in their entirety. Likewise, although our results show that forced sox9a/b expression in tfap2a-depleted foxd3zdf10 mutants is sufficient for the specification of craniofacial skeleton progenitors and their subsequent differentiation, the fact that craniofacial NC precursors are specified in sox9a;sox9b double mutants (Yan et al., 2005) might indicate that one or more different genes, whose expression is dependent on foxd3 and tfap2a function, are normally required as well for the initial specification of the NC craniofacial skeleton sublineage. In addition, the chromatophore rescue activity of sox9a/b is somewhat surprising given the phenotype of sox9a;sox9b double mutants (Yan et al., 2005). We did not detect rescue of NC sox10 expression in sox9a/b-injected, double deficient, 9-somite-stage embryos at the level of in situ hybridization, although this does not preclude low-level sox10 induction sufficient to drive chromatophore development in these embryos. It could also be possible that forced sox9a/b expression is sufficient to drive chromatophore development in the absence of sox10 even though the sox9a;sox9b double mutant phenotype indicates that they are not normally necessary (Yan et al., 2005). Lastly, unlike zebrafish sox10, which is entirely dispensable for NC induction (not shown) (Kelsh and Eisen, 2000), sox9a and sox9b have been shown to induce ectopic NC-like cells in zebrafish upon forced misexpression (Yan et al., 2005). However, this activity appears to be qualitatively slight, and although it possibly contributes to the robust craniofacial phenotype rescue observed, it would seem unlikely to entirely account for it. The foxd3zdf10;tfap2alow double mutant model provides an excellent experimental template for the identification of these additional regulators of NCC diversification. Taken together, however, based on the phenotypes of zebrafish SoxE family gene mutants and the robust phenotype rescue observed upon their forced expression in tfap2a-depleted foxd3zdf10 mutants, SoxE family genes are clearly principal regulators of NC sublineage specification genetically downstream of foxd3 and tfap2a. It will be important to determine in the future whether the genetic interactions between foxd3, tfap2a and SoxE genes are direct or indirect by using biochemical analyses.
We have shown that a foxd3-tfap2a-SoxE transcriptional network regulates the fate specification of NC sublineages. In addition, we (Stewart et al., 2006) and others (Kelsh and Eisen, 2000; Dutton et al., 2001; Knight et al., 2003; Barrallo-Gimeno et al., 2004; Yan et al., 2005; Montero-Balaguer et al., 2006) have documented additional consequences of the loss- or gain-of-function of these transcription factors during NC development. Specifically, zebrafish foxd3 (Montero-Balaguer et al., 2006; Stewart et al., 2006), tfap2a (Knight et al., 2003; Barrallo-Gimeno et al., 2004), sox10 (Kelsh and Eisen, 2000; Dutton et al., 2001), sox9a;sox9b (Yan et al., 2005) and foxd3;tfap2a (this study) mutants all display NC phenotypes that include limited cell death that is likely to contribute in a limited way to the mutant phenotypes, although these phenotypes vary widely in both their extent and spatiotemporal pattern between different mutants. Importantly, a similar range of defects has been demonstrated in experiments in other vertebrate models as well (see Gammill and Bronner-Fraser, 2003; Kos et al., 2001; Cheung et al., 2005; Sakai et al., 2006), although differences exist between these models and zebrafish, perhaps most noticeably regarding roles in NC induction (see, for example, Kelsh and Eisen, 2000). Thus, whereas our conclusion that the coordinate regulation of SoxE family gene expression by foxd3 and tfap2a is essential for the initial specification of NC sublineages, operationally defined by the absence of the expression of genes diagnostic of NC sublineages, the mechanism(s) underlying this developmental defect is currently not well defined. Conceptually, NCC death could deplete the numbers of NCCs available to be specified and develop, although in all of the zebrafish mutants relevant to this study, with the exception of foxd3zdf10;tfap2alow double mutants, significant numbers and types of derivatives successfully develop, and we have shown that significant numbers of NCCs persist in foxd3zdf10;tfap2alow double mutants. This suggests that NCC death does not entirely account for the foxd3zdf10;tfap2alow double mutant phenotype. Indeed, it seems just as likely that the failure in NC sublineage specification as a result of simultaneous foxd3;tfap2a loss of function might result in some cells that would normally be specified to adopt a specific sublineage fate adopting an alternative fate, cell death. Our results demonstrating limited phenotype rescue upon p53 morpholino injection do not distinguish between the alternative mechanisms described. Taken together, although cell death might underlie aspects of the foxd3zdf10;tfap2alow double mutant phenotype, it is unlikely to entirely account for it. Ultimately, it will be important to determine whether and to what extent defects in NC sublineage cell fate specification are the cause or consequence of NCC death observed in zebrafish mutant for the transcription factors investigated in this study.
In summary, using a genetic approach, we have demonstrated that the transcription factors foxd3 and tfap2a are required for the initiation of NCC diversification in zebrafish embryos through the synergistic regulation of the expression of SoxE family genes that function to differentially specify NC sublineages. These results also provide further evidence that the processes of NC induction and NC sublineage specification can be regulated independently. Our results identify a genetic network of transcriptional regulators that initiate NCC diversification. This network represents a minimal genetic scaffold to which additional regulators of NCC diversification can be appended to ultimately construct a comprehensive genetic network regulating the generation of NCC diversity.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/12/1987/DC1
Supplementary Material
We thank T. Schilling for sharing tfap2alow fish, J. Postlethwait and R. Kelsh for expression vectors, R. Stewart for technical advice and numerous colleagues for reagents used in this study. This research was supported by NIH GM076505 to P.D.H. with additional support from NIH P30-NS045758. Deposited in PMC for release after 12 months.
References
- Akimenko, M. A., Ekker, M., Wegner, J., Lin, W. and Westerfield, M. (1994). Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J. Neurosci. 14, 3475-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende, M. L. and Weinberg, E. S. (1994). The expression pattern of two zebrafish achaete-scute homolog (ash) genes is altered in the embryonic brain of the cyclops mutant. Dev. Biol. 166, 509-530. [DOI] [PubMed] [Google Scholar]
- An, M., Luo, R. and Henion, P. D. (2002). Differentiation and maturation of zebrafish dorsal root and sympathetic ganglion neurons. J. Comp. Neurol. 446, 267-275. [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno, A., Holzschuh, J., Driever, W. and Knapik, E. W. (2004). Neural crest survival and differentiation in zebrafish depends on mont blanc/tfap2a gene function. Development 131, 1463-1477. [DOI] [PubMed] [Google Scholar]
- Bi, W., Huang, W., Whitworth, D. J., Deng, J. M., Zhang, Z., Behringer, R. R. and de Crombrugghe, B. (2001). Haploinsufficiency of Sox9 results in defective cartilage primordial and premature skeletal mineralization. Proc. Natl. Acad. Sci. USA 98, 6698-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser, M. and Fraser, S. (1988). Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature 355, 161-164. [DOI] [PubMed] [Google Scholar]
- Budi, E. H., Patterson, L. B. and Parichy, D. M. (2008). Embryonic requirements for ErbB signaling in neural crest development and adult pattern formation. Development 135, 2603-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney, T. J., Dutton, K. A., Greenhill, E., Delfino-Machin, M., Dufourcq, P., Blader, P. and Kelsh, R. N. (2006). A direct role for Sox10 in specification of neural crest-derived sensory neurons. Development 133, 4619-4630. [DOI] [PubMed] [Google Scholar]
- Cheung, M., Chaboissier, M.-C., Mynett, A., Hirst, E., Schedl, A. and Briscoe, J. (2005). The transcriptional control of trunk neural crest induction, survival and delamination. Dev. Cell 8, 179-192. [DOI] [PubMed] [Google Scholar]
- Dottori, M., Gross, M. K., Labosky, P. and Goulding, M. (2001). The winged - helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 128, 4127-4138. [DOI] [PubMed] [Google Scholar]
- Dutton, K. A., Pauliny, A., Lopes, S. S., Elworthy, S., Carney, T. J., Rauch, J., Geisler, R., Haffter, P. and Kelsh, R. N. (2001). Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 128, 4113-4125. [DOI] [PubMed] [Google Scholar]
- Elworthy, S., Lister, J. A., Carney, T. J., Raible, D. W. and Kelsh, R. N. (2003). Transcriptional regulation of mitfa accounts for the sox10 requirement in zebrafish melanophore development. Development 130, 2809-2818. [DOI] [PubMed] [Google Scholar]
- Elworthy, S., Pinto, J. P., Pettifer, A., Cancela, M. L. and Kelsh, R. N. (2005). Phox2b function in the enteric nervous system is conserved in zebrafish and is sox10-dependent. Mech. Dev. 122, 659-669. [DOI] [PubMed] [Google Scholar]
- Frank, E. and Sanes, J. R. (1991). Lineage of neurons and glia in chick dorsal root ganglia: analysis in vivo with a recombinant retrovirus. Development 111, 895-908. [DOI] [PubMed] [Google Scholar]
- Gammill, L. S. and Bronner-Fraser, M. (2003). Neural crest specification: migrating into genomics. Nat. Rev. Neurosci. 4, 795-805. [DOI] [PubMed] [Google Scholar]
- Halloran, M. C., Sato-Maeda, M., Warren, J. T., Su, F., Lele, Z., Krone, P. H., Kuwada, J. Y. and Shoji, W. (2000). Laser-induced gene expression in specific cells of transgenic zebrafish. Development 127, 1953-1960. [DOI] [PubMed] [Google Scholar]
- Hanna, L. A., Foreman, R. K., Tarasenko, I. A., Kessler, D. S. and Labosky, P. A. (2002). Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes. Dev. 16, 2650-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henion, P. D. and Weston, J. A. (1997). Timing and pattern of cell fate restrictions in the neural crest lineage. Development 124, 4351-4359. [DOI] [PubMed] [Google Scholar]
- Herbarth, B., Pingault, V., Bondurand, N., Kuhlbrodt, K., Hermans-Borgmeyer, I., Puliti, A., Lemort, N., Goosens, M. and Wegner, M. (1998). Mutation of the Syr-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschprung disease. Proc. Natl. Acad. Sci. USA 95, 5161-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson, C. A., Moore, K. J., Nakayama, A., Steingrimsson, E., Copeland, N. G., Jenkins, N. A. and Arnheiter, H. (1993). Mutations at the mouse microthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 74, 395-404. [DOI] [PubMed] [Google Scholar]
- Hodgkinson, C. A., Nakayama, A., Li, H., Swenson, L. B., Opdecamp, K., Asher, J. H., Jr, Arnheiter, H. and Glaser, T. (1998). Mutations at the anopthalmic white locus in Syrian hamsters: haploinsufficiency in the Mitf gene mimics Waardenburg syndrome type 2. Hum. Mol. Genet. 7, 703-708. [DOI] [PubMed] [Google Scholar]
- Kapur, R. P. (1999). Early death of neural crest cells is responsible for total enteric aganglionosis in Sox10(Dom)/Sox10(DOM) mouse embryos. Pediatr. Dev. Pathol. 2, 559-569. [DOI] [PubMed] [Google Scholar]
- Kelsh, R. N. and Eisen, J. S. (2000). The zebrafish colourless gene regulates development of non-ectomesenchymal neural crest derivatives. Development 127, 515-525. [DOI] [PubMed] [Google Scholar]
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. and Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. [DOI] [PubMed] [Google Scholar]
- Kimmel, C. B., Miller, C. T., Kruze, G., Ullmann, B., BreMiller, R. A., Larison, K. D. and Snyder, H. C. (1998). The shaping of pharyngeal cartilages during early development of the zebrafish. Dev. Biol. 203, 245-263. [DOI] [PubMed] [Google Scholar]
- Knight, R. D., Nair, S., Nelson, S. S., Afshar, A., Javidan, Y., Geisler, R., Rauch, G. J. and Schilling, T. F. (2003). lockjaw encodes a zebrafish tfap2a required for early neural crest development. Development 130, 5755-5768. [DOI] [PubMed] [Google Scholar]
- Kos, R., Reedy, M. V., Johnson, R. L. and Erickson, C. A. (2001). The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development 128, 1467-1479. [DOI] [PubMed] [Google Scholar]
- LeDouarin, N. and Kalcheim, C. (1999). The Neural Crest. New York: Cambridge University Press.
- Levy, C., Khaled, M. and Fisher, D. E. (2006). MITF: master regulator of melanocyte development and melanoma oncogene. Trends. Mol. Med. 12, 406-414. [DOI] [PubMed] [Google Scholar]
- Lister, J. A., Robertson, C. P., Lepage, T., Johnson, S. L. and Raible, D. W. (1999). nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126, 3757-3767. [DOI] [PubMed] [Google Scholar]
- Lopes, S. S., Yang, X., Muller, J., Carney, T. J., McAdow, A. R., Rauch, G.-J., Jacoby, A. S., Hurst, L. D., Delfino-Machin, M., Hafter, P. et al. (2008). Leukocyte tyrosine kinase functions in pigment cell development. PLoS Genet. 4, e1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, M. E., Muller, F., Rudiger, R., Henion, P. D. and Rohrer, H. (2006). The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development 133, 4015-4024. [DOI] [PubMed] [Google Scholar]
- Mochii, M., Ono, T., Matsubara, Y. and Eguchi, G. (1998). Spontaneous transdifferentiation of quail pigmented epithelial cell is accompanied by a mutation in the Mitf gene. Dev. Biol. 196, 145-169. [DOI] [PubMed] [Google Scholar]
- Montero-Balaguer, M., Lang, M. R., Sachdev, S. W., Knappmeyer, C., Stewart, R. A., De La Guardia, A., Hatzopoulos, A. K. and Knapik, E. W. (2006). The mother superior mutation ablates foxd3 activity in neural crest progenitor cells and depletes neural crest derivatives in zebrafish. Dev. Dyn. 235, 3199-3212. [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama, Y., Akiyama, H., Rowitch, D. H. and de Crombrugghe, B. (2003). Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc. Natl. Acad. Sci. USA 100, 9360-9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay, G. M. (1996). Craniofacial defects in AP-2 null mutant mice. BioEssays 18, 785-788. [DOI] [PubMed] [Google Scholar]
- O'Brien, E. K., d'Alencon, C., Bonde, G., Li, W., Schoenebeck, J., Allende, M. L., Gelb, B. D., Yelon, D., Eisen, J. S. and Cornell, R. A. (2004). Transcription factor Ap-2alpha is necessary for development of embryonic melanophores, autonomic neurons and pharyngeal skeleton in zebrafish. Dev. Biol. 265, 246-261. [DOI] [PubMed] [Google Scholar]
- Parichy, D. M., Mellgren, E. M., Rawls, J. F., Lopes, S. S., Kelsh, R. N. and Johnson, S. L. (2000). Mutational analysis of endothelin receptor b1 (rose) during neural crest and pigment pattern development in the zebrafish Danio rerio. Dev. Biol. 227, 294-306. [DOI] [PubMed] [Google Scholar]
- Pohl, B. S. and Knochel, W. (2001). Overexpression of the transcriptional repressor FoxD3 prevents neural crest formation in Xenopus embryos. Mech. Dev. 103, 93-106. [DOI] [PubMed] [Google Scholar]
- Raible, D. W. and Eisen, J. S. (1994). Restriction of neural crest cell fate in the trunk of embryonic zebrafish. Development 120, 495-503. [DOI] [PubMed] [Google Scholar]
- Sakai, D., Suzuki, T., Osumi, N. and Wakamatsu, Y. (2006). Cooperative action of Sox9, Snail2 and PKA signaling in early neural crest development. Development 133, 1323-1333. [DOI] [PubMed] [Google Scholar]
- Sasai, N., Mizuseki, K. and Sasai, Y. (2001). Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development 128, 2525-2536. [DOI] [PubMed] [Google Scholar]
- Schilling, T. F. and Kimmel, C. B. (1994). Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120, 2945-2960. [DOI] [PubMed] [Google Scholar]
- Schorle, H., Meier, P., Buchert, M., Jaenisch, R. and Mitchell, P. J. (1996). Transcription factor Ap-2 is essential for cranial closure and craniofacial development. Nature 381, 235-238. [DOI] [PubMed] [Google Scholar]
- Sock, E., Pagon, R. A., Keymolen, K., Lissens, W., Wegner, M. and Scherer, G. (2003). Loss of DNA-dependent dimerization of the transcription factor SOX9 as a cause for campomelic dysplasia. Hum. Mol. Genet. 12, 1439-1447. [DOI] [PubMed] [Google Scholar]
- Southard-Smith, E. M., Kos, L. and Pavan, W. J. (1998). Sox10 mutation disrupts neural crest development in DOM Hirschprung mouse model. Nat. Genet. 18, 60-64. [DOI] [PubMed] [Google Scholar]
- Stewart, R. A., Arduini, B. L., Berghmans, S., George, R. E., Kanki, J. P., Henion, P. D. and Look, A. T. (2006). Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Dev. Biol. 292, 174-188. [DOI] [PubMed] [Google Scholar]
- Thisse, C., Thisse, B., Schilling, T. F. and Postlethwait, J. H. (1993). Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 119, 1203-1215. [DOI] [PubMed] [Google Scholar]
- Yan, Y. L., Willoughby, J., Liu, D., Crump, J. G., Wilson, C., Miller, C. T., Singer, A., Kimmel, C., Westerfield, M. and Postlethwait, J. H. (2005). A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development 132, 1069-1083. [DOI] [PubMed] [Google Scholar]
- Yelon, D., Ticho, B., Halpern, M. E., Ruvinsky, I., Ho, R. K., Silver, L. M. and Stainier, D. Y. (2000). The bHLH transcription factor Hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development 127, 2573-2582. [DOI] [PubMed] [Google Scholar]
- Zhang, J. A., Hagopian-Donaldson, S., Serbedzija, G., Elsemore, J., Plehn-Dujowich, D., McMahon, A. P., Flavell, R. A. and Williams, T. (1996). Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 381, 238-241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.