Abstract
Macaques, like humans, rapidly orient their attention in the direction other individuals are looking. Both cortical and subcortical pathways have been proposed as neural mediators of social gaze following, but neither pathway has been characterized electrophysiologically in behaving animals. To address this gap, we recorded the activity of single neurons in the lateral intraparietal area (LIP) of rhesus macaques to determine whether and how this area might contribute to gaze following. A subset of LIP neurons mirrored observed attention by firing both when the subject looked in the preferred direction of the neuron, and when observed monkeys looked in the preferred direction of the neuron, despite the irrelevance of the monkey images to the task. Importantly, the timing of these modulations matched the time course of gaze-following behavior. A second population of neurons was suppressed by social gaze cues, possibly subserving task demands by maintaining fixation on the observed face. These observations suggest that LIP contributes to sharing of observed attention and link mirror representations in parietal cortex to a well studied imitative behavior.
Keywords: gaze following, imitation, joint attention, mirror neurons, shared attention
People naturally and intuitively share attention with each other. In a laboratory setting, people respond more quickly to targets that are the object of another's attention, even when this social cuing is brief or consistently misleading (1–3). Monkeys' attention also follows the gaze of others (4), and the similar magnitude and time course of gaze following by rhesus macaques and humans (5) implicates shared neural mechanisms. The ability to follow gaze is believed to be an important foundation for theory of mind (6, 7); thus, the neural processes governing gaze following are relevant both to the evolution of social cognition (8–10) and to clinical disorders, such as autism, associated with social attention deficits (11–14). Although gaze following involves automatic “mirroring” of other's mental states, to our knowledge, mirror neurons (15, 16) for visual orienting have not previously been identified.
Current evidence suggests that identification of where other individuals are looking is accomplished by neurons along the superior temporal sulcus (STS) (17–20) and in the amygdala (21, 22). In primates, signals from these brain areas (19) ramify to multiple targets in the visual orienting system, including, within 1 or 2 steps, posterior parietal cortex [7A and lateral intraparietal area (LIP); see ref. 23], prefrontal cortex [supplemental eye field (SEF) and frontal (F)EF; see ref. 24], and subcortical visual areas [pulvinar nucleus of the thalamus (25), and superior colliculus (SC; see ref. 26)]. Neuroimaging studies indicate that perception of faces with averted gaze activates populations of neurons in the STS region (27, 28) and the amygdala (22), as well as the parietal cortex (28). These observations invite the simple hypothesis that gaze-following behavior is mediated by a relatively straightforward system, beginning with the STS and proceeding directly to the attention- and gaze-control networks. Although intuitively appealing, this model raises several important questions.
First, gaze-following behavior fits poorly into existing models of attention (1, 2), which dichotomize the underlying mechanism as either reflexively driven by exogenous stimuli or endogenously guided by internal goals (29, 30). Although there is some evidence that specific neural circuits mediate these processes (31–33), the precise contributions of neurons within different brain areas to exogenous, endogenous, and social attention (and, indeed, whether these processes are distinguishable at the neuronal level) remain unclear
Second, the fastest reported gaze-following behavior in monkeys is evoked at very short latencies (100 ms after gaze cue onset; see ref. 34), requiring the processing stream that discriminates gaze direction and relays this information to visual orienting areas to operate quite rapidly. Thus, although neuroimaging techniques can identify cortical areas sensitive to the direction of observed gaze, their temporal resolution is too coarse to determine whether these areas are capable of mediating fast gaze-following behavior. To date, the neural correlates of social gaze-evoked attention have only been explored by using brain imaging or neuropsychological techniques in humans (35–37).
Last, current neurophysiological models of visual orienting behavior posit some form of temporal integration mechanism (32, 33, 38). In such models, visual orienting is evoked when neuronal activity associated with shifting gaze to a particular location reaches a threshold level of firing. One appealing feature of such models is that they capture within a single framework the relationship between the strength of the neuronal response and both reaction time and the likelihood of orienting (39); thus, providing a good description of the relationship between orienting decisions and neuronal activity in brain areas associated with attention, including LIP (40). It is currently unclear whether social gaze cues influence LIP neurons in a manner consistent with these models.
In principle, these questions could be addressed by recording the activity of neurons in this putative social attention processing stream during spontaneous gaze following in controlled laboratory conditions (5, 34). To begin addressing these questions, we probed the impact of social gaze cues on the firing rates of LIP neurons in monkeys performing a simple visual orienting task, in which monkeys were required to maintain fixation on a monkey face with averted gaze, and then to shift their own gaze toward a peripheral target randomly illuminated either within or outside the direction of observed gaze. Previous studies have linked LIP activity to both covert and overt orienting of attention, with neuronal activity tracking visual saliency, saccade likelihood, and target value (41, 42). Our primary goals were to determine whether LIP neurons are sensitive to observed gaze direction and, if so, whether this sensitivity could mediate gaze-following behavior. We were particularly interested in whether the response dynamics were quick enough to mediate the rapid behavioral responses observed in a standard gaze-following probe (Fig. 1) (2, 5, 34). To our knowledge, no prior studies have linked the responses of single neurons to gaze-following behavior or reported the latency at which observed gaze direction is signaled by neurons in the brain. Although several prior studies have contrasted eye-contact with averted gaze (43), we found only one (44) that explicitly reported deictic signals (signals that “point out” specific spatiotemporal targets); the study was not optimized to examine the latency at which these signals arose.
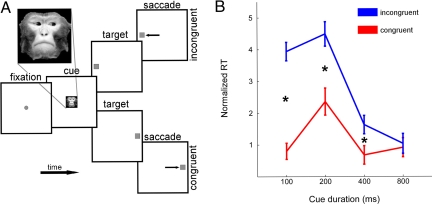
Fig. 1.
Visual orienting task and behavioral dynamics. (A) The impact of social gaze cues on the activity of single neurons in area LIP was probed while monkeys shifted gaze to a peripheral target after viewing an image of a familiar monkey looking toward the RF or away from it. Macaques first fixated a central yellow square (±3°) for 200–500 ms. The yellow square was then extinguished and a monkey face (Inset) was illuminated centrally for a variable duration (100, 200, 400, or 800 ms). If the monkey maintained fixation, the face was extinguished and a peripheral yellow square simultaneously illuminated at 1 of 2 fixed positions located symmetrically within, or opposite, the measured neuronal RF. Gaze shifts to the peripheral target within 350 ms were rewarded with a small squirt of juice. (B) Gaze following was observed after short (≤400 ms) face viewing durations. The average normalized saccade latency observed across all neurons and cue durations are here plotted for congruent (red) and incongruent (blue) cue conditions. Normalization was to the average response latency for all cue conditions for each given neuronal recording session, cue duration, and target location. Error bars represent SEM across sessions. Both the main effects of cue validity and cue duration were significant, with the interaction significant at P = 10−5. Effect size was significant by t test at 100, 200, and 400 ms (P = 3*10−10, 0.0002, and 0.03, respectively).
We found that activity in 30 of 106 neurons recorded in LIP (28%) was modulated by social gaze cues, even when these cues were presented outside their classical response fields (RFs), and despite the fact that optimal behavior in the task would completely ignore the cues (5). Of these, approximately half (43%) mirrored observed gaze, becoming more active both while directing attention toward a region of space and while observing other monkeys do the same. Also, the temporal dynamics of neuronal responses to social gaze cues predicted the time-course of gaze-following behavior. Other neurons were suppressed by gaze cues toward their RF, and may have acted to suppress task-irrelevant behavioral responses to observed gaze. These findings suggest that LIP has a role in behavioral responses to gaze (e.g., gaze following and shared attention). Although confirming a causal relationship would require techniques such as reversible inactivation or microstimulation, these correlational findings support a role for LIP in social mirroring of both orienting behavior and associated attentional states.
Results
Overall, monkeys followed gaze during physiological recordings, initiating saccades faster when a photographed monkey had also looked toward the target. Gaze-following behavior was strongest at short delays between cue onset and target appearance, as we have reported (5, 34). Monkeys showed significant gaze following for the shortest 3 cue durations (Fig. 1B; ANOVA, average normalized saccadic reaction time per neuron, by congruence X cue duration, P = 0.00001; posthoc t test of neuronwise effect size at 100 ms, P = 3*10−10; at 200 ms, P = 0.0002; at 400 ms, P = 0.03; and at 800 ms, P = 0.8). We have reported individual differences in both gaze-following magnitude and time course associated with dominance status (34), but the current study was not optimized to detect these differences and could not fully resolve them [ANOVA; congruence × subject identity, P = 0.0001; congruence × cue duration × subject identity, not significant (n.s.)]. All 4 monkeys showed stronger gaze-following behavior at shorter (< 400 ms) than longer (≥400 ms) social cue durations (paired t test, P = 0.0023), consistent with earlier reports (34). Because of their consistency and rapidity (5, 34), these fast gaze-following responses are of the greatest interest for the current study of neuronal response dynamics. Monkey Niko showed the strongest fast gaze following (mean = 4.0 ms; contributed 29% of neurons); followed by Sherry (mean = 2.5 ms; contributed 47% of neurons), Dart (mean = 2.1 ms; contributed 8.5% of neurons), and Otto (mean = 0.63 ms; contributed 15% of neurons).
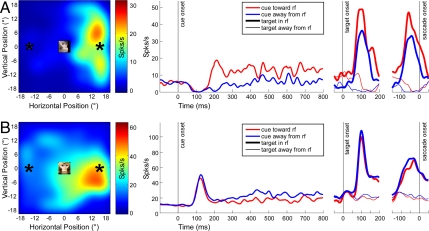
In total, 153 neurons were recorded, of which 106 were confirmed posthoc to strongly differentiate between targets located in their estimated RFs (“in RF”) and those reflected through the origin (“outside RF”) (t test with Bonferroni-corrected α = 0.05/153, over the interval 20–120 ms following target onset). Although faces subtended only the central 5° of visual space, were static, were presented outside the classical RF of the recorded neurons, and were irrelevant to the task of orienting for fluid rewards, the firing rates of some neurons were systematically modulated by observed gaze direction (Fig. 2). For example, Fig. 2A presents data for a neuron that increased firing following presentation of a monkey face gazing toward the right side of the monitor, the same direction preferred by the neuron when the subject oriented to a visual target during simple RF mapping trials. By contrast, other neurons fired more strongly when the observed monkey face was gazing away from the classical RF (Fig. 2B).
Fig. 2.
Single LIP neurons are sensitive to social gaze cues. (A) Example neuron showing firing rate enhancement by social gaze directed toward the RF. (B) Example neuron showing firing rate suppression by social gaze directed toward the RF. Response field plots (Left) illustrate firing rates for saccade targets across the visual field, recorded during an independent set of simple or delayed-saccade mapping trials. Schematic representation of cue image and target locations are superimposed on this map at scale; cue images did not intrude into the classical RFs of neurons. (Right) Neuronal activity as a function of time, synchronized to cue onset, target onset, and saccade onset, respectively. Cue and target location are indicated by color (red for cues gazing toward the RF; blue, away) and line thickness (thick lines for saccades toward the RF; thin, away). Gaze modulations were robust across time for both neurons.
Thirty (28%) of 106 neurons differentiated faces looking toward from those looking away from their RF (Fig. 3A and Fig. S1). Approximately half of these neurons showed systematic increases in firing rate (n = 13), whereas the other half showed systematic decreases in firing rate (n = 17), in response to faces gazing toward the RF. The distribution of neurons significantly enhanced, significantly suppressed, or failing to significantly differentiate gaze did not differ significantly across individuals (chi2, n.s.). Thus, area LIP appears to spontaneously receive information about where other individuals are looking, despite the fact that monkeys were not trained to discriminate these cues or associate them with rewards, and despite the fact that face cues did not predict the location of saccade targets and, thus, were irrelevant to the task.
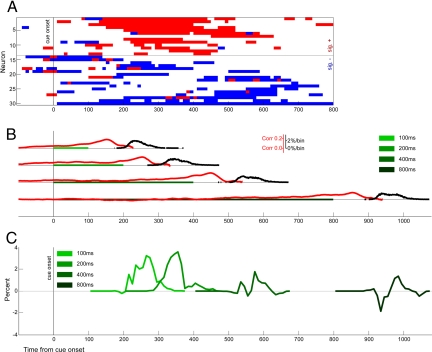
Fig. 3.
Population responses to social gaze cues anticipate gaze-following behavior. (A) Neural cue responses. Significant neuronal responses to observed gaze direction in 10-ms bins. Neurons enhanced by social gaze cues (red) are temporally clustered in the time windows for which gaze-following behavior is strongest, whereas those suppressed by social gaze cues maintain tonic decreases in activity throughout the fixation period. (B) Task dynamics: cue fixation, saccade preparation, and saccade latencies are shown for each cue duration. Green bars illustrate the duration of the cue fixation period, red curves indicate correlation of LIP activity with decreased saccade latency, and black curves indicate saccade onset density as a function of time. Thus, the red curves indicate the moment-to-moment correlation of observed LIP activity with decreased saccade latency, and range from nearly 0 to as high as 0.2 ≈30–50 ms before saccade initiation. Similarly, the black curves indicate when saccades were observed to begin, with a peak of ≈2% occurring in any given 1-ms bin. (C) Saccade latency distributions: differential saccade-onset density for congruently cued versus incongruent trials show early gaze following which later fades. We here attempt to indicate exactly when gaze following is first evidenced in behavior. To do this, we separately generated histograms of saccade onset time for congruent and incongruent responses, analogous to black curves in B. We then integrated these curves, and examined the difference between these cumulative histograms, to illustrate the precise times at which congruent saccades occur faster than incongruent. Thus, positive deflections indicate that more responses have occurred to congruently cued than incongruent trials, and negative deflections indicate the opposite. In summary, although suppressed neuronal responses are fairly uniform, the excitatory neuronal responses (A) are maximal while the 100–400 ms cue responses are being generated (B), the time period in which the largest behavioral effects are observed (C).
We next compared the time course of LIP social gaze cue sensitivity with the time course of behavioral gaze following. Looking exclusively at those trials in which a spatially neutral gray square appeared, rather than a directional gaze cue, we found that LIP population activity was negatively correlated with saccade latency from 148 ms prior through 115 ms after the time of cue offset/target onset (all trials, Fig. 3B; neutral only, Fig. S2). This finding is consistent with past observations of ramping LIP activity reaching a threshold ≈100–50 ms before initiation of a gaze shift toward the neuronal RF (38, 45). Note that these correlations do not necessarily imply that peak firing rates correlate with saccade latency; rather, we suspect these correlations may reflect a change in the latency of a short, stereotyped burst of activity, which in turn, correlates with saccade latency. Based on these data, in order for LIP to directly mediate gaze-following behavior, neurons in this area must be sensitive to social gaze cue direction in the 250-ms time window surrounding target onset and during subsequent saccade preparation.
In this experiment, as in earlier studies (5, 34), gaze-following behavior developed quickly, then faded. Intriguingly, we found that social gaze cues directed toward the RF most strongly excited neurons at latencies 100–500 ms after cue onset. In fact, nearly all neurons significantly enhanced by social gaze cues toward their RF differentiated between toward-RF and away-from-RF gazing faces in the period from 250 to 400 ms after cue presentation (Fig. 3A; also, see Fig. S1 Right). Thus, socially cued enhancements in LIP activity occurred in approximately the same time period in which we observed the strongest gaze-following behavior (Fig. 3). The temporal dynamics of socially cued modulation differed between gaze-activated and gaze-deactivated neurons [Kolmogorov–Smirinov (KS) test, P = 10−9; Fig. S1 Right]. Although socially cued suppression was uniform across the central cue period (KS test versus uniformity, P > 0.1), socially cued enhancement was significantly clustered in time (KS test versus uniformity, P = 10−11). This pattern suggests that, whereas social-gaze enhanced neurons contribute to gaze-following behavior, social-gaze suppressed neurons act to maintain fixation across the cue period and, thus, increase the likelihood the monkey will successfully complete the task.
To refine our understanding of social attention effects in LIP, we contrasted the latency with which neurons signaled image onset, the latency with which they distinguished social gaze cues from neutral gray squares, and the latency with which they distinguished social gaze cue direction (Fig. S1). We looked both at overall population responses and at subpopulations that were significantly enhanced or suppressed by image onset, image type, and cue direction, respectively. We found that latencies increased systematically. Image-independent responses to cue onset plateaued after 50 ms, presumably reflecting an overall change in luminance of the display. By contrast, distinctions between social gaze cues and a gray square control image arose between 60 ms (for those neurons that preferred large gray square) and 100 ms (for those neurons that preferred faces). Last, distinctions between social gaze cue directions arose last, with gaze-cued enhancements beginning between 100 and 200 ms, and gaze-cue suppression remaining fairly constant during the cue fixation period. These results are consistent with LIP receiving feed-forward information from successively higher levels of the visual system, with directional social gaze signals arriving relatively late. These findings are also consistent with the idea that LIP mediates both social salience assessment (46) and oculomotor reward contingencies related to task demands (42). We speculate that gaze-cue enhanced neurons signal the increased value of acquiring information about regions of space where other monkeys are looking. By contrast, we speculate that gaze-cue suppressed neurons contribute to active fixation required for successful task performance; to correctly complete the trial and receive juice reward, any overt gaze following must be suppressed, and fixation maintained, throughout the entire cue period.
Discussion
This report unifies past literature on mirror neurons, thought to participate in the imitation and interpretation of observed action (15, 16), with literature on gaze following, thought to mediate the sharing of attention between individuals (1, 2, 6, 9). Mirror neurons are motor neurons that discharge not only during enactment, but also during observation of a particular behavior (15, 16). LIP, although not classically a motor area, is active in gaze-related sensorimotor transformations (47–50), and its activity contributes to both overt (51) and covert shifts of attention (32, 41), and to maintenance of attention at fixation (52, 53). We here report that neurons in LIP respond not only when monkeys orient attention toward their RFs, but also when other monkeys are observed orienting in the same direction. These effects are detectable despite the irrelevance of social gaze cues to the behavioral task, and despite the fact that faces were presented outside the classical RFs of neurons. We find further support for gaze mirroring in the common modulation of gaze-following behavior (34, 54) and mirror system activity (55) by social relevance. Although only a small population of LIP neurons demonstrated mirroring behavior in this experiment, this number is consistent with past studies of mirror neurons in other areas. For example, in their initial description of mirror neurons in area F5, di Pellegrino et al. (15) identified 29 of 184 (16%) as having visuomotor mirror properties.
Also, we report that those neurons excited by gaze toward their RF were most strongly activated during the period in which the strongest gaze-following behavior was observed. Also, the pattern of neuronal activation associated with socially cued attention was broadly consistent with integrate-to-threshold models describing both exogenous and endogenous control of visual orienting (33, 38, 39). This evidence supports the notion that LIP neurons may contribute to the reflexive sharing of attention (neurotypical humans, see refs. 2, 56; other species, see refs. 5, 57, 58; clinical relevance, see refs. 14, 59). Although social gaze cue effects on neuronal activity were small, they were statistically significant even when driven merely by small, static, repetitive digital pictures. Although previously described mirror neurons in other areas are activated by the observation of specific behaviors performed by human actors (15, 16), LIP neurons here responded to the observation of static images of macaque faces presented on a computer monitor. Because gaze is intrinsically dynamic, and because averted gaze postures are rarely maintained, these cues depicted a sustained attentional state and, thus, implied a recent gaze shift. We anticipate that neuronal responses would be even more robust for dynamic social gaze cues, paralleling the phasic responses observed in other mirror neurons during observation of real-world movement (15, 16); also, we note that because we chose to use static images, low-level visual motion cannot account for the observed behavioral or neuronal responses.
We note several factors that militate for caution in interpretation of these results. Although our data show that LIP neurons are sensitive to social gaze early enough to mediate fast gaze-following behavior, we cannot confirm a causal role (60). Indeed, the activation of LIP neurons in response to observed gaze comes somewhat late in the preparatory window for 100-ms cue-duration saccades, despite the fact that gaze following of these cues is nearly as strong as gaze following of cues presented for 200 ms. We cannot currently exclude the possibility that activity in other brain areas also contributes to these fast gaze-following responses. In fact, modulations in the activity of LIP neurons may result from inputs from subcortical or frontal circuits that process social gaze cues. In this view, the observed modulations in LIP activity reflect the integration of social gaze cue information with calculations of salience (41) or reward (46) associated with acquiring behaviorally useful visual information. Alternatively, LIP may act to bind together observed conspecifics with the objects of their attention, operating in an analogous fashion to the spatial binding of coactivated RFs across saccades (61).
Indeed, although Calder et al. (28) have reported activation in human parietal cortex that differentiates the direction of observed gaze (see also refs. 35 and 36), other evidence suggests the posterior parietal cortex is not the only pathway through which gaze following may operate. For example, Vuilleumier (62) demonstrated that spatial neglect associated with parietal lesions in humans is ameliorated when social gaze cues are directed into the neglected hemifield. This observation suggests either that an intact parietal cortex is unnecessary for gaze-following behavior, or that the intrinsic saliency of social stimuli, like other motivational manipulations (63), can override parietal dysfunction. However, it is important to note that the lesions in that study likely spared portions of the parietal lobe, perhaps including the human homolog of LIP; thus, the results cannot rule out the possibility that areas homologous to LIP were intact and active in mediating the described gaze-following behavior. Conversely, lesions of right superior temporal gyrus (64), amygdala (65), or orbitofrontal cortex (66) each have been reported to disrupt gaze-following behavior. A subordinate role for LIP in gaze following would be consistent with the time course of microstimulation-evoked saccades across the gaze control network. Stimulation of LIP is 20–40 ms slower to evoke saccades than stimulation of the FEFs or the SC: FEF, 15–25 ms (67, 68); LIP, 30–50 ms (51, 69, 70); and SC, 13–20 ms (71, 72). Also, our observation of a population of neurons suppressed by social gaze cues suggests that in this task, LIP actively regulated the prepotent gaze-following response. Monkeys were trained extensively on this task, in which gaze direction is uncorrelated with future target location, and premature attempts to follow gaze abort fixation and preclude reward (5): under these conditions, optimal behavior would be produced by total suppression of gaze following.
Because LIP has been implicated in both exogenously and endogenously cued attention (31), it may seem unsurprising that neurons in this area also signal socially cued attention. As mentioned, however, gaze following is both faster than endogenous attention and more perceptually demanding than exogenous attention. As a result, gaze following has been hypothesized to rely on specialized mechanisms distinct from those mediating either endogenous or exogenous attention (6). In contrast with this hypothesis, our findings indicate that gaze-following behavior is influenced by one of the same systems governing both endogenous and exogenous orienting, and appears to be processed in a manner consistent with existing models of orienting behavior (32, 33, 38). Nonetheless, we recognize that further study will need to better quantify the dynamics of gaze mirroring throughout the attentional control network, and to disrupt this mirroring through targeted inactivations. By tracing neuronal activity from purely perceptual representations of gaze direction through behavioral readouts of attentional state, we may reveal not only how we read the intentions of others, but how we connect with the minds that animate them.
Materials and Methods
Subjects.
Four pair-housed male rhesus monkeys (Macaca mulatta) from our colony at the Duke University Medical Center served as subjects. All animals were originally reared in naturalistic social groups. To enhance motivation, subjects' water access was controlled outside of the experimental session. All procedures were approved by the Duke University Institutional Animal Care and Use Committee and were designed and conducted in compliance with the Public Health Service Guide for the Care and Use of Animals.
Recording.
All experiments were conducted by using a PC computer running custom software (http://www.ryklinsoftware.com). Monkeys viewed stimuli on a dark background on 24“ cathode ray tube (CRT) monitor positioned at ≈45-cm distance. Eye position was monitored by using a magnetic search coil surgically implanted beneath the conjunctiva of one eye and sampled at 500 Hz (73, 74) or via an Eyelink II optical gaze-tracking system. Head position was maintained with a surgically implanted stainless steel prosthesis (Crist) (75).
To permit electrophysiological recordings, macaques were additionally implanted with a stainless steel recording chamber (Crist) over posterior parietal cortex (LIP) (46, 48). Before each session, the chamber was aseptically opened, rinsed thoroughly with sterile saline, and fit with a plastic grid (Crist) (75). A 23-gauge hypodermic guide tube containing a tungsten steel 7–12MΩ electrode (Frederick Haer) was inserted through the grid; an X-Y micropositioner (Crist) and hydraulic microdrive (Kopf) were then mated to electrode and chamber. Electrophysiological recordings were amplified and filtered of line noise and search coil system interference (passband ≈500–5k Hz). Action potentials were identified in hardware (BAK; PLEXON) by time and amplitude criteria or by template-based spike sorting. The electrode was then lowered until visual or saccade-related activity was recognized on an audio monitor. As the monkey performed visually and memory-guided saccade trials, the electrode was lowered further at 2.5–20 μm/s until the waveform of at least 1 neuron could be isolated and its RF localized. Data were recorded by custom software (http://www.ryklinsoftware.com) and imported into Matlab for further analysis by custom scripts. All surgical procedures were performed aseptically, followed with appropriate analgesics and antibiotics, and in all other ways followed standard protocols described (5, 46).
Task.
Once a neuron had been isolated and spatially characterized, macaque subjects performed a modified Posner cuing task (5, 34, 76), in which they first fixated a central target, followed by a static, centrally presented social gaze cue. Each cue image consisted of a photograph of a familiar macaque gazing either toward or away from the mapped RF; photographs were 115 pixels square and subtended ≈5°. To minimize the impact of low-level stimulus features on behavioral and neural responses, we used 2 techniques. In half of the sessions, cue images were reflected across the vertical meridian to generate a feature-balanced set of social cues toward or away from the RF. In the other half, sets of ≈16 different cue images were chosen looking toward or opposite the RF, minimizing the contribution of any idiosyncratic visual features to deictic gaze responses. Gaze-modulated neurons were observed under both conditions. The direction faced by the cue image was randomly determined on each trial, and in each session cue images were selected so that one of the pair faced the RF of the neuron. Randomly, in one third of trials, a neutral gray square appeared instead of a social gaze cue; these trials allowed an independent measure of how LIP activity predicted saccade response time. After a variable duration (100, 200, 400, or 800 ms), the gaze cue abruptly offset, and a target appeared randomly either in the same or the opposite hemi-field as cue gaze. Target locations were chosen so that one target was in the RF of the neuron, whereas its complement was reflected through the origin to the spatially opposite location; gaze directions and target locations were independently randomized across each session. Subjects shifted gaze from fixation to this peripheral target as quickly as possible and maintained fixation for at least 300 ms to receive a juice reward.
Analysis.
Gaze following was operationalized as a decrease in reaction time to congruently cued versus incongruent stimuli (5, 34). Normalization in Fig. 1B was achieved by subtracting the mean RT toward each target for a given cue duration and recording session; error bars represent SE across sessions. Spikes were recorded continuously from 100 ms before task onset until task completion and were convolved with a 10-ms Gaussian smoothing window to preserve fine latency information while enhancing statistical power at low firing rates (45). To determine the relationship between neuronal activity and decreases in reaction time, we measured, for each neuron, the correlation between the ms-to-ms activity and decreased latency (calculated by subtracting the time of saccade onset from the time of target presentation).
Latency information was further analyzed by rebinning into 10-ms bins from 100 ms before cue presentation through the end of the cue period, and comparing spike counts by using Matlab's ranksum function (equivalent to a Mann–Whitney U function; e.g., as used in ref. 77). Three latencies each were tracked by using 2 different metrics. First, we checked all bins of all neurons to find which, if any, significantly differentiated (i) cue images relative to fixation baseline, (ii) faces relative to a neutral gray square, and (iii) faces looking toward the RF relative to those looking away. Bins that were significantly positive were distinguished from those that were significantly negative. We then applied the following latency metrics. First, we looked at the raw sum of neurons with significantly increased and with decreased firing rates across time, and recorded when either sum was above the binomial expectation (2-tailed α = 0.05). Second, we separately analyzed neurons that showed significant increases and decreases in activity, and tracked the time course of significant modulations across time for each of these subpopulations.
Neurons were considered significantly sensitive to a variable if they passed a permutation test designed as follows. The total number of bins significantly increased/decreased by a particular variable during the cue period had to exceed the 97.5th percentile total modulated by the presumably meaningless contrast of odd versus even trials. This threshold was set by permutation test, rather than by binomial distribution, to control for statistical dependencies between adjacent time points in a given recording session. For comparison of cue period activity to baseline, a slightly different permutation was appropriate. The threshold number of significant bins had to exceed the 97.5th percentile observed when ongoing activity was compared with a randomly determined 100-ms time window. Dynamics of socially cued modulations were tested by KS test over the time window 50–700 ms into the cue period.
Supplementary Material
Acknowledgments.
This work was supported by Autism Speaks/National Alliance for Autism Research (S.V.S.), National Institutes of Health (NIH) National Research Service Award (NRSA) postdoctoral fellowships (to S.V.S. and R.O.D.), an NIH NRSA predoctoral fellowship (to J.T.K.), NIH Grant MH066259 (to M.L.P.), and the Cure Autism Now Foundation (M.L.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900419106/DCSupplemental.
References
- 1.Driver J, Davis G, Ricciardelli P. Gaze perception triggers reflexive visuospatial orienting. Vis Cogn. 1999;6:509–540. [Google Scholar]
- 2.Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychol Bull Rev. 1998;5:490–495. [Google Scholar]
- 3.Langton SRH, Bruce V. Reflexive visual orienting in response to the social attention of others. Vis Cogn. 1999;6:541–567. [Google Scholar]
- 4.Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI. Gaze following and joint attention in rhesus monkeys (Macaca mulatta) J Comp Psychol. 1997;111:286–293. doi: 10.1037/0735-7036.111.3.286. [DOI] [PubMed] [Google Scholar]
- 5.Deaner RO, Platt ML. Reflexive social attention in monkeys and humans. Curr Biol. 2003;13:1609–1613. doi: 10.1016/j.cub.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Baron-Cohen S. How to build a baby that can read minds: Cognitive mechanisms in mindreading. Curr Psychol Cogn. 1994;13:513–552. [Google Scholar]
- 7.Perrett DI, Emery NJ. Understanding the intentions of others from visual social signals: Neurophysiological evidence. Curr Psychol Cogn. 1994;13:683–694. [Google Scholar]
- 8.Hare B, Tomasello M. Chimpanzees are more skillful in competitive than in cooperative cognitive tasks. Anim Behav. 2004;68:571–581. [Google Scholar]
- 9.Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behav Brain Sci. 2005;28:675–691. doi: 10.1017/S0140525X05000129. discussion 691–735. [DOI] [PubMed] [Google Scholar]
- 10.Tomasello M, Farrar MJ. Joint attention and early language. Child Dev. 1986;57:1454–1463. [PubMed] [Google Scholar]
- 11.Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J. Are children with autism blind to mentalistic significance of eyes? Brit J Dev Psychol. 1995;13:379–398. [Google Scholar]
- 12.Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- 13.Pelphrey KA, et al. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- 14.Ristic J, et al. Eyes are special but not for everyone: The case of autism. Brain Res Cogn Brain Res. 2005;24:715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 15.di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 16.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- 18.Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wicker B, Michel F, Henaff MA, Decety J. Brain regions associated with mutual gaze: A PET study. Neuroimage. 1998;8:221–227. doi: 10.1006/nimg.1998.0357. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Curr Biol. 2007;17:766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 22.Kawashima R, et al. The human amygdala plays an important role in gaze monitoring. A PET study. Brain. 1999;122:779–783. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- 23.Seltzer B, Pandya DN. Post-Rolandic cortical projections of the superior temporal sulcus in rhesus monkey. J Comp Neurol. 1991;312:625–640. doi: 10.1002/cne.903120412. [DOI] [PubMed] [Google Scholar]
- 24.Seltzer B, Pandya DN. Frontal lobe connections of the superior temporal sulcus in the rhesus monkey. J Comp Neurol. 1989;281:97–113. doi: 10.1002/cne.902810108. [DOI] [PubMed] [Google Scholar]
- 25.Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1997;379:313–332. [PubMed] [Google Scholar]
- 26.Fries W. Cortical projections to the superior colliculus in the macaque monkey: A retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- 27.Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the sts region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- 28.Calder AJ, et al. Separate coding of different gaze directions in the anterior superior temporal sulcus and inferior parietal lobule. Curr Biol. 2007;17:20–25. doi: 10.1016/j.cub.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonides J. In: Attention and Performance IX. Long JB, Baddeley AD, editors. Hillsdale, NJ: Erlbaum; 1981. [Google Scholar]
- 30.Muller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. J Exp Psychol Hum Percept Perform. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- 31.Corbetta M, Shulman GL. Control of goal-directed and stimulusdriven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 32.Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter RH. The neural control of looking. Curr Biol. 2000;10:R291–R293. doi: 10.1016/s0960-9822(00)00430-9. [DOI] [PubMed] [Google Scholar]
- 34.Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Curr Biol. 2006;16:R119–R120. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Hietanen JK, Nummenmaa L, Nyman MJ, Parkkola R, Hamalainen H. Automatic attention orienting by social and symbolic cues activates different neural networks: An fMRI study. Neuroimage. 2006;33:406–413. doi: 10.1016/j.neuroimage.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 36.Materna S, Dicke PW, Thier P. Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: A functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20:108–119. doi: 10.1162/jocn.2008.20.1.108. [DOI] [PubMed] [Google Scholar]
- 37.Hietanen JK, Leppanen JM, Nummenmaa L, Astikainen P. Visuospatial attention shifts by gaze and arrow cues: An ERP study. Brain Res. 2008;1215:123–136. doi: 10.1016/j.brainres.2008.03.091. [DOI] [PubMed] [Google Scholar]
- 38.Roitman JD, Shadlen MN. Response of neurons in posterior parietal cortex (area LIP) during a combined reaction-time direction-discrimination task. J Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gold JI, Shadlen MN. Banburismus and the brain: Decoding the relationship between sensory stimuli, decisions, and reward. Neuron. 2002;36:299–308. doi: 10.1016/s0896-6273(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 40.Ganguli S, et al. One dimensional dynamics of attention and decision making in LIP. Neuron. 2008;58:15–25. doi: 10.1016/j.neuron.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- 42.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 43.Perrett DI, et al. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc R Soc London B. 1985;223:293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- 44.De Souza WC, Eifuku S, Tamura R, Nishijo H, Ono T. Differential characteristics of face neuron responses within the anterior superior temporal sulcus of macaques. J Neurophysiol. 2005;94:1252–1266. doi: 10.1152/jn.00949.2004. [DOI] [PubMed] [Google Scholar]
- 45.Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free viewing visual search task. J Neurosci. 2006;26:3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein JT, Deaner RO, Platt ML. Neural correlates of social target value in macaque parietal cortex. Curr Biol. 2008;18:419–424. doi: 10.1016/j.cub.2008.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gnadt JW, Andersen RA. Memory related motor planning activity in parietal cortex of Macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- 48.Platt ML, Glimcher PW. Responses of intra-parietal neurons to saccadic targets and visual distractors. J Neurophysiol. 1997;78:1574–1589. doi: 10.1152/jn.1997.78.3.1574. [DOI] [PubMed] [Google Scholar]
- 49.Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- 50.Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: A review. Vision Res. 2000;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- 51.Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J Neurophysiol. 1998;80:1713–1735. doi: 10.1152/jn.1998.80.4.1713. [DOI] [PubMed] [Google Scholar]
- 52.Ben Hamed S, Duhamel JR. Ocular fixation and visual fixation activity in the monkey lateral intrparietal area. Exp Brain Res. 2002;142:512–528. doi: 10.1007/s00221-001-0954-z. [DOI] [PubMed] [Google Scholar]
- 53.Schiller PH, Tehovnik EJ. Look and see: How the brain moves your eyes about. Prog Brain Res. 2001;134:127–142. doi: 10.1016/s0079-6123(01)34010-4. [DOI] [PubMed] [Google Scholar]
- 54.Deaner RO, Shepherd SV, Platt ML. Familiarity accentuates gaze-cueing in women but not men. Biology Letters. 2007;3:64–67. doi: 10.1098/rsbl.2006.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kilner JM, Marchant JL, Frith CD. Modulation of the mirror system by social relevance. Soc Cogn Affect Neurosci. 2006;1:143–148. doi: 10.1093/scan/nsl017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychol Bull. 2007;133:694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 58.Itakura S. Gaze following and joint visual attention in nonhuman animals. Jpn Psych Res. 2004;46:216–226. [Google Scholar]
- 59.Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 60.Dinstein I, Thomas C, Behrmann M, Heeger DJ. A mirror up to nature. Curr Biol. 2008;18:R13–18. doi: 10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 62.Vuilleumier P. Perceived gaze direction in faces and spatial attention: A study in patients with parietal damage and unilateral neglect. Neuropsychologia. 2002;40:1013–1026. doi: 10.1016/s0028-3932(01)00153-1. [DOI] [PubMed] [Google Scholar]
- 63.Mesulam MM. Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akiyama T, et al. Gaze but not arrows: A dissociative impairment after right superior temporal gyrus damage. Neuropsychologia. 2006;44:1804–1810. doi: 10.1016/j.neuropsychologia.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 65.Akiyama T, et al. Unilateral amygdala lesions hamper attentional orienting triggered by gaze direction. Cereb Cortex. 2007;17:2593–2600. doi: 10.1093/cercor/bhl166. [DOI] [PubMed] [Google Scholar]
- 66.Vecera SP, Rizzo M. What are you looking at? Impaired “social attention” following frontal-lobe damage. Neuropsychologia. 2004;42:1657–1665. doi: 10.1016/j.neuropsychologia.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 67.Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- 68.Robinson DA, Fuchs AF. Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol. 1969;32:637–648. doi: 10.1152/jn.1969.32.5.637. [DOI] [PubMed] [Google Scholar]
- 69.Kurylo DD, Skavenski AA. Eye movements elicited by electrical stimulation of area PG in the monkey. J Neurophysiol. 1991;65:1243–1253. doi: 10.1152/jn.1991.65.6.1243. [DOI] [PubMed] [Google Scholar]
- 70.Shibutani H, Sakata H, Hyvarinen J. Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Exp Brain Res. 1984;55:1–8. doi: 10.1007/BF00240493. [DOI] [PubMed] [Google Scholar]
- 71.Robinson DA. Eye-movements evoked by collicular stimulation in alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- 72.Stanford TR, Freedman EG, Sparks DL. Site and parameters of microstimulation: Evidence for independent effects on the properties of saccades evoked from the primate superior colliculus. J Neurophysiol. 1996;76:3360–3381. doi: 10.1152/jn.1996.76.5.3360. [DOI] [PubMed] [Google Scholar]
- 73.Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- 74.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: An improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 75.Dean HL, Crowley JC, Platt ML. Visual and saccade-related activity in macaque posterior cingulate cortex. J Neurophysiol. 2004;92:3056–3068. doi: 10.1152/jn.00691.2003. [DOI] [PubMed] [Google Scholar]
- 76.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 77.Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci. 2002;22:5081–5090. doi: 10.1523/JNEUROSCI.22-12-05081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.