Abstract
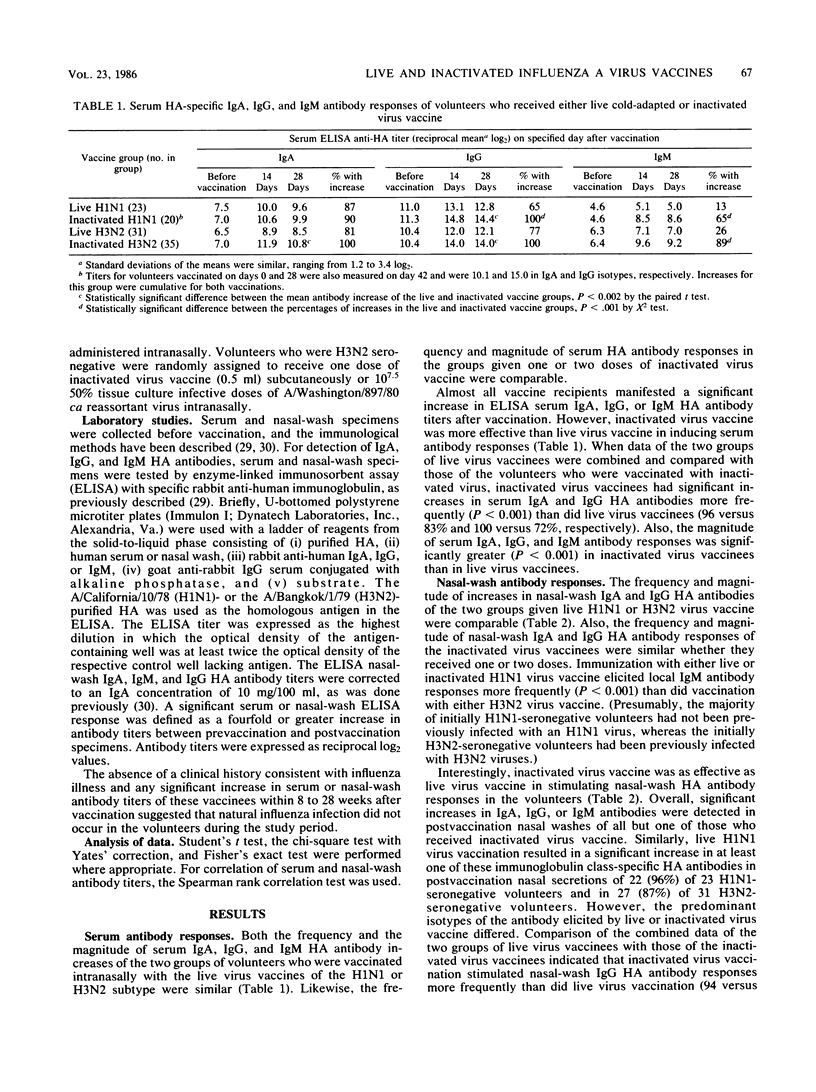
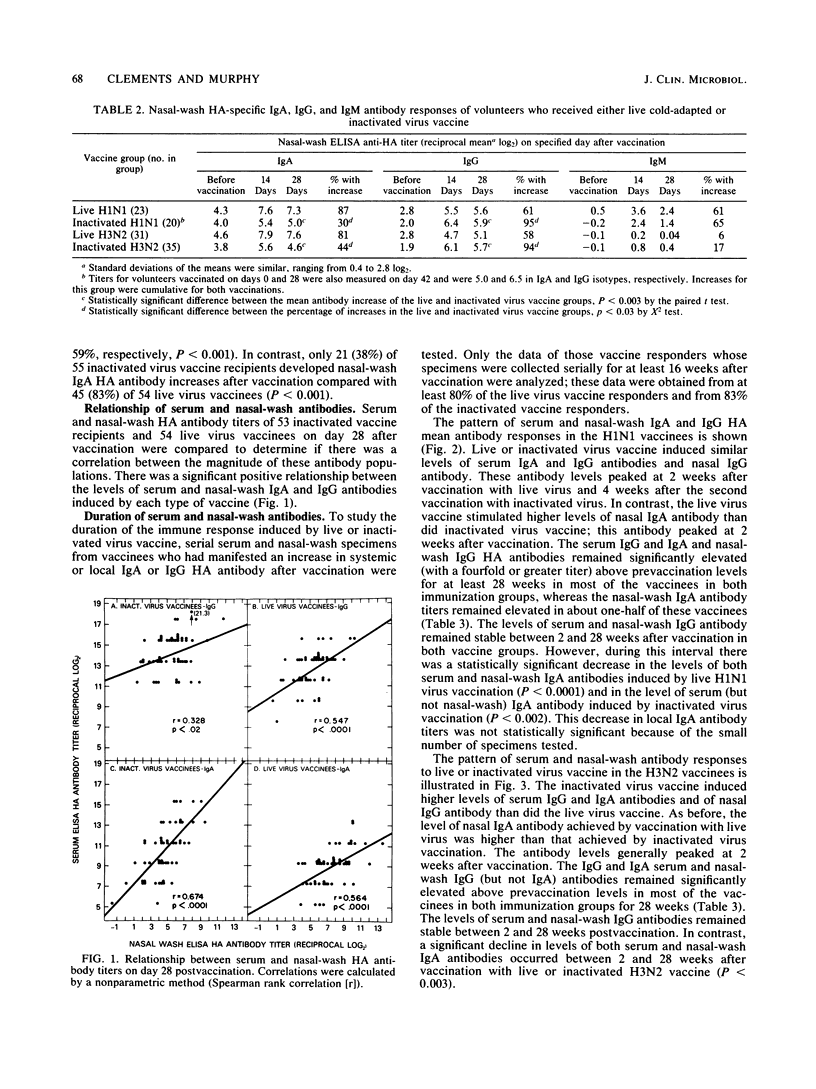
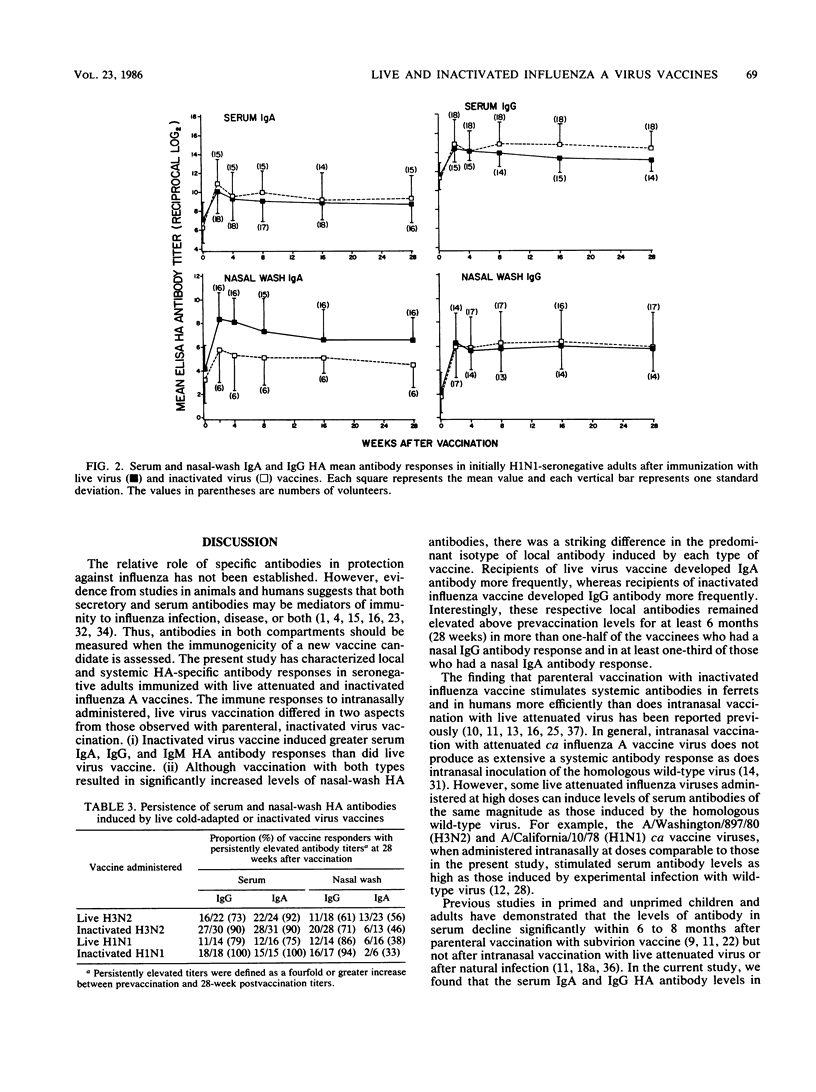
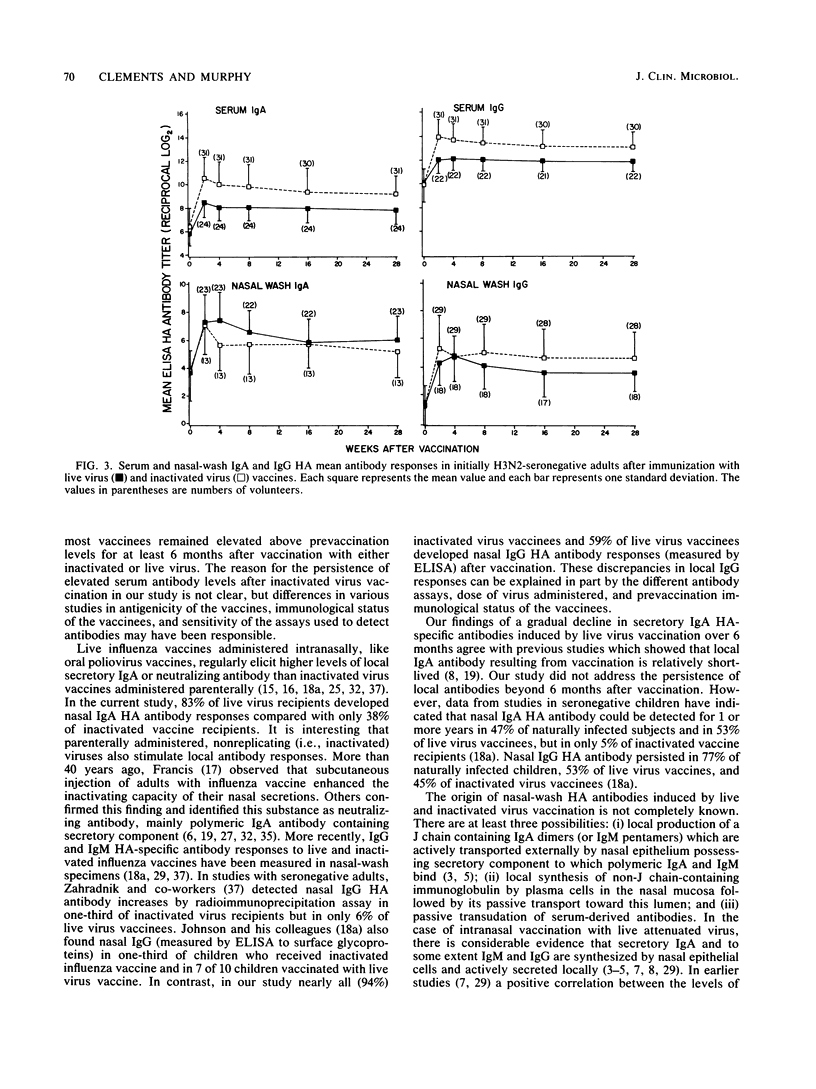
An enzyme-linked immunosorbent assay was used to measure nasal-wash and serum isotype-specific hemagglutinin antibody responses in 109 seronegative (hemagglutination-inhibiting titer less than or equal to 1:8) adults vaccinated intranasally with live attenuated A/Washington/897/80 (H3N2) or A/California/10/78 (H1N1) cold-adapted (ca) virus or with licensed subvirion vaccine subcutaneously. Live and inactivated virus elicited serum immunoglobulin A (IgA) responses in 83 and 96% of vaccinees, respectively, and elicited serum IgG responses in 72 and 100% of vaccinees. Inactivated virus induced higher titers of serum antibodies than did live virus and stimulated a nasal-wash IgG response more often than did live virus (94 versus 59%, P less than 0.01). In contrast, only 38% of inactivated virus vaccinees had local IgA responses compared with 83% of live virus vaccinees. Serum IgA and IgG and nasal IgG antibody titers remained elevated above prevaccination levels for at least 6 months in most of the live and inactivated vaccine responders, but the mean level of local IgA antibody induced by infection with live virus vaccine, in particular, decreased substantially. Considered in the context of previous work, the finding that live virus vaccine induced relatively long-lasting antibody in both local and serum compartments suggested that this vaccine may be a suitable alternative to inactivated vaccine for use in healthy persons.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber W. H., Small P. A., Jr Local and systemic immunity to influenza infections in ferrets. Infect Immun. 1978 Jul;21(1):221–228. doi: 10.1128/iai.21.1.221-228.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe R. B., Van Voris L. P. Cold-recombinant influenza A/California/10/78 (H1N1) virus vaccine (CR-37) in seronegative children: infectivity and efficacy against investigational challenge. J Infect Dis. 1984 May;149(5):735–740. doi: 10.1093/infdis/149.5.735. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology. 1974 Jun;26(6):1101–1114. [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Murphy B. R., Radl J., Haaijman J. J., Mestecky J. Subclass distribution and molecular form of immunoglobulin A hemagglutinin antibodies in sera and nasal secretions after experimental secondary infection with influenza A virus in humans. J Clin Microbiol. 1985 Aug;22(2):259–264. doi: 10.1128/jcm.22.2.259-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlington D. B., Clements M. L., Meiklejohn G., Phelan M., Murphy B. R. Hemagglutinin-specific antibody responses in immunoglobulin G, A, and M isotypes as measured by enzyme-linked immunosorbent assay after primary or secondary infection of humans with influenza A virus. Infect Immun. 1983 Aug;41(2):540–545. doi: 10.1128/iai.41.2.540-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. T., Waldmann T. A., Rossen R. D., Douglas R. G., Jr, Couch R. B. Changes in IgA and IgG concentrations in nasal secretions prior to the appearance of antibody during viral respiratory infection in man. J Immunol. 1970 Sep;105(3):584–591. [PubMed] [Google Scholar]
- Clark A., Potter C. W., Jennings R., Nicholl J. P., Langrick A. F., Schild G. C., Wood J. M., Tyrrell D. A. A comparison of live and inactivated influenza A (H1N1) virus vaccines. 1. Short-term immunity. J Hyg (Lond) 1983 Jun;90(3):351–359. doi: 10.1017/s0022172400028989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A., Potter C. W., Jennings R., Nicholl J. P., Langrick A. F., Schild G. C., Wood J. M., Tyrrell D. A. A comparison of live and inactivated influenza A (H1N1) virus vaccines. 2. Long-term immunity. J Hyg (Lond) 1983 Jun;90(3):361–370. doi: 10.1017/s0022172400028990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Maassab H. F., Murphy B. R. Dose response of influenza A/Washington/897/80 (H3N2) cold-adapted reassortant virus in adult volunteers. J Infect Dis. 1984 May;149(5):814–815. doi: 10.1093/infdis/149.5.814. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Murphy B. R. Advantage of live attenuated cold-adapted influenza A virus over inactivated vaccine for A/Washington/80 (H3N2) wild-type virus infection. Lancet. 1984 Mar 31;1(8379):705–708. doi: 10.1016/s0140-6736(84)92222-0. [DOI] [PubMed] [Google Scholar]
- Clements M. L., O'Donnell S., Levine M. M., Chanock R. M., Murphy B. R. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983 Jun;40(3):1044–1051. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton R. J., Clark A., Potter C. W. Immunity to influenza in ferrets. XIV: Comparative immunity following infection or immunization with live or inactivated vaccine. Br J Exp Pathol. 1981 Jun;62(3):297–307. [PMC free article] [PubMed] [Google Scholar]
- Francis T., Jr A RATIONALE FOR STUDIES IN THE CONTROL OF EPIDEMIC INFLUENZA. Science. 1943 Mar 12;97(2515):229–235. doi: 10.1126/science.97.2515.229. [DOI] [PubMed] [Google Scholar]
- Hoskins T. W., Davies J. R., Smith A. J., Miller C. L., Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979 Jan 6;1(8106):33–35. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- Human influenza: aspects of the immune response to vaccination. Ann Intern Med. 1969 Aug;71(2):369–398. doi: 10.7326/0003-4819-71-2-369. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Jr, Feldman S., Thompson J. M., Mahoney J. D., Wright P. F. Comparison of long-term systemic and secretory antibody responses in children given live, attenuated, or inactivated influenza A vaccine. J Med Virol. 1985 Dec;17(4):325–335. doi: 10.1002/jmv.1890170405. [DOI] [PubMed] [Google Scholar]
- Lazar A., Okabe N., Wright P. F. Humoral and cellular immune responses of seronegative children vaccinated with a cold-adapted influenza A/HK/123/77 (H1N1) recombinant virus. Infect Immun. 1980 Mar;27(3):862–866. doi: 10.1128/iai.27.3.862-866.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman S. J., Wright P. F., Patil K. D. Antibody decline in children following A/New Jersey/76 influenza virus immunization. J Pediatr. 1980 Feb;96(2):271–274. doi: 10.1016/s0022-3476(80)80823-7. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M., Appleyard G., Brand C. M., Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984 Apr;14(4):350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- Maassab H. F., Cox N. J., Murphy B. R., Kendal A. P. Biological, genetic and biochemical characterization of a cold-adapted recombinant A/Victoria/3/75 virus and its evaluation in volunteers. Dev Biol Stand. 1977 Jun 1;39:25–31. [PubMed] [Google Scholar]
- Mann J. J., Waldman R. H., Togo Y., Heiner G. G., Dawkins A. T., Kasel J. A. Antibody response in respiratory secretions of volunteers given live and dead influenza virus. J Immunol. 1968 Apr;100(4):726–735. [PubMed] [Google Scholar]
- Meyer H. M., Jr, Hopps H. E., Parkman P. D., Ennis F. A. Review of existing vaccines for influenza. Am J Clin Pathol. 1978 Jul;70(1 Suppl):146–152. [PubMed] [Google Scholar]
- Mostow S. R., Schoenbaum S. C., Dowdle W. R., Coleman M. T., Kaye H. S., Hierholzer J. C. Studies on inactivated influenza vaccines. II. Effect of increasing dosage on antibody response and adverse reactions in man. Am J Epidemiol. 1970 Oct;92(4):248–256. doi: 10.1093/oxfordjournals.aje.a121204. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Clements M. L., Madore H. P., Steinberg J., O'Donnell S., Betts R., Demico D., Reichman R. C., Dolin R., Maassab H. F. Dose response of cold-adapted, reassortant influenza A/California/10/78 virus (H1N1) in adult volunteers. J Infect Dis. 1984 May;149(5):816–816. doi: 10.1093/infdis/149.5.816. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Nelson D. L., Wright P. F., Tierney E. L., Phelan M. A., Chanock R. M. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982 Jun;36(3):1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Phelan M. A., Nelson D. L., Yarchoan R., Tierney E. L., Alling D. W., Chanock R. M. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol. 1981 Mar;13(3):554–560. doi: 10.1128/jcm.13.3.554-560.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Rennels M. B., Douglas R. G., Jr, Betts R. F., Couch R. B., Cate T. R., Jr, Chanock R. M., Kendal A. P., Maassab H. F., Suwanagool S. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect Immun. 1980 Aug;29(2):348–355. doi: 10.1128/iai.29.2.348-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puck J. M., Glezen W. P., Frank A. L., Six H. R. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. J Infect Dis. 1980 Dec;142(6):844–849. doi: 10.1093/infdis/142.6.844. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Cogliano R. C., Shands J. W., Jr, Small P. A., Jr Serum antibody prevents lethal murine influenza pneumonitis but not tracheitis. Infect Immun. 1979 Sep;25(3):992–997. doi: 10.1128/iai.25.3.992-997.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel R. P., Hendley J. O., Sande M. A., Gwaltney J. M., Jr Revised (1972-1973) bivalent influenza vaccine. Serum and nasal antibody responses to parenteral vaccination. JAMA. 1973 Oct 22;226(4):435–438. [PubMed] [Google Scholar]
- Wright P. F., Okabe N., McKee K. T., Jr, Maassab H. F., Karzon D. T. Cold-adapted recombinant influenza A virus vaccines in seronegative young children. J Infect Dis. 1982 Jul;146(1):71–79. doi: 10.1093/infdis/146.1.71. [DOI] [PubMed] [Google Scholar]
- Zahradnik J. M., Kasel J. A., Martin R. R., Six H. R., Cate T. R. Immune responses in serum and respiratory secretions following vaccination with a live cold-recombinant (CR35) and inactivated A/USSR/77 (H1N1) influenza virus vaccine. J Med Virol. 1983;11(4):277–285. doi: 10.1002/jmv.1890110403. [DOI] [PubMed] [Google Scholar]


