Abstract
The Prader–Willi syndrome (PWS) genetic interval contains several brain-expressed small nucleolar (sno)RNA species that are subject to genomic imprinting. In vitro studies have shown that one of these snoRNA molecules, h/mbii-52, negatively regulates editing and alternative splicing of the serotonin 2C receptor (5htr2c) pre-RNA. However, the functional consequences of loss of h/mbii-52 and subsequent increased post-transcriptional modification of 5htr2c are unknown. 5HT2CRs are important in controlling aspects of cognition and the cessation of feeding, and disruption of their function may underlie some of the psychiatric and feeding abnormalities seen in PWS. In a mouse model for PWS lacking expression of mbii-52 (PWS-IC+/−), we show an increase in editing, but not alternative splicing, of the 5htr2c pre-RNA. This change in post-transcriptional modification is associated with alterations in a number of 5HT2CR-related behaviours, including impulsive responding, locomotor activity and reactivity to palatable foodstuffs. In a non-5HT2CR-related behaviour, marble burying, loss of mbii-52 was without effect. The specificity of the behavioural effects to changes in 5HT2CR function was further confirmed using drug challenges. These data illustrate, for the first time, the physiological consequences of altered RNA editing of 5htr2c linked to mbii-52 loss that may underlie specific aspects of the complex PWS phenotype and point to an important functional role for this imprinted snoRNA.
INTRODUCTION
RNA editing is increasingly being recognized as a novel post-transcriptional mechanism to modify the function of an encoded gene. In the brain, a number of neurotransmitter receptor pre-mRNA species have been identified as subject to RNA editing processes (1–3). The serotonin 2C receptor (5HT2CR) pre-RNA is subject to adenosine-to-inosine editing at five sites (A, B, C, D, E) within the alternatively spliced exon Vb. As inosine behaves as a guanine in translation, editing causes a change in the amino acid sequence in the second intracellular loop of the encoded receptor protein, which in turn results in a less functional 5HT2CR (1). The level of editing of the 5htr2c pre-mRNA has been shown to be sensitive to pharmacological and behavioural manipulations of serotonin levels (4–6) and is altered in psychiatric disease states (7,8). Also, genetic manipulations that lead to extreme levels of editing can have behavioural effects (9).
A key regulator of post-transcriptional modification of 5htr2c pre-mRNA is the small nucleolar (sno)RNA, hbii-52. hbii-52 has an 18nt complementary anti-sense box to the edited region of the 5htr2c pre-mRNA and was first identified as one of several snoRNA species found in the Prader–Willi syndrome (PWS) imprinting cluster (10). In vitro studies have demonstrated that this snoRNA is important in modulating both RNA editing (11) and alternative splicing (12,13) of 5htr2c pre-mRNA. Furthermore, studies of post mortem PWS brain have indicated that when loss of expression of hbii-52 occurs, this is correlated with an increase in RNA editing of 5htr2c pre-mRNA (13). 5HT2CRs play many important roles in the brain, and as such abnormal post-transcriptional modifications of 5htr2c pre-mRNA due to loss of hbii-52 may underlie some of the PWS behavioural phenotype, in particular, aspects of psychiatric illness. However, as yet no one has directly assessed the physiological consequences of hbii-52 loss.
Utilizing an imprinting centre deletion model of PWS (14), we were able to examine the behavioural consequences of an alteration in RNA editing of the 5htr2c pre-mRNA as a result of the loss of expression of mbii-52, the murine homologue of hbii-52. Our data point to an important and novel role for this imprinted snoRNA in regulating brain serotonin effects on behaviour via its action on 5htr2c.
RESULTS
Altered RNA editing but not alternative splicing of 5htr2c in PWS-IC+/− brain
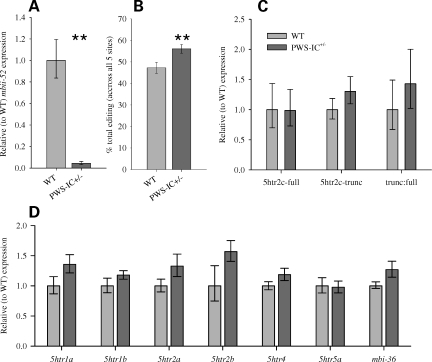
First, it was important to demonstrate a clear change in the molecular processing of 5htr2c pre-RNA in the PWS-IC+/− brain and link these changes with the loss of the snoRNA mbii-52 in this model. As expected, quantitative real-time PCR (qPCR) analysis revealed a significantly reduced expression of mbii-52 in adult PWS-IC+/− brain (single hemisphere) samples (Fig. 1A). We then went on to examine the extent of RNA editing of the 5htr2c pre-RNA and found an increase in overall levels of A-to-I editing (Fig. 1B). However, qPCR analyses of the full- and truncated-splice variants of 5htr2c revealed no difference in expression levels or splice variant ratios between PWS-IC+/− and controls (Fig. 1C). Furthermore, qPCR revealed no significant changes in expression of several other important 5HT receptor types (Fig. 1D). Similarly, there was no change in the expression of the snoRNA mbi-36 (Fig. 1D), which is transcribed from the second intron of 5htr2c and has been suggested to play an antagonistic role to mbii-52 (10).
Figure 1.
Molecular analysis. (A) Expression of mbii-52 was almost completely abolished in the brains of PWS-IC+/− mice (n = 8) compared with WT controls (n = 8); Student's t-test, P = 0.002. There was an increase in overall levels of editing of 5htr2c pre-RNA (B); ANOVA, F1,146 = 7.28, P = 0.008. However, there was no change in the expression levels of the full-length (full) and truncated (trunc) splice variants (C); Student's t-test; full, P = 0.33; trunc, P = 0.25. There was no significant change in expression levels of several other important serotonin receptor genes or the snoRNA mbi-36, which is transcribed from within the second intron of the 5htr2c gene (D); Student's t-test, P = 0.28. Data shown are the mean for each group ± SEM; **P < 0.001.
mbii-52 has a complementary anti-sense box specifically to the 5htr2c pre-RNA. We would therefore expect post-transcriptional modifications only at this RNA species. This was clearly demonstrated by examining the degree of editing of another neurotransmitter receptor pre-RNA that is subject to post-transcriptional modification, Glur2. There were no differences in the percentage levels of editing of Glur2 between PWS-IC+/− (84.1%) and wild-type (WT; 82.6%) adult hemi-brain samples (χ2, P = 0.82).
PWS-IC+/− mice show increased 5HT2cR-mediated impulsivity in the 5-choice serial reaction time task
In order to examine the behavioural consequences of increased editing, we assessed the performance of the PWS-IC+/− mice in the 5-choice serial reaction time task (5-CSRTT). We chose 5-CSRTT because it is an extensively characterized cognitive task that uses nose poke responses to brief flashes of light presented pseudorandomly across an array of five locations to measure aspects of attention (response accuracy and omissions) and impulse control (premature responding) in rodents (15). Moreover, the task is well defined in terms of the role of neurotransmitter systems, and pharmacological manipulation of 5HT2C receptors in the 5-CSRTT produces distinct and robust effects on impulse control (16–18). The rodent 5-CSRTT is analogous to continuous performance tasks in humans and as such measures behaviours of relevance to aspects of psychiatric disease (15,19,20).
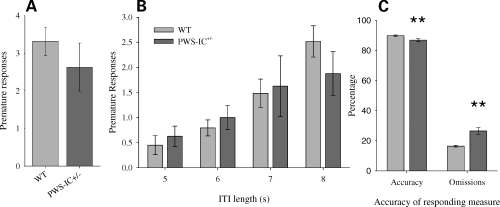
PWS-IC+/− and WT control mice acquired the task to performance criteria (>80% accuracy and <25% omissions). Under baseline, non-drugged conditions, premature responding during the inter-trial interval (ITI; when the subject is waiting for the light stimulus presentation to occur) was not different between PWS-IC+/− mice and WT controls, either at the baseline ITI of 5 s (Fig. 2A) or under conditions where the ITI was increased within the session to encourage the tendency to make an impulsive response (Fig. 2B). There were, however, small but significant differences between the PWS-IC+/− mice and WT controls in behavioural indices of attentional functioning, with the PWS-IC+/− mice showing relatively worse performance in discriminative accuracy measures and percentage omissions (Fig. 2C).
Figure 2.
Behavioural measures of performance in the 5-CSRTT. There were no differences between PWS-IC+/− (n = 16) and WT (n = 29) in the number of premature responses in the 5-CSRTT under baseline, non-drugged conditions (A); Students t-test, P = 0.31. Similarly, there were no differences in premature response number under conditions where the ITI was increased to induce impulsive responding (B); ANOVA, no main effect of GENOTYPE (F1,43 = 0.015, P = 0.904), no GENOTYPE × MANIPULATION interaction (F2.5,108.4 = 0.89, P = 0.43). Although PWS-IC+/− achieved criteria (>80% accuracy and <25% omissions), under baseline conditions they displayed small, but significant, impairments in measures of attentional function (C); Student's t-test; accuracy P = 0.004; omissions, P < 0.001. Data shown are the mean for each group ± SEM; **P < 0.001.
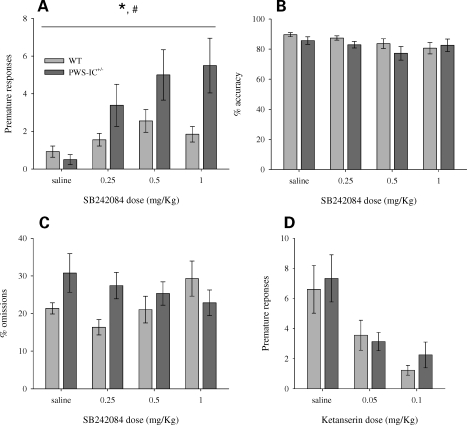
We then went on to examine 5HT2CR-mediated behaviour in the task directly by examining the response of PWS-IC+/− mice to the 2C-specific antagonist, SB242084. This drug has been shown to increase the number of premature responses in the 5-CSRTT in both rats (18) and mice (16). As expected, SB242084 systematically increased premature responding in a dose-dependent manner across both groups (Fig. 3A). However, this effect was more pronounced in PWS-IC+/− mice, where these subjects made nearly twice as many premature responses compared with WTs at the higher doses (Fig. 3A). The effect of SB242084 was specific to premature responding as measures of attention in the task were unaltered (Fig. 3B and C). To further test the specificity of serotonin-mediated interactions on premature responding, we then examined the response of both groups to ketanserin, a 5HT2AR antagonist that has weak affinity for 2C, and has been shown to reduce the number of premature responses in the 5-CSRTT (17). Ketanserin dose-dependently reduced premature responding equally in both the PWS-IC+/− and WT mice (Fig. 3D), indicating no differential 5HT2AR function in the PWS-IC+/− mice.
Figure 3.
Pharmacological manipulation of behavioural measures of performance in the 5-CSRTT. Premature responding in the 5-CSRTT was increased across both groups by the 5HT2CR antagonist, SB242084 (A). However, this effect was enhanced in PWS-IC+/− mice (n = 10) relative to WT controls (n = 25); ANOVA main effect of GENOTYPE, F1,33 = 5.69, P = 0.023; GENOTYPE × DOSE interaction F2.6,84.5 = 3.08, P = 0.039 (#P < 0.05). The effects of SB242084 on performance were specific to premature responding as measures of accuracy were unaffected (B); ANOVA, no effect of DOSE (F2.2,76.3 = 1.6, P = 0.26). Similarly, SB242084 had no effects on percentage omissions (C); ANOVA, no effect of DOSE (F2.4,72.9 = 1.65, P = 0.19). The differential effect on premature responding seen in PWS-IC+/− mice was specific to antagonism of 5HT2CR, as dosing with ketanserin, a 5HT2AR antagonist that has weak affinity for 5HT2CR, reduced premature responses equally across both groups (D); ANOVA, main effect of DOSE (F1.7,60.8 = 11.18, P < 0.001), no DOSE = GENOTYPE interaction (F1.7,60.8=0.105, P = 0.87). Data shown are the mean for each group ± SEM; *Main effect (of DOSE) P < 0.05, #Interaction P < 0.05.
PWS-IC+/− mice show increased locomotor activity in the presence of food reward
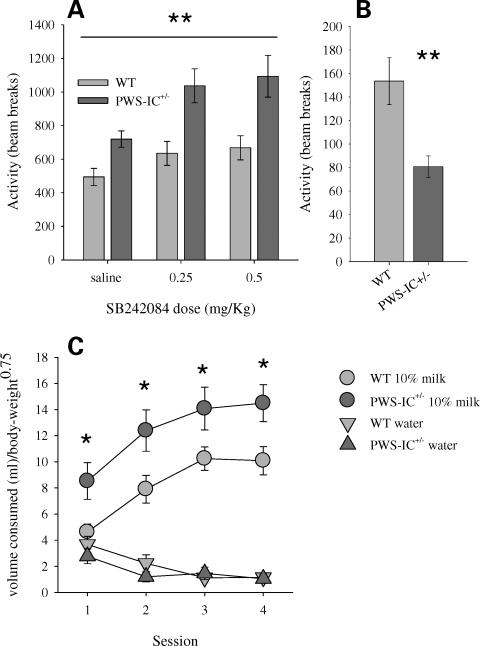
Analysis of beam breaks in the 5-CSRTT operant boxes suggested that PWS-IC+/− mice were more active than WT controls and that the 5HT2CR antagonist SB242084, which increased activity in both groups, had an enhanced effect in the PWS-IC+/− mice (Fig. 4A). This finding was even more striking given the fact that when locomotor activity (LMA) was measured outside the operant boxes in simple activity cages, where no food reward was present, the PWS-IC+/− mice were hypoactive compared with WT animals (Fig. 4B). We surmised that the increased activity displayed by the PWS-IC+/− mice in the operant boxes may reflect an enhanced arousal to the presence of the food reward (in this case 10% condensed milk solution). Support for this idea was provided by the 2-choice preference test, where the animals had to choose between water and the 10% condensed milk used in the 5-CSRTT. Indicative of an enhanced reactivity to palatable foodstuffs the PWS-IC+/− mice acquired a preference for the 10% condensed milk more rapidly and consumed more of the 10% condensed milk when normalized for body weight (Fig. 4C).
Figure 4.
LMA response to, and consumption of food rewards. LMA was greater in PWS-IC+/− mice compared with WT during the 5-CSRTT, and this was further enhanced by the 5HT2CR antagonist (SB242084) (A); ANOVA, main effect of GENOTYPE, F1,34 = 8.57, P = 0.006. However, in simple activity cages, where no food reward is present, PWS-IC+/− mice (n = 20) were hypoactive compared with WT controls (n = 19) (B); Student's t-test, P < 0.001. The idea that PWS-IC+/− mice show more motivational arousal by food rewards is supported by data from the 2-choice consumption test (C). Over successive daily sessions, PWS-IC+/− mice (n = 16) consumed more of the palatable foodstuff (10% condensed milk) than WT controls (n = 14), when normalized for body weight (body weight0.75); ANOVA, main effect of GENOTYPE, F1,26 = 20.7, P < 0.001. Data shown are the mean for each group ± SEM; *P < 0.05; **Main effect P < 0.001.
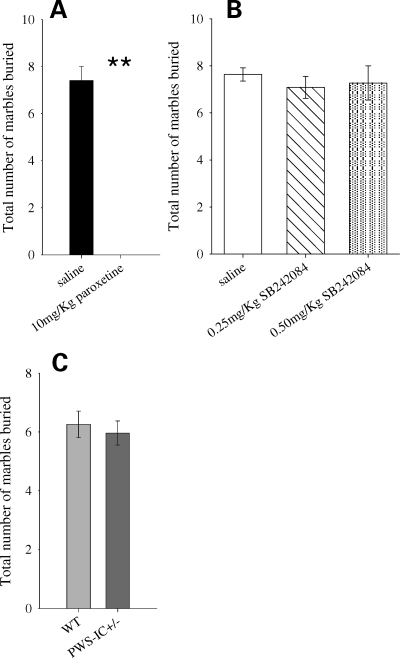
PWS-IC+/− mice show no effects on marble burying behaviour
As an additional test of the extent to which the effects of the loss of mbii-52 on behaviour were specific to 5HT2CR, we assessed the PWS-IC+/− mice in another behavioural task, the marble burying task, which was sensitive to general manipulations of serotonin but not to 5HT2CR manipulations. In line with other studies (21,22), we first demonstrated that the task in our hands was sensitive to dosing with a serotonin-selective re-uptake inhibitor (SSRI) and that marble burying behaviour was decreased (Fig. 5A). However, the task was insensitive to antagonism of 5HT2CR, as treatment with SB242084 at doses that produce behavioural changes in the 5-CSRTT had no effect on marble burying (Fig. 5B). PWS-IC+/− mice demonstrated equivalent behaviour to WT controls in the marble burying task (Fig. 5C).
Figure 5.
Marble burying behaviour. Marble burying behaviour in normal animals was abolished in response to general manipulations of serotonin levels by the SSRI paraxotine (n = 6) (A); Student's t-test, P < 0.001. However, the 5HT2CR antagonist SB242084 had no effect on burying behaviour (B); ANOVA, no main effect of dose F2,32 = 0.29, P = 0.75. Marble burying was equivalent between PWS-IC+/− (n = 10) and WT animals (n = 9) (C); Student's t-test, P = 0.63. Data shown are the mean for each group ± SEM; **P < 0.001.
PWS-IC+/− mice show no difference in whole tissue monoamine levels
We then went on to examine whether there were any general differences in whole tissue monoamine levels in the PWS-IC+/− mice in two key brain regions of relevance to the neural circuitry mediating impulse control, namely the pre-frontal cortex and ventral striatum. There were no systematic differences between PWS-IC+/− and WT mice in tissue levels of serotonin, dopamine or noradrenaline (Table 1).
Table 1.
Whole tissue monoamine levels in pre-frontal cortex and ventral striatum of WT (n = 8) and PWS-IC+/− (n = 6) mice
| Pre-frontal cortex |
Ventral striatum |
|||||
|---|---|---|---|---|---|---|
| WT (±SEM) | PWS-IC+/− (±SEM) | P-value (t-value) | WT (±SEM) | PWS-IC+/− (±SEM) | P-value (t-value) | |
| Serotonin (pmol/mg) | 0.145 (0.004) | 0.140 (0.007) | 0.59 (t12 = 0.55) | 0.127 (0.016) | 0.139 (0.008) | 0.57 (t12 = 0.57) |
| Dopamine (pmol/mg) | 0.033 (0.022) | 0.018 (0.002) | 0.57 (t12 = 0.58) | 0.360 (0.079) | 0.442 (0.074) | 0.47 (t12 = 0.47) |
| Noradrenaline (pmol/mg) | 0.139 (0.007) | 0.140 (0.009) | 0.95 (t12 = 0.57) | 0.120 (0.008) | 0.124 (0.009) | 0.76 (t12 = 0.31) |
DISCUSSION
Using the PWS-IC+/− mouse model, we have demonstrated the behavioural consequences of an alteration in editing of 5htr2c pre-RNA due to loss of expression of the imprinted regulatory snoRNA mbii-52. Specifically, we show evidence that altered 5HT2CR function in brain occurring via this novel molecular mechanism is associated with effects on impulsive responding, LMA and reactivity to palatable food rewards. A non-5HT2CR-mediated, but serotonin-sensitive behaviour, the marble burying task, was unaltered in the PWS-IC+/− mice.
The PWS-IC+/− mouse model we used has a deletion of the imprinting control region of the PWS interval. This results in the lack of expression of a number of paternally expressed/maternally repressed genes in this interval (23), including mbii-52 (10). Although only mbii-52 has the 18nt complementary anti-sense box to the edited region of the 5htr2c pre-mRNA, we controlled for potentially confounding effects by, in the first instance, examining specific behaviours in the 5-CSRTT that were known to be sensitive to manipulations of 5HT2CR. The specificity of the behavioural effects observed were underlined by the effects of selective drug compounds, whereby the selective 5HT2CR antagonist SB242084 produced differential changes in behavioural indices of impulse control between PWS-IC+/− mice and controls, but the 5HT2AR antagonist ketanserin had opposite but equal effects between the groups. Furthermore, the marble burying task, in which there was no effect of the 5HT2CR antagonist, and in which the PWS-IC+/− mice and controls performed equally, suggested that general serotonergic function remains intact in the mutant mice. Additional evidence of no general change in serotonergic function was supported by gene expression data, indicating no systematic changes in the expression of other important 5HTR subtype genes and no general change in monoamine whole tissue levels in either the pre-frontal cortex or the ventral striatum.
The degree of change in editing of the 5htr2c pre-mRNA we found in the PWS-IC+/− adult brain was relatively subtle (10% increase) but comparable to the change found in human PWS brain (13). Additionally, although in vitro studies point to a role for mbii-52 in alternative splicing (13), we found no apparent change in levels of 5htr2c splice variants in the PWS-IC+/− model, again findings comparable with data from human PWS brain (13). mbii-52 and 5htr2c generally show overlapping expression, including in the frontal cortical regions, ventral striatum (nucleus accumbens, NAc) and hippocampus (10,24). However, this is not true for all brain structures, with complete absence of mbii-52 expression in the choroid plexus (10,11). Consequently, our data may simply reflect the overall, net level of change in whole adult brain, and it may be important to assess the degree of both editing and alternative splicing in discrete brain regions and at different developmental time points. Nevertheless, it is clear that, despite an almost complete absence of mbii-52 expression in the brain, the 5htr2c pre-mRNA does not become fully edited. We suggest two possible reasons for this: either the level of editing we observe is the highest that can physiologically be achieved or there are additional, as yet unidentified, regulators of these processes that remain intact in the absence of mbii-52. Whatever the situation at the molecular level, the subtlety and specific nature of the post-transcriptional modifications we see indicate that behavioural functions are very sensitive to any change in 5htr2c pre-mRNA editing.
The neurobiological mechanisms underlying the behavioural effects in the PWS-IC+/− mice are unknown but the established inhibitory modulation of dopamine release by the binding of serotonin to 5HT2CR located within the ventral tegmental area and the NAc may be of relevance (25). Thus, increased editing of the 5htr2c pre-RNA found in PWS-IC+/− mice would lead to a receptor of decreased efficacy and in turn an increase in dopamine release. Such an effect would be consistent with data showing that 5HT2CRs in the NAc are important for regulating impulse control in the 5-CSRTT (26) and would also be consistent with the increased locomotor response to food rewards seen in the PWS-IC+/− mice, an effect that was disproportionately enhanced by the selective 5HT2CR antagonist SB242084 and accompanied by data showing that PWS-IC+/− mice were quicker to develop a preference for a palatable foodstuff and consumed more of it when normalized for body weight differences. To test this idea directly, future work needs to address dopamine release (as opposed to whole tissue levels, which are unaltered) in the NAc of the PWS-IC+/− mice. However, it is important to point out that 5HT2CRs with decreased efficacy in other brain systems (e.g. the hypothalamus) also have the potential to contribute to altered feeding behaviour (27,28).
Recently, evidence from a small number of single-patient cases (29,30) with localized deletions within paternal 15q11-q13, and studies of a deletion mouse model (31), appear to have ruled out hbii-52 as a primary causal gene for PWS, at least as defined by the early life phenotypes and/or the later hyperphagia. Instead, these and other studies (32) suggest that the snoRNA HBII-85 is critical for these core phenotypes. Nevertheless, expression of hbii-52 is lost in the vast majority of PWS cases. Therefore, it is highly likely that loss of hbii-52 expression in PWS and the subsequent alterations to post-transcriptional modification of 5htr2c contribute to the complex behavioural phenotype seen in PWS, which extends beyond eating abnormalities into a number of other psychopathologies (33,34). 5HT2CRs regulate many serotonin-mediated behavioural functions (25) including aspects of anxiety (35) and the pain response (36); the latter also giving rise to correlative changes in levels of RNA editing (37). Clearly, it is important that future work address the extent to which h/mbii-52 and altered levels of 5htr2c pre-mRNA editing contribute to other neurobiologically dissociable functions of 5HT2CRs. The present data are the first illustration of a role in regulating behaviour for the snoRNA mbii-52 and also add to the increasing literature relating imprinted genes to important brain functions (38).
MATERIALS AND METHODS
Animal subjects
The generation and genetic characteristics of the imprinting centre deletion have been described in detail previously (23,39). Briefly, the deletion is 35 kb in length, including Snrpn exons 1–6, and extending 16 kb 5′ of the Snrpn gene. PWS-IC+/− animals suffer from early postnatal lethality on a pure C57BL/6J background, so it was necessary to breed PWS-IC−/+-positive males to outbred strain females (CD1) and, as previously described (14), selectively cull WT littermates (identified on the basis of their increased size 48 h after birth) leaving only one or two per WT/litter. Animals were weaned at approximately 4 weeks of age and were single-sexed group housed with WT littermates (2–5 animals per cage). Experimental cohorts consisted of male and female animals, and behavioural testing began when animals were aged 7–11 weeks, finishing when animals were 40–50 weeks old. The pharmacological validation of marble burying behaviour was performed in WT mice of the same genetic background (CD1 X C57BL/6) bred in Cardiff University. Animals were subject to a 12 h light/dark cycle (lights on at 7 a.m.), and had ad libitum access to standard lab chow and water unless stated otherwise. For part of the experiment, water was restricted to 2 h access per day (given after testing). Although on restricted water access, standard laboratory chow was available ad libitum. This regime maintained the subjects at ≈90% of free-feeding body weight. All procedures were conducted in accordance with the requirements of the UK Animals (Scientific Procedures) Act 1986.
RNA editing and qPCR analysis
RNA from dissected brain hemispheres was isolated using standard Trizol methods, and reverse-transcribed using SuperScript® III First-Strand Synthesis SuperMix for qRT-PCR. For RNA editing analysis, cDNA samples from eight separate brains (four WT and four PWS-IC+/−) were subject to PCR using specific primers that span the edited region of the 5htr2c gene (see Supplementary Material, Table S1), and separately, the edited region of the Glur2 gene (see Supplementary Material, Table S1). PCR products were cloned, and recombinant plasmids were introduced into bacteria, the transformations from each animal yielding >200 clones expressing recombinant plasmid DNA. Twenty of these were pseudorandomly picked to process plasmid DNA for nucleotide sequencing. The proportion of edited and non-edited 5htr2c RNA can be determined from the relative abundance of each of the possible sequences (4,5).
Real-time qPCR analysis of gene expression (see Supplementary Material, Table S1 for detail of primer sequences) was performed using a Rotorgene 6000 coupled with a CAS1200 automated set up, and utilizing standard consumables (Corbett Research, Cambridge, UK). PCR reactions were carried out using custom-designed primers (see Supplementary Material) and Quantace SensiMix NoRef (Bioline). Real-time qPCR data were analysed using the ΔCt method as described previously (40). Briefly, individual PCR reaction data are normalized to a housekeeping gene (in this case dynein and 18s rRNA) giving a value known as ΔCt. Statistical analysis was performed on these values. The data were processed further to show how the PWS-IC values vary with respect to WT (ΔΔCt) and allow simpler graphical representation after transformation (2−ΔΔCt).
5-Choice serial reaction time task
2-Choice consumption test
Prior to testing, all animals were handled daily for 2 weeks and their body weight monitored. After this time, the animals were placed on a 20-h water restriction schedule for 4 days, and then on a 22-h water restriction for a further 10 days until body weight had stabilized. Animals were then tested individually for their consumption of palatable foodstuff (10% solution of condensed milk; Nestle Ltd, UK) outside their home cages in a 2-choice test. Briefly, the subjects were given five, separate 10-min sessions over 5 days with an excess of either water or 10% milk solution presented in two small bowls (apart from the first habituation session where two samples of water were presented) that were secured with Velcro to the base of the testing cage. The bowls were weighed before and after each session to provide a measure of the volume of each consumed; any contamination of the solutions in the bowls (faeces, etc.) was removed with tweezers prior to weighing. The main measure was volume consumed of water and milk, normalized to body weight0.75 in order to account for difference in size between PWS-IC+/− and WT animals.
5-CSRTT apparatus, behavioural shaping and trial design
The use of the 5-CSRTT to assay aspects of attention and impulse control in mice has been fully described elsewhere (20,41). Testing took place in a 9-hole box modified for use in mice, with four alternate holes in the horizontal array covered. Shaping involved training mice to press a Perspex panel opposite the array of holes in order to gain access to reinforcer. During the 5-CSRTT, trials were initiated by a panel push. This resulted in a 5-s ITI after which a stimulus light was randomly presented in one of the five uncovered holes. A nose poke by the mouse in the illuminated hole (i.e. a correct response) resulted in the presentation of 20 µl of reinforcer behind the Perspex panel and collection of this reward initiated a second trial. An incorrect response (i.e. a response in a hole in which a light was not presented), an omission (i.e. no response during the duration of the stimulus + 5 s) or a premature response (i.e. a nose poke prior to the onset of a stimulus light) resulted in a 5-s time-out period in which the ‘house lights’ were illuminated. The time-out period could be terminated through a panel push, which started a new trial.
Training to baseline performance
Immediately following shaping, the stimulus duration was set for 32 s; this was gradually reduced to a baseline stimulus duration of 0.8 s in the sequence 32, 16, 8, 4, 2, 1.8, 1.6, 1.4, 1.2, 1.0 and 0.8 s. The stimulus duration for a given mouse was reduced to the next level once it had met performance criteria over two consecutive days (>25 completed trials, >80% accuracy, i.e. ratio of correct:total responses and <25% omissions, i.e. no response to <25% of all trials initiated); during the course of training, no other parameters were altered. Baseline acquisition performance was set as criterion performance observed at a stimulus duration of 0.8 s.
Behavioural measures
The following measures from the 5-CSRTT are reported here: discriminative % response accuracy (percentage correct commissions; the primary measure of attention), % omissions (omitted trials as a percentage of total trial number; reflecting possible failures of detection and/or motivational/motor deficits), number of premature responses (indexing aspects of impulse control), beam breaks (indexing LMA during the session).
Task and pharmacological manipulations at stable baseline
Upon stabilization of baseline performance, a variety of manipulations designed to influence dissociable aspects of attention and response control were performed. Each manipulation was performed after 2 consecutive days of stable baseline performance. The 5-CSRTT manipulation performed was ‘long ITI’ (of 5, 6, 7 and 8 s), which was designed to increase premature responding. Additionally, in order to demonstrate the effects of ketanserin on reducing premature responding, the animals were run on a session where the ITI was 10 s throughout (the ITI at baseline is 5 s).
SB242084 (Sigma-Aldrich, UK) was dissolved in 0.9% saline solution and injected s.c. 10 min prior to testing. All animals received all doses in a pseudorandom order. Ketanserin (Sigma-Aldrich) was also dissolved in 0.9% saline solution, but was administered i.p. 10 min before testing.
LMA testing
Spontaneous LMA was measured using a battery of bespoke activity cages fitted with infrared beams linked to an Acorn computer. The dimensions of activity cages were 210 × 360 × 200 mm3 (width × length × height), with the infrared beams situated at 30 mm from either end and at 10 mm above the floor of the cage. Data were collected in 5-min bins over a period of 2 h under red illumination. The subjects were assessed at the same time on three successive days. Data presented here is from the third and final day of testing when the subjects have habituated to the test arena.
Marble burying behaviour
Mice were individually placed in a clean opaque plastic box (45 × 28 × 15 cm3) filled with sawdust to a depth of 4–5 cm. During the test, the box was covered with a clear plastic lid with gaps at each end to allow air to circulate. The box contained eight opaque red marbles placed equidistant in the one-half of the box (exact positioning of the marbles was achieved by using a cardboard grid template) on top of the sawdust. Two separate pharmacological experiments were performed, where prior to each session animals were administered either vehicle (0.9% saline) or drug. Experiment 1, animals were dosed (i.p.) with vehicle or 10 mg/kg of the SSRI paroxetine s.c. (Sigma-Aldrich, UK); experiment 2, animals were dosed (s.c.) with vehicle, 0.25 and 0.5 mg/kg SB242084. All sessions were video-taped and the total number of marbles buried in each 2-min bin recorded post hoc. The total test period was 16 min. Other measures were also obtained using Ethovision (Noldus, UK). The test was run under low-level illumination.
Analysis of whole tissue monoamines
Animals were sacrificed by exposure to a rising concentration of carbon dioxide and cervical dislocation. The brains were rapidly removed and dissected according to two key regions of interest: the pre-frontal cortex and ventral striatum. Tissue aliquots were derived from both hemispheres and homogenized in 200 µl of 0.2 M perchloric acid by an ultrasonic cell disruptor (Microson, UK). Levels of NA, DA and 5-HT were determined in the supernatant by reversed-phase, high-performance liquid chromatography, as described previously (42).
Statistics
All statistics were analysed using SPSS 12.0.2 (SPSS, US). Data were analysed by Student's t-test, ANOVA or where appropriate χ2, with the main between-subject factors being GENOTYPE (PWS-IC+/− or WT). Other between-subject factors included SSRI (0, 10 mg/kg) and SB (0, 0.25, 0.5 mg/kg of SB242084). Where necessary, the following within-subject factors were also analysed: ITI (5, 6, 7, 8 s); DOSE (0, 0.25, 0.5 1.0 mg/kg of SB242084 or 0, 0.05, 0.10 mg/kg of ketanserin); SESSION (day 1–4 of 2-choice milk test). For repeated-measures analyses, Mauchly's test of sphericity of the covariance matrix was applied. Huynh-Feldt corrections were applied as necessary, and adjusted degrees of freedom are provided.
FUNDING
This work was supported by a Neuroscience and Mental Health Interdisciplinary Research Group studentship to C.M.D. D.R. was the recipient of a Research Council UK Dorothy Hodgkin Postgraduate award (in collaboration with GlaxoSmithKline). A.S.G. was supported by a Wellcome Trust ‘Value in People’ award. L.S.W. was supported by a University of Cardiff LINK award and is a member of the MRC Co-operative on Imprinting in Health and Disease. A.R.I. is the Beebe Trust Research Fellow.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
Supplementary Material
REFERENCES
- 1.Burns C.M., Chu H., Rueter S.M., Hutchinson L.K., Canton H., Sanders-Bush E., Emeson R.B. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 2.Sommer B., Kohler M., Sprengel R., Seeburg P.H. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 3.Ohlson J., Pedersen J.S., Haussler D., Ohman M. Editing modifies the GABA(A) receptor subunit alpha3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurevich I., Englander M.T., Adlersberg M., Siegal N.B., Schmauss C. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J. Neurosci. 2002;22:10529–10532. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englander M.T., Dulawa S.C., Bhansali P., Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J. Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakae A., Nakai K., Tanaka T., Hagihira S., Shibata M., Ueda K., Masimo T. The role of RNA editing of the serotonin 2C receptor in a rat model of oro-facial neuropathic pain. Eur. J. Neurosci. 2008;27:2373–2379. doi: 10.1111/j.1460-9568.2008.06205.x. [DOI] [PubMed] [Google Scholar]
- 7.Gurevich I., Tamir H., Arango V., Dwork A.J., Mann J.J., Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- 8.Dracheva S., Patel N., Woo D.A., Marcus S.M., Siever L.J., Haroutunian V. Increased serotonin 2C receptor mRNA editing: a possible risk factor for suicide. Mol. Psychiatry. 2008;13:1001–1010. doi: 10.1038/sj.mp.4002081. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara Y., Grimberg A., Teegarden S., Mombereau C., Liu S., Bale T.L., Blendy J.A., Nishikura K. Dysregulated editing of serotonin 2C receptor mRNAs results in energy dissipation and loss of fat mass. J. Neurosci. 2008;28:12834–12844. doi: 10.1523/JNEUROSCI.3896-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavaille J., Buiting K., Kiefmann M., Lalande M., Brannan C.I., Horsthemke B., Bachellerie J.P., Brosius J., Huttenhofer A. Identification of brain-specific and imprinted small nucleolar RNA genes exhibiting an unusual genomic organization. Proc. Natl Acad. Sci. USA. 2000;97:14311–14316. doi: 10.1073/pnas.250426397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitali P., Basyuk E., Le Meur E., Bertrand E., Muscatelli F., Cavaille J., Huttenhofer A. ADAR2-mediated editing of RNA substrates in the nucleolus is inhibited by C/D small nucleolar RNAs. J. Cell Biol. 2005;169:745–753. doi: 10.1083/jcb.200411129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flomen R., Knight J., Sham P., Kerwin R., Makoff A. Evidence that RNA editing modulates splice site selection in the 5-HT2C receptor gene. Nucleic Acids Res. 2004;32:2113–2122. doi: 10.1093/nar/gkh536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishore S., Stamm S. The snoRNA HBII-52 regulates alternative splicing of the serotonin receptor 2C. Science. 2006;311:230–232. doi: 10.1126/science.1118265. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain S.J., Johnstone K.A., DuBose A.J., Simon T.A., Bartolomei M.S., Resnick J.L., Brannan C.I. Evidence for genetic modifiers of postnatal lethality in PWS-IC deletion mice. Hum. Mol. Genet. 2004;13:2971–2977. doi: 10.1093/hmg/ddh314. [DOI] [PubMed] [Google Scholar]
- 15.Robbins T.W. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher P.J., Tampakeras M., Sinyard J., Higgins G.A. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- 17.Talpos J.C., Wilkinson L.S., Robbins T.W. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J. Psychopharmacol. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- 18.Winstanley C.A., Theobald D.E., Dalley J.W., Glennon J.C., Robbins T.W. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- 19.Davies W., Humby T., Isles A.R., Burgoyne P.S., Wilkinson L.S. X-monosomy effects on visuospatial attention in mice: a candidate gene and implications for Turner syndrome and attention deficit hyperactivity disorder. Biol. Psychiatry. 2007;61:1351–1360. doi: 10.1016/j.biopsych.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Lambourne S.L., Humby T., Isles A.R., Emson P.C., Spillantini M.G., Wilkinson L.S. Impairments in impulse control in mice transgenic for the human FTDP-17 tauV337M mutation are exacerbated by age. Hum. Mol. Genet. 2007;16:1708–1719. doi: 10.1093/hmg/ddm119. [DOI] [PubMed] [Google Scholar]
- 21.Njung'e K., Handley S.L. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice: a putative test for anxiolytic agents. Br. J. Pharmacol. 1991;104:105–112. doi: 10.1111/j.1476-5381.1991.tb12392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinomiya K., Fujii Y., Sugimoto Y., Azuma N., Tokunaga S., Kitazumi K., Kamei C. Effect of paroxetine on marble-burying behavior in mice. Methods Find Exp. Clin. Pharmacol. 2005;27:685–687. doi: 10.1358/mf.2005.27.10.948883. [DOI] [PubMed] [Google Scholar]
- 23.Yang T., Adamson T.E., Resnick J.L., Leff S., Wevrick R., Francke U., Jenkins N.A., Copeland N.G., Brannan C.I. A mouse model for Prader–Willi syndrome imprinting-centre mutations. Nat. Genet. 1998;19:25–31. doi: 10.1038/ng0598-25. [DOI] [PubMed] [Google Scholar]
- 24.Rogelj B., Hartmann C.E., Yeo C.H., Hunt S.P., Giese K.P. Contextual fear conditioning regulates the expression of brain-specific small nucleolar RNAs in hippocampus. Eur. J. Neurosci. 2003;18:3089–3096. doi: 10.1111/j.1460-9568.2003.03026.x. [DOI] [PubMed] [Google Scholar]
- 25.Giorgetti M., Tecott L.H. Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur. J. Pharmacol. 2004;488:1–9. doi: 10.1016/j.ejphar.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Robinson E.S., Dalley J.W., Theobald D.E., Glennon J.C., Pezze M.A., Murphy E.R., Robbins T.W. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology. 2008;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y., Jones J.E., Kohno D., Williams K.W., Lee C.E., Choi M.J., Anderson J.G., Heisler L.K., Zigman J.M., Lowell B.B., et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam D.D., Przydzial M.J., Ridley S.H., Yeo G.S., Rochford J.J., O'Rahilly S., Heisler L.K. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149:1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Runte M., Varon R., Horn D., Horsthemke B., Buiting K. Exclusion of the C/D box snoRNA gene cluster HBII-52 from a major role in Prader–Willi syndrome. Hum. Genet. 2005;116:228–230. doi: 10.1007/s00439-004-1219-2. [DOI] [PubMed] [Google Scholar]
- 30.Sahoo T., Del Gaudio D., German J.R., Shinawi M., Peters S.U., Person R.E., Garnica A., Cheung S.W., Beaudet A.L. Prader–Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat. Genet. 2008;40:719–721. doi: 10.1038/ng.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding F., Prints Y., Dhar M.S., Johnson D.K., Garnacho-Montero C., Nicholls R.D., Francke U. Lack of Pwcr1/MBII-85 snoRNA is critical for neonatal lethality in Prader–Willi syndrome mouse models. Mamm. Genome. 2005;16:424–431. doi: 10.1007/s00335-005-2460-2. [DOI] [PubMed] [Google Scholar]
- 32.Ding F., Li H.H., Zhang S., Solomon N.M., Camper S.A., Cohen P., Francke U. SnoRNA Snord116 (Pwcr1/MBII-85) deletion causes growth deficiency and hyperphagia in mice. PLoS ONE. 2008;3:e1709. doi: 10.1371/journal.pone.0001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soni S., Whittington J., Holland A.J., Webb T., Maina E., Boer H., Clarke D. The course and outcome of psychiatric illness in people with Prader–Willi syndrome: implications for management and treatment. J. Intellect. Disabil. Res. 2007;51:32–42. doi: 10.1111/j.1365-2788.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- 34.Holland A.J., Whittington J.E., Butler J., Webb T., Boer H., Clarke D. Behavioural phenotypes associated with specific genetic disorders: evidence from a population-based study of people with Prader–Willi syndrome. Psychol. Med. 2003;33:141–153. doi: 10.1017/s0033291702006736. [DOI] [PubMed] [Google Scholar]
- 35.Heisler L.K., Zhou L., Bajwa P., Hsu J., Tecott L.H. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007;6:491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 36.Honda M., Uchida K., Tanabe M., Ono H. Fluvoxamine, a selective serotonin reuptake inhibitor, exerts its antiallodynic effects on neuropathic pain in mice via 5-HT2A/2C receptors. Neuropharmacology. 2006;51:866–872. doi: 10.1016/j.neuropharm.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 37.Nakae A., Nakai K., Tanaka T., Takashina M., Hagihira S., Shibata M., Ueda K., Mashimo T. Serotonin 2C receptor mRNA editing in neuropathic pain model. Neurosci. Res. 2008;60:228–231. doi: 10.1016/j.neures.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson L.S., Davies W., Isles A.R. Genomic imprinting effects on brain development and function. Nat. Rev. Neurosci. 2007;8:832–843. doi: 10.1038/nrn2235. [DOI] [PubMed] [Google Scholar]
- 39.Chamberlain S.J., Brannan C.I. The Prader–Willi syndrome imprinting center activates the paternally expressed murine Ube3a antisense transcript but represses paternal Ube3a. Genomics. 2001;73:316–322. doi: 10.1006/geno.2001.6543. [DOI] [PubMed] [Google Scholar]
- 40.Isles A.R., Davies W., Burrmann D., Burgoyne P.S., Wilkinson L.S. Effects on fear reactivity in XO mice are due to haploinsufficiency of a non-PAR X gene: implications for emotional function in Turner's syndrome. Hum. Mol. Genet. 2004;13:1849–1855. doi: 10.1093/hmg/ddh203. [DOI] [PubMed] [Google Scholar]
- 41.Humby T., Laird F.M., Davies W., Wilkinson L.S. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur. J. Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 42.Dalley J.W., Theobald D.E., Eagle D.M., Passetti F., Robbins T.W. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.