Abstract
Inactivating mutations in the retinoid isomerase (RPE65) or lecithin:retinol acyltransferase (LRAT) genes cause Leber congenital amaurosis (LCA), a severe visual impairment in humans. Both enzymes participate in the retinoid (visual) cycle, the enzymatic pathway that continuously generates 11-cis-retinal, the chromophore of visual pigments in rod and cone photoreceptor cells needed for vision. We investigated human RPE65–LCA patients and mice with visual cycle abnormalities to determine the impact of chronic chromophore deprivation on cones. Young patients with RPE65 mutations showed foveal cone loss along with shortened inner and outer segments of remaining cones; cone cell loss also was dramatic in young mice lacking Rpe65 or Lrat gene function. To selectively evaluate cone pathophysiology, we eliminated the rod contribution to electroretinographic (ERG) responses by generating double knockout mice lacking Lrat or Rpe65 together with an inactivated rod-specific G protein transducin gene (Gnat1−/−). Cone ERG responses were absent in Gnat1−/−Lrat−/− mice which also showed progressive degeneration of cones. Cone ERG responses in Gnat1−/−Rpe65−/− mice were markedly reduced and declined over weeks. Treatment of these mice with the artificial chromophore pro-drug, 9-cis-retinyl acetate, partially protected inferior retinal cones as evidenced by improved ERGs and retinal histochemistry. Gnat1−/− mice chronically treated with retinylamine, a selective inhibitor of RPE65, also showed a decline in the number of cones that was ameliorated by 9-cis-retinyl acetate. These results suggest that chronic lack of chromophore leads to progressive loss of cones in mice and humans. Therapy for LCA patients should be geared toward early adequate delivery of chromophore to cone photoreceptors.
INTRODUCTION
Leber congenital amaurosis (LCA) is a group of inherited retinal diseases that causes severe visual impairment in early human life with eventual blindness (1,2). Among the many genes associated with LCA are RPE65 (retinal pigment epithelium-specific protein 65 kDa) and LRAT (lecithin:retinol acyltransferase). The gene products are enzymes involved in continuous production of the chromophore, 11-cis-retinal, through a series of metabolic transformations called the retinoid cycle (3).
The importance of RPE65 for rod photoreceptor function and integrity was demonstrated in the Rpe65−/− mouse (4) and RPE65-mutant dog (5). It was that cone photoreceptor cells in the Rpe65−/− mouse degenerate more rapidly than rod photoreceptors (6), and similar observations were published for the Lrat−/− mouse (7,8). In double knockout Rpe65−/−Rho−/− mice, the addition of chromophore increased proper transport of cone opsins to outer segments (OSs) while partially preserving cone structure and function in the compromised retina lacking rods due to elimination of rhodopsin (Rho) (7). The R91W Rpe65 mutant associated with human LCA but expressed in mice (9) exhibited slow production of chromophore, cone pigment mislocalization and cone degeneration, but when this mutant was expressed on a Rho−/−-background, partial restoration of cone function occurred, suggesting a competition between rods and cones for 11-cis-retinal (10). These are critical findings because of both the importance of cones for human high resolution spatial vision and color perception (11) and the need to evaluate cone status in any potential therapy for LCA (12). Children with RPE65–LCA manifest cone photoreceptor loss in the first decade of life. The central retinal RPE layer of normal primate retina also shows higher retinoid isomerase activity than the more peripheral RPE, so we speculated that early cone photoreceptor loss in RPE65–LCA indicates that robust RPE65-based visual chromophore production is important for cones (13). Residual cone structure and function could be supported by a retinal-based alternative pathway for chromophore production (14).
Availability of animal models for LCA led to rapid development of gene transfer and pharmacological interventions to restore vision as a prelude for treating these diseases in humans (15–18). Moreover, the outcomes of the first human gene transfer trials in the RPE65 form of LCA have recently been reported (19–21). Rod and cone function was restored in the retinal area of gene transfer (22).
Here, we used high resolution in vivo microscopy to study cone cell morphology in young patients lacking functional RPE65. Pure cone cell electroretinographic (ERG) responses were recorded and retinal morphology was examined in mice lacking either Rpe65 or Lrat together with genetically disabled rod function, Gnat1−/−. Progressive degeneration of cones in Gnat1−/−Rpe65−/− and Gnat1−/−Lrat−/− mice was ameliorated by treatment with the pro-drug, 9-cis-retinyl acetate (17,23). We also used retinylamine treatment of Gnat1−/− mice to determine if chronic depletion of chromophore by a retinoid cycle inhibitor leads to cone degeneration (24–27).
RESULTS
Foveal cone abnormalities in the early decades of life of human LCA due to RPE65 mutations
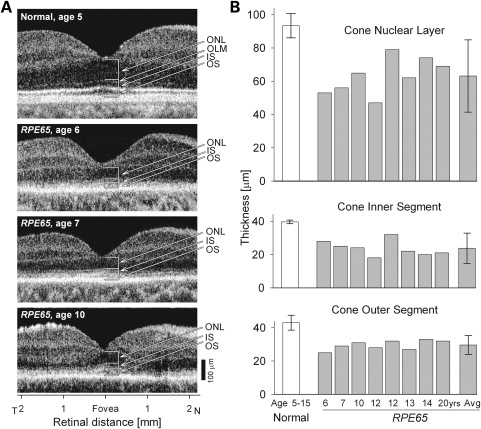
High resolution optical imaging revealed the cone-rich fovea of the normal human retina in cross-sections (Fig. 1A). The cone photoreceptor cell layer (ONL, outer nuclear layer) and the inner segment (IS) and OS laminae could be identified at this resolution. Representative RPE65–LCA patients at ages 6, 7 and 10 had reduced foveal ONL as well as abnormally thinned IS and OS layers (Fig. 1A). Quantified foveal cone ONL, IS and OS laminae in a group of five normal subjects (age range, 5–15 years) were compared with eight similar-aged RPE65–LCA patients (age range, 6–20 years) (Fig. 1B). All three cone parameters were significantly different in RPE65–LCA patients when compared with normal (for ONL, IS and OS, P < 0.001), suggesting loss of a subset of foveal cone cells and abnormal structure of the retained cells.
Figure 1.
Foveal cone morphology of young LCA patients with RPE65 mutations. (A) Cross-sectional scans along the horizontal meridian of the central retina of a normal child (upper panel) and three children with LCA due to RPE65 mutations (lower panels). Arrows and brackets indicate ONL, outer nuclear layer; OLM, outer limiting membrane; IS, inner segment; OS, outer segment. (B) Mean foveal ONL, IS and OS thickness in a group of young subjects with normal vision (ages 5–15 years) and in eight young patients with RPE65–LCA (ages 6–20 years). Mean values for the parameters from the RPE65–LCA group are also shown (Avg). Error bars represent ± 2 SD.
Early onset cone cell degeneration in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice
Retinal structure
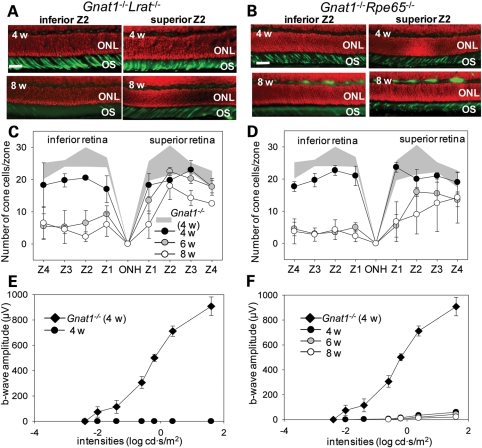
We counted the number of cones in four zones (width 100 µm) of the inferior and superior retina. In 4-week-old double knockout animals, decreased numbers of cone photoreceptor cells were evident in Gnat1−/−Lrat−/− mice (inferior retina, 75.6 ± 6.0%, superior retina 85.6 ± 9.4%, Fig. 2A, upper panel and C) and Gnat1−/−Rpe65−/− mice (inferior retina, 79.6 ± 5.8%; superior retina, 92.3 ± 7.4%, Fig. 2B upper panel, D) when compared with Gnat1−/− mice of the same age. Interestingly, severe degeneration of cone cells was most prominent in the inferior retina of double knockout mice by 6 weeks of age (Gnat1−/−Lrat−/− mice, 35.7 ± 12.7%; Gnat1−/−Rpe65−/− mice, 20.3 ± 2.6%) when compared with that noted in 4-week-old Gnat1−/− mice. In contrast, progressive cone cell death was relatively slow in the superior retina of both double knockout strains (Gnat1−/−Lrat−/− mice, 94.0 ± 17.0%, Gnat1−/−Rpe65−/− mice, 53.2 ± 15.5%) by 6 weeks of age (Fig. 2C and D). Some progression of cone cell death was seen in the superior retina of these animals between 6 and 8 weeks of age (Gnat1−/−Lrat−/− mice, 66.5 ± 16.3%; Gnat1−/−Rpe65−/− mice, 50.2 ± 14.6%), whereas no further cone cell death was detected in the inferior retina of either strain between these ages (Gnat1−/−Lrat−/− mice, Fig. 2A, lower panel and C; Gnat1−/−Rpe65−/− mice, Fig. 2B, lower panel and D). Cone cell death progressed slightly faster in Gnat1−/−Rpe65−/− than that in Gnat1−/−Lrat−/− mice. At 6 months of age, only a few cone cells were seen in peripheral areas of the superior retina in both strains, whereas no such cell death was observed in Gnat1−/− mice (data not shown).
Figure 2.
Histological and functional evaluation of cone photoreceptor cells in Gnat1−/−Rpe65−/− and Gnat1−/−Lrat−/− mice. (A and B) Cone photoreceptor cells were stained with PNA (green, cone outer segments) and DAPI (red, nuclei) and representative images of immunohistochemical (IHC) sections from the second zone from the center (Z2) in the superior and inferior retina are shown. Cone photoreceptor cells in both the superior and inferior retina of 4-week-old (4 w) Gnat1−/−Lrat−/− mice (A, upper panel) and Gnat1−/−Rpe65−/− mice (B, upper panel) were slightly decreased. However, severe cone photoreceptor cell death was observed in the inferior retinas of both double knockout strains at 8 weeks of age, even though cone photoreceptor cells in the superior retina were still maintained (A and B, lower panels). ONL, outer nuclear layer; OS, outer segment. Bar indicates 10 µm. (C and D) Populations of cone photoreceptor cells were quantified in 4-, 6- and 8-week-old mice at four zones in the superior and inferior retina, respectively, and age-related changes in these cone cell numbers are shown. Cones were markedly reduced in both strains between 4 and 6 weeks of age, most prominently in the inferior retina when compared with both those in 4-week-old Gnat1−/− mice, and those in 4-week-old double knockout mice of both strains (P < 0.0001). (E and F) Scotopic single flash ERG responses were obtained from Gnat1−/−Lrat−/− mice and Gnat1−/−Rpe65−/− mice at 4, 6 and 8 weeks of age and compared with 4-week-old Gnat1−/− mice. ERG b-wave amplitudes are plotted. ERG responses were severely reduced in both double knockout strains when compared with those of Gnat1−/− mice. No responses were obtained from Gnat1−/−Lrat−/− mice at these sequential ages; only the data obtained from 4 weeks of age are plotted (E). Gnat1−/−Rpe65−/− mice showed markedly diminished but measurable responses at higher light intensities (F, P < 0.0001). Bars indicate SDs, n = 4–7.
Retinal function
To determine the function of cone photoreceptor cells in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice, we recorded ERG responses. Interestingly, no functional ERG responses were obtained from Gnat1−/−Lrat−/− mice at 4 weeks of age (Fig. 2E), even though more than ∼70% of the cone photoreceptors were present (in the sampled retinal regions) when compared with Gnat1−/− mice (Fig. 2A and C). Lrat−/− single knockout mice demonstrated residual ERG responses (28). Gnat1−/−Rpe65−/− mice also showed a discrepancy between ERG recordings and histological assessments, but residual responses at high light intensities were recorded at 4, 6 and 8 weeks of age (∼6.5% at 4 weeks of age; ∼5.9% at 6 weeks of age; ∼3.6% at 8 weeks of age compared with responses at 1.6 log cd s/m2 in Gnat1−/− mice at 4 weeks of age Fig. 2F).
Treatment with 9-cis-retinyl acetate reduces cone cell death in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice
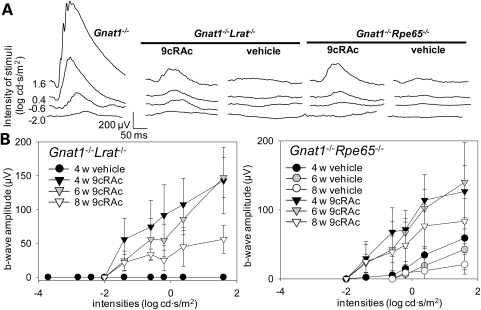
Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice were treated with the artificial pro-drug, 9-cis-retinyl acetate, from post-partum day 10 (P10) to P21 by periodic intraperitoneal (PI) injection and then from P22 to P56 by oral gavage (see Materials and Methods for regimen). Mice tolerated the treatment without loss of weight or gross changes in behavior. Levels of 9-cis-retinal determined in the eyes of Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice were similar to those found in Gnat1−/−mice at 4 weeks of age (Gnat1−/−Lrat−/− mice, 460.6 ± 15.0 pmol/eye; Gnat1−/−Rpe65−/− mice, 481.0 ± 89.2 pmol/eye; Gnat1−/− mice, 471.4 ± 7.1 pmol/eye, respectively) and these levels were maintained during the remaining experimental period (data not shown). No significant difference in cone photoreceptor cell numbers was observed between 9-cis-retinyl acetate-treated groups and vehicle-treated control mice of both strains at 4 weeks of age and the cone population was maintained at the 4 weeks level when both 9-cis-retinyl acetate-treated double knockout strains reached 6 weeks of age (Fig. 3C and D). In contrast, the comparable vehicle-treated control double knockout strains had a substantial loss of cones, most notably in the inferior retina, by 6 weeks of age that did not change significantly by 8 weeks of age (Fig. 3C and D). However, by 8 weeks of age the number of cones was also reduced in the 9-cis-retinyl acetate-treated double knockout animals (Gnat1−/−Lrat−/− mouse, 55.7 ± 2.5% in the inferior retina, 71.1 ± 16.1% in the superior retina, Fig. 3A and C; Gnat1−/−Rpe65−/− mice, 46.8 ± 10.8% in the inferior retina, 73.9 ± 21.2% in the superior retina, Fig. 3B and D), although not to the extent found in vehicle-treated controls.
Figure 3.
Histological evaluation of cone photoreceptor cells in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice after 9-cis-retinyl acetate (9cRAc) treatment. Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice were treated with 9-cis-retinyl acetate (from P10 to P21, 1.0 µg/g body weight; from P22 to P56, 50 µg/g body weight; see Materials and Methods for regimen) and cone photoreceptor cells were quantified immunohistochemically at 6 and 8 weeks (6–8 w) of age by PNA (green, outer segments) and DAPI (red, nuclei) staining. Representative images from Z2 in the superior and the inferior retina of 8-week-old 9-cis-retinyl acetate-treated and vehicle-treated Gnat1−/−Lrat−/− mice (A) and Gnat1−/−Rpe65−/− mice (B) are shown. Cone photoreceptor cells were better preserved in retinas of 9-cis-retinyl acetate-treated mice (upper panels) than in vehicle-treated controls (lower panels). Populations of cone photoreceptor cells also were significantly greater, especially in the inferior retina of both strains when compared with vehicle-treated controls at 6 and 8 weeks of age (Gnat1−/−Lrat−/− mice, C; Gnat1−/−Rpe65−/− mice, D). ONL, outer nuclear layer; OS, outer segment. For (A and B) bars indicate 10 µm. For (C and D) bars indicate SDs. n = 4–7, P < 0.0001 for inferior retina.
ERG recordings were used to evaluate effects on retinal function of 9-cis-retinyl acetate treatment in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice. In contrast to Rpe65−/− mice, Lrat−/− mice do not store retinoids in the liver or in the RPE (23,28). To adequately compare ERG responses of these two strains of mice, fully regenerated visual pigments should be tested. Thus, photo-bleaching of regenerated visual pigment in both these strains of mice was minimized to compare the effects of 9-cis-retinyl acetate treatment. Therefore, these mice were maintained in the dark during treatment. The choice of Gnat1−/− mice also enabled us to evaluate cone-specific ERGs under scotopic conditions along with the corresponding retinal morphology and compare the findings with those obtained in inbred strains such as C57BL/6 or 129SV mice. As shown in Fig. 4A, treatment with 9-cis-retinyl acetate partially preserved b-waves in both double knockout strains and there was significant functional improvement when compared with vehicle control groups (Gnat1−/−Lrat−/− mice: 15.7 ± 5.4% at 4 weeks of age, 20.2 ± 4.1% at 6 weeks of age and 9.6 ± 3.5% at 8 weeks of age; Gnat1−/−Rpe65−/− mice: 15.3 ± 2.7% at 4 weeks of age, 19.1 ± 7.9%, at 6 weeks of age and 14.6 ± 5.6% at 8 weeks of age when compared with responses of Gnat1−/− mice at 4, 6 and 8 weeks of age, respectively, to light at 1.6 log cd s/m2; Fig. 4B, N = 4–7 for each time point, P < 0.0001). These observations suggest that 9-cis-retinoids can regenerate cone visual pigments in photoreceptor cells and help retain cone function in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mouse retina.
Figure 4.
Functional evaluation of cone photoreceptor cells in Gnat1−/−Rpe65−/− and Gnat1−/−Lrat−/− mice after 9-cis-retinyl acetate (9cRAc) treatment. ERG responses were obtained in 4-, 6- and 8-week-old Gnat1−/−Rpe65−/− and Gnat1−/−Lrat−/− mice on the day following the last day of treatment with 9-cis-retinyl acetate (from P10 to P21, IP injection of 1.0 µg/g body weight; from P22 to P56, gavage with 50 µg/g body weight; see Materials and Methods for regimen). (A) Representative traces of scotopic single flash ERG recordings are shown for Gnat1−/− and for both strains of 4-week-old double knockout mice either treated with 9-cis-retinyl acetate or vehicle solution. Comparison of these recordings shows that both double knockout strains had reduced responses relative to the Gnat1−/− mouse and that treatment with 9-cis-retinyl acetate partially preserved ERG responses in both double knockout strains. (B) ERG b-wave amplitudes indicate that treatments with 9-cis-retinyl acetate improved responses in Gnat1−/−Lrat−/− mice (left panel) and Gnat1−/−Rpe65−/− mice (right panel) at 4, 6 and 8 weeks of age. No b-wave responses were detected in vehicle-treated Gnat1−/−Lrat−/− mice at 4, 6 and 8 weeks of age (only data from 4-week-old Gnat1−/−Lrat−/− mice are presented). Gnat1−/−Rpe65−/− mice showed some residual responses during the experimental period. Bars indicate SDs; n = 4–7, P < 0.0001.
9-cis-Retinyl acetate preserves cone cell function in retinylamine-treated Gnat1−/− mice with induced chromophore deficiency
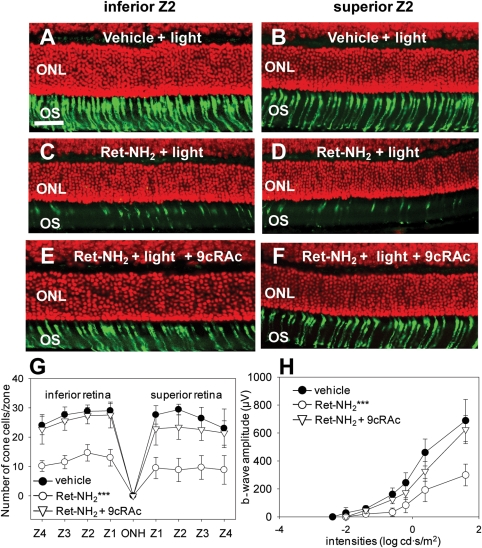
Considering the positive effects of 9-cis-retinyl acetate treatment in the mouse genetic models of LCA, we speculated that chromophore deficiency might explain the cone cell dysfunction and death observed in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice. To test this hypothesis, we produced a phenotype mimicking 11-cis-retinal deficiency in Gnat1−/− mice. Mice were gavaged with retinylamine followed 6 h later by strong light bleaching (500 cd/m2 for 30 min). Then they were maintained in the dark for 1 week (from days 1–7). This regimen was shown to decrease 11-cis-retinal levels in the eyes of such mice to ∼5% at day 2 and ∼25% by day 7 (27). In the present study, this protocol was repeated once, i.e. mice were gavaged again on day 8 with the same dose of retinylamine followed by bleaching 6 h later and then dark-adapted for another week (days 8 –14) before being studied. The effects of 11-cis-retinal deficiency on cone cell structure and function in Gnat1−/− mice then were evaluated on day 14 by the same procedures used for Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice. This 2-week-protocol caused major cone cell degeneration of both the inferior and the superior retina (Fig. 5C, inferior retina; D, superior retina; G, cone cell number) as well as substantial retinal dysfunction (Fig. 5H) in Gnat1−/− mice depleted of chromophore by treatment with retinylamine. Bleaching combined with vehicle instead of retinylamine failed to affect cone cell morphology (Fig. 5A, inferior retina; B, superior retina), cone cell numbers (Fig. 5G) or ERG responses (Fig. 5H) when compared with untreated control Gnat1−/− mice. To evaluate possible cone cell preservation effects of 9-cis-retinyl acetate treatment in this model, we gavaged these retinylamine-treated animals with 9-cis-retinyl acetate during periods of dark-adaptation after bleaching on days 2, 4, 6, 9, 11 and 13 during the 2 weeks experimental period and evaluated them on day 14. The cone photoreceptor cell population was maintained in the retina of mice treated with 9-cis-retinyl acetate (Fig. 5E, inferior retina; F, superior retina; G, cone cell number) and ERG recordings provided results consistent with the histological findings (Fig. 5H). These observations clearly indicate that the level of visual chromophore is critical for cone cell survival and function. Moreover, treatment of chromophore-depleted Gnat1−/− mice with the artificial pro-drug chromophore, 9-cis-retinal acetate, can partially preserve cone cell structure and function.
Figure 5.
Cone photoreceptor cell degeneration due to chromophore deficiency in Gnat1−/− mice is prevented by 9-cis-retinyl acetate (9cRAc) administration. To determine whether the cone photoreceptor cell death observed in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice might be induced by 11-cis-retinal deficiency, a phenotype mimicking this condition was produced in Gnat1−/− mice by administration of the retinoid cycle inhibitor, retinylamine. Four-week-old Gnat1−/− mice were gavaged with retinylamine (Ret-NH2)(250 µg/g body weight) followed 6 h later by strong light bleaching (500 cd/m2 for 30 min) on day 1 and maintained in the dark for a week (from days 1–7); the same regimen was repeated during the following week (from days 8–14). Cone photoreceptor cell death and function were evaluated by viewing retinal histology (PNA, green for cone photoreceptors; DAPI, red for nuclei), counting cone cell numbers, and recording ERGs on day 14. To determine the effects of artificial chromophore replenishment on cone photoreceptor cells, mice undergoing the 2 week retinylamine/bleaching chromophore depletion regimen were supplemented with 9-cis-retinyl acetate gavaged at 50 µg/g body weight three times a week (day 2, 4, 6, 9, 11 and 13). Cone photoreceptor immunohistochemistry, populations and function were then evaluated on day 14. No significant effects on cone photoreceptor immunohistochemistry (A and B, bar indicates 10 µm), population (G) or ERG responses (H) were observed after light exposure in Gnat1−/− vehicle-treated control mice, whereas chromophore deprivation by retinylamine gavage and light exposure induced drastic cone photoreceptor degeneration in both the inferior and the superior retina (C, D, G ***, P < 0.0001) of Gnat1−/− mice. Retinal dysfunction in these animals was also evidenced by ERG recordings (H ***, P < 0.0001). However, cone photoreceptor immunohistochemistry, populations and function were maintained in 9-cis-retinyl acetate-treated Gnat1−/− mice comparably to vehicle-treated control animals (E, F, G, H). OS, outer segments; ONL, outer nuclear layer. Bars indicate SDs; n =3–6/each group.
DISCUSSION
Early onset of cone photoreceptor cell death has been observed in several mouse models of human LCA (6–9,29–33) and in human RPE65–LCA as well (13,34). Moreover, we have reported that 9-cis-retinoids are effective prophylactic agents for the treatment of retinal degeneration in mouse models of LCA and other human retinopathies (17,18,23,35,36). Here, we confirm and extend the observations of early loss of cones in young RPE65–LCA patients with higher resolution imaging and in double knockout Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice. The effect of 9-cis-retinyl acetate was evaluated on cone photoreceptor histology and function in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice, and the results were compared with those found in Gnat1−/− mice with disabled rod signaling. Pharmacological inhibition of the retinoid cycle followed by exposure to bright light also led to cone loss in Gnat1−/− mice that was largely prevented by supplementation with 9-cis-retinyl acetate, an artificial chromophore pro-drug.
Differences in retinal degeneration between Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice
At first glance, cone histology and electrophysiology appeared similar in Gnat1−/−Rpe65−/− and Gnat1−/−Lrat−/− mice, but there were subtle differences. Cones in Gnat1−/−Rpe65−/− mice seemed to degenerate faster in both the inferior and superior retina. Residual production of chromophore or formation of 9-cis-retinal (37) may lead to generation of functional cone visual pigments that induce faster degeneration than when the retina is devoid of retinoids such as in Lrat−/− mice (23,28,38), or more rapid degeneration might result from altered physiology of RPE cells in Rpe65−/− mice as they accumulate large amounts of retinoids in retinosomes (38), structures that store retinyl esters. Greater damage to the inferior than the superior retina found in many animal models supports the first contention because the inferior retina is exposed to higher levels of illumination. However, significant differences in subsets of genes in the inferior versus superior retina may also contribute to this difference because these genes can modify cellular responses of photoreceptors (39). Cone cell death was observed to progress similarly in untreated Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice irrespective of light exposure and S cones were more susceptible to 11-cis-retinal deficiency than M/L cones (data not shown).
Preservation of cones in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice treated with 9-cis-retinyl acetate
Consistent with previous results (6–8), treatment with the artificial pro-drug chromophore, 9-cis-retinyl acetate, appeared to preserve retinal histology more effectively in Gnat1−/−Rpe65−/− than in Gnat1−/−Lrat−/− mice, possibly because of superior initial cone status in the Gnat1−/−Rpe65−/− animals. Thus, 4-week-old Gnat1−/−Rpe65−/− mice retained ERG responses, whereas Gnat1−/−Lrat−/− did not (Fig. 4B and C), but artificial chromophore treatment preserved cone function in both double knockout strains as evidenced by their similar ERG responses (Fig. 4B and C).
Deprivation of chromophore and cone degeneration
Chronic deprivation of chromophore through the RPE65 pathway resulting from treatment with the retinoid inhibitor, retinylamine, leads to the demise of cone photoreceptors in Gnat1−/− mice (24). This observation suggests that the classic visual cycle is essential for cone cell health, even if cone visual pigments are generated by an alternative pathway (40,41). It also confirms that cones are much more sensitive than rods to the lack of chromophore. This increased sensitivity could arise from differences in vectorial transport between cone opsins and rod opsin. The chromophore-free opsin of rhodopsin is properly transported to rod OSs, whereas chromophore-free cone pigments and other transduction proteins mislocalize to synaptic terminals (7). Importantly, this anomaly can be largely prevented by administration of the chromophore precursor, 9-cis-retinyl acetate, suggesting specificity for retinoid treatment and supporting efficacy data for certain retinoids used for the experimental treatment of transgenic animals.
Implications for human trials involving chromophore inhibition and supplementation of the retinoid cycle
Two opposing pharmacological treatments have been suggested for retinal degenerations related to a malfunctioning retinoid cycle (3). First, therapies have been proposed to prevent accumulation of toxic all-trans-retinal and its condensation products. Our recent data suggest that all-trans-retinal is the major responsible toxic agent and its condensation products are more likely to represent surrogate markers due to reduced clearance of this aldehyde (42). It has been proposed that retinal degenerations resulting from accumulation of toxic pigments, such as Stargardt disease due to ABCA4 mutations, can be treated pharmacologically by inhibiting the retinoid cycle or limiting the supply of vitamin A to the eyes (3). However, considering the importance of retinoids for maintaining cone cell function as shown here, the benefits of reducing toxic all-trans-retinal production along with its condensation products could be outweighed by more rapid cone cell degeneration.
Supplementation with native or artificial chromophore also has been suggested as a remedy for impaired synthesis of visual chromophore (17,18,23,36). Cis-retinoids administered systemically appear to be helpful in maintaining not only rods, but also cones, as demonstrated here. There does not appear to be any major drawback to this treatment with respect to the structure and function of the retina. Short-term clinical safety trials of these artificial chromophore pro-drugs are underway in normal volunteers (clinicaltrials.gov: NCT00765427).
Another strategy to restore the function of enzymes involved in chromophore regeneration has been focal sub-retinal gene therapy. Proof-of-concept studies have been performed in small and large animal models of human genetic diseases resulting in retinoid cycle blockade and the results indicate increases in retinal-visual function (12,15,16,43). Human RPE65–LCA has recently been treated with gene replacement therapy that has been shown to be safe and efficacious after focal sub-retinal delivery of a vector-gene (19–22). The next steps for this mode of treatment will determine the safety of increasing the area of injection and dose, ‘second eye treatments’ in the same individual, and the value of more efficient or specific vectors. Trials in younger and younger patients are being performed on the basis of the assumption that there will be a greater measurable benefit due to less degeneration, even though the human disease is characterized by a major loss of photoreceptors in the first decade of life (13,34).
The current study also adds experimental data to support the finding that cone loss occurs early in the life of both humans with RPE65–LCA and relevant mouse models. Also shown is the potential value of early chromophore supplementation in maintaining the structure and function of cones. Another simple suggestion is that early detection of RPE65–LCA should be followed promptly by sub-retinal gene therapy, no matter how young the patient. However, there are currently a number of concerns associated with sub-retinal vector gene delivery that need to be addressed. Examples include surgical trauma to the macula–fovea (19,21), the amount of retina that can be detached without post-operative complications, possible immune reactions to a persistent vector (44) and the safety of enrolling individuals with high baseline serum antibody levels to the adeno-associated viral vector. We suggest that prophylactic therapy with oral retinoids starting at an early age be seriously considered if proven safe in normal subjects over a wide spectrum of ages. This strategy could preserve cone (and rod) structure and function, while candidacy for ocular delivery of vector-genes is assessed. Additionally, there are large numbers of adults with RPE65–LCA who have partial residual vision that may be worth treating (45). A pharmacologically based provocative test would support the need for either ocular gene delivery or possibly chronic oral administration of retinoids as a substitute or supplement to gene therapy.
MATERIALS AND METHODS
Human studies
LCA patients with RPE65 mutations (n = 8; ages 6–20 years) and normal subjects (n = 5; ages 5–15 years) were studied (13,34,45). Informed consent or assent was obtained; all procedures followed guidelines in the Declaration of Helsinki and were approved by the institutional review board of the University of Pennsylvania.
Optical coherence tomography
Cross-sectional images of retina were obtained with optical coherence tomography (OCT). Principles of the method and our recording and analytical techniques have been published (34,46,47). Data were acquired with ultra-high speed and high resolution (∼5 µm axial, 15 µm lateral) imaging by using frequency domain OCT (RTVue-100, Optovue Inc., Fremont, CA, USA). Horizontal scans (4096 longitudinal reflectivity profiles, LRPs, covering 4.5 mm) crossing the anatomical fovea were obtained. Post-acquisition processing of OCT data at the fovea was performed with custom programs (MATLAB 6.5, MathWorks, Natick, MA, USA). Measurements of retinal laminar thicknesses were made by using the average of seven foveal LRPs corresponding to a nominal lateral sampling distance of ∼8 µm. ONL thickness was defined as the major intraretinal signal trough delimited by the signal slope maxima (34,45–47). IS and OS layer thicknesses were defined on the basis of the amalgamation of current hypotheses dealing with the correspondence between OCT signals and histologically defined layers (46,48–51). Specifically, IS thickness was defined to extend from the scleral boundary of the ONL (immediately vitreal to the signal peak presumed to correspond to the outer limiting membrane) to the next major signal peak in the scleral direction (presumed to correspond to the IS/OS boundary). OS thickness was defined to extend from the IS/OS boundary to the next major signal peak in the scleral direction. This latter peak is first of a complex of multiple peaks and it presumably corresponds to reflections originating from OS tips (48).
Animals
Mouse lines
Lrat−/− mice were generated and genotyped as described previously (28). Rpe65−/− mice (4) were provided by T. Michael Redmond (Laboratory of Retinal Cell and Molecular Biology, National Eye Institute). Gnat1−/− mice (52) were the generous gift from Janet Lem (Tufts University, Boston). Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− double knockout mice were generated by cross-breeding Gnat1−/− mice with Lrat−/− or Rpe65−/− mice, respectively; progeny were genotyped as described previously (17,28,53). Only mice with the leucine variant at the 450 amino acid position of RPE65 were used.
Mouse husbandry
All mice were housed in the animal facility at the School of Medicine, Case Western Reserve University, where they were maintained either under complete darkness or in a 12 h light (∼10 lux)/12 h dark cyclic environment. Manipulations in the dark were done under dim red light transmitted through a Kodak no. 1 safelight filter (transmittance > 560 nm). All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the School of Medicine, Case Western Reserve University Animal Care and Use Committee.
Pharmacological therapy
9-cis-Retinal was purchased from Sigma-Aldrich (St Louis, MO, USA) and both 9-cis-retinyl acetate and retinylamine were prepared under dim red light by methods previously described (23,24).
Cone photoreceptor preservation in Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/−double knockout mice
9-cis-Retinyl acetate was dissolved in absolute ethanol (10% final concentration) to which vehicle solution (10% fatty acid-free bovine serum albumin in 0.9% NaCl) was added as previously described for a similar treatment (30). Each litter of Gnat1−/−Lrat−/− and Gnat1−/−Rpe65−/− mice was randomly divided into groups that either received 9-cis-retinyl acetate or control vehicle solution injections. Mice were injected IP with 9-cis-retinyl acetate every third day for a total of four times, starting at post-natal day 10 (P10) at a dose of 1.0 µg/g body weight (at P10, P13, P16, P19). At P22, oral gavage with 9-cis-retinyl acetate in soybean oil (Spectrum Chemical MFG, Corp., CA, USA) at a dose of 50 µg/g body weight was initiated and given every second day until P28. After P28, mice were gavaged with the same dose once a week until P56 (8 weeks of age). Mice in the control group were PI with the same solution lacking 9-cis-retinyl acetate and gavaged with soybean oil alone by the same regimen used in retinoid-treated mice. Mice given 9-cis-retinyl acetate together with their control littermates were housed in the dark until the end of the experiment.
Cone photoreceptor preservation in Gnat1−/− mice with persistent chromophore deprivation
Four-week-old Gnat1−/− mice were gavaged with retinylamine at a dose of 250 µg/g body weight and 6 h later were bleached with strong light (500 cd/m2) for 30 min. Starting the next day, the same mice were gavaged with 9-cis-retinyl acetate in soybean oil at a dose of 50 µg/g body weight three times every second day. Two days after the last 9-cis-retinyl acetate gavage, Gnat1−/− mice were again gavaged with retinylamine at a dose of 250 µg/g body weight and 6 h later were bleached with strong light (500 cd/m2) for 30 min. Starting the next day, the same mice were gavaged with 9-cis-retinyl acetate in soybean oil at a dose of 50 µg/g body weight three times every second day. Control mice received soybean oil vehicle according to the same schedule.
Immunohistochemistry
For immunohistochemistry, freshly removed mouse eyes were immersion-fixed for 6 h in freshly prepared 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4 and processed for OCT (Miles) embedment. Immunohistochemical staining procedures employed were previously described (54). Briefly, cross-sections of mouse eyecups were pre-incubated with peanut agglutinin (Invitrogen) or 4′-6-diamidino-2-phenylindole. Signals were detected with either a Cy3 conjugated secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA) or an Alexa488 conjugated secondary antibody (Invitrogen). Sections were analyzed with a Leica TCS SP2 confocal microscope (Leica).
Cell counts
Cone photoreceptor cells were counted in four zones (Z1–Z4) placed at equal distances between the central (within 100 µm of the optic nerve head) and peripheral (within 100 µm of the retinal edge) retina. Zones in the superior and inferior retina were counted separately. Numbers of cells in each zone were averaged and the data were statistically analyzed by one-way ANOVA.
Electroretinograms
All ERG studies were performed using previously published methods (55). Briefly, mice under a safety light were anesthetized by IP injection of 20 µl/g body weight of 6 mg/ml ketamine and 0.44 mg/ml xylazine diluted with 10 mm sodium phosphate, pH 7.2, containing 100 mm NaCl. Pupils were dilated with 1% tropicamide. A contact lens electrode was placed on the eye and a reference electrode and ground electrode were positioned on the ear and tail, respectively. ERGs were recorded with a computerized system, UTAS E-3000 (LKC Technologies, Inc.).
Single-flash recording
Durations of white light flash stimuli (from 20 µs to 1 ms) were adjusted to provide a range of illumination intensities (from −3.7 to 1.6 log cd s/m2). Three to five recordings were made at sufficient intervals (from 10 s to 10 min) between flash stimuli to allow recovery from any photo-bleaching effects. Statistical analysis of responses was carried out with the one-way ANOVA test.
FUNDING
This work was supported by the National Institutes of Health (NIH grants K08EY019031, EY09339, EY13203, EY017280 and P30 EY11373); Foundation Fighting Blindness; Macula Vision Research Foundation; Hope for Vision; Research to Prevent Blindness and Ohio Lions Research Foundation.
ACKNOWLEDGEMENTS
We are grateful to Dr M. Redmond (NEI) for Rpe65−/− mice and Dr J. Lem for Gnat1−/− mice (Tufts University). We also thank Dr L.T. Webster, (Case Western Reserve University) for his comments on the manuscript, Dr A. Sumaroka (University of Pennsylvania) for OCT analyses and S. Roos, M.S. Matosky, K. Okano and M. Singer (Case Western Reserve University) for their technical support and advice about some of the procedures employed.
Conflict of Interest statement. University of Washington, Acucela Inc., Retinagenix Inc. and QLT Inc. may commercialize some of the technology described in this work. K.P., A.M. and T.M. were consultants for QLT Inc. and Acucela Inc. K.P. is a co-founder of Retinagenix Inc.
REFERENCES
- 1.Stone E.M. Leber congenital amaurosis—a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2007;144:791–811. doi: 10.1016/j.ajo.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 2.den Hollander A.I., Roepman R., Koenekoop R.K., Cremers F.P. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Travis G.H., Golczak M., Moise A.R., Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redmond T.M., Yu S., Lee E., Bok D., Hamasaki D., Chen N., Goletz P., Ma J.X., Crouch R.K., Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre G.D., Baldwin V., Pearce-Kelling S., Narfstrom K., Ray K., Acland G.M. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol. Vis. 1998;4:23. [PubMed] [Google Scholar]
- 6.Znoiko S.L., Rohrer B., Lu K., Lohr H.R., Crouch R.K., Ma J.X. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the rpe65−/− mouse at early ages. Invest. Ophthalmol. Vis. Sci. 2005;46:1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H., Fan J., Li S., Karan S., Rohrer B., Palczewski K., Frederick J.M., Crouch R.K., Baehr W. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J. Neurosci. 2008;28:4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan J., Rohrer B., Frederick J.M., Baehr W., Crouch R.K. Rpe65−/− and Lrat−/− mice: comparable models of leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2008;49:2384–2389. doi: 10.1167/iovs.08-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samardzija M., von Lintig J., Tanimoto N., Oberhauser V., Thiersch M., Reme C.E., Seeliger M., Grimm C., Wenzel A. R91W mutation in Rpe65 leads to milder early-onset retinal dystrophy due to the generation of low levels of 11-cis-retinal. Hum. Mol. Genet. 2008;17:281–292. doi: 10.1093/hmg/ddm304. [DOI] [PubMed] [Google Scholar]
- 10.Samardzija M., Tonimoto N., Kostic C., Beck S., Oberhauser V., Joly S., Thirsch M., Fahl E., Arsenijevic Y., von Lintig J., et al. In conditions of limited chomophore supply rods entrap 11-cis-retinal leading to loss of cone function and cell death. Hum. Mol. Genet. 2009;18:1266–1275. doi: 10.1093/hmg/ddp026. [DOI] [PubMed] [Google Scholar]
- 11.Rodieck R.W. The First Steps in Seeing. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- 12.Jacobson S.G., Aleman T.S., Cideciyan A.V., Sumaroka A., Schwartz S.B., Windsor E.A., Traboulsi E.I., Heon E., Pittler S.J., Milam A.H., et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc. Natl Acad. Sci. USA. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson S.G., Aleman T.S., Cideciyan A.V., Heon E., Golczak M., Beltran W.A., Sumaroka A., Schwartz S.B., Roman A.J., Windsor E.A., et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc. Natl Acad. Sci. USA. 2007;104:15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mata N.L., Radu R.A., Clemmons R.C., Travis G.H. Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acland G.M., Aguirre G.D., Ray J., Zhang Q., Aleman T.S., Cideciyan A.V., Pearce-Kelling S.E., Anand V., Zeng Y., Maguire A.M., et al. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 16.Acland G.M., Aguirre G.D., Bennett J., Aleman T.S., Cideciyan A.V., Bennicelli J., Dejneka N.S., Pearce-Kelling S.E., Maguire A.M., Palczewski K., et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Hooser J.P., Aleman T.S., He Y.G., Cideciyan A.V., Kuksa V., Pittler S.J., Stone E.M., Jacobson S.G., Palczewski K. Rapid restoration of visual pigment and function with oral retinoid in a mouse model of childhood blindness. Proc. Natl Acad. Sci. USA. 2000;97:8623–8628. doi: 10.1073/pnas.150236297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Hooser J.P., Liang Y., Maeda T., Kuksa V., Jang G.F., He Y.G., Rieke F., Fong H.K., Detwiler P.B., Palczewski K. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J. Biol. Chem. 2002;277:19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr, Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M., et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N., et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 21.Hauswirth W., Aleman T.S., Kaushal S., Cideciyan A.V., Schwartz S.B., Wang L., Conlon T., Boye S.L., Flotte T.R., Byrne B., et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 2008:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cideciyan A.V., Aleman T.S., Boye S.L., Schwartz S.B., Kaushal S., Roman A.J., Pang J.J., Sumaroka A., Windsor E.A., Wilson J.M., et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl Acad. Sci. USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batten M.L., Imanishi Y., Tu D.C., Doan T., Zhu L., Pang J., Glushakova L., Moise A.R., Baehr W., Van Gelder R.N., et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golczak M., Kuksa V., Maeda T., Moise A.R., Palczewski K. Positively charged retinoids are potent and selective inhibitors of the trans–cis isomerization in the retinoid (visual) cycle. Proc. Natl Acad. Sci. USA. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golczak M., Imanishi Y., Kuksa V., Maeda T., Kubota R., Palczewski K. Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J. Biol. Chem. 2005;280:42263–42273. doi: 10.1074/jbc.M509351200. [DOI] [PubMed] [Google Scholar]
- 26.Golczak M., Maeda A., Bereta G., Maeda T., Kiser P.D., Hunzelmann S., von Lintig J., Blaner W.S., Palczewski K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J. Biol. Chem. 2008;283:9543–9554. doi: 10.1074/jbc.M708982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda A., Maeda T., Golczak M., Imanishi Y., Leahy P., Kubota R., Palczewski K. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol. Pharmacol. 2006;70:1220–1229. doi: 10.1124/mol.106.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batten M.L., Imanishi Y., Maeda T., Tu D.C., Moise A.R., Bronson D., Possin D., Van Gelder R.N., Baehr W., Palczewski K. Lecithin–retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J. Biol. Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohrer B., Crouch R. Rod and cone pigment regeneration in RPE65−/− mice. Adv. Exp. Med. Biol. 2006;572:101–107. doi: 10.1007/0-387-32442-9_16. [DOI] [PubMed] [Google Scholar]
- 30.Rohrer B., Lohr H.R., Humphries P., Redmond T.M., Seeliger M.W., Crouch R.K. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest. Ophthalmol. Vis. Sci. 2005;46:3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y., Moiseyev G., Takahashi Y., Ma J.X. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest. Ophthalmol. Vis. Sci. 2006;47:1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- 32.Wenzel A., von Lintig J., Oberhauser V., Tanimoto N., Grimm C., Seeliger M.W. RPE65 is essential for the function of cone photoreceptors in NRL-deficient mice. Invest. Ophthalmol. Vis. Sci. 2007;48:534–542. doi: 10.1167/iovs.06-0652. [DOI] [PubMed] [Google Scholar]
- 33.Bemelmans A.P., Kostic C., Crippa S.V., Hauswirth W.W., Lem J., Munier F.L., Seeliger M.W., Wenzel A., Arsenijevic Y. Lentiviral gene transfer of RPE65 rescues survival and function of cones in a mouse model of Leber congenital amaurosis. PLoS Med. 2006;3:e347. doi: 10.1371/journal.pmed.0030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson S.G., Cideciyan A.V., Aleman T.S., Sumaroka A., Windsor E.A., Schwartz S.B., Heon E., Stone E.M. Photoreceptor layer topography in children with leber congenital amaurosis caused by RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2008;49:4573–4577. doi: 10.1167/iovs.08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda A., Maeda T., Palczewski K. Improvement in rod and cone function in mouse model of Fundus albipunctatus after pharmacologic treatment with 9-cis-retinal. Invest. Ophthalmol. Vis. Sci. 2006;47:4540–4546. doi: 10.1167/iovs.06-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda T., Maeda A., Leahy P., Saperstein D.A., Palczewski K. Effects of long-term administration of 9-cis-retinyl acetate on visual function in mice. Invest. Ophthalmol. Vis. Sci. 2009;50:322–333. doi: 10.1167/iovs.08-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan J., Rohrer B., Moiseyev G., Ma J.X., Crouch R.K. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc. Natl Acad. Sci. USA. 2003;100:13662–13667. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imanishi Y., Batten M.L., Piston D.W., Baehr W., Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulte D., Furukawa T., Peters M.A., Kozak C.A., Cepko C.L. Misexpression of the Emx-related homeobox genes cVax and mVax2 ventralizes the retina and perturbs the retinotectal map. Neuron. 1999;24:541–553. doi: 10.1016/s0896-6273(00)81111-3. [DOI] [PubMed] [Google Scholar]
- 40.Schonthaler H.B., Lampert J.M., Isken A., Rinner O., Mader A., Gesemann M., Oberhauser V., Golczak M., Biehlmaier O., Palczewski K., et al. Evidence for RPE65-independent vision in the cone-dominated zebrafish retina. Eur. J. Neurosci. 2007;26:1940–1949. doi: 10.1111/j.1460-9568.2007.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mata N.L., Ruiz A., Radu R.A., Bui T.V., Travis G.H. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-Retinol. Biochemistry. 2005;44:11715–11721. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeda A., Maeda T., Golczak M., Chou S., Desai A., Hoppel C.L., Matsuyama S., Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 2009 doi: 10.1074/jbc.M900322200. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aleman T.S., Jacobson S.G., Chico J.D., Scott M.L., Cheung A.Y., Windsor E.A., Furushima M., Redmond T.M., Bennett J., Palczewski K., et al. Impairment of the transient pupillary light reflex in Rpe65(−/−) mice and humans with leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2004;45:1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
- 44.Stieger K., Schroeder J., Provost N., Mendes-Madeira A., Belbellaa B., Meur G.L., Weber M., Deschamps J.Y., Lorenz B., Moullier P., et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol. Ther. 2008;17:516–523. doi: 10.1038/mt.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobson S.G., Aleman T., Cideciyan A.V., Roman A.J., Sumaroka A., Windsor E.A., Schwartz S.B., Heon E., Stone E.M. Defining the residual vision in Leber congenital amaurosis caused by RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2008 doi: 10.1167/iovs.08-2696. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y., Cideciyan A.V., Papastergiou G.I., Banin E., Semple-Rowland S.L., Milam A.H., Jacobson S.G. Relation of optical coherence tomography to microanatomy in normal and rd chickens. Invest. Ophthalmol. Vis. Sci. 1998;39:2405–2416. [PubMed] [Google Scholar]
- 47.Jacobson S.G., Cideciyan A.V., Aleman T.S., Pianta M.J., Sumaroka A., Schwartz S.B., Smilko E.E., Milam A.H., Sheffield V.C., Stone E.M. Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum. Mol. Genet. 2003;12:1073–1078. doi: 10.1093/hmg/ddg117. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Cense B., Rha J., Jonnal R.S., Gao W., Zawadzki R.J., Werner J.S., Jones S., Olivier S., Miller D.T. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. Opt. Express. 2006;14:4380–4394. doi: 10.1364/OE.14.004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez E.J., Hermann B., Povazay B., Unterhuber A., Sattmann H., Hofer B., Ahnelt P.K., Drexler W. Ultrahigh resolution optical coherence tomography and pancorrection for cellular imaging of the living human retina. Opt. Express. 2008;16:11083–11094. doi: 10.1364/oe.16.011083. [DOI] [PubMed] [Google Scholar]
- 50.Potsaid B., Gorczynska I., Srinivasan V.J., Chen Y., Jiang J., Cable A., Fujimoto J.G. Ultrahigh speed spectral/Fourier domain OCT ophthalmic imaging at 70 000 to 312 500 axial scans per second. Opt. Express. 2008;16:15149–15169. doi: 10.1364/oe.16.015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gotzinger E., Pircher M., Geitzenauer W., Ahlers C., Baumann B., Michels S., Schmidt-Erfurth U., Hitzenberger C.K. Retinal pigment epithelium segmentation by polarization sensitive optical coherence tomography. Opt. Express. 2008;16:16410–16422. doi: 10.1364/oe.16.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calvert P.D., Krasnoperova N.V., Lyubarsky A.L., Isayama T., Nicolo M., Kosaras B., Wong G., Gannon K.S., Margolskee R.F., Sidman R.L., et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc. Natl Acad. Sci. USA. 2000;97:13913–13918. doi: 10.1073/pnas.250478897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maeda A., Maeda T., Sun W., Zhang H., Baehr W., Palczewski K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc. Natl Acad. Sci. USA. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maeda T., Lem J., Palczewski K., Haeseleer F. A critical role of CaBP4 in the cone synapse. Invest. Ophthalmol. Vis. Sci. 2005;46:4320–4327. doi: 10.1167/iovs.05-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maeda A., Maeda T., Imanishi Y., Kuksa V., Alekseev A., Bronson J.D., Zhang H., Zhu L., Sun W., Saperstein D.A., et al. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J. Biol. Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]