Abstract
In HIV-1-infected individuals on currently recommended antiretroviral therapy (ART), viremia is reduced to <50 copies of HIV-1 RNA per milliliter, but low-level residual viremia appears to persist over the lifetimes of most infected individuals. There is controversy over whether the residual viremia results from ongoing cycles of viral replication. To address this question, we conducted 2 prospective studies to assess the effect of ART intensification with an additional potent drug on residual viremia in 9 HIV-1-infected individuals on successful ART. By using an HIV-1 RNA assay with single-copy sensitivity, we found that levels of viremia were not reduced by ART intensification with any of 3 different antiretroviral drugs (efavirenz, lopinavir/ritonavir, or atazanavir/ritonavir). The lack of response was not associated with the presence of drug-resistant virus or suboptimal drug concentrations. Our results suggest that residual viremia is not the product of ongoing, complete cycles of viral replication, but rather of virus output from stable reservoirs of infection.
Keywords: antiretroviral drug intensification, HIV-1 persistance, viral reservoir
In HIV type 1 (HIV-1) infection, plasma virus levels have proven to be an important indicator of viral replication, risk of disease progression, and response to therapy (1–7). Initial studies of changes in viremia in response to antiretroviral drugs demonstrated exponential reductions occurring in at least 2 distinct phases (1, 2, 4). These phases of decay have half-lives of ≈1 day and ≈14 days, reflecting the lifespan of HIV-infected CD4+ T lymphoblasts and that of a second, longer-living population of infected cells, respectively. Within several weeks, plasma virus levels fall to below the limit of detection of HIV-1 RNA assays approved for patient management (50 copies per milliliter of plasma). On the basis of these studies, there was initial optimism that HIV-1 could be eradicated with prolonged antiretroviral therapy (ART) (7).
Since the initial studies, several discoveries have tempered hope for eradication. The first was the identification of a long-lived latent reservoir of HIV-1 in resting CD4+ T cells (8, 9). Latently infected cells persist in patients on ART who have suppression of viremia to levels below the detection limit of clinical assays (10–12). With a half-life estimated at 44 months, this compartment cannot be eliminated within the lifespan of most infected individuals (13–15). A second discovery was that most patients whose HIV-1 RNA levels were suppressed by ART to <50 copies per milliliter were actually viremic at a low level (16). Novel quantitative techniques, including the single-copy assay (SCA), can quantify HIV-1 viremia at levels down to <1 copy per milliliter (17), allowing a more detailed analysis of the viral decay kinetics on ART (18, 19). Studies using the SCA revealed that the initial 2-phase decline in viremia (7) is followed by a prolonged third phase of decay occurring over months. Subsequently, there appears to be a stable fourth phase during which there is no appreciable decay (19). The median level of the residual viremia during this fourth phase is ≈1.5 copies per milliliter.
One way to probe the source of low-level viremia in patients on ART is by intensification of therapy with an additional, potent antiretroviral drug not previously used by the patient. On the one hand, residual viremia may be the result of virus released from latently infected cells whose proviruses have become transcriptionally active, and from other long-lived, chronically infected cellular reservoirs. In these cases, the level of viremia would not be affected by intensification of ART because release of virions from cells infected before therapy would be unaffected by currently available antiretroviral drugs, which only block new cycles of viral replication. Further, the released viruses would not be expected to productively infect other cells in the presence of drugs that block new cycles of replication. This explanation for the source of residual viremia is consistent with the clinical observation that patients who are adherent to ART can maintain durable suppression of viremia without virological failure (20–22). In addition, analysis of rebound viremia in patients interrupting antiretroviral therapy has demonstrated no evidence of ongoing genetic variability during suppression (23), consistent with the absence of viral genetic adaptation or new drug resistance mutations in patients on suppressive ART (24–26).
Alternatively, residual viremia could arise from ongoing, new cycles of viral replication resulting from incomplete inhibitory activity or penetration of antiretrovirals. Evidence for ongoing replication comes from studies suggesting that treatment intensification reduces the level of residual viremia (27) and the frequency of intermittently measurable viremia (blips) (28). The implication of this work is that current ART may not fully suppress viral replication. Given that ongoing replication may lead to the development of resistance, the inability of ART to completely suppress viral replication could compromise long-term virological and clinical outcomes in treated patients. Treatment intensification should reduce residual viremia coming from ongoing cycles of replication in drug-accessible compartments.
To determine the contribution of ongoing viral replication to residual viremia in patients on ART, we carried out 2 independent treatment intensification studies and measured changes in viremia by using the SCA with a limit of detection of 1 copy per milliliter (17). Our studies included patients on suppressive regimens and used 1 of 3 different potent, Food and Drug Administration-approved antiretroviral agents, new to each patient, for intensification. Separate analyses of the 2 studies and analysis of the pooled data revealed that treatment intensification did not reduce the level of residual viremia. This result does not support ongoing, complete cycles of replication as the principal source of persistent viremia.
Results
Study Participants.
At the Johns Hopkins Hospital (JHH), 10 patients were enrolled into the atazanavir/ritonavir (ATV/r) intensification study; at the National Institutes of Health (NIH), 7 patients were enrolled into a study of treatment intensification with either efavirenz (EFV) or lopinavir/ritonavir (LPV/r). Two patients discontinued the studies before completing intensification and were excluded from further analysis: 1 patient in the ATV/r intensification study who requested to be discontinued before drug intensification for personal reasons, and 1 patient who discontinued EFV intensification because of fatigue. None of the study patients suffered serious adverse events. Although all 15 patients had viral RNA levels ≥1 copy per milliliter during at least one screening visit, only 9 participants had viral RNA levels ≥1 copy per milliliter for the majority of preintensification time points. These 9 patients were used for the primary analysis of treatment intensification (Table 1). Secondary analyses that included the 6 other patients (Table S1) were also performed.
Table 1.
Demographic factors and clinical characteristics
Participants had an average CD4+ T cell count of 565 cells per microliter (range, 367–799 cells per microliter) and suppression of viremia on a stable ART for an average of 4 years (range, 0.7–10.0 years). NIH participants were younger than JHH participants (mean, 41.8 vs. 50.8 years) and had a higher representation of men who have sex with men as an HIV-1 risk factor (75% vs. 60%). All participants were on ART consisting of a nucleoside reverse transcriptase inhibitor (NRTI) backbone and either a nonnucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor (PI).
Effect of Treatment Intensification on Residual Viremia.
Before intensification, participants had repeated measurements of viremia with the SCA. The median level of viremia in the participants was 3 copies per milliliter, consistent with previous reports (18, 19). Treatment intensification was carried out with 1 of 3 regimens (ATV/r, LPV/r, or EFV), all of which are potent antiretroviral agents recommended for initial ART (29). The drug chosen for intensification was, in all cases, from a class (PI or NNRTI) not previously used by the participant. Treatment intensification was conducted for either 4 (EFV and LPV/r intensification) or 8 (ATV/r intensification) weeks.
Combined results for the 5 participants at JHH intensified with ATV/r are summarized in Fig. 1A. Median levels between samples taken before, during, or after the ATV/r intensification were 3, 5, and 2 copies per milliliter, respectively. Results from each of these 5 participants are shown individually in Fig. 1B. Interestingly, patient JHH-3 demonstrated median plasma HIV-1 RNA levels of 30 copies per milliliter, significantly higher than those of the other patients, who demonstrated median levels of <10 copies per milliliter (Fig. 1B, JHH-3). At times, levels of viremia in patient JHH-3 were close to the detection limit of standard assays (50 copies per milliliter), and this observation was consistent with the patient's prestudy history of frequent “blips” (intermittent viremia >50 copies per milliliter). Although the level of residual viremia in this patient was relatively high, this pattern did not change during intensification (Fig. 1B, JHH-3). Furthermore, the level of residual viremia did not decrease in the other 4 patients as a consequence of ATV/r intensification (Fig. 1B).
Fig. 1.
Intensification does not reduce residual viremia in patients on ART. (A) Median HIV-1 RNA levels of the 5 JHH patients at multiple sampling points before, during, and after intensification with ATV/r. (B) Levels of viremia in the JHH patients intensified with ATV/r. (C) Median HIV-1 RNA levels of the 4 NIH patients at multiple sampling points before, during, and after intensification with EFV and LPV/r. (D) Levels of viremia in NIH patients intensified with either EFV or LPV/r. Open symbols represent measurements below the limit of detection (1 copy per milliliter).
Results for the 4 NIH participants intensified with EFV or LPV/r are summarized in Fig. 1 C and D. Median HIV-1 RNA levels for the 4 participants were 3, 6, and 5 copies per milliliter before, during, and after intensification, respectively. As with the ATV/r group, the frequency of detectable viremia did not change during the intensification interval and study follow-up.
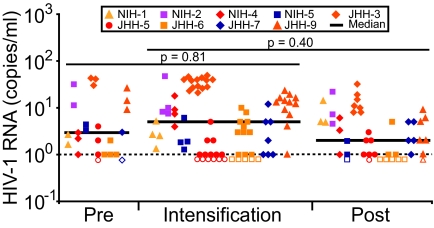
Fig. 2 shows the HIV-1 RNA results for JHH and NIH participants combined (n = 9). Median levels of viremia during the intensification periods were not significantly different from preintensification and postintensification HIV-1 RNA levels (P = 0.81 and 0.40, respectively).
Fig. 2.
ART intensification does not reduce HIV-1 viremia. HIV-1 RNA levels for all time points for all 9 patients. Each set of colored symbols represents HIV-1 RNA values obtained for the indicated patient during each phase of the study and corresponds to the symbols used in Fig. 1. Open symbols represent measurements below the limit of detection (1 copy per milliliter). Levels of viremia during intensification were not statistically different from preintensification and postintensification levels.
Variation in viremia was present in individual patients during the study period, with 5 patients exhibiting slight declines in the slope of viremia (−0.0034 to −0.62 copies per milliliter per week) and increases in the slope of viremia in 4 patients (0.006–0.67 copies per milliliter per week) during the entire study period. Importantly, however, in each of the 9 patients, the slope of viremia during the intensification period was not significantly different from zero, indicating there was no evidence of decline in viremia as a consequence of drug intensification.
To summarize, in neither the aggregate results nor in any of the 9 individual study subjects could a statistically significant difference in median viremia levels be detected before, during, or after treatment intensification.
Six participants excluded from the primary analysis because of inconsistently detectable viremia in the preintensification period (Fig. S1 and Table S1) were included in a secondary analysis. Inclusion of these participants did not change the results of the comparisons; there were no significant differences in median HIV-1 RNA levels before, during, or after intensification (P = 0.18 and 0.09, respectively).
Levels of Intensifying Drugs in Plasma.
To assess whether the participants received adequate doses of drug, plasma concentrations of the intensifying drugs were measured before, during, and after treatment intensification (Fig. 3).
Fig. 3.
Therapeutic concentrations of intensifying drug were achieved during the intensification period. (A) ATV. (B and C) EFV. (D) LPV. Open symbols represent measurements below the LOQ for respective drugs.
In patients taking EFV, ATV was boosted with ritonavir (RTV) as recommended in Department of Health and Human Services guidelines (30). During the intensification interval, levels of the intensifying ATV in our study population were maintained at a median concentration of 1.018 μg/mL (range, <0.088 to 2.69 μg/mL), which is 47-fold more than IC90 of ATV in primary CD4+ T cells (31) (Fig. 3A). Levels of EFV used in prestudy regimens of the JHH participants were not altered during periods of intensification (Fig. 3B). In patients intensified with LPV or EFV, the levels of LPV (range, 5.50–5.98 μg/mL) and EFV (range, 1.82–2.37 μg/mL) were within the therapeutic range during the intensification periods (Fig. 3 C and D). Levels of ATV, LPV, and EFV returned to below detection limits after completing intensification, demonstrating that viral RNA levels obtained during the postintensification period were the result of the baseline regimen. These results demonstrate that therapeutic concentrations of intensification drugs were achieved in study participants during intensification. Small fluctuations in HIV-1 RNA levels did not correlate with changes in intensifying drug concentration.
Sequence Analysis of the pro Gene in ATV-Intensified Patient Virus.
To ensure that a lack of decay of residual viremia after intensification was not associated with drug-resistant virus, we isolated HIV-1 RNA from plasma specimens obtained from patients in the ATV/r intensification study and sequenced pro by using sensitive amplification techniques (24–26, 32). In 4 patients with frequently detectable residual viremia in the range of 1–50 copies per milliliter, we were able amplify and sequence 1–6 independent, clonal amplicons per patient (26).
Clonal sequence analysis confirmed that HIV-1 sequences were patient-specific. None of the patients had plasma virus with major ATV resistance mutations I50L, I84V, or N88S (33) (Fig. S2). Some patients had polymorphisms considered to be minor resistance mutations for ATV; however, these variants are common in drug-naïve patients, and individually they do not appear to confer resistance to ATV (34).
Discussion
Currently recommended ART is capable of suppressing plasma HIV-1 RNA to levels <50 copies per milliliter for long periods, but cell-free virions can be found for many years in the plasma of most patients on ART. It has been unclear whether low-level residual viremia is primarily derived from complete cycles of viral replication that continue despite ART, and it has been frequently suggested that a study of antiretroviral intensification may clarify this critical question (16, 35). By using an RT-PCR assay capable detecting 1 copy of HIV RNA per milliliter, we found that levels of residual viremia were not affected by intensification of ART with an additional, potent antiretroviral drug. Moreover, this observation was not the result of suboptimal exposure to the intensifying drugs or the presence of drug-resistant virus. The study was conducted at 2 different sites with 3 different intensifying agents, and the results did not vary according to research site, intensifying drug class, or intensifying drug.
This result contradicts a previous study of ART intensification that suggested a contribution of ongoing HIV-1 replication to residual viremia (27). That study, however, was conducted in patients who were on a nonstandard 2-drug ART regimen (efavirenz and indinavir), which may have been incompletely suppressive relative to current 3-drug regimens. Interestingly, preintensification viral RNA levels were higher than those of our patients, and decreased during intensification to within the range of viral RNA levels detected in our study. The effects of an intensifying agent on residual viremia in patients on standard of care therapy have not been previously described. All study participants were on combination antiretroviral regimens with at least 3 active antiretrovirals and had maintained viral suppression below the clinical limit of detection for an average of 4.8 years. Moreover, all of the patients were undergoing combination antiretroviral therapy with at least 2 NRTIs and either a PI or an NNRTI. In the present study, we hypothesized that if active cycles of HIV-1 replication were contributing to viremia, there would be a substantial effect on levels of viremia during the period of intensification observable on a time scale approximating the half-life of actively infected cells (1–2 days), as documented for antiretroviral regimens, including monotherapy (1). In our study, comparison of HIV-1 viremia before, during, and after intensification revealed no detectable effect of this kind, in clear contradiction to our hypothesis.
Previous reports have suggested that transiently detectable viremia (blips) may be a surrogate for ongoing replication (28). In this regard, JHH-3 had a comparatively high level of residual viremia (median, 30 copies per milliliter; range, 8–49 copies per milliliter) and a history of 7 episodes of blips during the 3 years before study enrollment. Nevertheless, addition of ATV/r did not reduce the level of viremia during the period of intensification. The history of frequent blips in this patient is thus likely to be related to his relatively high steady-state level of residual viremia and not to ongoing viral replication. In support of this view, Nettles et al. (32) proposed that blips may be a consequence of biological and statistical variation around a mean HIV-1 RNA level just below the detection limit, representing output from stable viral reservoirs.
One potential limitation of our study is that we cannot exclude the possible contributions of viral replication in putative “drug sanctuary” sites to residual viremia. The central nervous system (36, 37) (CNS) and genitourinary (GU) tract (38) are regarded as distinct compartments of HIV-1 infection in which antiretroviral penetration may be reduced (39–45). The recent CHARTER study (46) measured levels of ATV in cerebrospinal fluid (CSF); even with RTV boosting, CSF levels were ≈100-fold lower than plasma levels and were lower than the reported ATV IC50. Additional studies from this group (45) have rank-ordered penetration of antiretrovirals into CSF; some older drugs (e.g., indinavir) have higher relative penetration than newer agents, such as ATV and tenofovir. With regard to the drugs used for intensification in our study, LPV/r was considered to have a high CNS-penetration effectiveness score (45). Based on ratios of penetration into sanctuary sites and clinically determined trough plasma concentrations (BMS EFV package insert), we estimate EFV levels in CSF and semen to be 1.8-fold and 30-fold higher, respectively, than the IC90 (6 ng/mL) (31). Thus, these 2 drugs may give better CNS penetration than ATV/r. It is also important to note that the real penetration of any antiretroviral into the brain has not been extensively studied, and studies using CSF as a surrogate show that CSF may not accurately reflect levels of virus or drug penetration in brain parenchyma (47). In addition, NNRTIs and PIs are strongly protein-bound, and the reduction in drug levels in compartments such as CSF may be partially compensated by the substantial reduction in protein concentration in this site (48). With regard to the GU tract, previous studies have shown that individuals on EFV-based ART have undetectable (<5 copies per milliliter) HIV-1 RNA levels in semen while maintaining low but measurable levels (between 5 and 50 copies per milliliter) in plasma (16). Clearly, the issue of drug sanctuary sites requires further study and remains an important caveat in the interpretation of intensification studies. However, it should be emphasized that whatever the differences in the abilities of the intensification agents to penetrate the CNS and GU tract, such potential differences were not reflected in the outcomes of intensification; levels of residual viremia were not reduced with any intensification agent. Collectively, available evidence suggests that detectable HIV-1 RNA in the CNS and GU compartments during ART is uncommon and that viral replication in these compartments is unlikely to make a major contribution to residual viremia in most treated patients.
A second potential limitation was study size. We investigated antiretroviral intensification in a relatively small group of 9 patients, and it is possible that a larger survey would identify patients with viremia suppressible on intensification. In our study, 0 of 9 evaluable participants experienced a decrease in HIV-1 RNA levels, which is not consistent with an intensifying antiretroviral drug effect on active cycles of HIV replication.
Our studies enrolled a total of 15 participants, and all patients exhibited variability in viremia during the study period. In 6 participants, the majority of determinations before intensification and during intensification were near or below the limit of detection of the SCA (Fig. S1 and Table S1), making it difficult to assess the impact of intensification. Consequently, these 6 participants were excluded from our primary analysis. Such exclusion may have introduced bias because patients with undetectable values after intensification, who would show evidence of decline, would be eliminated. This exclusion did not appear to create a bias, however, because an aggregate analysis of all 15 patients revealed no significant differences in median HIV-1 RNA values before, during, and after intensification. Thus, intensification did not have a statistically significant effect on the level of residual viremia either in primary or secondary analysis. The cumulative experience of intrapatient variability in participants will be useful in informing design and sample size estimates for future trials.
Finally, our analyses are based on the assumption that cells successfully infected in the presence of ART have the same kinetic properties as those in untreated patients. If the cells responsible for residual viremia live substantially longer than a few days after infection, then our ability to detect a significant decline might have been limited by the relatively short duration of treatment intensification. We consider this possibility very unlikely, however, because it would require that antiretroviral drugs inhibit infection of short-lived but not long-lived cells.
Although we did not detect significant changes in viremia over time during the intensification period, detectable increases in residual viremia on ART have been identified in studies of antiretroviral simplification. Recently, in collaboration with Wilkin and colleagues, we detected unequivocal increases in residual viremia after simplification of standard combination ART to ATV/r alone. Increases in plasma HIV-1 RNA occurred 4–12 weeks before viremia became detectable (>50 copies per milliliter) by commercial assays. These results indicate that the SCA used in the current study can detect changes in residual HIV-1 RNA in patients who simplify to a less potent regimen (49).
In summary, studies of HIV-1 RNA kinetics have provided insights into the rate of viral replication, disease progression, and the response to therapy (1–7). Here, we show that intensification of ART does not reduce residual viremia in patients with HIV-1 RNA levels <50 copies per milliliter. This finding is consistent with qualitative studies showing that the residual virus is genetically stable and lacks new resistance mutations (24–26) with quantitative studies suggesting that the level of residual viremia is a reflection of the size of stable compartments established before therapy (18, 19) and with observations of consistently low levels of residual viremia in patients on different ART regimens (18). The absence of further decline in viremia with antiretroviral intensification studies supports our thesis that standard combination therapy is maximally suppressive. Because our findings are consistent with the hypothesis that residual viremia primarily represents output from stable reservoirs of infection, strategies for identifying and targeting these reservoirs may be the next important step in eliminating the persistence of HIV-1 infection.
Materials and Methods
Subjects.
HIV-infected patients were recruited for 2 independent intensification studies at the JHH and the NIH. Subjects at JHH received intensification with RTV-boosted ATV, and NIH study patients received intensification with EFV or RTV-boosted LPV.
Atazanavir Intensification Study.
The JHH study recruited HIV-1-infected adults (ages 18–60 years) who had suppression of viremia to <50 copies per milliliter for >6 months on ART, had never been treated with protease inhibitors, and had CD4+ T cell counts >200 cells per microliter for >6 months. Subjects were excluded if they had any known genotypic resistance to ATV, reported the use of medications contraindicated for coadministration with ATV, had hepatitis C coinfection, were significantly anemic, or had a history of ongoing alcohol abuse, illicit drug use, or medical noncompliance. The study was approved by the Johns Hopkins Institutional Review Board (IRB), and patients enrolled January 2007 to January 2008.
Efavirenz and Lopinavir Intensification Study.
The NIH study used similar recruitment criteria, except that there was no upper age limit for eligibility, and hepatitis C coinfection was not an exclusion criterion (one patient had a history of hepatitis C). None of the enrolled patients had been exposed to the class of drug used for intensification. The study was approved by the National Institute of Allergy and Infectious Diseases IRB, and patients enrolled March 2006 to October 2007.
The ATV intensification study was conducted over a 16-week interval. Baseline viremia assessments were performed for each study patient over 4 consecutive weeks (study weeks 1–4). Daily ATV (300 mg) coadministered with RTV (100 mg) was then added beginning in week 5 and continuing through week 12, during which twice-weekly plasma HIV-1 RNA measurements were performed. During the postintensification phase of the study (weeks 13–16), ATV and RTV were discontinued, and twice-weekly measurements were continued.
During the treatment intensification period (weeks 5–12), the patients informed the study coordinator of medication adherence, new medications, and observed symptoms by using a weekly questionnaire. The patients were assessed at weeks 6, 10, and 14 for symptoms and signs of drug toxicity or excessive phlebotomy. Additionally, they were physically examined by the study safety officer at baseline and on weeks 9 and 14 of the protocol. Plasma ATV levels were assessed on a weekly basis to confirm adherence to and absorption of the study medication.
The EFV and LPV/RTV intensification studies included a 30-day intensification period. Each patient contributed baseline samples before undergoing the 30-day interval of regimen intensification. The patients underwent blood sampling at days 7, 14, 21, 29, and 30 on the protocol. The intensifying drug was discontinued after day 30, followed by an optional 30-day washout period. Samples for drug level measurements were obtained before, during, and after intensification.
Drug dosage adjustments recommended by the Department of Health and Human Services guidelines for the new drug additions were made. NIH-1, taking unboosted ATV 400 mg/day, was switched to boosted ATV therapy (ATV 300 mg/day and RTV 100 mg/day) during intensification with EFV 600 mg/day. NIH-3 and NIH-6, who intensified LPV/r-containing regimens with EFV, increased LPV/r dosing from 400 mg of LPV and 100 mg of RTV twice daily to 600 mg of LPV and 150 mg of RTV twice daily during the intensification. NIH-4 and NIH-5 underwent intensification with LPV/r at a dose of 600 mg of LPV and 150-mg ritonavir.
Specimen Collection.
Seven milliliters of plasma was apportioned for plasma HIV-1 RNA measurements, and the remaining plasma (typically 4–5 mL) was saved for sequencing analysis and drug level testing. All plasma specimens were immediately frozen and stored at −80 °C until use.
Quantification of Residual Viremia.
Levels of residual viremia were measured at JHH and NIH by using the SCA, an ultrasensitive HIV-1 RNA assay, as described elsewhere (17). Both sites determined a limit of quantification (LOQ) of 1 copy per milliliter (17) for the SCA by using HIV-1 virus standards of a known concentration obtained through the NIH AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases: HIV-1 VQA RNA Quantification Standard from the Division of AIDS Virology Quality Assurance (VQA) Program. At both sites, the methodology for the determination of the LOQ was based on a study demonstrating the 50 copies per milliliter LOQ for the ultrasensitive viral RNA assay used in clinical settings (50). By using the same virus standards and methodology for validation of the LOQ, we ensured that measurements conducted at both sites are in agreement and accurate.
Sequence Analysis of Plasma Virus.
To ensure that only virion RNA was measured, virus particles from plasma specimens (3–7 mL) were collected by ultracentrifugation at 170,000 × g for 30 min at 4 °C. Virus particles were resuspended, lysed, and viral RNA extracted and subjected to a 2-step nested RT-PCR in 7 separate reactions as described elsewhere (24–26, 32). PCR products were cloned by using the TOPO cloning system (Invitrogen), and at least 6 colonies derived from 1 RT-PCR were sequenced to analyze clonality.
Drug Level Monitoring.
ATV, LPV, and EFV concentrations were determined by using validated HPLC-UV assays by C. Flexner at Johns Hopkins University as described previously (51, 52). Briefly, ATV and LPV were extracted from plasma by using a liquid–liquid extraction, and EFV was extracted by using protein precipitation. Interday and intraday accuracies and precisions ranged from 94.9% to 111.1% and 0.4% to 6.4%, respectively, for all analytes.
Statistical Analysis.
Based on our experience with patients on suppressive antiviral therapy, we anticipated that there would be a substantial number of measurements below the 1 copy per milliliter LOQ, even using our sensitive HIV RNA assay. Thus, we planned for several different analyses to evaluate the impact of therapy intensification. We first considered plasma HIV-1 RNA level as a binary variable (above or below 1 copy per milliliter) and used repeated-measures regression models with generalized estimating equation techniques to determine whether the proportion <1 copy per milliliter increased over time, adjusting for the correlation within individuals. Second, we also used parametric mixed regression models with left censoring limits that enabled us to more fully model the distribution of the HIV-1 RNA data. We used an extension of the model used by Hughes (53) for repeated measures as implemented by Thiebaut (54). In this model, we assume log HIV RNA values can be described by a normal distribution but incorporate both censoring below the 1 copy per milliliter limit and correlation from repeated measurements using a random intercept term. Permutation tests yielded similar conclusions.
Slopes of HIV-1 viremia were obtained by linear regression; the t test statistic (t = b/SE, where b is the slope of the regression line and SE is the standard error of the slope) was calculated, from which the P value was computed.
Supplementary Material
Acknowledgments.
We are grateful to the participants who volunteered for this study. We thank the laboratory of C. Flexner for performing drug level monitoring. We thank R. Burke and S. Stallings for assistance and thank J. Kovacs, R. Davey, M. Polis, H. Masur, V. Boltz, and H. C. Lane for helpful discussions. J.M.C. was a Research Professor of the American Cancer Society, with support from the George Kirby Foundation. J.W.M. received support through Science Applications International Corporation Subcontract 25XS119.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903107106/DCSupplemental.
References
- 1.Ho DD, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 2.Wei X, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 3.Mellors JW, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 4.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: Virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 5.Gulick RM, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 6.Hammer SM, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS clinical trials group 320 study team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 7.Perelson AS, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 8.Chun TW, et al. In vivo fate of HIV-1-infected T cells: Quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 9.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 10.Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 12.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 13.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 14.Siliciano JD, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 15.Strain MC, et al. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: Intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci USA. 2003;100:4819–4824. doi: 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dornadula G, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. J Am Med Assoc. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 17.Palmer S, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldarelli F, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3:e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer S, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105:3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paterson DL, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 21.Maggiolo F, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials. 2007;8:282–292. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- 22.Nachega JB, et al. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 23.Joos B, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci USA. 2008;105:16725–16730. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermankova M, et al. HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/mL receiving combination therapy. J Am Med Assoc. 2001;286:196–207. doi: 10.1001/jama.286.2.196. [DOI] [PubMed] [Google Scholar]
- 25.Persaud D, et al. Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. J Virol. 2004;78:968–979. doi: 10.1128/JVI.78.2.968-979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey JR, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80:6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Havlir DV, et al. Productive infection maintains a dynamic steady state of residual viremia in human immunodeficiency virus type 1-infected persons treated with suppressive antiretroviral therapy for five years. J Virol. 2003;77:11212–11219. doi: 10.1128/JVI.77.20.11212-11219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramratnam B, et al. Intensification of antiretroviral therapy accelerates the decay of the HIV-1 latent reservoir and decreases, but does not eliminate, ongoing virus replication. J Acquir Immune Defic Syndr. 2004;35:33–37. doi: 10.1097/00126334-200401010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Hammer SM, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the international AIDS society-USA panel. J Am Med Assoc. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 30.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Washington, DC: Department of Health and Human Services; 2008. pp. 1–128. [Google Scholar]
- 31.Shen L, et al. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat Med. 2008;14:762–766. doi: 10.1038/nm1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nettles RE, et al. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. J Am Med Assoc. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 33.Colonno R, et al. Identification of I50L as the signature atazanavir (ATV)-resistance mutation in treatment-naive HIV-1-infected patients receiving ATV-containing regimens. J Infect Dis. 2004;189:1802–1810. doi: 10.1086/386291. [DOI] [PubMed] [Google Scholar]
- 34.Kozal MJ, et al. Extensive polymorphisms observed in HIV-1 clade B protease gene using high-density oligonucleotide arrays. Nat Med. 1996;2:753–759. doi: 10.1038/nm0796-753. [DOI] [PubMed] [Google Scholar]
- 35.Ramratnam B, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6:82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 36.Peeters MF, et al. Comparison of human immunodeficiency virus biological phenotypes isolated from cerebrospinal fluid and peripheral blood. J Med Virol. 1995;47:92–96. doi: 10.1002/jmv.1890470117. [DOI] [PubMed] [Google Scholar]
- 37.Clements JE, et al. The central nervous system is a viral reservoir in simian immunodeficiency virus-infected macaques on combined antiretroviral therapy: A model for human immunodeficiency virus patients on highly active antiretroviral therapy. J Neurovirol. 2005;11:180–189. doi: 10.1080/13550280590922748-1. [DOI] [PubMed] [Google Scholar]
- 38.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 39.Foudraine NA, et al. Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet. 1998;351:1547–1551. doi: 10.1016/S0140-6736(98)07333-4. [DOI] [PubMed] [Google Scholar]
- 40.Aweeka F, et al. Failure to detect nelfinavir in the cerebrospinal fluid of HIV-1-infected patients with and without AIDS dementia complex. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:39–43. doi: 10.1097/00042560-199901010-00006. [DOI] [PubMed] [Google Scholar]
- 41.Kravcik S, et al. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J Acquir Immune Defic Syndr. 1999;21:371–375. [PubMed] [Google Scholar]
- 42.Tashima KT, et al. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J Infect Dis. 1999;180:862–864. doi: 10.1086/314945. [DOI] [PubMed] [Google Scholar]
- 43.Taylor S, et al. Penetration of efavirenz into the male genital tract: Drug concentrations and antiviral activity in semen and blood of HIV-1-infected men. AIDS. 2001;15:2051–2053. doi: 10.1097/00002030-200110190-00022. [DOI] [PubMed] [Google Scholar]
- 44.van Leeuwen E, et al. Penetration of atazanavir in seminal plasma of men infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2007;51:335–337. doi: 10.1128/AAC.00774-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letendre S, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Best BM, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS. 2009;23:83–87. doi: 10.1097/QAD.0b013e328317a702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis. 2008;197(Suppl 3):S294–S306. doi: 10.1086/533419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalvass JC, Maurer TS, Pollack GM. Use of plasma and brain unbound fractions to assess the extent of brain distribution of 34 drugs: Comparison of unbound concentration ratios to in vivo P-glycoprotein efflux ratios. Drug Metab Dispos. 2007;35:660–666. doi: 10.1124/dmd.106.012294. [DOI] [PubMed] [Google Scholar]
- 49.Wilkin TJ, et al. Regimen simplification to atazanavir-ritonavir alone as maintenance antiretroviral therapy: Final 48-week clinical and virologic outcomes. J Infect Dis. 2009;199:866–871. doi: 10.1086/597119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun R, et al. Ultrasensitive reverse transcription-PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1998;36:2964–2969. doi: 10.1128/jcm.36.10.2964-2969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamotte C, Peytavin G, Farinotti R. Determination of nelfinavir, a potent HIV protease inhibitor, and its active metabolite M8 in human plasma by high-performance liquid chromatography with photodiode-array detection. J Chromatogr B Biomed Sci Appl. 1999;735:159–170. doi: 10.1016/s0378-4347(99)00419-3. [DOI] [PubMed] [Google Scholar]
- 52.Nettles RE, et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42:1189–1196. doi: 10.1086/501458. [DOI] [PubMed] [Google Scholar]
- 53.Hughes JP. Mixed effects models with censored data with application to HIV RNA levels. Biometrics. 1999;55:625–629. doi: 10.1111/j.0006-341x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 54.Thiebaut R, Jacqmin-Gadda H. Mixed models for longitudinal left-censored repeated measures. Comput Methods Programs Biomed. 2004;74:255–260. doi: 10.1016/j.cmpb.2003.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.