Summary
The kidney develops in a specific position along the anterior-posterior axis. All vertebrate kidney tissues are derived from the intermediate mesoderm (IM), and early kidney genes such as Lim1 and Pax2 are expressed in amniotes posterior to the sixth somite axial level. IM cells anterior to this level do not express kidney genes owing to changes in their competence to respond to kidney-inductive signals present along the entire axis. We aimed to understand the molecular mechanisms governing the loss of competence of anterior IM cells and the formation of the anterior border of the kidney morphogenetic field. We identified the dorsal neural tube as the potential kidney-inductive tissue and showed that activin, a secreted morphogen, is necessary but insufficient for Lim1 induction and establishment of the kidney field. Activin or activin-like and BMP signaling cascades are activated along the entire axis, including in anterior non-kidney IM, suggesting that competence to respond to these signals involves downstream or other components. Detailed expression pattern analysis of Hox genes during early chick development revealed that paralogous group four genes share the same anterior border as the kidney genes. Ectopic expression of Hoxb4 in anterior non-kidney IM, either by retinoic acid (RA) administration or plasmid-mediated overexpression, resulted in ectopic kidney gene expression. The anterior expansion of Lim1 expression was restrained when Hoxb4 was co-expressed with a truncated form of activin receptor. We suggest a model in which the competence of IM cells to respond to TGFβ signaling and express kidney genes is driven by RA and mediated by Hoxb4.
Keywords: Kidney development, Pronephros, Intermediate mesoderm, Anterior-posterior patterning, Activin, Retinoic acid, Hox genes
INTRODUCTION
A morphogenetic field is described as a group of cells that is competent to respond to the biochemical influences of surrounding tissues and consequently differentiates into specific morphological structures. The morphogenetic field has definitive boundaries that determine the eventual position of molecular events leading to its derivatives. In vertebrate embryos, the kidney morphogenetic field (KMF) arises within the intermediate mesoderm (IM) and is characterized by a specific profile of gene expression, including Lim1 (also known as Lhx1) (Fujii et al., 1994) and Pax2 (Dressler et al., 1990). The major patterning events specifying the kidney lineage take place during, and shortly after, gastrulation (Cartry et al., 2006; James and Schultheiss, 2003; Mauch et al., 2000). While still in the primitive streak (PS), the prospective IM is committed to its kidney fate but requires extrinsic signals in order to become specified (Barak et al., 2005). The initial specification towards IM fate occurs at stages HH5-7 (Hamburger and Hamilton, 1992), shortly after prospective IM cells leave the PS and migrate anteriorly. By stage 8, IM cells are located in their final position and start to express both Lim1 and Pax2. This expression is restricted to IM located posterior to the sixth somite level (Fujii et al., 1994; James and Schultheiss, 2003; Mauch et al., 2000), consistent with specific tissue structures that give rise to the pronephric duct primordium (Hiruma and Nakamura, 2003).
Little is known about the signals governing IM specification. Several studies have demonstrated the role of neighboring tissues in IM specification, mainly in relation to the mediolateral axis (Barak et al., 2005; James and Schultheiss, 2003; James and Schultheiss, 2005; Mauch et al., 2000; Seufert et al., 1999). Considerable evidence from studies carried out in zebrafish, Xenopus and avian embryos has shown a role for BMP, activin and retinoic acid (RA) signaling in early events of kidney specification and pronephros induction. In the Xenopus animal cap assay, activin can induce the expression of Xlim-1 and of the related gene Xlmx-1b (Haldin et al., 2003; Taira et al., 1992). Combining activin and RA results in a synergistic effect on Xlim-1 induction and can induce the formation of pronephric tubules (Moriya et al., 1993; Osafune et al., 2002; Taira et al., 1992). Similarly, mouse embryonic stem cells respond to activin and RA by upregulation of IM markers (Kim and Dressler, 2005). Moreover, both zebrafish and Xenopus Lim1 genes contain an activin response element (ARE) in their first intron, which, at least in zebrafish embryos, is required for the expression of this gene (Rebbert and Dawid, 1997; Watanabe et al., 2002). Previous studies suggest a role for BMP signaling in specifying IM cells and the induction of IM genes (James and Schultheiss, 2005; Obara-Ishihara et al., 1999).
Barak et al. (Barak et al., 2005) recently provided a cellular mechanism to explain the formation of the anterior border of the KMF. Two main themes emerged from this study. The first proposed the presence of kidney-inductive signals along the entire axis, including in the anterior non-kidney IM regions. The second theme suggested that IM cells lying anterior to the sixth somite level do not express kidney genes as a result of changes in their competence to respond to these inductive signals. Based on these results, Barak and co-workers proposed a model in which changes in cell competence determine the formation of the anterior border of kidney gene expression. These changes occur in a narrow time window in early developmental stages during the migration of the cells to their final destination in the anterior IM. Therefore, despite the presence of such inductive factors in the anterior environment, IM cells do not express the kidney program. By contrast, cells that migrate later do not lose their competence to respond to inductive signals and are specified as kidney tissue. In the current study, we aimed to discover the molecular mechanism governing the loss of competence of early gastrulating IM cells and, thereby, the formation of the anterior border of the KMF.
MATERIALS AND METHODS
Embryo culture
Fertile White Leghorn chicken and Japanese quail (Coturnix coturnix japonica) eggs were incubated at 38°C in a humidified incubator until embryos reached HH3-15 (Hamburger and Hamilton, 1992). For explant analysis, chick and quail embryonic tissues were cultured in type I collagen gels (Münsterberg et al., 1995; Schultheiss et al., 1995) suffused with PS culture media: DMEM-F12 supplemented with 5% fetal calf serum (Beth-Ha'emek, Israel), 5 μg/ml human transferrin (Gibco), 100 μg/ml conalbumin (Sigma), 1× insulin-transferrin-selenium (Gibco), 1% Pen-Strep and 1% l-glutamine. In experiments using activin and RA, recombinant activin A (R&D Systems) or all-trans RA (Sigma) were added to the culture medium to a final concentration of 10 ng/ml or 10 μM, respectively. For in vivo manipulations, chick embryos were grown in modified New culture as previously described (Barak et al., 2005).
RT-PCR and restriction site polymorphism analysis
A detailed description of the RT-PCR procedure, including primer sequences, and restriction site analysis details are available upon request.
Implantation of RA beads
DEAE beads were soaked in 10 μM RA or 1.7 M DMSO prior to in vivo implantation into the migratory pathway of prospective anterior IM cells of stage 4 embryos.
Expression plasmids and electroporation
Expression plasmids for Hoxb4 were kindly provided by Olivier Pourquié (Iimura and Pourquie, 2006). A dominant-negative form of Xenopus activin receptor 1 (Hemmati-Brivanlou and Melton, 1992) was obtained from Abraham Fainsod and subcloned into pCIG (Megason and McMahon, 2002). Electroporation was carried out as described (Wilm et al., 2004). Detailed protocols are available upon request.
Whole-mount in situ hybridization, sectioning and immunohistochemistry
Whole-mount RNA in situ hybridization (ISH) was performed as described (Schultheiss et al., 1995) using probes for chick Lim1 (Tsuchida et al., 1994) and chick Hoxb4 (Morrison et al., 1995). Embryos were cryosectioned as described (Barak et al., 2005). Immunohistochemistry was performed on cryosections and paraffin sections using the following primary antibodies: Lim1 (Developmental Studies Hybridoma Bank), GFP (Molecular Probes), phospho-Smad1/5/8 and phospho-Smad2 (Cell Signaling Technology). Secondary antibodies were Cy3- or Cy2-conjugated anti-mouse or anti-rabbit IgG (Jackson ImmunoResearch). Detailed immunohistochemistry protocols are available upon request. Images were collected on a Leica DMIRE2 microscope with a Leica DC300fx camera or on a Zeiss Axiovert 200 fluorescent microscope with an Axiocam HS.
RESULTS
Identifying the source tissue of the kidney-inductive signals
In order to identify potential early kidney inducers, we first aimed to identify the kidney-inductive tissue. Previous studies demonstrated a role for adjacent paraxial tissues in the positive regulation of kidney genes in posterior IM (James and Schultheiss, 2003; James and Schultheiss, 2005; Mauch et al., 2000; Seufert et al., 1999). Moreover, Barak et al. (Barak et al., 2005) showed that kidney-inductive signals are secreted from tissues medial to the IM along the entire axis. Neither ectoderm nor endoderm is required for pronephros induction during early IM development stages (Mauch et al., 2000). Thus, the source of medial inductive signals could be the paraxial mesoderm, the neural tube (NT), the notochord, or any combination thereof.
Inductive signals for kidney genes are also present in the anterior regions at relatively late stages, when the somites are already formed. These signals are sufficient for the induction of kidney properties in prospective IM regions from the PS of stage 6 embryos (PS6) in vivo (Barak et al., 2005). Thus, we sought to recombine PS6 with midline tissues of stage 10 embryos and to culture them in isolation from any surrounding tissues. Kidney gene expression in the cultured tissues was analyzed by RT-PCR. In order to distinguish between genes expressed in the responding tissue (PS6) and the presumed inductive tissue (midline tissues), we recombined tissues from chick and quail embryos. Restriction site polymorphism between chick and quail PCR products enables one to distinguish between genes expressed in the responding tissue from those expressed in the inductive tissue, as previously described (Schultheiss et al., 1995). Based on chicken sequences, we designed a set of primers for the amplification of partial sequences of Gapdh, Lim1 and Pax2 transcripts. We then amplified selected regions of these genes by RT-PCR from stage 12 quail embryo cDNAs. Since we found restriction sites specific to the chick sequences only, we used the chick embryo as a donor for the prospective posterior IM of PS6 and the quail as the source of midline tissues. Detection of digested products indicated that the genes analyzed are expressed in the tissue derived from the chick embryo (Fig. 1A).
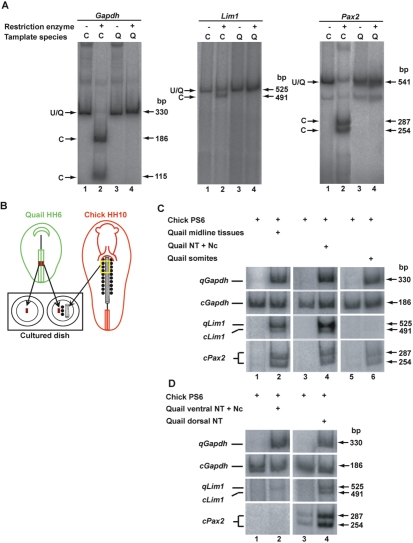
Fig. 1.
The dorsal neural tube secretes kidney-inductive signals. (A) Discrimination between chick and quail RT-PCR products by restriction site analysis. Each set of PCR primers was used to amplify cDNA from stage 12 chick (C) or quail (Q) embryos. PCR products were analyzed directly (lanes 1, 3) or following digestion by restriction enzymes that specifically recognize the chick sequences (lanes 2, 4). The identity of the bands and their sizes are labeled (U, uncut). (B) Scheme describing the experimental design. (C) Two parallel PS6 regions from chick embryos were isolated and cultured for 24 hours with or without isolated anterior midline tissues [neural tube (NT), notochord and somites 2-4] or NT and notochord (NT+Nc) or somites 2-4 of stage 10 quail embryos. Lim1 and Pax2 expression was analyzed by RT-PCR followed by restriction enzyme digestion (see A). Amplification of Gapdh transcript was used to compare RNA levels in each sample. For Pax2 transcripts, only the digested products are shown. (D) Two parallel PS6 regions from chick embryos were cultured for 24 hours with or without dorsal NT or ventral NT plus notochord (NT+Nc) of stage 10 quail embryos. Gapdh, Lim1 and Pax2 expression was analyzed as described in B. Genes are from quail (q) or chick (c).
First, we verified the relevance and reliability of the in vitro system by examining the ability of isolated midline tissues (NT, notochord and somites 2-4) to induce kidney gene expression in PS6. Two parallel regions of chick PS6 were cultured for 24 hours, either alone or together with anterior midline tissues from the stage 10 quail embryo (Fig. 1B). When cultured alone, no Lim1 or Pax2 expression was detected in PS6 (Fig. 1C, lane 1, n=10/10). However, co-culturing chick PS6 together with anterior midline tissues from quail embryos resulted in the upregulation of both Lim1 and Pax2 expression in the grafted PS6 (Fig. 1C, lane 2, n=10; 7/10 for Lim1 and Pax2). These results are in agreement with those of Barak et al. (Barak et al., 2005) and confirm the in vitro model.
Next, we dissected the midline into axial and paraxial tissues. NT and notochord or somites isolated from quail embryos were recombined with chick PS6 and cultured in vitro for 24 hours. RT-PCR analyses followed by restriction digestion revealed upregulation of both Lim1 and Pax2 expression in PS6 cultured in the presence of axial tissues (Fig. 1C, lanes 3 and 4, n=18; 9/18 and 12/18 for Lim1 and Pax2, respectively). By contrast, co-culturing chick PS6 with quail somites showed a differential induction of Lim1 and Pax2 expression (Fig. 1C, lanes 5 and 6, n=7; faint expression of Lim1 was observed in 1/7 cases and 6/7 expressed Pax2). Taken together, these results indicate that both the axial and paraxial tissues secrete a factor(s) that can induce Pax2 expression in kidney precursor tissues, whereas Lim1 expression is regulated by factors that emanate from the NT or notochord.
Finally, we sought to identify the precise region in the NT that is the source of the inductive signals. NT isolated from quail embryos was dissected along the rostrocaudal axis into dorsal and ventral halves. The dorsal half contained the roof plate, whereas the ventral half contained the floor plate and the notochord. Neither Lim1 nor Pax2 expression was detected in PS6 cultured with the ventral part of the NT (Fig. 1D, lanes 1 and 2, n=5; 0/5 for both Lim1 and Pax2). By contrast, co-culturing chick PS6 and the dorsal half of the NT resulted in the upregulation of both Lim1 and Pax2 expression in the grafted PS (Fig. 1D, lanes 3 and 4, n=6; 5/6 and 3/6 for Lim1 and Pax2, respectively). These findings are in agreement with the observation reported by Mauch et al. (Mauch et al., 2000), whereby the floor plate and notochord are not required for in vivo pronephros induction.
TGFβ signaling is involved in the specification of the kidney morphogenetic field
Several signaling molecules are secreted from the dorsal NT (i.e. from the neural folds prior to NT closure and later from the roof plate). Among these are members of the TGFβ family, including activin, Bmp4 and Bmp7 (Connolly et al., 1995; Liem et al., 1997; Liem et al., 1995; Tam, 2001). The expression of these TGFβ family proteins in the neural plate at early developmental stages is in close proximity to migrating kidney IM precursor cells (James and Schultheiss, 2003) (Fig. 2A). Moreover, previous studies suggested a role for activin and BMPs in early kidney induction (see Introduction). Therefore, we sought to investigate the role of activin and the activation patterns of both activin and BMP signaling in relation to the KMF anterior border.
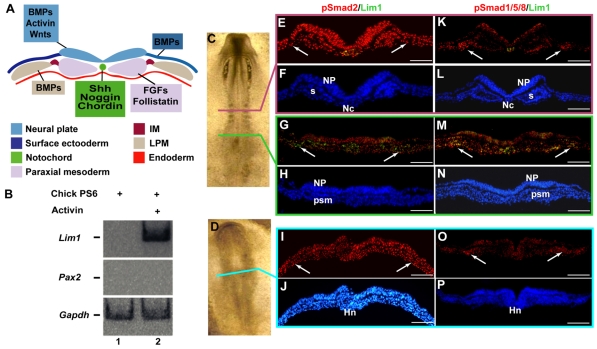
Fig. 2.
TGFβ signaling in intermediate mesoderm specification. (A) Scheme summarizing the architecture of tissues and their morphogens at the time and location the kidney morphogenetic field is specified. (B) Two parallel PS6 regions from chick embryos were isolated and cultured for 24 hours with or without 10 ng/ml activin. Gapdh, Lim1 and Pax2 expression was analyzed by RT-PCR. (C-P) Profile of Smad2 and Smad1/5/8 activation during intermediate mesoderm (IM) specification. Stage 8 (C) and stage 6 (D) chick embryos were fixed and sectioned, followed by double immunostaining using anti-phospho-Smad2 (E,G,I) or anti-phospho-Smad1/5/8 (K,M,O) together with anti-Lim1/2 antibodies. DAPI staining is shown in F,H,J,L,N,P. Analysis was performed at the second somite level of stage 8 chick embryos (magenta line in C), and at the level of Hensen's node of stage 8 (green line in C) and stage 6 (turquoise line in D) chick embryos. IM is indicated by arrows. Hn, Hensen's node; Nc, notochord; NP, neural plate; psm, pre-segmented mesoderm; s, somite. Scale bars: 100 μm.
In order to examine the role of activin in inducing early kidney markers, we first cultured PS6 with or without activin. Lim1 expression was upregulated in PS6 cultured in the presence of activin (Fig. 2B, lane 2, n=12; 7/12). By contrast, Pax2 expression was not induced in grafted tissues in the presence of activin (Fig. 2B, lane 2, n=12; 0/12).
In order to analyze the endogenous activation pattern of activin signaling during early kidney formation, we utilized antibodies that specifically recognize the phosphorylated form of Smad2 (phospho-Smad2), the intracellular mediator of activin-like molecules (including activin and nodal). Double immunostaining with anti-phospho-Smad2 and anti-Lim1 revealed ubiquitous activation of activin-like signaling in the three germ layers (Fig. 2C-J). The observed ubiquitous activation of phospho-Smad2 at early developmental stages would seem to contradict an expected morphogen gradient. However, as our detection procedure was not performed quantitatively we cannot rule out the existence of a mediolateral phospho-Smad2 gradient. Cross-sections through anterior and posterior regions of stage 8 embryos (Fig. 2C,E-H) showed Smad2 phosphorylation along the entire IM axis, regardless of the KMF anterior border, as marked by anti-Lim1 staining. Cross-sections of stage 6 embryos at the level of Hensen's node revealed activation of Smad2 in prospective anterior non-kidney IM (IM6) (Fig. 2D,I-J, arrows). Control cross-sections of stage 10 embryos showed restricted activation patterns of activin (data not shown), consistent with results obtained by Faure et al. (Faure et al., 2002) and confirming the specificity of the antibody.
BMPs have been shown to play a role in pronephros induction and pronephric duct maintenance (James and Schultheiss, 2005; Obara-Ishihara et al., 1999). Faure et al. used anti-phospho-Smad1/5/8 antibodies to examine the endogenous patterns of BMP signaling during early chick development (Faure et al., 2002). Although their study was detailed, these authors did not present data concerning BMP signaling activation during IM specification. As shown in Fig. 2K-N, BMP signaling is activated specifically in IM cells along the entire axis of stage 8 embryos, regardless of the anterior border of early kidney markers. Double immunostaining with anti-phospho-Smad1/5/8 and anti-Lim1 clearly showed that BMP signaling is activated in both anterior and posterior IM regions where Lim1 is expressed differentially (Fig. 2K,M, arrows). Cross-sections through the Hensen's node region of stage 6 embryos revealed specific activation of BMP signaling in lateral mesoderm, including the prospective anterior IM regions (Fig. 2O,P).
These results support previous studies that suggested a role for activin and BMPs in kidney induction. However, the activation patterns of activin-like and BMP signaling cannot explain the formation of the KMF anterior border; thus, the involvement of downstream or other factors might be required for this process.
Hoxb4 expression pattern during IM specification
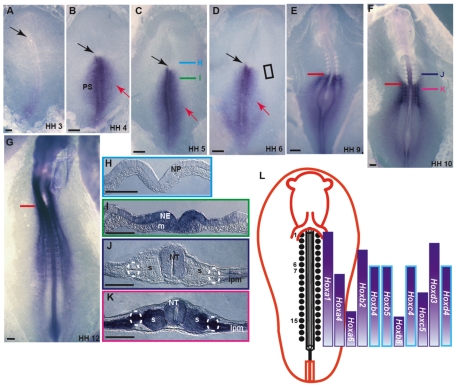
The Hox gene family is a well-known candidate for implementing border formation along the anterior-posterior (A-P) axis (Pearson et al., 2005). The Hox code with respect to the IM A-P axis is unknown. In order to gain a better understanding of the potential role of Hox genes in patterning the IM, we studied in detail the expression pattern of several Hox genes at the level of the sixth somite by whole-mount in situ hybridization (ISH) (Fig. 3; data not shown). Here we focus on the Hoxb4 expression pattern, as the anterior expression boundary of this gene in the paraxial mesoderm was shown to be situated at the sixth somite level (Bel-Vialar et al., 2002). Hoxb4 expression was first detected at stage 3, with weak expression at the PS posterior edge (Fig. 3A). At stages 4-6, Hoxb4 PS expression was intensified and could be detected along the entire length of the PS, excluding Hensen's node. Expression of this gene was also detected in mesodermal cells migrating from the PS (Fig. 3B-D, arrows). Cross-sections through anterior regions of stage 5 embryos showed no Hoxb4 expression (Fig. 3H). However, sections through the prospective IM region in the PS showed expression of the gene in the PS groove, prospective neuroepithelium and in migrating mesodermal cells, but not in the ectoderm (Fig. 3I). At stage 9, the anterior border of Hoxb4 expression in both the NT and all mesodermal tissues (including IM) was at the sixth somite level (Fig. 3E). By stage 10, expression in the NT and the lateral plate mesoderm (LPM) shifted anteriorly and could be detected anterior to the sixth somite level (Fig. 3F), a phenomenon that was preserved at later developmental stages (Fig. 3G). Cross-sections through anterior regions of stage 10 embryos showed Hoxb4 expression in the NT and the LPM, whereas in posterior regions it was detected in the NT, in all mesodermal tissues excluding the notochord, and in ectoderm and endoderm (Fig. 3J,K). The anterior IM border of Hoxb4 expression was associated with the sharply defined anterior border of kidney gene expression. As summarized in Fig. 3L, Hoxb4, Hoxb5, Hoxc4 and Hoxd4 transcripts were expressed in the IM from the sixth somite level posteriorly and thus potentially constitute the Hox code involved in kidney gene regulation.
Fig. 3.
Hoxb4 expression patterns during early chick development. (A-G) Whole-mount RNA in situ hybridization (ISH) using a Hoxb4 probe. Hamburger and Hamilton (HH) stages are indicated. Black arrows mark Hensen's node. Red arrows in B-D point to gastrulating cells expressing Hoxb4. Red lines mark the sixth somite axial level. (A-F) Ventral view; (G) dorsal view. (H-K) Cross-sections at the level of H-K in C,F. Dashed circles mark the IM (J,K). (L) Scheme of the expression patterns of several Hox genes in the IM of a chick embryo. Bars with a blue margin represent Hox genes that have an anterior boundary at the sixth somite level. NE, neuroepithelium; m, mesoderm; NT, neural tube; s, somite; LPM, lateral plate mesoderm. Scale bars: 100 μm.
RA induces anterior expansion of Lim1 and Hoxb4 expression exclusively in the IM
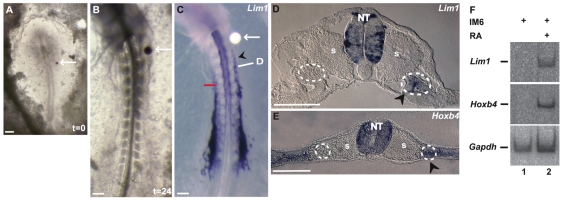
RA is a known regulator of Hox gene expression and its role in A-P patterning has been investigated extensively (reviewed by Duester, 2007; Maden, 2006). In addition, RA has been shown to be involved in the induction of pronephric cell fate (Kim and Dressler, 2005; Moriya et al., 1993; Osafune et al., 2002). These observations, together with the above results that showed the IM anterior border of several Hox genes, prompted us to hypothesize a role for RA in controlling the expression of early kidney genes and determining the KMF anterior border. In order to investigate this hypothesis, RA-soaked beads were implanted into the migratory pathway of prospective anterior IM cells of stage 4 embryos (Fig. 4A, arrow). Following 24 hours of incubation, the beads reached a position in or next to the anterior IM (Fig. 4B, arrow). The embryos were fixed and analyzed for Lim1 and Hoxb4 expression. As shown in Fig. 4C, ectopic expression of Lim1 was observed in the anterior IM as compared with the control contralateral side (arrowhead, n=18; 16/18). Moreover, the anterior expansion of Lim1 expression was restricted to IM cells, as confirmed in cross-sections (Fig. 4D, arrowhead), despite non-specific diffusion of RA from the bead. Analyzing Hoxb4 expression in RA-treated embryos revealed, as predicted, anterior expansion of this gene in the IM on the experimental side (Fig. 4E, arrowhead, n=12; 10/12). In several cases, the anterior expansion of Hoxb4 was also observed in the lateral somite region (data not shown). In addition, RA induced the expression of Lim1 and Hoxb4 in grafted IM6 (Fig. 4F), a tissue previously shown not to be competent to respond to kidney-inductive signals (Barak et al., 2005).
Fig. 4.
Retinoic acid induces anterior Lim1 and Hoxb4 expansion in the IM. (A) A retinoic acid (RA)-soaked bead (arrow) was implanted in the migratory pathway of IM cells of a stage 4 chick embryo. (B) Twenty-four hours after implantation, the bead reached a location in the anterior IM (arrow). (C) Whole-mount ISH for Lim1 reveals specific IM anterior expansion of expression (arrowhead) as compared with the contralateral side. The red line marks the sixth somite border. The arrow marks the RA-soaked bead. (D) Cross-section through the region indicated by the bar in C clearly shows that Lim1 expansion is specific to the IM of the experimental side (arrowhead). (E) Cross-section of another embryo through the anterior IM close to the RA bead shows upregulation of Hoxb4 expression in IM tissues (arrowhead) as compared with the contralateral control side. IM is encircled by dashed lines. (F) Two parallel IM6 regions were isolated and cultured for 24 hours with or without 10 μM RA. Lim1, Hoxb4 and Gapdh expression was analyzed by RT-PCR. NT, neural tube; s, somite. Scale bars: 100 μm.
The specific expression of Lim1 in anterior IM cells suggests the requirement of an additional endogenous factor(s) that, in combination with RA, creates the precise conditions for kidney cell fate.
Overexpression of Hoxb4 expands Lim1 and Pax2 expression in anterior IM
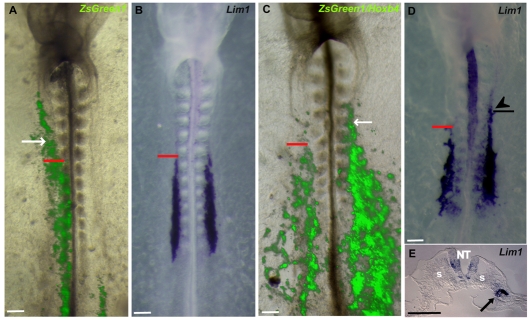
The ability of RA to ectopically induce Lim1 and Hoxb4 expression in anterior non-kidney IM raised two possibilities: (1) that both genes are regulated independently by RA; and (2) that Hoxb4 mediates RA control of Lim1. In order to investigate the second possibility, we overexpressed Hoxb4 in anterior IM regions. The pCIZ-Hoxb4 construct was electroporated into the prospective IM region of stage 3 embryo PS (PS3). Control embryos were electroporated with an empty vector. Following 24 hours of incubation, expression of the ZsGreen1 fluorescent reporter gene was observed in control embryos in IM anterior to the sixth somite level (Fig. 5A, arrow). No alteration in normal Lim1 expression was observed in these embryos (Fig. 5B; Table 1). By contrast, overexpression of the vector containing Hoxb4 resulted in anterior ectopic expression of Lim1 in correlation with the presence of the plasmid in anterior IM cells (Fig. 5C,D, arrow and arrowhead, respectively; Table 1). Cross-sections through anterior regions confirmed Lim1 expression in IM cells on the experimental side (Fig. 5E, arrow). These results suggest a role for Hoxb4 in Lim1 regulation in IM cells and raise the possibility that Hoxb4 is involved in the specification of the anterior border of the KMF. In agreement with this, Pax2 expression was also expanded anteriorly in IM cells of embryos overexpressing Hoxb4 (Table 1; data not shown).
Fig. 5.
Overexpression of Hoxb4 results in anterior Lim1 expansion. (A-D) pCIZ control (A,B) or Hoxb4 expression (C,D) vector driving internal ribosome entry site 2 (IRES2)-ZsGreen1 expression was electroporated into the prospective IM region of PS3. Eighteen hours after electroporation, ZsGreen1 expression marking the location of the control (A) or Hoxb4 expression (C) vector could be visualized in the anterior IM (arrow; red line marks the sixth somite border). (B,D) ISH reveals specific IM anterior expansion of Lim1 expression only in embryos electroporated with the Hoxb4 expression vector (arrowhead in D), and not in embryos expressing the control vector (B). (E) Cross-section through the region indicated by the arrowhead in D showing that Lim1 expansion is specific to the IM of the experimental side (arrow). NT, neural tube; s, somite. Scale bars: 100 μm.
Table 1.
Summary of electroporation experiments
|
Expression in anterior IM
|
|||
|---|---|---|---|
| Expression plasmid | Lim1 | Pax2 | Reduction in endogenous Lim1 expression |
| pCIZ | 0/3 | na | - |
| pCIZ-Hoxb4 | 14/17 | 1/2 | - |
| pCIRX | 0/2 | 0/3 | - |
| pCIRX-Hoxb4 | 3/5 | 5/5 | - |
| pCIG + pCIRX-Hoxb4 | 3/3 | na | - |
| pCIG-dnXAR1 + pCIRX-Hoxb4 | 0/4 | na | 4/4 |
| pCIG-dnXAR1 | - | na | 4/4 |
Data show the number of embryos that exhibit anterior expansion of Lim1 or Pax2 expression/total number of cases. Reduction in endogenous Lim1 expression was detected only in the experiment in which dnXAR1 was electroporated. na, not analyzed.
Activin-like signaling is necessary but insufficient for Lim1 induction in IM cells
The above results, obtained from two different experimental setups, revealed that both activin and Hoxb4 are able to induce Lim1 expression. Furthermore, we showed that RA upregulates both Lim1 and Hoxb4 in anterior non-kidney mesoderm. Thus, both activin and RA, as signaling molecules, can regulate Lim1 expression. Moreover, because the activation pattern of activin-like signaling is along the entire axis and the expression pattern of Hoxb4 is restricted to the sixth somite level in the paraxial mesoderm and IM, it is unlikely that activin is upstream of Hoxb4. Therefore, we hypothesized that activin or activin-like molecules and Hoxb4 work in coordination to promote kidney field formation. In order to assess this hypothesis, we co-expressed Hoxb4 with a dominant-negative form of Xenopus activin receptor 1 (dnXAR1) in the IM. Control experiments in which the Hoxb4 expression vector (pCIRX-Hoxb4) was co-electroporated with pCIG (an empty vector) into the prospective IM region of PS3 resulted in extended Lim1 expression in anterior non-kidney IM (Fig. 6A-D; Table 1), as expected. By contrast, no ectopic expression of Lim1 was observed anterior to the sixth somite level in embryos co-electroporated with both Hoxb4 and pCIG-dnXAR1 vectors. The presence of Hoxb4 in anterior IM was confirmed by detection of the dsRed reporter gene (Fig. 6E-H, left side; Table 1). Moreover, endogenous Lim1 expression was abolished in the posterior IM on the contralateral side, where both plasmids were expressed extensively (Fig. 6E-H, right-hand side). Cross-sections through anterior (Fig. 6I) and posterior (Fig. 6J) regions confirmed the absence of Lim1 expression in IM cells expressing the dnXAR1 plasmid, as revealed by anti-GFP staining (Fig. 6I,J, arrowheads). Similar results were obtained from experiments in which dnXAR1 was expressed in posterior IM alone (Table 1; data not shown).
Fig. 6.
Activin or activin-like signaling is necessary but insufficient for Lim1 induction in IM cells. (A-H) Hoxb4 expression vector driving IRES2-dsRed expression was co-electroporated with pCIG control vector (A-D) or with dnXAR1 expression vector driving IRES2-GFP expression (E-H) into the prospective IM region of PS3. The red line marks the sixth somite border. (A,E) Eighteen hours after electroporation, expression of dsRed could be visualized in both anterior (arrows) and posterior IM. GFP expression marks the location of the control vector (B) or of dnXAR1 expression (F). (C,G) Merged images of Hoxb4 expression together with the control (C) or dnXAR1 (G) vector. (D) ISH for Lim1 reveals specific IM anterior expansion of expression (arrowhead) in cells that express Hoxb4 together with the control vector. (H) No anterior expansion of Lim1 was detected in the IM of embryos co-expressing Hoxb4 and dnXAR1 (open arrowhead), and the endogenous expression of Lim1 in the posterior IM was diminished (white arrowhead). (I,J) The embryo in H was cross-sectioned at the indicated levels and immunostained using anti-GFP antibodies. Merged fields are shown. (I) An anterior cross-section shows that IM cells expressing dnXAR1 (represented by GFP staining) do not express Lim1 (open arrowhead). (J) A posterior cross-section shows that IM cells expressing dnXAR1 do not express endogenous Lim1 (white arrowhead) as compared with IM cells on the contralateral side. (K) Two parallel IM6 regions were isolated and cultured for 24 hours with or without 10 ng/ml activin. Expression of Lim1, Hoxb4 and Gapdh was analyzed by RT-PCR. NT, neural tube; s, somite. Scale bars: 100 μm.
In order to verify whether activin is also sufficient for Lim1 induction, activin was added to IM6 cultures in which Hoxb4 is normally not expressed (Fig. 6K, lane 1; Fig. 3D, boxed). Neither Lim1 nor Hoxb4 expression was detected following incubation, in contrast to the experiment described in Fig. 2B, in which activin was added to cultures of PS6 tissue in which Hoxb4 is normally expressed (Fig. 3D). Taken together, our results suggest that activin or activin-like molecules are necessary but insufficient as signaling molecules for Lim1 induction and the establishment of the KMF.
DISCUSSION
One of the major goals of the interdisciplinary field of evolutionary development is to discover the developmental mechanisms involved in changes leading to innovations in the body plan. In a previous study (Barak et al., 2005), we proposed the term `posteriorization shift' of the KMF to illustrate changes in the field position along the A-P axis observed in various chordates. For example, gene expression pattern analysis in amphioxus reveals AmphiPax2/5/8 and AmphiLim1/5 in Hatschek's nephridium at the anterior region of the pharynx (Czerny et al., 1997; Langeland et al., 2006). In the study by Barak et al. (Barak et al., 2005), we pointed to a major cellular mechanism that provides an explanation for the posterior shift of the KMF. According to this mechanism, kidney-inductive signals are secreted from midline tissues along the entire axis, including anterior non-kidney-generating IM, supporting the idea that in the past the kidney field was positioned in the most anterior segments of the body. However, no expression of kidney genes is observed in anterior IM owing to the loss of competence of gastrulating cells to respond to kidney-inductive signals. The current study further advances our understanding of the molecular mechanism underlying the loss of competence of anterior IM cells.
The dorsal NT retains kidney-inductive properties
Our results reveal that the NT roof plate can induce both Lim1 and Pax2 expression in PS6. Consistent with our results, Taira et al. (Taira et al., 1994) showed that Xlim-1 induction in pronephros precursor cells in the lateral mesoderm of Xenopus exogastrulae requires interaction with the ectoderm. Although these authors discussed the resemblance of this phenomenon to the requirement for a NT-derived signal for muscle cell differentiation in the somite, they dismissed the possibility that the ectodermal signal for pronephric Xlim-1 expression arises from the NT, as the pronephros is not in direct contact with this tissue in Xenopus embryos. By contrast, our findings are in agreement with the fact that during cell migration, the prospective kidney-generating IM is in close proximity to the neural plate (James and Schultheiss, 2003) (Fig. 2A).
Previous studies have demonstrated a role for medial tissues in kidney induction and suggested a pivotal role for the adjacent paraxial mesoderm in this process (Mauch et al., 2000; Seufert et al., 1999). However, none of these studies excluded experimentally the possibility of dorsal NT induction of the pronephros. For example, Seufert and colleagues (Seufert et al., 1999) did not address the possibility that nervous system defects resulting from UV-induced ventralization might account for both somite and pronephros loss, and based their conclusion that the neural tissue is unlikely to be the source of kidney-inductive signals on `preliminary data' (Seufert et al., 1999). Similarly, separation between the somites and IM resulted in separation of the IM from the NT as well, and dissection between the notochord and the somites left the NT roof plate on the somite side, resulting in pronephros induction (Mauch et al., 2000). Taken together, the experimental evidence obtained in these studies does not contradict our conclusion regarding the kidney-inductive properties retained by the dorsal NT.
Activin-like signaling is required but insufficient for kidney induction
One of the factors secreted from the NT roof plate (Liem et al., 1997) that is a good candidate for a pronephros induction factor is activin. Activin is also expressed in the neural plate of stage 5-8 embryos (Connolly et al., 1995), which, at these stages, is in immediate contact with migrating kidney precursor cells. Limited evidence exists for the involvement of activin in early kidney induction. In the current study, we provided additional support for the activin-like signaling requirement in this process, suggesting that it is required for Lim1 expression in IM cells and functions in a cell-autonomous manner. These results are in agreement with the observation that both zebrafish and Xenopus Lim1 genes contain an ARE in their first intron, which can direct the expression of this gene (Rebbert and Dawid, 1997; Watanabe et al., 2002).
Our results also show that activin-like signaling is insufficient for kidney induction. This assertion is based on two complementary observations: the activation pattern of phospho-Smad2 along the entire IM axis (Fig. 2) and the lack of Lim1 induction in isolated IM6 cultured in the presence of activin. The latter is consistent with the observation that IM6 is not a competent tissue and cannot respond to kidney-inductive signals in vivo (Barak et al., 2005). Thus, although cells in the anterior IM are able to initiate the activin-like intracellular signaling cascade, they fail to convert this signal into effective Lim1 induction. These findings suggest that Lim1 induction in IM cells in response to activin or activin-like molecules requires the involvement of other factors operating in coordination with activin-like signaling. A good candidate would be RA, which was previously shown to cooperate with activin in forming pronephric tubules and inducing Xlim-1 expression in the Xenopus animal cap assay (Moriya et al., 1993; Osafune et al., 2002; Taira et al., 1992).
RA signaling in pronephros development: direct or indirect induction?
Several lines of evidence suggest a role for RA signaling in early kidney development (Cartry et al., 2006; Moriya et al., 1993; Osafune et al., 2002; Taira et al., 1992; Taira et al., 1994; Wingert et al., 2007). In agreement with these studies, we demonstrate here the involvement of RA signaling in determining the KMF anterior border. There are several possible mechanisms by which RA might regulate Lim1 expression: the first suggests that RA regulates Lim1 expression directly. This mechanism is supported by the study of Cartry et al. (Cartry et al., 2006), who showed that RA induces Xlim-1 expression in the presence of a protein synthesis inhibitor. The second mechanism suggests that RA regulates Lim1 indirectly via Hoxb4 induction in IM cells. Our observation that ectopic expression of Hoxb4 in anterior IM cells results in anterior expansion of Lim1 expression, similar to the effect obtained by RA, strongly supports this mechanism. Several studies in Xenopus embryos further corroborate this second possibility. It has been shown that activin-induced Xlim-1 expression in RA-treated animal caps is eliminated by administering a protein synthesis inhibitor, suggesting an indirect mode of action (Tadano et al., 1993). Furthermore, Taira et al. (Taira et al., 1994) compared the expression of Hox genes and Xlim-1 in control and RA-treated embryos and noted that: (1) RA expands the normal expression domains that are common to both the Hox and Xlim-1 genes; (2) RA induction of Hox genes, including Hoxb4 and Hoxb5, appears to precede the appearance of RA-induced Xlim-1; and (3) the endogenous expression domains of Hoxb4 and Hoxb5 overlap with those of Xlim-1 in the pronephros. The indirect mechanism further predicts that RA may confer IM cells with the competence to respond to activin-like signals by inducing Hoxb4 expression in these cells.
Potential role of Hox genes in determining the KMF anterior border
It is well known that Hox genes regulate the specification of regional identity along the embryonic A-P axis and therefore also the positioning of structures along this axis. Although embryonic kidneys are highly patterned along the A-P axis, only a few studies have investigated the role that Hox genes play during kidney development and all in relation to metanephros development (Mugford et al., 2008; Patterson and Potter, 2003; Wellik et al., 2002). In the current study, we have shown that Hox genes belonging to paralogous group four, including Hoxb4, Hoxc4 and Hoxd4, as well as Hoxb5, share the same anterior border at the sixth somite level with early kidney genes, suggesting a role in kidney gene regulation. Indeed, ectopic expression of Hoxb4 in anterior IM domains results in anterior expansion of the kidney field, similar to results obtained using Hoxd11, which exerted metanephric fate in the mesonephros (Mugford et al., 2008). Our results raise an obvious question: is Hoxb4 required for kidney gene expression? The answer would probably be no, as no kidney phenotypes were reported in Hoxb4 mutant mice (Ramirez-Solis et al., 1993), nor in compound mutants for three members of paralogous group four (Horan et al., 1995b). These results are somewhat expected as considerable redundancy exists among Hox genes in general and paralogous group four in particular (Horan et al., 1995a; Horan et al., 1995b). It is plausible that in Hoxb4 mutants, subtle changes in the pronephros position would be evident and that the anterior border of the kidney gene expression would be shifted moderately posteriorly. Analysis of early kidney gene expression in Hoxb4 mutants or in compound mutants is required in order to verify this hypothesis.
Modes of gene regulation mediated by Hoxb4
The many roles of Hox genes during embryonic development and aspects of their structure and molecular function have been studied extensively. However, our knowledge of the downstream genes directly regulated by Hox transcription factors is largely incomplete (Pearson et al., 2005; Svingen and Tonissen, 2006). A major difficulty in predicting Hox target genes is that the Hox-response elements are rather elusive. Several direct target genes of Hoxb4 have been identified in Xenopus and mouse embryos, including Hoxb4 itself, Hoxb3, Hoxb5, RAR-β, RAS-related protein-1, FLASH and Iroquois 5 (Gould et al., 1997; Morgan et al., 2004; Morsi El-Kadi et al., 2002; Serpente et al., 2005; Theokli et al., 2003). In the current study, we have shown that Hoxb4 upregulates the expression of both Lim1 and Pax2, although evidence for direct regulation was not provided.
Another perception emerging from our study is that Hoxb4 is insufficient for the induction of Lim1 expression. This idea is based on the result that the upregulation of Lim1 mediated by Hoxb4 is inhibited in IM cells that also express truncated activin receptor. These results might suggest cooperation between activin-like signaling and Hoxb4 in Lim1 activation, which is in agreement with accumulating evidence for cooperation between Smads and Hox proteins in target gene activation (Grieder et al., 1997; Grienenberger et al., 2003; Marty et al., 2001) and repression (Shi et al., 1999; Walsh and Carroll, 2007; Yang et al., 2000). Most of this evidence has come from studies in D. melanogaster that demonstrated that Hox binding sites are located in the vicinity of, or within, areas known as Dpp/TGFβ-response elements (Grieder et al., 1997; Marty et al., 2001; Walsh and Carroll, 2007). Interestingly, Grieder et al. (Grieder et al., 1997) have shown that the activity of a Dpp/TGFβ-responsive enhancer is stimulated by Dpp signaling only upon binding of the Hox protein Labial together with its co-factor Extradenticle. These authors suggested that a tissue-specific response to Dpp could be generated through synergistic effects on an enhancer carrying both Dpp- and Hox-responsive sequences (Grieder et al., 1997). This statement is in complete agreement with our hypothesis that the induction of kidney genes within the IM in response to TGFβ signaling requires the participation of downstream factors such as Hoxb4.
A molecular mechanism to explain the formation of the kidney morphogenetic field anterior border
In the current study, we have provided several lines of evidence that suggest a molecular mechanism to explain the cellular model of kidney field anterior border formation proposed by Barak et al. (Barak et al., 2005). We suggest that the kidney-inductive signals are members of the TGFβ superfamily and that competence to respond to these signals is driven by RA and is mediated by Hoxb4 (Fig. 7). The model proposes that at least three different morphogen gradients participate in the establishment of the KMF. Along the mediolateral axis, a gradient of active BMP signaling is established from lateral to medial. Activin-like signals are secreted from the dorsal NT and establish a gradient in the opposite direction. These two mediolateral gradients are established along the entire A-P axis. The precise levels of BMP and activin-like signaling appropriate for kidney gene induction are obtained only in the IM domain. Along the A-P axis, a gradient of RA signaling is established from posterior to anterior, leading to the assembly of Hox gene expression domains. Hoxb4 is expressed in both the paraxial mesoderm and the IM from the sixth somite level posteriorly. Only IM cells that express Hoxb4 and that meet the appropriate levels of BMP and activin-like signaling can execute the kidney program.
Fig. 7.
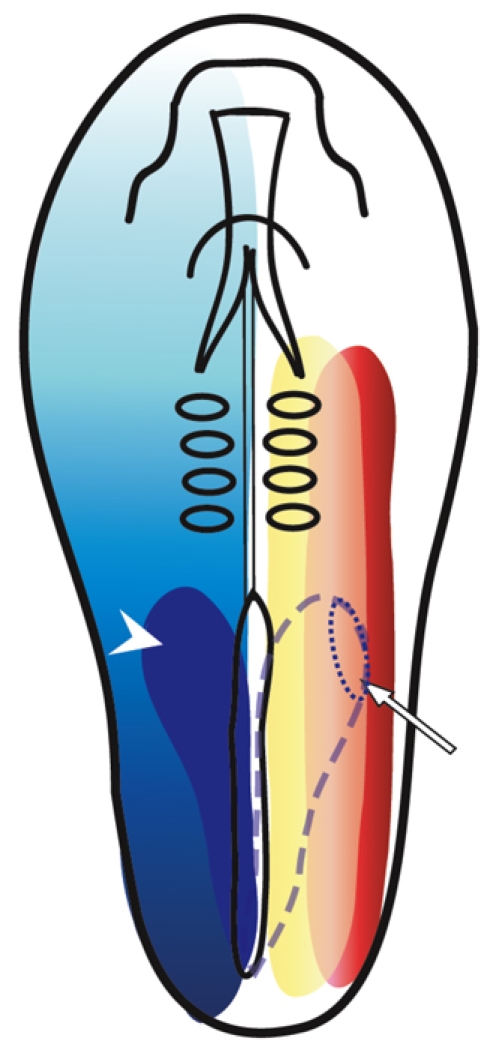
Scheme illustrating the molecules that constitute the kidney morphogenetic field in a stage 8 chick embryo. The overlap between three different morphogen gradients determines the domain of kidney gene expression. Red illustrates the lateral-medial gradient of BMP signaling, yellow the mediolateral gradient of activin-like signaling, and blue the posterior-anterior gradient of RA that generates the expression domain of Hoxb4 (dark-blue domain on the left side, arrowhead). The kidney morphogenetic field (dotted line, arrow) is established in the overlapping domain of BMP and activin-like signaling and Hoxb4 expression (dashed line on the right).
The expression patterns of Hoxb4 during early gastrulation stages support the prediction that Hoxb4 confers IM cells with the competence to respond to kidney-inductive signals. In agreement with the ability of prospective IM cells of either PS4 or PS6 to respond to kidney-inductive signals, Hoxb4 is expressed along the entire length of the PS at these developmental stages. However, during prospective IM cell migration from PS4 to their final position in anterior IM6, they move from the Hoxb4 expression domain to a Hoxb4-deprived domain, correlating with the loss of competence. Prospective IM cells of PS6 retain the expression of this gene during their migration to their final position in posterior IM. Therefore, the Hoxb4 spatiotemporal expression profile is a key regulator of cell competence and hence of the formation of the distinct anterior border of the KMF. The molecular mechanisms regulating the loss of Hoxb4 expression during early gastrulation remain to be determined.
We thank Thomas Schultheiss, Dale Frank, Dalit Sela-Donenfeld, Adi Salzberg and members of the Reshef laboratory for discussion of various aspects of this study. We also thank Olivier Pourquié, Abraham Fainsod and Thomas Schultheiss for expression plasmids and probes. This study was supported by the Fogarty International Research Collaboration Award (NIH 1RO3 TW006864-01) to Thomas Schultheiss and R.R. and grants to R.R. from the Israel Cancer Research Fund and Israel Science Foundation (461/03-17.2). Deposited in PMC for release after 12 months.
References
- Barak, H., Rosenfelder, L., Schultheiss, T. M. and Reshef, R. (2005). Cell fate specification along the anterior-posterior axis of the intermediate mesoderm. Dev. Dyn. 232, 901-914. [DOI] [PubMed] [Google Scholar]
- Bel-Vialar, S., Itasaki, N. and Krumlauf, R. (2002). Initiating Hox gene expression: in the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development 129, 5103-5115. [DOI] [PubMed] [Google Scholar]
- Cartry, J., Nichane, M., Ribes, V., Colas, A., Riou, J. F., Pieler, T., Dolle, P., Bellefroid, E. J. and Umbhauer, M. (2006). Retinoic acid signalling is required for specification of pronephric cell fate. Dev. Biol. 299, 35-51. [DOI] [PubMed] [Google Scholar]
- Connolly, D. J., Patel, K., Seleiro, E. A., Wilkinson, D. G. and Cooke, J. (1995). Cloning, sequencing, and expressional analysis of the chick homologue of follistatin. Dev. Genet. 17, 65-77. [DOI] [PubMed] [Google Scholar]
- Czerny, T., Bouchard, M., Kozmik, Z. and Busslinger, M. (1997). The characterization of novel Pax genes of the sea urchin and Drosophila reveal an ancient evolutionary origin of the Pax2/5/8 subfamily. Mech. Dev. 67, 179-192. [DOI] [PubMed] [Google Scholar]
- Dressler, G. R., Deutsch, U., Chowdhury, K., Nornes, H. O. and Gruss, P. (1990). Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 109, 787-795. [DOI] [PubMed] [Google Scholar]
- Duester, G. (2007). Retinoic acid regulation of the somitogenesis clock. Birth Defects Res. C Embryo Today 81, 84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure, S., de Santa Barbara, P., Roberts, D. J. and Whitman, M. (2002). Endogenous patterns of BMP signaling during early chick development. Dev. Biol. 244, 44-65. [DOI] [PubMed] [Google Scholar]
- Fujii, T., Pichel, J. G., Taira, M., Toyama, R., Dawid, I. B. and Westphal, H. (1994). Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev. Dyn. 199, 73-83. [DOI] [PubMed] [Google Scholar]
- Gould, A., Morrison, A., Sproat, G., White, R. A. and Krumlauf, R. (1997). Positive cross-regulation and enhancer sharing: two mechanisms for specifying overlapping Hox expression patterns. Genes Dev. 11, 900-913. [DOI] [PubMed] [Google Scholar]
- Grieder, N. C., Marty, T., Ryoo, H. D., Mann, R. S. and Affolter, M. (1997). Synergistic activation of a Drosophila enhancer by HOM/EXD and DPP signaling. EMBO J. 16, 7402-7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienenberger, A., Merabet, S., Manak, J., Iltis, I., Fabre, A., Berenger, H., Scott, M. P., Pradel, J. and Graba, Y. (2003). Tgfbeta signaling acts on a Hox response element to confer specificity and diversity to Hox protein function. Development 130, 5445-5455. [DOI] [PubMed] [Google Scholar]
- Haldin, C. E., Nijjar, S., Masse, K., Barnett, M. W. and Jones, E. A. (2003). Isolation and growth factor inducibility of the Xenopus laevis Lmx1b gene. Int. J. Dev. Biol. 47, 253-262. [PubMed] [Google Scholar]
- Hamburger, V. and Hamilton, H. L. (1992). A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 195, 231-272. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou, A. and Melton, D. A. (1992). A truncated activin receptor inhibits mesoderm induction and formation of axial structures in Xenopus embryos. Nature 359, 609-614. [DOI] [PubMed] [Google Scholar]
- Hiruma, T. and Nakamura, H. (2003). Origin and development of the pronephros in the chick embryo. J. Anat. 203, 539-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan, G. S., Kovacs, E. N., Behringer, R. R. and Featherstone, M. S. (1995a). Mutations in paralogous Hox genes result in overlapping homeotic transformations of the axial skeleton: evidence for unique and redundant function. Dev. Biol. 169, 359-372. [DOI] [PubMed] [Google Scholar]
- Horan, G. S., Ramirez-Solis, R., Featherstone, M. S., Wolgemuth, D. J., Bradley, A. and Behringer, R. R. (1995b). Compound mutants for the paralogous hoxa-4, hoxb-4, and hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes Dev. 9, 1667-1677. [DOI] [PubMed] [Google Scholar]
- Iimura, T. and Pourquie, O. (2006). Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature 442, 568-571. [DOI] [PubMed] [Google Scholar]
- James, R. G. and Schultheiss, T. M. (2003). Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev. Biol. 253, 109-124. [DOI] [PubMed] [Google Scholar]
- James, R. G. and Schultheiss, T. M. (2005). Bmp signaling promotes intermediate mesoderm gene expression in a dose-dependent, cell-autonomous and translation-dependent manner. Dev. Biol. 288, 113-125. [DOI] [PubMed] [Google Scholar]
- Kim, D. and Dressler, G. R. (2005). Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J. Am. Soc. Nephrol. 16, 3527-3534. [DOI] [PubMed] [Google Scholar]
- Langeland, J. A., Holland, L. Z., Chastain, R. A. and Holland, N. D. (2006). An amphioxus LIM-homeobox gene, AmphiLim1/5, expressed early in the invaginating organizer region and later in differentiating cells of the kidney and central nervous system. Int. J. Biol. Sci. 2, 110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem, K. F., Jr, Tremml, G., Roelink, H. and Jessell, T. M. (1995). Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell 82, 969-979. [DOI] [PubMed] [Google Scholar]
- Liem, K. F., Jr, Tremml, G. and Jessell, T. M. (1997). A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91, 127-138. [DOI] [PubMed] [Google Scholar]
- Maden, M. (2006). Retinoids and spinal cord development. J. Neurobiol. 66, 726-738. [DOI] [PubMed] [Google Scholar]
- Marty, T., Vigano, M. A., Ribeiro, C., Nussbaumer, U., Grieder, N. C. and Affolter, M. (2001). A HOX complex, a repressor element and a 50 bp sequence confer regional specificity to a DPP-responsive enhancer. Development 128, 2833-2845. [DOI] [PubMed] [Google Scholar]
- Mauch, T. J., Yang, G., Wright, M., Smith, D. and Schoenwolf, G. C. (2000). Signals from trunk paraxial mesoderm induce pronephros formation in chick intermediate mesoderm. Dev. Biol. 220, 62-75. [DOI] [PubMed] [Google Scholar]
- Megason, S. G. and McMahon, A. P. (2002). A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129, 2087-2098. [DOI] [PubMed] [Google Scholar]
- Morgan, R., Nalliah, A. and Morsi El-Kadi, A. S. (2004). FLASH, a component of the FAS-CAPSASE8 apoptotic pathway, is directly regulated by Hoxb4 in the notochord. Dev. Biol. 265, 105-112. [DOI] [PubMed] [Google Scholar]
- Moriya, N., Uchiyama, H. and Asashima, A. (1993). Induction of pronephric tubules by activin and retinoic acid in presumptive ectoderm of Xenopus laevis. Dev. Growth Differ. 35, 123-128. [DOI] [PubMed] [Google Scholar]
- Morrison, A., Chaudhuri, C., Ariza-McNaughton, L., Muchamore, I., Kuroiwa, A. and Krumlauf, R. (1995). Comparative analysis of chicken Hoxb-4 regulation in transgenic mice. Mech. Dev. 53, 47-59. [DOI] [PubMed] [Google Scholar]
- Morsi El-Kadi, A. S., in der Reiden, P., Durston, A. and Morgan, R. (2002). The small GTPase Rap1 is an immediate downstream target for Hoxb4 transcriptional regulation. Mech. Dev. 113, 131-139. [DOI] [PubMed] [Google Scholar]
- Mugford, J. W., Sipila, P., Kobayashi, A., Behringer, R. R. and McMahon, A. P. (2008). Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev. Biol. 319, 396-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münsterberg, A. E., Kitajewski, J., Bumcrot, D. A., McMahon, A. P. and Lassar, A. B. (1995). Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 9, 2911-2922. [DOI] [PubMed] [Google Scholar]
- Obara-Ishihara, T., Kuhlman, J., Niswander, L. and Herzlinger, D. (1999). The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 126, 1103-1108. [DOI] [PubMed] [Google Scholar]
- Osafune, K., Nishinakamura, R., Komazaki, S. and Asashima, M. (2002). In vitro induction of the pronephric duct in Xenopus explants. Dev. Growth Differ. 44, 161-167. [DOI] [PubMed] [Google Scholar]
- Patterson, L. T. and Potter, S. S. (2003). Hox genes and kidney patterning. Curr. Opin. Nephrol. Hypertens. 12, 19-23. [DOI] [PubMed] [Google Scholar]
- Pearson, J. C., Lemons, D. and McGinnis, W. (2005). Modulating Hox gene functions during animal body patterning. Nat. Rev. Genet. 6, 893-904. [DOI] [PubMed] [Google Scholar]
- Ramirez-Solis, R., Zheng, H., Whiting, J., Krumlauf, R. and Bradley, A. (1993). Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell 73, 279-294. [DOI] [PubMed] [Google Scholar]
- Rebbert, M. L. and Dawid, I. B. (1997). Transcriptional regulation of the Xlim-1 gene by activin is mediated by an element in intron I. Proc. Natl. Acad. Sci. USA 94, 9717-9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss, T. M., Xydas, S. and Lassar, A. B. (1995). Induction of avian cardiac myogenesis by anterior endoderm. Development 121, 4203-4214. [DOI] [PubMed] [Google Scholar]
- Serpente, P., Tumpel, S., Ghyselinck, N. B., Niederreither, K., Wiedemann, L. M., Dolle, P., Chambon, P., Krumlauf, R. and Gould, A. P. (2005). Direct crossregulation between retinoic acid receptor {beta} and Hox genes during hindbrain segmentation. Development 132, 503-513. [DOI] [PubMed] [Google Scholar]
- Seufert, D. W., Brennan, H. C., DeGuire, J., Jones, E. A. and Vize, P. D. (1999). Developmental basis of pronephric defects in Xenopus body plan phenotypes. Dev. Biol. 215, 233-242. [DOI] [PubMed] [Google Scholar]
- Shi, X., Yang, X., Chen, D., Chang, Z. and Cao, X. (1999). Smad1 interacts with homeobox DNA-binding proteins in bone morphogenetic protein signaling. J. Biol. Chem. 274, 13711-13717. [DOI] [PubMed] [Google Scholar]
- Svingen, T. and Tonissen, K. F. (2006). Hox transcription factors and their elusive mammalian gene targets. Heredity 97, 88-96. [DOI] [PubMed] [Google Scholar]
- Tadano, T., Otani, H., Taira, M. and Dawid, I. B. (1993). Differential induction of regulatory genes during mesoderm formation in Xenopus laevis embryos. Dev. Genet. 14, 204-211. [DOI] [PubMed] [Google Scholar]
- Taira, M., Jamrich, M., Good, P. J. and Dawid, I. B. (1992). The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 6, 356-366. [DOI] [PubMed] [Google Scholar]
- Taira, M., Otani, H., Jamrich, M. and Dawid, I. B. (1994). Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development 120, 1525-1536. [DOI] [PubMed] [Google Scholar]
- Tam, P. (2001). The early neural plate rules over the mesoderm. Dev. Cell 1, 3-4. [DOI] [PubMed] [Google Scholar]
- Theokli, C., Morsi El-Kadi, A. S. and Morgan, R. (2003). TALE class homeodomain gene Irx5 is an immediate downstream target for Hoxb4 transcriptional regulation. Dev. Dyn. 227, 48-55. [DOI] [PubMed] [Google Scholar]
- Tsuchida, T., Ensini, M., Morton, S. B., Baldassare, M., Edlund, T., Jessell, T. M. and Pfaff, S. L. (1994). Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 79, 957-970. [DOI] [PubMed] [Google Scholar]
- Walsh, C. M. and Carroll, S. B. (2007). Collaboration between Smads and a Hox protein in target gene repression. Development 134, 3585-3592. [DOI] [PubMed] [Google Scholar]
- Watanabe, M., Rebbert, M. L., Andreazzoli, M., Takahashi, N., Toyama, R., Zimmerman, S., Whitman, M. and Dawid, I. B. (2002). Regulation of the Lim-1 gene is mediated through conserved FAST-1/FoxH1 sites in the first intron. Dev. Dyn. 225, 448-456. [DOI] [PubMed] [Google Scholar]
- Wellik, D. M., Hawkes, P. J. and Capecchi, M. R. (2002). Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 16, 1423-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm, B., James, R. G., Schultheiss, T. M. and Hogan, B. L. (2004). The forkhead genes, Foxc1 and Foxc2, regulate paraxial versus intermediate mesoderm cell fate. Dev. Biol. 271, 176-189. [DOI] [PubMed] [Google Scholar]
- Wingert, R. A., Selleck, R., Yu, J., Song, H. D., Chen, Z., Song, A., Zhou, Y., Thisse, B., Thisse, C., McMahon, A. P. et al. (2007). The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 3, 1922-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Ji, X., Shi, X. and Cao, X. (2000). Smad1 domains interacting with Hoxc-8 induce osteoblast differentiation. J. Biol. Chem. 275, 1065-1072. [DOI] [PubMed] [Google Scholar]