Abstract
Recent phylogenetic studies have revealed the major role played by the uplift of the Andes in the extraordinary diversification of the Neotropical flora. These studies, however, have typically considered the Andean uplift as a single, time-limited event fostering the evolution of highland elements. This contrasts with geological reconstructions indicating that the uplift occurred in discrete periods from west to east and that it affected different regions at different times. We introduce an approach for integrating Andean tectonics with biogeographic reconstructions of Neotropical plants, using the coffee family (Rubiaceae) as a model group. The distribution of this family spans highland and montane habitats as well as tropical lowlands of Central and South America, thus offering a unique opportunity to study the influence of the Andean uplift on the entire Neotropical flora. Our results suggest that the Rubiaceae originated in the Paleotropics and used the boreotropical connection to reach South America. The biogeographic patterns found corroborate the existence of a long-lasting dispersal barrier between the Northern and Central Andes, the “Western Andean Portal.” The uplift of the Eastern Cordillera ended this barrier, allowing dispersal of boreotropical lineages to the South, but gave rise to a huge wetland system (“Lake Pebas”) in western Amazonia that prevented in situ speciation and floristic dispersal between the Andes and Amazonia for at least 6 million years. Here, we provide evidence of these events in plants.
Keywords: biogeography, Neotropical biodiversity, Rubiaceae
The uplift of the tropical Andes in the Neogene had a profound impact on the history of the South American continent. It changed the course of the Amazon system from flowing northwestwards to the modern system that flows to the Atlantic side (1, 2) and affected the climate of the region by forming the only barrier to atmospheric circulation in the Southern Hemisphere (3). Recent phylogenetic studies have shown that the Andean orogeny had also a major role in the evolution of the Neotropical flora. The Neotropics hold the highest plant species diversity in the world (4). This richness has traditionally been explained in terms of environmental factors (5), but lately, more integrative explanations have been advanced that emphasize the role of historical and evolutionary factors in the shaping of Neotropical diversity (6, 7). The “tropical conservatism hypothesis,” for example, argues that there are more plant species in the Neotropics simply because more lineages originated and diversified there, owing to the long-term climatic stability of the region and the tendency of species to retain their climatic niches over evolutionary time (7, 8). It is now also clear that part of this richness has been gained by the migration of lineages from other biogeographic regions (6). For instance, pantropically distributed plant families such as Malpighiaceae, Fabaceae, and Annonaceae (6, 9, 10, 11) originated at temperate latitudes as part of the former “boreotropical flora” (12–14) and subsequently entered the Neotropics via the mountain ranges of Central America and the newly formed Northern Andes. One point in common to these hypotheses is the key role that the formation of the tropical Andes would have played in the historical diversification of the Neotropical flora (15). Recent phylogenetic studies have shown that the Andean uplift acted both as a dispersal route for boreotropical lineages (16, 17) and as a driver in promoting rapid diversification, via allopatric speciation and ecological displacement, in highland (16–19) and montane (11) habitats.
Fewer studies, however, have documented the impact of the Andean uplift on the lowland Amazonian flora. Clearly, the uplift must have affected these taxa by forming a new biotic barrier and profoundly changing the hydrology and climate of the region (20). Furthermore, previous biogeographic studies on Andean radiations have typically considered the Andean orogeny as a single, time-limited event, usually in connection with the final (Miocene to Pleistocene) uplift of the Andes (11, 19). This contrasts with geological reconstructions indicating that the uplift took place in discrete periods, progressing from south to north and from west to east and affecting different regions at different times (2, 3, 21, 22). Episodic marine incursions, related to global sea level rises during the extensional tectonic phases that followed periods of major uplift, had a dramatic impact in the drainage patterns of the region, as evidenced by paleogeographic and paleontological evidence (1, 2, 23–28). These marine incursions have been discussed in relation to their role as a pathway in the evolutionary transition from marine to freshwater habitats of Neotropical fishes (24, 29), but they could also have acted as barriers to dispersal or as vicariance events fragmenting the ranges of terrestrial animals and plants. It seems surprising that, despite increasingly detailed geological reconstructions (2, 24, 26–28), thus far no study has attempted to document the effect of these events on the evolution of the Neotropical flora. Generally, detailed reconstructions have been hampered by the lack of resolution in many Andean species-rich clades (19). Current biogeographic methods require well-resolved phylogenies, and uncertainty in phylogenetic relationships makes it difficult to reconstruct the specific sequence of geological vicariance and speciation events.
Here, we use an integration of phylogenetic, biogeographic, and molecular dating methods to reconstruct the evolutionary history of tribes Cinchoneae and Isertieae, which together form one of the major clades of Neotropical Rubiaceae. The distribution of this clade spans highland and montane habitats (the Andes, the Guiana, and the Brazilian Shields), as well as lowland tropical forests (the Amazonia and Chocó). It thus offers a unique opportunity to disentangle the evolutionary processes underlying botanical evolution in the region. Our results reveal an extraordinary level of congruence between the evolution of the Neotropical Rubiaceae and the progressive west-to-east Andean uplift, which brought about a series of marine incursions and lacustrine systems that blocked the dispersal of plants and shaped the distribution of the modern flora.
Study Group
The coffee family (Rubiaceae) is the fourth largest family of flowering plants, with some 13,100 species in 611 genera and 3 subfamilies (30, 31). Although cosmopolitan in distribution, its highest diversity is distinctly confined to the tropics. Subfamily Rubioideae is pantropically distributed and comprises some highly diverse groups in the Neotropics (e.g., Palicoureeae and Spermacocae), but it is otherwise concentrated to the Old World where it probably originated (32, 33). Subfamily Ixoroideae shows a similar pattern, because it comprises a species-rich Neotropical clade (the “Condaminae–Calycophylleae” alliance) but is otherwise concentrated in the Paleotropics. Contrastingly, except for tribe Naucleeae, the large subfamily Cinchonoideae is predominantly Neotropical. In tropical South America, Cinchonoideae is represented by sister tribes Cinchoneae and Isertieae, which have been shown to build a strongly supported clade (34) and is sometimes treated as a single tribe (30). Comprising some 130 species of small trees and shrubs divided into 11 genera [see supporting information (SI) Table S1], the Cinchoneae and Isertieae are important ecological components of a wide array of habitats. Some species are also economically important as a source of quinine. The distribution of Isertieae is concentrated in the lowlands of the Amazon basin and eastern Guiana, whereas Cinchoneae species are mainly confined to the highland and montane habitats of the Northern and Central Andes, reaching up to 3,300 m (Figs. S1 and S2).
Results and Discussion
Gentry (35), following Raven and Axelrod (36), listed the Neotropical Rubiaceae as a Gondwana-derived group, evolving in isolation since the separation of South America from Africa. This hypothesized origin predicts that (i) the group has a minimal age of 100 Ma (37), and (ii) Old and New World lineages are reciprocally monophyletic (6). In contrast, the competing hypothesis of boreotropical origin predicts that (i) South American groups are derived from northern relatives with Old World taxa as sister groups, (ii) the divergence between Old and New World groups occurred between 40 and 50 Ma, i.e., around the Eocene climatic optimum, which favored the exchange of tropical floristic elements between these land masses (38), and (iii) Early Tertiary fossils have been found in North America, Europe, or Asia (6).
Our biogeographic reconstruction (Fig. 1I and II) corroborates a boreotropical origin in all 3 predictions: (i) our phylogeny (Fig. S3) shows the Neotropical sister tribes Cinchoneae and Isertieae nested within a clade of mainly Central American and Antillean tribes, together sister to the essentially African tribe Naucleeae and the Paleotropical subfamily Ixoroideae; (ii) our divergence time estimates place the most recent common ancestor (MRCA) of the Rubiaceae in the Early Paleocene (66.1 Ma, 63.0–68.8; see Table S2), whereas the minimum age of Cinchonoideae is estimated as only 51.3 (47.8–54.6) Ma, well after the last known island chain between Africa and South America possibly existed [Late Cretaceous, ≈88 Ma (37)]; and finally, (iii) several Cephalanthus fossils indicate the presence of the tribe Naucleeae in Europe from the Late Eocene (39) (see SI Text). Until the Late Eocene or Early Oligocene, a continuous belt of boreotropical vegetation covered much of southern North America, southern Eurasia, and northwestern Africa (40). At that time, plant migration through direct land connection or across limited water gaps (6) could have been possible through the North Atlantic “Thulean” land bridge (13, 41) or through the Early–Mid Tertiary “Beringian land bridge” (42). Although climates during the Early Eocene were warmer than today (38), Beringia was in a considerably higher paleolatitude than the Thulean bridge. Dispersal of boreotropical elements is therefore considered more likely across the North Atlantic during this period (13, 42), which is also supported by the fact that the oldest Cephalanthus fossils have been found in Europe (39). Together, these lines of evidence strongly suggest that the Rubiaceae used the corridors provided by boreotropical vegetation and the North Atlantic land bridge as a pathway to reach North America in the Late Paleocene/Early Eocene (Fig. 2I).
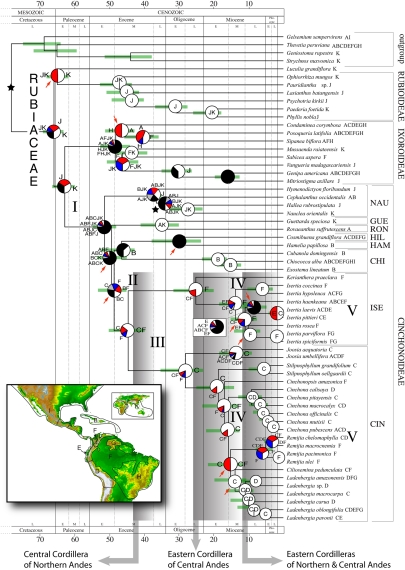
Fig. 1.
Combined chronogram and biogeographic analysis of Neotropical Rubiaceae. The tree is the 50% majority-rule consensus (with compatible groups added) from the Bayesian analysis, with branches proportional to absolute ages (in millions of years) calculated from mean branch lengths of 6,000 Bayesian trees. Green bars indicate 95% confidence intervals of node ages estimated from 1,000 trees randomly sampled from the Bayesian stationary distribution. Node charts show the relative probabilities of alternative ancestral distributions obtained by integrating dispersal-vicariance analysis (DIVA) optimizations over the 1,000 Bayesian trees; the first 4 areas with highest probability are colored according to their relative probability in the following order: white > red > blue > gray; any remaining areas (usually frequencies <0.01) are collectively given with black color. Stars indicate calibration points. Red arrows indicate clades with a posterior probability <0.90. Present ranges for each species are given after the species name. Brackets identify subfamilies and tribes: CHI, Chiococceae; CIN, Cinchoneae; GUE, Guettardeae; HAM, Hamelieae; HIL, Hillieae; ISE, Isertieae; NAU, Naucleeae; RON, Rondeletieae. Shaded boxes indicate approximate periods of Andean uplift phases. The biogeographic interpretation of events I–V is summarized in Fig. 2. (Inset) Areas used in the biogeographic analysis. A, Central America, B, West Indies; C, Northern Andes; D, Central Andes; E, Chocó; F, Amazonia; G, The Guiana Shield; H, Southeastern South America; I, Temperate North America; J, Africa; K, Australasia. Topographic map from the National Geophysical Data Center (www.ngdc.noaa.gov).
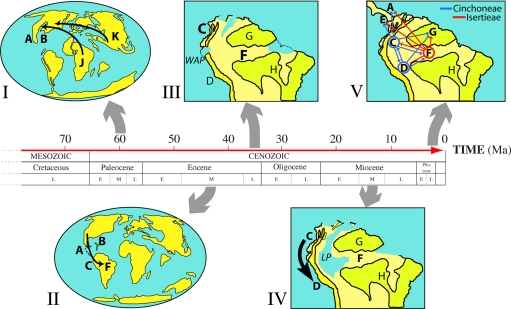
Fig. 2.
Spatiotemporal evolution of the Neotropical Rubiaceae. (I) Paleocene: Rubiaceae ancestors use the boreotropical route to reach North America from the Paleotropics. (II) Early Eocene: Dispersal into South America, presumably facilitated by occasional island chains. (III) Late Eocene: North Andean and Amazonian lineages become isolated by marine incursions such as the Western Andean Portal (WAP). (IV) Middle Miocene: The gradual uplift of the Eastern Cordillera creates a huge watershed, Lake Pebas (LP). It also closes the WAP, enabling dispersal of plant lineages from the Northern to the Central Andes. (V) The Pebas system drains, promoting land dispersal of several lineages and rapid speciation of terrestrial plants in western Amazonia. Area codings as in Fig. 1. (Maps I–II are based on C. R. Scotese's PALEOMAP project (www.scotese.com); maps III–V modified from refs. 2 and 28).
From North America, dispersals to South America (Fig. 2II) may have been facilitated by the proto-Greater Antilles in the Early Eocene and later by the Greater Antilles and the Avies Ridge around the Eocene/Oligocene boundary [GAARlandia, 33–35 Ma (43)]. Our divergence time estimates suggest that the ancestors of Cinchonoideae arrived in northwestern South America around the Early/Middle Eocene (49.2 Ma, 44.9–53.1; node 26 in Fig. S4). At that time, sea levels some 50 m above today's (44) created a marine incursion from the Caribbean that limited land dispersal eastwards (1, 24) and another incursion from the Pacific that blocked dispersal to the south (24, 45, 46) (Fig. 2III). Lowland areas were covered by closed-canopy tropical rainforests (47). Most of the Andes had not yet been formed, except for some low mountains in the regions now corresponding to the Central and Southern Andes (3, 21). Interestingly, the MRCA of tribes Isertieae and Cinchoneae is reconstructed as being lowland-adapted (see Fig. S5). But starting in the Middle Eocene, the Andean orogeny went through a major phase of mountain building, sometimes referred to as the Incaic II (21, 48). This phase was longitudinally widespread, and in the northern region it caused uplift of the Central Cordillera (21, 49). The newly formed montane habitats must have acted as an ecological barrier to lowland taxa, and this seems to explain the geographic disjunction (Andes vs. Amazonia) between the MRCAs of tribes Cinchoneae and Isertieae (Fig. 1III and Fig. S5).
In the case of Isertieae, their MRCA is most likely reconstructed as being confined to lowland Amazonia (Fig. 2III), where it first radiated in the Late Oligocene, giving rise to the genera Kerianthera and Isertia (Fig. 1III and Fig. S5). Diversification in Isertieae occurred mainly in the Middle and Late Miocene, which is strikingly coincident with the uplift of the Eastern Cordillera in the Northern Andes [Fig. 1IV; (2, 21)]. For tribe Cinchoneae, ecological adaptation to higher altitudes seems to have been the key to its diversification, with most speciation events confined to montane habitats in the Northern and Central Andes (Fig. 1 III and IV and Fig. S5).
Western Andean Portal.
Most optimizations indicate that the MRCA of Cinchoneae was confined to the Northern Andes, presumably in the new habitats created by the Central Cordillera (Fig. 1III). From the Eocene to the Middle Miocene, some studies have proposed that marine incursions from the Pacific invaded a lowland corridor between the Northern and Central Andes at the latitude of southern Ecuador/northern Peru [≈3–5°S (1, 2, 45, 46, 50)], termed the “Western Andean Portal” (WAP, Fig. 2III) or “Guayaquil Gap” (23). Indication for these incursions comes from fossil occurrences of marine organisms and palynomorphs (23, 46) and paleosedimentary evidence (1, 2, 50). The WAP is suggested to have been uplifted, and marine incursions ended, in connection with the uplift of the Eastern Cordilleras of the Central and Northern Andes from the Middle Miocene onwards (13–11 Ma) (2, 21, 50).
Our biogeographic reconstruction of subfamily Cinchonoideae (Fig. 1III) corroborates the existence of a dispersal barrier between the Northern and the Central Andes coincident with the WAP, and provides indication for the persistence of this barrier until the Middle Miocene. Throughout the Oligocene and Early Miocene, all ancestral area reconstructions in tribe Cinchoneae occur—partly or exclusively—in the Northern Andes (Fig. 1III). The uplift of the WAP is then observed as at least 5 independent migrations from the Northern to the Central Andes within the genera Cinchona and Ladenbergia (Fig. 1IV). All 5 events are dated as occurring around the Middle/Late Miocene (12–10 Ma, Fig. S4 and Table S2), almost simultaneously with the suggested end of marine incursions in the WAP (1, 2, 21, 50).
Several questions remain unanswered concerning the geographic extent and duration of marine settings in the WAP (24, 28). What is clear, however, is that it constituted an important and long-lasting biogeographic barrier, as evidenced by the fact that many montane taxa exhibit endemism centers in either side of the WAP (see SI Text for an expanded discussion), suggesting that species in those groups were not able to cross the WAP during their time of radiation. In plants, this pattern has been recognized in many families, such as Campanulaceae, Calceolariaceae, Tropaeolaceae, Loasaceae, Passifloraceae, Alstroemeriaceae, and Grossulariaceae (see SI Text for references). In birds, this area has long been recognized as a turnover point between the Northern and Central Andean biogeographic regions of endemism (51).
Lake Pebas.
Until the end of the Oligocene (≈24 Ma), a fluvial system referred to as the paleo-Orinoco dominated the drainage of northwestern Amazonia and the foreland Andean basins toward Lake Maracaibo, on the Caribbean coast. Then, in the Early Miocene (≈23 Ma) geotectonic changes in the Amazon Basin associated with the ongoing uplift of the Eastern Cordillera in the Central Andes (28) caused western Amazonia to gradually become submerged, from south to north and from west to east. This process created a huge (>1 million km2) system of long-lived lakes and wetlands from at least 17 to 11 Ma, known as “Lake Pebas” or the “Pebas Sea” (24–28). Whether it was a purely fluvio-lacustrine (fresh water) system or whether marine settings were also present is still a matter of debate (24, 27, 28). However, most authors agree that western Amazonia was completely flooded by some kind of wetland system from at least the Middle to the Late Miocene and that this system was connected to the Caribbean marine incursion in the north (Fig. 2IV). From the Late Miocene onwards (11–7 Ma), there was a new period of rapid mountain uplift, affecting mainly the Eastern Andean Cordilleras [sometimes termed Quechua phases II and III (21, 49)]. This presumably caused the western margin of the Guiana Shield to emerge, which closed the Caribbean connection of the paleo-Orinoco, shifted the drainage of the Amazon Basin eastwards, and lead to the demise of Lake Pebas (27, 28) (Fig. 2V). Aquatic conditions, however, seem to have persisted in western-central Amazonia until at least 7 Ma, when the modern Amazon system came into place (28).
Previous studies have provided indications to the potential role played by Lake Pebas as a pathway for marine organisms to disperse into fresh water biotopes, based on fossil occurrences of mollusks and DNA phylogenies of Neotropical fishes (23, 24, 26). On the other hand, if this wetland system was as large and interconnected as suggested, it should also have acted as a biotic barrier to the dispersal of terrestrial organisms between the Andes and the eastern Amazonian and Guiana regions.
Indeed, the existence of a dispersal barrier between the Andes and lowland Amazonia during the Middle Miocene is corroborated by our biogeographic reconstructions (Fig. 1IV). Until the Early/Middle Miocene boundary, several ancestors in tribe Cinchoneae are inferred to have been widespread in both of those areas. But after that—coinciding with the time Lake Pebas is proposed to have existed—all ancestral lineages in Cinchoneae and Isertieae appear to have occupied either the Andes or Amazonia, but not both. Most endemic species of Isertieae are currently found in Guiana and eastern Amazonian lowlands (Fig. S1A), whereas western-central Amazonian distributions are mainly represented by widespread species, suggesting that these distributions are the result of recent range expansion, probably after the drying of Lake Pebas. Similarly, several ancestral nodes in Remijia are reconstructed as occurring on both sides of the barrier (Fig. 1V), but these nodes are dated after the Miocene/Pliocene boundary (≈5.3 Ma, Fig. S4) and thus postdate the closing of the Pebas wetland system. The final reestablishment of land connections between the Andes and Amazonia is evidenced by at least 7 independent colonization events in Cinchoneae and Isertieae from the Late Miocene onwards (Fig. 1V). Eventually, the emergence of new lands in Central America after the closing of the Panama Isthmus (3.5 Ma) (21, 52) provided suitable areas for northwards dispersal of South American lineages. Species such as Joosia umbellifera and Isertia haenkeana, which are now widespread in Central America, probably dispersed soon after the establishment of that land connection (Fig. 2V).
Materials and Methods
Phylogenetic analyses were performed under parsimony and Bayesian methods as implemented in PAUP* (53) and MrBayes3 (54). We used representative genera of 4 families of Gentianales as outgroup, based on evidence that the Rubiaceae are the sister group to the rest of Gentianales (e.g., ref. 55). The ingroup included representatives of all Rubiaceae subfamilies, with focus on subfamily Cinchonoideae in which all tribes were represented (Fig. 1). The final dataset comprised 62 species and 5,894 characters, derived from matK, rbcL, ITS1–5.8S–ITS2, trnL-F, and rps16 (Table S3). Thus, our dataset comprised some 3–3.5 times more characters than similar studies (e.g., refs. 9 and 19), which resulted in a robust phylogeny where 80% of all tree nodes were strongly supported (Bayesian posterior probability values, pp ≥0.95 or jackknife ≥85%). Dataset and trees are available from TreeBase (www.treebase.org) accession nos. S2334 and M4437. Evolutionary rates were estimated with the Penalized Likelihood algorithm implemented in r8s (56). Accurate fossil calibration has been suggested as a key factor in age estimation (57). Here, absolute ages were estimated by using fossil fruits and seeds of Cephalanthus, a genus with an exceptionally rich and reliable fossil record from the Late Eocene onwards (Fig. S4). Additionally, a maximum age constraint of 78 Ma was independently enforced on the basal node of the tree based on the crown age of Gentianales (58). Finally, to incorporate topological and branch length uncertainty in our age estimates, 1,000 trees randomly sampled from the Bayesian stationary distribution were independently dated and results summarized to obtain median values and 95% credibility intervals of node ages (Fig. S4 and Table S2). See SI Text for a detailed description of the phylogenetic and dating methods used.
Eleven areas (Fig. 1 Inset) were defined for the biogeographic analysis based on the extant distribution patterns of Rubiaceae and on geological history (3, 21). Whenever possible, we tried to maximize congruence with other biogeographic studies in South America (59, 60). Dispersal-vicariance analysis [DIVA (61, 62)] was used to infer ancestral distributions and historical events involved in the biogeographic history of Rubiaceae. To overcome the uncertainty associated with phylogenetic estimation, we use an approach that averages DIVA biogeographic and temporal reconstructions over a Bayesian sample of highly probable trees (in this case n = 1,000), generating credibility support values for alternative phylogenetic relationships (63). Integrating over the posterior distribution of trees often reveals preference for a single or more restricted set of solutions, thus reducing the uncertainty in DIVA optimizations (63).
Incomplete taxon sampling may cause problems for historical inference methods because it reduces the accuracy of ancestral state reconstructions (see ref. 64 for a different view). This is particularly problematic in a large family such as Rubiaceae, spanning nearly all continents. To overcome this problem, we used encompassing areas outside the Neotropics, where most of Ixoroideae (as well as the first diverging clade in Cinchonoideae, the tribe Naucleeae) are found. We also estimated the geographic bias in our sample for the Neotropical tribes Cinchoneae and Isertieae, the main focus of this article. Results showed that our taxon sampling is fairly representative of the actual distribution of the species within each genus and tribe (see Table S4, Fig. S6, and SI Text for a detailed discussion of distribution patterns in these tribes). In addition, to test the sensitivity of our DIVA reconstructions to missing taxa, we performed a series of heuristic simulations in which we added hypothetical taxa from underrepresented areas to key nodes in the phylogeny of these 2 tribes. DIVA ancestral reconstructions proved to be very stable to the addition of missing taxa, at least for the nodes involved in the WAP and Lake Pebas scenario (Fig. S7). We also examined the effect of missing taxa on divergence time estimations by randomly deleting taxa from the original sample and calculating divergence times on these reduced datasets. As Linder et al. (65) suggested, PL proved to be largely insensitive to taxon sampling, and for most nodes the estimated ages were within the 95% confidence interval from the complete dataset (Fig. S8; see SI Text for more details on simulations). This gives us confidence that the biogeographic scenario presented here (Fig. 2) would not be significantly altered by the addition of missing taxa.
Supplementary Material
Acknowledgments.
We are indebted to F. Wesselingh, C. Hoorn, R. Eriksson, E. M. Friis, P. Endress, S. Schultka, B. Bremer, C. Rydin, E. Kowalski, T. Sempere, L. Kinoshita, K. Yamamoto, T. Eriksson, M. Sanderson, K. Suguio, R. Bemerguy, M. Pirie, O. Seberg, J. Ohlson, B. Oxelman, and N. Wikström for invaluable advice and to V. Aldén for technical assistance. This manuscript has been greatly improved thanks to the suggestions of 2 anonymous reviewers and the editor. We wish to dedicate this study to the memory of Lennart Andersson, who initiated the project and participated actively in it until his death in January 2005. This work was supported by grants from the Swedish Research Council (to C. P.), from the Royal Swedish Academy of Sciences, the Royal Society of Arts and Sciences in Gothenburg, Kungliga och Hvitfeldtska Stiftelsen, Carl Tryggers Stiftelse, Helge Ax:son Johnsons Stiftelse, and University of Gothenburg (to A.A.), and from the Program “Ramon y Cajal” of the Spanish Ministry of Education and Science and the “Biogeography Working Group” supported by NESCent (National Science Foundation Grant EF-0423641) (to I.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.H.T. is a guest editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper has been deposited in the GenBank database (accession nos. DQ448595–DQ448612).
This article contains supporting information online at www.pnas.org/cgi/content/full/0811421106/DCSupplemental.
References
- 1.Hoorn C. Marine incursions and the influence of Andean tectonics on the Miocene depositional history of northwestern Amazonia: Results of a palynostratigraphic study. Palaeogeogr Palaeocl. 1993;105:267–309. [Google Scholar]
- 2.Hoorn C, Guerrero J, Sarmiento GA, Lorente MA. Andean tectonics as a cause of changing drainage patterns in Miocene northern South America. Geology. 1995;23:237–240. [Google Scholar]
- 3.Gregory-Wodzicki KM. Uplift history of the central and northern Andes: A review. Geol Soc Am Bull. 2000;112:1091–1105. [Google Scholar]
- 4.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 5.Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. Proc Natl Acad Sci USA. 2007;104:5925–5930. doi: 10.1073/pnas.0608361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pennington RT, Dick CW. The role of immigrants in the assembly of the South American rainforest tree flora. Philos Trans R Soc London Ser B. 2004;359:1611–1622. doi: 10.1098/rstb.2004.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donoghue MJ. A phylogenetic perspective on the distribution of plant diversity. Proc Natl Acad Sci USA. 2008;105:11549–11555. doi: 10.1073/pnas.0801962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiens JJ, Donoghue MJ. Historical biogeography, ecology, and species richness. Trends Ecol Evol. 2004;19:639–644. doi: 10.1016/j.tree.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Davis CC, Bell CD, Mathews S, Donoghue MJ. Laurasian migration explains Gondwanan disjunctions: Evidence from Malpighiaceae. Proc Natl Acad Sci USA. 2002;99:6833–6837. doi: 10.1073/pnas.102175899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson JE, Chatrou LW, Mols JB, Erkens RHJ, Pirie MD. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Philos Trans R Soc London Ser B. 2004;359:1495–1508. doi: 10.1098/rstb.2004.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pirie MD, Chatrou LW, Mols JB, Erkens RHJ, Oosterhof J. Andean-centred genera in the short-branch clade of Annonaceae: Testing biogeographical hypothesis using phylogeny reconstruction and molecular dating. J Biogeogr. 2006;33:31–46. [Google Scholar]
- 12.Tiffney BH. Perspectives on the origin of the floristic similarity between eastern Asia and eastern North America. J Arnold Arbor. 1985a;66:73–94. [Google Scholar]
- 13.Tiffney BH. The Eocene North Atlantic land bridge: Its importance in Tertiary and modern phytogeography of the Northern Hemisphere. J Arnold Arbor. 1985b;66:243–273. [Google Scholar]
- 14.Wolfe JA. Some aspects of plant geography of the Northern Hemisphere during the Late Cretaceous and Tertiary. Ann Mo Bot Gard. 1975;62:264–279. [Google Scholar]
- 15.Moore BR, Donoghue MJ. Correlates of diversification in the plant clade Dipsacales: Geographic movements and evolutionary innovations. Am Nat. 2007;170:S28–S55. doi: 10.1086/519460. [DOI] [PubMed] [Google Scholar]
- 16.Bell CD, Donoghue MJ. Phylogeny and biogeography of Valerianaceae (Dipsacales) with special reference to the South American valerians. Organisms Divers Evol. 2005;5:147–159. [Google Scholar]
- 17.Winkworth RC, Donoghue MJ. Viburnum phylogeny based on combined molecular data: Implications for taxonomy and biogeography. Am J Bot. 2005;92:653–666. doi: 10.3732/ajb.92.4.653. [DOI] [PubMed] [Google Scholar]
- 18.von Hagen KB, Kadereit JW. The diversification of Halenia (Gentianaceae): Ecological opportunity versus key innovation. Evolution (Lawrence, Kans) 2003;57:2507–2518. [PubMed] [Google Scholar]
- 19.Hughes C, Eastwood R. Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proc Natl Acad Sci USA. 2006;103:10334–10339. doi: 10.1073/pnas.0601928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brumfield RT, Edwards SV. Evolution into and out of the Andes: A Bayesian analysis of historical diversification in Thamnophilus ant shrikes. Evolution (Lawrence, Kans) 2007;61:346–367. doi: 10.1111/j.1558-5646.2007.00039.x. [DOI] [PubMed] [Google Scholar]
- 21.Taylor DW. Paleobiogeographic relationships of Andean angiosperms of Cretaceous to Pliocene age. Palaeogeogr Palaeocl Palaeoecol. 1991;88:69–84. [Google Scholar]
- 22.Garzione CN, et al. Rise of the Andes. Science. 2008;320:1304–1307. doi: 10.1126/science.1148615. [DOI] [PubMed] [Google Scholar]
- 23.Nuttal CP. A review of the Tertiary non-marine molluscan faunas of the Pebasian and other inland basins of north-western South America. Bull Brit Mus Nat Hist Geol. 1990;45:165–371. [Google Scholar]
- 24.Lundberg JG, et al. In: Phylogeny and Classification of Neotropical Fishes. Malabarba LR, Reis RE, Vari RP, Lucena ZM, Lucena CAS, editors. Porto Alegre, Brazil: Edipucrs; 1998. pp. 13–48. [Google Scholar]
- 25.Wesselingh FP, et al. Lake Pebas: A palaeoecological reconstruction of a Miocene, long-lived lake complex in western Amazonia. Cainozoic Res. 2002;1:35–81. [Google Scholar]
- 26.Wesselingh FP. Miocene long-lived lake Pebas as a stage of mollusc radiations, with implications for landscape evolution in western Amazonia. Scripta Geol. 2006;133:1–17. [Google Scholar]
- 27.Wesselingh FP, Salo JA. A Miocene perspective on the evolution of the Amazonian biota. Scripta Geol. 2006;133:439–458. [Google Scholar]
- 28.Wesselingh FP, et al. In: Amazonia: Landscape and species evolution. Hoorn C, Wesselingh FP, editors. Oxford: Blackwell Publishing; 2010. in press. [Google Scholar]
- 29.Lovejoy NR, Albert JS, Crampton WGR. Miocene marine incursions and marine/freshwater transitions: Evidence from Neotropical fishes. J S Am Earth Sci. 2006;21:5–13. [Google Scholar]
- 30.Robbrecht E, Manen JF. The major evolutionary lineages of the coffee family (Rubiaceae, angiosperms). Combined analysis (nDNA and cpDNA) to infer the position of Coptosapelta and Luculia, and supertree construction based on rbcL, rps16, trnL-trnF and atpB-rbcL data. A new classification in two subfamilies, Cinchonoideae and Rubioideae. Syst Geogr Pl. 2006;76:85–146. [Google Scholar]
- 31.Govaerts R, Frodin DG, Ruhsam M, Bridson DM, Davis AP. World Checklist and Bibliography of Rubiaceae. Kew, UK: The Trustees of the Royal Botanic Gardens; 2007. [Google Scholar]
- 32.Manen JF, Dessein S, De Block P, Robbrecht E. Towards an evolutionary scenario for the coffee family (Rubiaceae, angiosperms): Further insights from a supertree. Scripta Bot Belg. 2006;40:47. [Google Scholar]
- 33.Sonké B, Dessein S, Taedoumg H, Groeninckx I, Robbrecht E. A new species of Colletoecema (Rubiaceae) from southern Cameroon with a discussion of relationships among basal Rubioideae. Blumea. 2008;53:533–547. [Google Scholar]
- 34.Andersson L, Antonelli A. Phylogeny of the tribe Cinchoneae (Rubiaceae), its position in Cinchonoideae, and description of a new genus, Ciliosemina. Taxon. 2005;54:17–28. [Google Scholar]
- 35.Gentry AH. Neotropical floristic diversity: Phytogeographical connections between Central and South America, Pleistocene climatic fluctuations, or an accident of the Andean orogeny? Ann Mo Bot Gard. 1982;69:557–593. [Google Scholar]
- 36.Raven PH, Axelrod DI. Angiosperm biogeography and past continental movements. Ann Mo Bot Gard. 1974;61:539–673. [Google Scholar]
- 37.Morley RJ. Interplate dispersal routes for megathermal angiosperms. Perspec Plant Ecol Evol Syst. 2003;6:5–20. [Google Scholar]
- 38.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to the present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 39.Mai DH, Walther H. The Upper Eocene floras of the Weisselster basin and adjacent areas. (in German) Abh Staat Mus Miner Geol Dresden. 1985;33:1–260. [Google Scholar]
- 40.Lavin M, Luckow M. Origins and relationships of tropical North America in the context of the boreotropics hypothesis. Am J Bot. 1993;80:1–14. [Google Scholar]
- 41.McKenna MC. In: Structure and Development of the Greenland-Scotland Bridge: New Concepts and Methods. Bott MHP, Saxov S, Talwani M, Thiede J, editors. New York: Plenum; 1983. pp. 351–395. [Google Scholar]
- 42.Sanmartín I, Enghoff H, Ronquist F. Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biol J Linnean Soc. 2001;73:345–390. [Google Scholar]
- 43.Iturralde-Vinent MA, MacPhee RDE. Paleogeography of the Caribbean region: Implications for Cenozoic biogeography. Bull Am Mus Nat Hist. 1999;238:1–95. [Google Scholar]
- 44.Miller KG, et al. The phanerozoic record of global sea-level change. Science. 2005;310:1293–1298. doi: 10.1126/science.1116412. [DOI] [PubMed] [Google Scholar]
- 45.Steinmann M, Hungerbühler D, Seward D, Winkler W. Neogene tectonic evolution and exhumation of the southern Ecuadorian Andes; a combined stratigraphy and fission-track approach. Tectonophysics. 1999;307:255–276. [Google Scholar]
- 46.Santos C, Jaramillo C, Bayona G, Rueda M, Torres V. Late Eocene marine incursion in north-western South America. Palaeogeogr Palaeocl. 2008;264:140–146. [Google Scholar]
- 47.Morley RJ. Origin and Evolution of Tropical Rain Forests. Chichester, UK: Wiley; 2000. [Google Scholar]
- 48.Noble DC, McKee EH, Mourier T, Mégard F. Cenozoic stratigraphy, magmatic activity, compressive deformation, and uplift in northern Peru. Bull Geol Soc Am. 1990;102:1105–1113. [Google Scholar]
- 49.Irving EM. Structural evolution for the northernmost Andes, Colombia. USGS. 1975;846:1–47. [Google Scholar]
- 50.Hungerbühler D, et al. Neogene stratigraphy and Andean geodynamics of southern Ecuador. Earth Sci Rev. 2002;57:75–124. [Google Scholar]
- 51.Stotz DF, Fitzpatrick JW, Parker TA, Moskovits DK. Neotropical Birds: Ecology and Conservation. Chicago: University of Chicago Press; 1996. [Google Scholar]
- 52.Briggs JC. The genesis of Central America: Biology versus geophysics. Glob Ecol Biogeogr. 1994;4:169–172. [Google Scholar]
- 53.Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods) Sunderland, MA: Sinauer; 2002. Ver 4.0b10. [Google Scholar]
- 54.Ronquist F, Huelsenbeck JP. MrBayes3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 55.Albach DC, Soltis PS, Soltis DE, Olmstead RG. Phylogenetic analysis of asterids based on sequences of four genes. Ann Mo Bot Gard. 2001;88:163–212. [Google Scholar]
- 56.Sanderson MJ. Estimating absolute rates of molecular evolution and divergence times: A penalized likelihood approach. Mol Biol Evol. 2002;19:101–109. doi: 10.1093/oxfordjournals.molbev.a003974. [DOI] [PubMed] [Google Scholar]
- 57.Sauquet H, et al. Contrasted patterns of hyperdiversification in Mediterranean hotspots. Proc Natl Acad Sci USA. 2009;106:221–225. doi: 10.1073/pnas.0805607106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bremer K, Friis EM, Bremer B. Molecular phylogenetic dating of asterid flowering plants shows early Cretaceous diversification. Syst Bot. 2004;53:496–505. doi: 10.1080/10635150490445913. [DOI] [PubMed] [Google Scholar]
- 59.Cracraft J. Deep-history biogeography, retrieving the historical pattern of evolving continental biotas. Syst Zool. 1988;37:221–236. [Google Scholar]
- 60.Posadas PE, Estévez JM, Morrone JJ. Distributional patterns and endemism areas of vascular plants in the Andean subregion. Fontqueria. 1997;48:1–9. [Google Scholar]
- 61.Ronquist F. DIVA version 1.1. Computer program and manual available from Uppsala University. 1996 www.ebc.uu.se/systzoo/research/diva/diva.html.
- 62.Ronquist F. Dispersal-vicariance analysis: A new approach to the quantification of historical biogeography. Syst Biol. 1997;46:195–203. [Google Scholar]
- 63.Nylander JAA, Olsson U, Alström P, Sanmartín I. Accounting for phylogenetic uncertainty in biogeography: A Bayesian approach to dispersal-vicariance analysis of the Thrushes (Aves: Turdus) Syst Biol. 2008;57:257–268. doi: 10.1080/10635150802044003. [DOI] [PubMed] [Google Scholar]
- 64.Li G, Steel M, Zhang L. More taxa are not necessarily better for the reconstruction of ancestral character states. Syst Biol. 2008;57:647–653. doi: 10.1080/10635150802203898. [DOI] [PubMed] [Google Scholar]
- 65.Linder HP, Hardy CR, Rutschmann F. Taxon sampling effects in molecular clock dating: An example from the African Restionaceae. Mol Phylogen Evol. 2005;35:569–582. doi: 10.1016/j.ympev.2004.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.