Abstract
A model of awareness based on interoceptive salience is described, which has an endogenous time base that might provide a basis for the human capacity to perceive and estimate time intervals in the range of seconds to subseconds. The model posits that the neural substrate for awareness across time is located in the anterior insular cortex, which fits with recent functional imaging evidence relevant to awareness and time perception. The time base in this model is adaptive and emotional, and thus it offers an explanation for some aspects of the subjective nature of time perception. This model does not describe the mechanism of the time base, but it suggests a possible relationship with interoceptive afferent activity, such as heartbeat-related inputs.
Keywords: insula, awareness, homeostasis, subjective, emotion, interoception
1. Introduction
I am a functional neuroanatomist who has studied ascending pathways for pain and temperature sensation. Although I have published no reports on the pattern of brain activation during the mental estimation of time, the editors of this special issue were aware that the research I had performed had led to a proposal for a structural model of human subjective awareness that involves a time-based representation of successive emotional moments, and they asked me to describe how this model could substantialize the perception of time. Briefly, this model posits a cortical basis for subjective awareness in which a serial set of representations of all feelings at each immediate moment (‘now’) extends across a finite period of present time (‘the specious moment’). The progression of emotional feelings at successive moments across time effectively provides a cinemascopic view of the sentient self. The endogenous temporal index in this model is subjective and emotionally flexible. As I will explain, the functional anatomical evidence that supports this model indicates that the proposed substrate for awareness probably exists in the anterior insular cortex (AIC). That location fits with functional imaging evidence indicating that the AIC contains a crucial neural component for the perception of time itself by humans.
I first described this homeostatic model of human awareness in a book chapter written 2 years ago (Craig 2008) and I elaborated further in a more recent paper that reviewed a surfeit of functional imaging studies that are parsimonious with this model (Craig 2009). Here I describe briefly the anatomical background for the model, then I describe the model in more detail and relate it to certain aspects of time perception and, finally, I suggest a few research directions that might produce further insights into our awareness of time.
2. The functional anatomic basis for the model
This proposal for a homeostatic model of awareness grew out of research on the central representation of feelings from the body (Craig 2002). I used single-cell recordings and high-resolution anterograde and retrograde tracing techniques to study specifically nociceptive, thermoreceptive, muscle-sensitive and itch-sensitive cells in lamina I of the spinal cord and to trace their projections in the spinal cord, brainstem and thalamus. These studies revealed a phylogenetically novel ascending sensory pathway from lamina I (in the spinal cord) that joined with another novel pathway from the solitary nucleus (in the brainstem) in primates to form projections not only to all autonomic cardiorespiratory integration regions in the spinal cord and brainstem, but also to a specific thalamo-cortical relay nucleus that is modality specific and topographically organized in a unique manner. The subsequent recognition that this pathway fundamentally represents the long-missing homeostatic afferent side of the autonomic nervous system led to the insight that the terminus of this novel pathway in the posterior insular cortex of primates provides the basis for the sense of the physiological condition of the entire body, which I termed ‘interoception’ (Craig 2002). This ‘interoceptive cortex’ includes somatotopic representations of the activity in small-diameter primary afferent sensory fibres that generate numerous individually mapped and distinct ‘feelings’ from the body, such as first ‘pricking’ pain, second ‘burning’ pain, cool, warm, itch, muscle ache, sensual touch, thirst, hunger, taste, ‘air hunger’ and other visceral sensations.
Functional imaging studies provide unequivocal corroboration of this pathway in humans by demonstrating graded activation of the dorsal posterior insular cortex associated with these feelings (e.g. Williamson et al. 1997; Craig et al. 2000; Drzezga et al. 2001; Del Parigi et al. 2002; Olausson et al. 2002, 2005; Keltner et al. 2006). Discrete lesions of the dorsal posterior insula in the human brain produce complete loss of such feelings on the contralateral side of the body (Schmahmann & Leifer 1992; Greenspan et al. 1999). Microelectrode stimulation of either the thalamic relay nucleus or the dorsal posterior insula can elicit reports of discretely perceived interoceptive feelings in awake human patients (Davis et al. 1999; Lenz et al. 1999; Ostrowsky et al. 2002; for additional references see Craig 2002, 2008, 2009). The imaging evidence indicates that the primary interoceptive regions in the posterior insular cortex are re-represented and integrated, first in the homolateral mid-insula and then in the anterior insula, where activation correlates with subjective feelings rather than objective stimulus characteristics (Craig et al. 2000; Craig 2002, 2009).
In sub-primates, homeostatic afferent activity is processed in the brainstem (primarily in the parabrachial nucleus) and the forebrain receives only a highly integrated representation of such activity. The novel appearance of the homeostatic afferent thalamo-cortical pathway in primates probably resulted from the enormous encephalization that took place at the onset of primate evolution. The generation of a high-resolution cortical image of the homeostatic condition of the body paralleled the evolutionary development in mammals of limbic behavioural control by each individual's autonomic condition in place of control by primordial olfactory signals (Heimer & Van Hoesen 2006). Cortical integration of high-resolution information on the state of the body provides improved autonomic control, and thus improved survival of the individual and the species, as well as the opportunity for the evolution of further advancements in the integration of the individual's condition in order to guide behaviour even more efficiently. Such advanced integration apparently led to improved conspecific emotional communication in humanoid primates (Dunbar & Shultz 2007) and the development of a representation in the AIC of the ‘sentient self’ across time.
3. A structural model of human awareness across time
In this model, the cortical representation of the sentient self in the AIC is based on the integration of salience across all conditions in the individual's body and in the physical and emotional environment at each moment of time. The salience of any factor is determined by its significance for the maintenance and advancement of the individual and the species. In other words, a physical or environmental object or condition is salient if it has significance for the organism's survival. At the most fundamental level of existence, the conditions that affect survival are important for the maintenance of the health of the physical body (and ultimately the brain), which is the energy-efficient neurobiological process of homeostasis. In this model, therefore, the neural substrates responsible for sentience across time are based on the neural representation of the physiological condition of the body, which is consistent with the essence of the James–Lange theory of emotion and Damasio's ‘somatic marker’ hypothesis (Craig 2002, 2009). Importantly, the main homeostatic (autonomic) control function for the maintenance of the physiological condition of the body is cardiorespiratory activity, yet homeostasis integrates across all physiological and autonomic conditions to achieve an optimal balance (Janig 2006).
The phylogenetically novel homeostatic afferent pathway from lamina I and the solitary nucleus in primates provides the basis for the sense of the physiological condition of the entire body in the posterior insular cortex. This engenders distinct feelings from the body that have a fundamental homeostatic basis in their concomitant autonomic (cardiorespiratory) sequelae. The neural construction of these feelings seems to provide an important template for the construction of all feelings. The primary neural representations of the state of the body are re-represented and integrated in the mid-insula and, again, in the anterior insula (on the left or right side or both, depending on the source of the activity; see below). The mid-insula also receives convergent activity associated with salient environmental stimuli of many sensory modalities (apparently via higher order sensory regions, the temporal pole, the frontal cortex and the amygdala), and it receives direct modulation from the ventral striatum (nucleus accumbens; Menon & Levitin 2005), which provides important incentive/hedonic signals for the integration of salience. The few available studies of the anatomical connections of the insular cortex in primates and other mammals indicate that it is interconnected with the amygdala, the hypothalamus, the anterior cingulate cortex (ACC) and the orbitofrontal cortex, and that its primordial role is descending control of homeostatic afferent integration in the brainstem parabrachial nucleus (Mesulam & Mufson 1982a,b; Mufson & Mesulam 1982; Augustine 1985, 1996; Vogt & Pandya 1987; Chikama et al. 1997; Mufson et al. 1997; Saper 2002; Fudge et al. 2005).
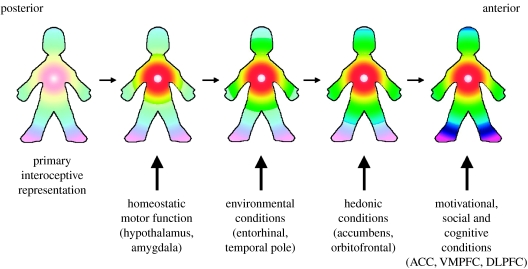
There is a general posterior-to-anterior processing gradient for increasing complexity in the frontal cortex (Amodio & Frith 2006; Koechlin & Jubault 2006), which is consistent with the evidence for a posterior-to-anterior progression in the insula and with the enormous expansion of the anterior insula across humanoid primates. The posterior-to-mid-to-anterior progression of neural processing through the insula provides a foundation for the sequential integration of the primary homeostatic condition of the body with the salient features of the sensory environment and then with the motivational, hedonic and social conditions that are represented in the other interconnected parts of the brain. As illustrated in figure 1, the sequential integration in this model is elaborated on the fundamental neural construct of the homeostatic feelings from the body, anchored by the homeostatic cardiorespiratory responses. The model proposes that this progression ultimately leads to the integration of salience across all conditions in a unified meta-representation of the ‘global emotional moment’. This processing stage thus constitutes an image of ‘the material me’ or the sentient self at the immediate moment of time—‘now’.
Figure 1.
A cartoon illustrating how the hypothesized integration of salient activity progresses from the posterior insula (left) through the mid-insula to the anterior insula (right). The primary interoceptive representations of the distinct feelings from the body in the dorsal posterior insula provide a somatotopic foundation and a template for the construction of all feelings. It is anchored by the homeostatic effects of each feeling on cardiorespiratory function, as indicated by the focus of the colours in the chest. The salient homeostatic, environmental, hedonic, motivational, social and cognitive factors are progressively integrated by the indicated inputs from other parts of the brain to produce an ultimate ‘global emotional moment’, which represents the sentient self at one moment of time. VMPFC, ventromedial prefrontal cortex; DLPFC, dorsolateral prefrontal cortex.
In this model, the quantal structural unit that engenders a global emotional moment is anatomically replicated to produce a finite set of repeated meta-representations of global emotional moments, and an endogenous time base indexes this set to produce a cinemascopic representation of ‘me’ across time. As illustrated in figure 2, this structure (a ‘meta-memory’ of salience across time) can provide the basis for the continuity of subjective emotional awareness within a finite present (Craig 2008, 2009).
Figure 2.
A cartoon illustrating the representation of time in the proposed model of awareness. (a) How a series of global emotional moments that are indexed across a finite period of present time, from the past into the anticipated future, can produce a cinemascopic ‘image’ of the sentient self that is continuous across a moving window of present time is shown. (b) This structure is depicted during a period of high emotional salience and arousal, when global emotional moments are rapidly ‘filled up’. The accumulation of global emotional moments produces a subjective dilation of time, and objective time appears to ‘stand still’.
The available functional imaging evidence indicates that storage buffers for such global emotional moments must be present in order to enable immediate comparisons of past and anticipated future feelings with the present feelings. Such comparators are directly indicated by studies of risk prediction and anticipation (Seymour et al. 2004; Preuschoff et al. 2008). One interesting implication of a comparative buffer in this model is that the interaction between a master comparator buffer and the time series of global emotional moments might be experienced introspectively as an ‘observer’ that nonetheless cannot ‘see’ itself, reminiscent of discussions of phenomenal consciousness (James 1890).
In this model, each global emotional moment is generated by the feelings from the body, the environment and the social context in real time. Anticipatory global emotional moments in the immediate future (which are important for predicting future emotional states contingent on present actions and behaviours), however, must be generated by stored representations of expectations and internal models of behaviour acquired by experience. Thus, expectations of events at particular future times must be based on past experience, and the system must be calibrated by comparisons with objective reality. Interestingly, the right AIC displays graded sensitivity to unexpected cross-modal disparities in time synchronization (see below).
An anatomical correlate of these hypothetical meta-representations of global emotional moments/behaviours might be provided by the so-called Von Economo neurons (VENs) that are selectively located in the anterior insula and the anterior cingulate cortices of humanoid primates; they are especially numerous only in adult humans but are not present at all in monkeys (Nimchinsky et al. 1999; Allman et al. 2005). The significance of VENs is discussed in prior papers (Craig 2008, 2009).
The hypothesis that the AIC engenders awareness is supported by recent imaging studies from a broad range of fields (as reviewed and discussed in Craig 2009). First, the AIC seems to contain a representation of the sentient self; it is activated during subjective feelings associated with any physical stimulus and every emotion (e.g. Craig et al. 2000; Damasio et al. 2000; Hennenlotter et al. 2005; Jabbi et al. 2007), and it is selectively active in association with self-generated movements and feelings of body ownership and movement agency (e.g. Mutschler et al. 2007; Tsakiris et al. 2007). In addition, studies of self-recognition (as in the mirror test for self-awareness) indicate that the AIC is selectively activated when a subject identifies himself/herself in a photo, consistent with the idea that the feeling of identifying with the self-image (conditioned by feelings of ownership of movements and emotional expressions in a mirror) requires a mental representation of the sentient self (Devue et al. 2007; de Waal 2008). A study of the attentional blink (Kranczioch et al. 2005), in which a second target cannot be perceived if it occurs too quickly following an initial target in a series of rapid visual stimuli, demonstrated activation of the AIC and the neighbouring inferior frontal gyrus when the second target was correctly detected at the shortest intervals (100–200 ms) but not when it was not perceived. Studies of visual and auditory bistable percepts indicate clearly that subjective awareness of a particular percept correlates with the activation of the AIC and the neighbouring inferior frontal cortex (e.g. Sterzer & Kleinschmidt 2007). A study of subjective recognition of ambiguous visual images that were slowly revealed reported that slowly increasing activation occurred in higher order visual regions, but a sudden burst of activation occurred in the AIC and the adjacent inferior frontal cortex when the subjects signalled the ‘moment-of-recognition’ that is coincident with awareness of the percept (Ploran et al. 2007). Strong evidence for this hypothesis was provided by a study of subjective awareness of one's own erroneous eye movements (Klein et al. 2007), which reported a direct correlation only with activation in the AIC (and not the ACC). Interestingly, a study of subjective awareness of mental processes (the ‘feeling-of-knowing’ inherent in memory recall; Kikyo et al. 2002) reported a selective and graded activation of the AIC and the ACC. Finally, this model is strongly supported by recent clinical studies which reported that degeneration of VENs in the AIC and the ACC is selectively associated with the loss of emotional awareness and self-conscious behaviours in patients with fronto-temporal dementia (FTD; Seeley et al. 2006, 2007; Sturm et al. 2006).
The ACC is intimately interconnected with the AIC and is co-activated in most, but not all, such studies. I view the AIC and the ACC as complementary sensory and motor limbic cortices that work together and provide the substrates for the feelings and motivations, respectively, that make up all emotions and emotional behaviours (Craig 2002, 2008, 2009). This fits with the dual lamina I spinothalamic projection to both the insula and the ACC, the respective descending projections from the insula and the ACC to sensory (parabrachial nucleus) and motor (periaqueductal grey) brainstem regions, the overall anatomical organization of the frontal cortex into sensory and motor networks (Ongur et al. 2003) and the evolutionarily ancient limbic role of the cingulate cortex in integrated behavioural control. This perspective is also consistent with a global view of the organization of the limbic system (Heimer & Van Hoesen 2006). Recent functional imaging studies have indicated that the AIC and the ACC work together for cognitive control as well as emotional processing (e.g. Cole & Schneider 2007; Thielscher & Pessoa 2007), although, at most times, the right AIC may actually lead (Sridharan et al. 2008) while the left AIC monitors behaviour (Klein et al. 2007), prior to activation of the ACC. Most notably, the AIC is activated without ACC involvement in studies that focus on subjective awareness (e.g. Craig et al. 2000; Klein et al. 2007; Tsakiris et al. 2007; Preuschoff et al. 2008).
4. Time perception in the homeostatic model of the sentient self
If a representation of the sentient self across time does exist in the AIC, as proposed, with an endogenous time base that indexes a sequential progression of global emotional moments, then studies of the human perception of time intervals in the range of seconds to subseconds should reveal activation specifically in the AIC. This prediction is confirmed by functional imaging studies of time perception, many of which reported activation in this region (e.g. Rao et al. 2001; Coull 2004; Lewis & Miall 2006; Livesey et al. 2007). Such activation was variously ascribed to the ‘ventrolateral prefrontal cortex’, the ‘frontal operculum’, the inferior frontal gyrus or the anterior insula, and few comments were made on the possible functional significance of such activation. The imaging evidence is also consistent with the putative involvement of dopaminergic modulation of the putamen and fronto-striatal circuits in human time perception (see Droit-Volet & Meck 2007).
The most recent fMRI study of time perception provides strong corroboration for the present hypothesis. Livesey et al. (2007) designed an experiment in which task difficulty was manipulated in order to isolate time estimation from other task-related cognitive demands, because they had noted that prior studies of time perception might have been confounded by several cognitive factors. The authors found five small loci of activation specifically associated with time perception in the range of subseconds to seconds: one in the dorsal putamen on both sides, another in the inferior parietal cortex on the left side only and another at the junction of the anterior insula with the frontal operculum on both sides. They suggested that the focus in the bilateral AIC must be ‘of central importance’ for time perception. They mentioned that their findings relate to an internal-clock model of mental timekeeping, in which pulses from an internal time base are counted and then compared with stored representations of the target time interval. Assuming that the site in the left inferior parietal cortex is associated with counting, then their results suggest that the AIC and the putamen bilaterally constitute a unique mental real-time clock that is used to measure the passage of time in the external environment.
The observations of Livesey et al. (and the prior imaging studies) support the view that human awareness of time specifically involves the AIC. This conclusion is consistent with the proposal described above that an endogenous time base in the AIC indexes the passage of global emotional moments across a finite period of the immediate present. This convergence of evidence and model suggests that the same endogenous time base that is used to index the progression of global emotional moments is also used for subjective time estimation. If the strong heartbeat-related activation of the dorsal putamen (Ito & Craig 2008; Pollatos et al. 2007) is used to index the shifting emotional moments in the AIC, for example, then the comparative buffer in the AIC could be used to compare the feeling associated with a prior time interval with the feeling of the present interval across the range of the meta-memory, i.e. in the range of seconds to subseconds. This suggestion is consistent also with the subjective slowing of time produced by an injection of clonidine, which causes a decrease in sympathetic tone (see Coull 2004).
The role of sequential time in this model of awareness has interesting implications. One especially fascinating aspect is that this structure can provide an emergent basis for the uniquely human faculty of music. That is, if music is viewed as the rhythmic temporal progression of emotionally laden moments, which this model directly instantiates, then this model provides a ready basis for the neural representation of music as a coherent emotional construct. This idea is supported directly by functional imaging and clinical evidence indicating a specific role of the AIC in the emotional appreciation of music and in music making (Ackermann & Riecker 2004; see Craig 2008, 2009). In addition, a lesion of the anterior insula can eliminate emotional involvement with music (Griffiths et al. 2004). A positron emission tomography (PET) study showed selective activation of the left AIC associated with rhythm by using occasional mistimed intervals in well-known short melodies (Platel et al. 1997), which is directly relevant to both music and time perception. The emergent capacity for music in this model can also explain the primal emotional effects of communal music making, because it indicates that music inherently involves the core of awareness. (In fact, recognition that the AIC is uniquely associated with emotion, movement, music and time suggested the formulation of this model; see Craig 2008.)
Whether the quantal units of the global emotional moments are arranged sequentially within the AIC or rather are flexibly recruited from a common resource pool in the AIC by the endogenous indexing time base, the fact that the integration of emotional salience is the fundamental feature of this model implies that the rate of subjective time passage must be adaptive. Thus, another interesting implication of this model is that the progression of emotional moments across time is inherently subjective, emotional and flexible. This can explain the well-known phenomenon of subjective time dilation during an intensely emotional period (see also Wittmann & Paulus 2008), such as during an accident (Tse et al. 2004; van Wassenhove et al. 2008) or a parachute jump (Campbell & Bryant 2007). In this model, a high rate of salience accumulation would ‘fill up’ global emotional moments quickly, because the information capacity for salience of each individual neural representation of a global emotional moment must be finite. Thus, the rate of passage of global emotional moments must effectively speed up during an intensely emotional period and time in the objective world would appear to ‘stand still’ to the subjective observer. The illustration in figure 2b suggests how this might be implemented by the model.
Of course, the converse situation also applies; that is, during periods of low emotional salience, when we are disengaged, large intervals of time in the objective world can appear to pass quickly. Curiously, during a period of low emotional salience when little is happening, if we become bored and turn our attention inwards, then our awareness seems to outpace the real world and objective time seems to pass excruciatingly slowly. This might also be explained with the same structural model, because subjective emotional moments of self-referenced interoceptive salience are then passing by more quickly than the static level of low salience in the objective world. However, quite remarkably, during periods when we experience intense pleasant feelings, for example in the company of a loving partner, real time can seem to pass by too quickly rather than too slowly (Droit-Volet & Meck 2007). The model of sequential global emotional moments, as described thus far, cannot explain this situation, because this is also a period of high attentional engagement and emotional salience, such as during an accident, and yet with the opposite effect on subjective time. A solution to this potential contradiction involving the asymmetric representation of emotions in the left and right forebrain is proposed further below.
Others have suggested that the evolution of deliberate social signalling or intentional emotional interaction between individual humanoid primates required the development of a reflective awareness of me across time that can compare the effects of my actions now, in the past and in the future (Frith & Frith 2007; Lewis 2008). This perspective implies again that the endogenous time base must be sensitive to emotional salience. For example, the development of long-distance emotional relationships, in which interactions occur only rarely, requires that an emotional moment be extended across different time scales. The same flexibility would be needed for the emotional appreciation of the pattern of seasonal variations of vegetation, climate and the stars.
In spite of the need for emotional flexibility, objective timing is crucially important for the AIC. This fact is demonstrated by studies showing that the AIC is selectively sensitive to time synchronicity. For example, in one PET study, the AIC displayed a graded response to a cross-modal timing mismatch between auditory and visual stimuli that are normally synchronous (e.g. a speaking mouth) at intervals of 150 ms and less (Bushara et al. 2001). This neural activation correlated directly with the subjects' ability to detect and respond to such a mismatch. Interestingly, studies of inspection time, the attentional blink and rapid visual search all indicate a similar value for the shortest time period that can produce 100 per cent reliability in human visual awareness (Deary et al. 2004; Kranczioch et al. 2005). (Of course, stimulus pairs in any single sensory system can be discriminated with much finer temporal resolution.) For example, an fMRI study of inspection time reported that subjects' ability to detect visual asymmetry in a briefly displayed image decreased progressively from 100 per cent to chance levels for presentation times shorter than 150 ms. The strikingly progressive increase in activation in the AIC (and the ACC) at these brief presentation times is consistent with the occasional behavioural need for heightened awareness of the environment in real time (Deary et al. 2004).
5. Functional asymmetry in the AIC
Functional imaging evidence indicates that physical stimuli that drive sympathetic activity, such as temperature and pain, cause activation predominantly in the right AIC (Craig et al. 2000; Brooks et al. 2002), whereas stimuli that drive afferents in parasympathetic nerves, such as stomach distension and gustation (Stephan et al. 2003; Veldhuizen et al. 2007) preferentially activate the mid-insula and the AIC on the left side. Similarly, electrical stimulation of the right insula clinically can cause tachycardia, whereas stimulation of the left insula can cause bradycardia (Oppenheimer et al. 1992; see also Wittling et al. 1998). Such evidence suggests that the homeostatic model of interoceptive integration that leads to awareness uses the right and left insular cortices to process homeostatic afferent activity asymmetrically. A functional opponent organization provides an optimally energy-efficient control mechanism (such as the antagonistic muscles across a joint), and the opponency of the sympathetic and parasympathetic halves of the autonomic nervous system (although incomplete), particularly in the innervation of the heart, provides a primordial basis for an opponent homeostatic organization in the forebrain. Further evidence for this proposition is provided by the effects of barbiturate injection in the left and right carotid arteries in the Wada test; injection on the left side (which anaesthetizes the left forebrain and releases the right forebrain) can cause tachycardia and negative mood, whereas injection in the right carotid can cause bradycardia and positive mood (Heilman 2000).
I have proposed that the homeostatic asymmetry in the insular cortex of the two sides can provide a basis for a well-known model of the forebrain asymmetry of emotion (Craig 2005), in which positive affect, affiliative feelings and approach behaviours (energy nourishment) are associated with the left forebrain, and negative affect, arousing feelings and avoidance behaviours (energy expenditure) are associated with the right forebrain (Davidson 2004). This model implies that the AIC on the two sides of the brain differs, and the functional imaging evidence supports an association of the right AIC with negative and arousing feelings and the left AIC with positive and affiliative feelings (see Craig 2005, 2009). For example, the right AIC is activated while viewing an untrustworthy face, whereas the left AIC is activated while viewing a trustworthy face (Winston et al. 2002). Of course, under most circumstances, both sides are co-active, just as the coordinated co-activation of the sympathetic and parasympathetic nerves in the ongoing autonomic control of the heart. To my mind, the interactions between the respective AICs on the two sides of the brain required for the formation of a single unified sense of self deserves intense study, because it may provide insight into the basis for emotional imbalance.
Interestingly, there is striking evidence that the AIC on the two sides also differ with respect to timing. Ackermann & Riecker (2004) reported that covert singing activates the AIC bilaterally, but when the tempo was slow (less than 3 Hz), the right AIC was predominantly activated, and when the tempo was fast (more than 3 Hz), the left AIC was predominantly activated. Other evidence suggests that the right AIC at times directs forebrain processing (Sridharan et al. 2008) and the left AIC monitors outcomes (Klein et al. 2007), but perhaps these roles reverse in circumstances of positive affect.
With regard to time perception, these considerations might suggest a solution to the apparent contradiction described above in the asymmetric effects of strong negative and positive emotions on time perception (Droit-Volet & Meck 2007). That is, the psychophysical evidence indicates that if we are emotionally engaged in a negative situation subjective time dilates, whereas in a positive situation subjective time contracts. In this asymmetric model of homeostatic emotion, if our awareness is heightened in a sympathetically arousing situation, energy is expended, global emotional moments accumulate rapidly in the right AIC, subjective time dilates and objective time seems to stand still. On the other hand, if our awareness is heightened in a soothing affiliative situation, the left forebrain predominates and parasympathetic tone increases heart rate variability, and perhaps while positive emotional energy is being absorbed into global emotional moments in the left AIC, the global emotional moments in both the left and right AIC progress slowly, because there is little sympathetic salience, so that subjective time contracts (seems to run slow) and objective time passes much too quickly.
This model of emotional asymmetry predicts that if we are aroused and engaged in a negative or challenging setting, then the right AIC is more active and objective time intervals are overestimated, but if we are peacefully content in an affiliative setting, then the left AIC is more active and time intervals are underestimated. This model of asymmetry similarly predicts opposite effects of interoceptive conditions on time perception; for example, if hyperthermia causes subjective time dilation (see Wittmann 2009), then hypothermia could cause subjective time contraction, and if pain causes subjective time dilation, then slow breathing (activating vagal bronchopulmonary afferents) could cause time contraction.
6. Conclusion
The structural model for awareness localized in the AIC that I have described is supported by a large number of studies, as briefly described above and reviewed in more detail elsewhere (Craig 2009). The concordance between the hypothesized time base in this model of awareness and recent functional imaging evidence on forebrain sites involved in the perception of time (Livesey et al. 2007) supports the idea that the AIC is a crucial locus in the human brain for the perception of time. The AIC may contain a subjective, emotionally sensitive mechanism for representing salient factors in our bodies and our environments on a moment to moment basis. The time base that is used to sequentially index the passage of these moments and to detect synchrony in the environment may provide the time base that humans use to estimate time intervals in the range of seconds to subseconds. These ideas fit with the subjective nature of time perception and with the emotional and interoceptive dependence of time estimation in humans (see Droit-Volet & Gil 2009 and Wittmann 2009).
These considerations suggest several potentially fruitful research directions. Studies of interactions between subjective time, emotion and music could directly address this model and reveal new interactions that define the substrate that underlies our awareness. Identification of the mechanism for the timing of sequential global emotional moments in the AIC is needed, because the present model does not specify how the endogenous time base is instantiated. The possibility that the strong influence of the heartbeat on the AIC and the dorsal putamen (Critchley et al. 2004; Pollatos et al. 2007; Ito & Craig 2008) might be important for the endogenous time base needs to be examined. The homeostatic model suggests that interoceptive feelings from the body provide the basis for the construct of the sentient self in global emotional moments, and so this model suggests potential explanations for the dependence of subjective time perception on interoceptive signals, such as body temperature (see Wittmann 2009). The interactions between the AIC on the two sides are potentially highly significant, and the predictions from this model of asymmetry described above need to be tested. Similarly, the identification of the buffer used for comparisons of feelings (and time intervals) remains to be accomplished. I look forward to future advances in this field.
Acknowledgments
I am grateful for the continuous support of the Barrow Neurological Foundation and for the comments of the editors and the anonymous reviewers.
Footnotes
One contribution of 14 to a Theme Issue ‘The experience of time: neural mechanisms and the interplay of emotion, cognition and embodiment’.
References
- Ackermann H., Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89:320–328. doi: 10.1016/S0093-934X(03)00347-X. doi:10.1016/S0093-934X(03)00347-X [DOI] [PubMed] [Google Scholar]
- Allman J.M., Watson K.K., Tetreault N.A., Hakeem A.Y. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn. Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. doi:10.1016/j.tics.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. doi:10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Augustine J.R. The insular lobe in primates including humans. Neurol. Res. 1985;7:2–10. doi: 10.1080/01616412.1985.11739692. [DOI] [PubMed] [Google Scholar]
- Augustine J.R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. doi:10.1016/S0165-0173(96)00011-2 [DOI] [PubMed] [Google Scholar]
- Brooks J.C., Nurmikko T.J., Bimson W.E., Singh K.D., Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. doi:10.1006/nimg.2001.0974 [DOI] [PubMed] [Google Scholar]
- Bushara K.O., Grafman J., Hallett M. Neural correlates of auditory–visual stimulus onset asynchrony detection. J. Neurosci. 2001;21:300–304. doi: 10.1523/JNEUROSCI.21-01-00300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L.A., Bryant R.A. How time flies: a study of novice skydivers. Behav. Res. Ther. 2007;45:1389–1392. doi: 10.1016/j.brat.2006.05.011. doi:10.1016/j.brat.2006.05.011 [DOI] [PubMed] [Google Scholar]
- Chikama M., McFarland N.R., Amaral D.G., Haber S.N. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J. Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M.W., Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. doi:10.1016/j.neuroimage.2007.03.071 [DOI] [PubMed] [Google Scholar]
- Coull J.T. fMRI studies of temporal attention: allocating attention within, or towards, time. Brain Res. Cogn. Brain Res. 2004;21:216–226. doi: 10.1016/j.cogbrainres.2004.02.011. doi:10.1016/j.cogbrainres.2004.02.011 [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig A.D. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn. Sci. 2005;9:566–571. doi: 10.1016/j.tics.2005.10.005. doi:10.1016/j.tics.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Craig A.D. Interoception and emotion. In: Lewis M., Haviland-Jones J.M., Barrett L.F., editors. Handbook of emotions. 3rd edn. Guilford Publications; New York, NY: 2008. pp. 272–288. [Google Scholar]
- Craig A.D. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. doi:10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Craig A.D., Chen K., Bandy D., Reiman E.M. Thermosensory activation of insular cortex. Nat. Neurosci. 2000;3:184–190. doi: 10.1038/72131. doi:10.1038/72131 [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. doi:10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L.L., Parvizi J., Hichwa R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. doi:10.1038/79871 [DOI] [PubMed] [Google Scholar]
- Davidson R.J. Well-being and affective style: neural substrates and biobehavioural correlates. Phil. Trans. R. Soc. Lond. B. 2004;359:1395–1411. doi: 10.1098/rstb.2004.1510. doi:10.1098/rstb.2004.1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K.D., Lozano A.M., Manduch M., Tasker R.R., Kiss Z.H.T., Dostrovsky J.O. Thalamic relay site for cold perception in humans. J. Neurophysiol. 1999;81:1970–1973. doi: 10.1152/jn.1999.81.4.1970. [DOI] [PubMed] [Google Scholar]
- Deary I.J., Simonotto E., Meyer M., Marshall A., Marshall I., Goddard N., Wardlaw J.M. The functional anatomy of inspection time: an event-related fMRI study. Neuroimage. 2004;22:1466–1479. doi: 10.1016/j.neuroimage.2004.03.047. doi:10.1016/j.neuroimage.2004.03.047 [DOI] [PubMed] [Google Scholar]
- Del Parigi A., Chen K., Salbe A.D., Gautier J.F., Ravussin E., Reiman E.M., Tataranni P.A. Tasting a liquid meal after a prolonged fast is associated with preferential activation of the left hemisphere. Neuroreport. 2002;13:1141–1145. doi: 10.1097/00001756-200207020-00014. doi:10.1097/00001756-200207020-00014 [DOI] [PubMed] [Google Scholar]
- Devue C., Collette F., Balteau E., Degueldre C., Luxen A., Maquet P., Brédart S. Here I am: the cortical correlates of visual self-recognition. Brain Res. 2007;1143:169–182. doi: 10.1016/j.brainres.2007.01.055. doi:10.1016/j.brainres.2007.01.055 [DOI] [PubMed] [Google Scholar]
- de Waal F.B. The thief in the mirror. PLoS Biol. 2008;19:e201. doi: 10.1371/journal.pbio.0060201. doi:10.1371/journal.pbio.0060201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droit-Volet S., Gil S. The time–emotion paradox. Phil. Trans. R. Soc. B. 2009;364:1943–1953. doi: 10.1098/rstb.2009.0013. doi:10.1098/rstb.2009.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droit-Volet S., Meck W.H. How emotions colour our perception of time. Trends Cogn. Sci. 2007;11:504–513. doi: 10.1016/j.tics.2007.09.008. doi:10.1016/j.tics.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Drzezga A., et al. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain. 2001;92:295–305. doi: 10.1016/s0304-3959(01)00271-8. doi:10.1016/S0304-3959(01)00271-8 [DOI] [PubMed] [Google Scholar]
- Dunbar R.I., Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. doi:10.1126/science.1145463 [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. Social cognition in humans. Curr. Biol. 2007;17:R724–R732. doi: 10.1016/j.cub.2007.05.068. doi:10.1016/j.cub.2007.05.068 [DOI] [PubMed] [Google Scholar]
- Fudge J.L., Breitbart M.A., Danish M., Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J. Comp. Neurol. 2005;490:101–118. doi: 10.1002/cne.20660. doi:10.1002/cne.20660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan J.D., Lee R.R., Lenz F.A. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain. 1999;81:273–282. doi: 10.1016/S0304-3959(99)00021-4. doi:10.1016/S0304-3959(99)00021-4 [DOI] [PubMed] [Google Scholar]
- Griffiths T.D., Warren J.D., Dean J.L., Howard D. ‘When the feeling's gone’: a selective loss of musical emotion. J. Neurol. Neurosurg. Psychiatry. 2004;75:344–345. [PMC free article] [PubMed] [Google Scholar]
- Heilman K.M. Emotional experience: a neurological model. In: Lane R.D., Nadel L., editors. Cognitive neuroscience of emotion. Oxford University Press; New York, NY: 2000. pp. 328–344. [Google Scholar]
- Heimer L., Van Hoesen G.W. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci. Biobehav. Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. doi:10.1016/j.neubiorev.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Hennenlotter A., Schroeder U., Erhard P., Castrop F., Haslinger B., Stoecker D., Lange K.W., Ceballos-Baumann A.O. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26:581–591. doi: 10.1016/j.neuroimage.2005.01.057. doi:10.1016/j.neuroimage.2005.01.057 [DOI] [PubMed] [Google Scholar]
- Ito S., Craig A.D. Striatal projections of the vagal-responsive region of the thalamic parafascicular nucleus in macaque monkeys. J. Comp. Neurol. 2008;506:301–327. doi: 10.1002/cne.21513. doi:10.1002/cne.21513 [DOI] [PubMed] [Google Scholar]
- Jabbi M., Swart M., Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. doi:10.1016/j.neuroimage.2006.10.032 [DOI] [PubMed] [Google Scholar]
- James, W. 1890 The principles of psychology. See http://psychclassics.yorku.ca.James/Principles/index.htm
- Janig W. Cambridge University Press; Cambridge, UK: 2006. The integrative action of the autonomic nervous system. [Google Scholar]
- Keltner J.R., Furst A., Fan C., Redfern R., Inglis B., Fields H.L. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J. Neurosci. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. doi:10.1523/JNEUROSCI.4463-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikyo H., Ohki K., Miyashita Y. Neural correlates for feeling-of-knowing: an fMRI parametric analysis. Neuron. 2002;36:177–186. doi: 10.1016/s0896-6273(02)00939-x. doi:10.1016/S0896-6273(02)00939-X [DOI] [PubMed] [Google Scholar]
- Klein T.A., Endrass T., Kathmann N., Neumann J., von Cramon D.Y., Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. doi:10.1016/j.neuroimage.2006.11.014 [DOI] [PubMed] [Google Scholar]
- Koechlin E., Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. doi:10.1016/j.neuron.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Kranczioch C., Debener S., Schwarzbach J., Goebel R., Engel A.K. Neural correlates of conscious perception in the attentional blink. Neuroimage. 2005;24:704–714. doi: 10.1016/j.neuroimage.2004.09.024. doi:10.1016/j.neuroimage.2004.09.024 [DOI] [PubMed] [Google Scholar]
- Lenz F.A., Jaeger C.J., Seike M.S., Lin Y.C., Reich S.G., DeLong M.R., Vitek J.L. Thalamic single neuron activity in patients with dystonia: dystonia-related activity and somatic sensory reorganization. J. Neurophysiol. 1999;82:2372–2392. doi: 10.1152/jn.1999.82.5.2372. [DOI] [PubMed] [Google Scholar]
- Lewis M. The emergence of human emotions. In: Lewis M., Haviland-Jones J.M., Barrett L.F., editors. Handbook of emotions. 3rd edn. Guilford Press.; New York, NY: 2008. pp. 304–319. [Google Scholar]
- Lewis P.A., Miall R.C. A right hemispheric prefrontal system for cognitive time measurement. Behav. Process. 2006;71:226–234. doi: 10.1016/j.beproc.2005.12.009. doi:10.1016/j.beproc.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Livesey A.C., Wall M.B., Smith A.T. Time perception: manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia. 2007;45:321–331. doi: 10.1016/j.neuropsychologia.2006.06.033. doi:10.1016/j.neuropsychologia.2006.06.033 [DOI] [PubMed] [Google Scholar]
- Menon V., Levitin D.J. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28:175–184. doi: 10.1016/j.neuroimage.2005.05.053. doi:10.1016/j.neuroimage.2005.05.053 [DOI] [PubMed] [Google Scholar]
- Mesulam M.-M., Mufson E.J. Insula of the old world monkey. III. Efferent cortical output and comments on function. J. Comp. Neurol. 1982a;212:38–52. doi: 10.1002/cne.902120104. doi:10.1002/cne.902120104 [DOI] [PubMed] [Google Scholar]
- Mesulam M.-M., Mufson E.J. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J. Comp. Neurol. 1982b;212:1–22. doi: 10.1002/cne.902120102. doi:10.1002/cne.902120102 [DOI] [PubMed] [Google Scholar]
- Mufson E.J., Mesulam M.M. Insula of the old world monkey. II. Afferent cortical input and comments on the claustrum. J. Comp. Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. doi:10.1002/cne.902120103 [DOI] [PubMed] [Google Scholar]
- Mufson, E. J., Sobreviela, T. & Kordower, J. H. 1997 Chemical neuroanatomy of the primate insula cortex: relationship to cytoarchitectonics, connectivity, function and neurodegeneration. In Handbook of chemical neuroanatomy: the primate nervous system, part I, vol. 13 (eds F. E. Bloom, A. Bjorklund & T. Hokfelt), pp. 377–454. New York, NY: Elsevier Science.
- Mutschler I., Schulze-Bonhage A., Glauche V., Demandt E., Speck O., Ball T. A rapid sound–action association effect in human insular cortex. PLoS ONE. 2007;2:e259. doi: 10.1371/journal.pone.0000259. doi:10.1371/journal.pone.0000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky E.A., Gilissen E., Allman J.M., Perl D.P., Erwin J.M., Hof P.R. A neuronal morphologic type unique to humans and great apes. Proc. Natl Acad. Sci. USA. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. doi:10.1073/pnas.96.9.5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H., et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5:900–904. doi: 10.1038/nn896. doi:10.1038/nn896 [DOI] [PubMed] [Google Scholar]
- Olausson H., Charron J., Marchand S., Villemure C., Strigo I.A., Bushnell M.C. Feelings of warmth correlate with neural activity in right anterior insular cortex. Neurosci. Lett. 2005;389:1–5. doi: 10.1016/j.neulet.2005.06.065. doi:10.1016/j.neulet.2005.06.065 [DOI] [PubMed] [Google Scholar]
- Ongur D., Ferry A.T., Price J.L. Architectonic subdivision of the human orbital and medial prefrontal cortex. J. Comp. Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. doi:10.1002/cne.10609 [DOI] [PubMed] [Google Scholar]
- Oppenheimer S.M., Gelb A., Girvin J.P., Hachinski V.C. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K., Magnin M., Ryvlin P., Isnard J., Guenot M., Mauguière F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb. Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. doi:10.1093/cercor/12.4.376 [DOI] [PubMed] [Google Scholar]
- Platel H., Price C., Baron J.C., Wise R., Lambert J., Frackowiak R.S., Lechevalier B., Eustache F. The structural components of music perception. A functional anatomical study. Brain. 1997;120:229–243. doi: 10.1093/brain/120.2.229. doi:10.1093/brain/120.2.229 [DOI] [PubMed] [Google Scholar]
- Ploran E.J., Nelson S.M., Velanova K., Donaldson D.I., Petersen S.E., Wheeler M.E. Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. J. Neurosci. 2007;27:11 912–11 924. doi: 10.1523/JNEUROSCI.3522-07.2007. doi:10.1523/JNEUROSCI.3522-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O., Gramann K., Schandry R. Neural systems connecting interoceptive awareness and feelings. Hum. Brain Mapp. 2007;28:9–18. doi: 10.1002/hbm.20258. doi:10.1002/hbm.20258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K., Quartz S.R., Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J. Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. doi:10.1523/JNEUROSCI.4286-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.M., Mayer A.R., Harrington D.L. The evolution of brain activation during temporal processing. Nat. Neurosci. 2001;4:317–323. doi: 10.1038/85191. doi:10.1038/85191 [DOI] [PubMed] [Google Scholar]
- Saper C.B. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. doi:10.1146/annurev.neuro.25.032502.111311 [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Leifer D. Parietal pseudothalamic pain syndrome: clinical features and anatomic correlates. Arch. Neurol. 1992;49:1032–1037. doi: 10.1001/archneur.1992.00530340048017. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Carlin D.A., Allman J.M., Macedo M.N., Bush C., Miller B.L., Dearmond S.J. Early frontotemporal dementia targets neurons unique to apes and humans. Ann. Neurol. 2006;60:660–667. doi: 10.1002/ana.21055. doi:10.1002/ana.21055 [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Allman J.M., Carlin D.A., Crawford R.K., Macedo M.N., Greicius M.D., DeArmond S.J., Miller B.L. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis. Assoc. Disord. 2007;21:S50–S57. doi: 10.1097/WAD.0b013e31815c0f14. doi:10.1097/WAD.0b013e31815c0f14 [DOI] [PubMed] [Google Scholar]
- Seymour B., O'Doherty J.P., Dayan P., Koltzenburg M., Jones A.K., Dolan R.J., Friston K.J., Frackowiak R.S. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. doi:10.1038/nature02581 [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl Acad. Sci. USA. 2008;105:12 569–12 574. doi: 10.1073/pnas.0800005105. doi:10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan E., Pardo J.V., Faris P.L., Hartman B.K., Kim S.W., Ivanov E.H., Daughters R.S., Costello P.A., Goodale R.L. Functional neuroimaging of gastric distention. J. Gastrointest. Surg. 2003;7:740–749. doi: 10.1016/s1091-255x(03)00071-4. doi:10.1016/S1091-255X(03)00071-4 [DOI] [PubMed] [Google Scholar]
- Sterzer P., Kleinschmidt A. A neural basis for inference in perceptual ambiguity. Proc. Natl Acad. Sci. USA. 2007;104:323–328. doi: 10.1073/pnas.0609006104. doi:10.1073/pnas.0609006104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Rosen H.J., Allison S., Miller B.L., Levenson R.W. Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain. 2006;129:2508–2516. doi: 10.1093/brain/awl145. doi:10.1093/brain/awl145 [DOI] [PubMed] [Google Scholar]
- Thielscher A., Pessoa L. Neural correlates of perceptual choice and decision making during fear–disgust discrimination. J. Neurosci. 2007;27:2908–2917. doi: 10.1523/JNEUROSCI.3024-06.2007. doi:10.1523/JNEUROSCI.3024-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M., Hesse M.D., Boy C., Haggard P., Fink G.R. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cereb. Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. doi:10.1093/cercor/bhl131 [DOI] [PubMed] [Google Scholar]
- Tse P.U., Intriligator J., Rivest J., Cavanagh P. Attention and the subjective expansion of time. Percept. Psychophys. 2004;66:1171–1189. doi: 10.3758/bf03196844. [DOI] [PubMed] [Google Scholar]
- van Wassenhove V., Buonomano D.V., Shimojo S., Shams L. Distortions of subjective time perception within and across senses. PLoS ONE. 2008;3:e1437. doi: 10.1371/journal.pone.0001437. doi:10.1371/journal.pone.0001437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen M.G., Bender G., Constable R.T., Small D.M. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem. Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. doi:10.1093/chemse/bjm025 [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Pandya D.N. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. doi:10.1002/cne.902620208 [DOI] [PubMed] [Google Scholar]
- Williamson J.W., Nobrega A.C.L., Mccoll R., Mathews D., Winchester P., Friberg L., Mitchell J.H. Activation of the insular cortex during dynamic exercise in humans. J. Physiol. 1997;503:277–283. doi: 10.1111/j.1469-7793.1997.277bh.x. doi:10.1111/j.1469-7793.1997.277bh.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J.S., Strange B.A., O'Doherty J., Dolan R.J. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat. Neurosci. 2002;5:277–283. doi: 10.1038/nn816. doi:10.1038/nn816 [DOI] [PubMed] [Google Scholar]
- Wittling W., Block A., Genzel S., Schweiger E. Hemisphere asymmetry in parasympathetic control of the heart. Neuropsychologia. 1998;36:461–468. doi: 10.1016/s0028-3932(97)00129-2. doi:10.1016/S0028-3932(97)00129-2 [DOI] [PubMed] [Google Scholar]
- Wittmann M. The inner experience of time. Phil. Trans. R. Soc. B. 2009;364:1955–1967. doi: 10.1098/rstb.2009.0003. doi:10.1098/rstb.2009.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M., Paulus M.P. Decision making, impulsivity and time perception. Trends Cogn. Sci. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. doi:10.1016/j.tics.2007.10.004 [DOI] [PubMed] [Google Scholar]