Abstract
Individuals time as if using a stopwatch that can be stopped or reset on command. Here, we review behavioural and neurobiological data supporting the time-sharing hypothesis that perceived time depends on the attentional and memory resources allocated to the timing process. Neuroimaging studies in humans suggest that timekeeping tasks engage brain circuits typically involved in attention and working memory. Behavioural, pharmacological, lesion and electrophysiological studies in lower animals support this time-sharing hypothesis. When subjects attend to a second task, or when intruder events are presented, estimated durations are shorter, presumably due to resources being taken away from timing. Here, we extend the time-sharing hypothesis by proposing that resource reallocation is proportional to the perceived contrast, both in temporal and non-temporal features, between intruders and the timed events. New findings support this extension by showing that the effect of an intruder event is dependent on the relative duration of the intruder to the intertrial interval. The conclusion is that the brain circuits engaged by timekeeping comprise not only those primarily involved in time accumulation, but also those involved in the maintenance of attentional and memory resources for timing, and in the monitoring and reallocation of those resources among tasks.
Keywords: attention, distracter, interval timing, intertrial interval, resources, time sharing
1. Introduction
Humans and other animals are able to time events as if they use an internal clock that functions as a stopwatch. The traditional paradigm of interval timing—the stopwatch paradigm—is that pulses emitted at regular intervals by a pacemaker are stored in an accumulator whose content represents the current subjective time (Church 1984; Gibbon et al. 1984). Support for this paradigm comes from data in bees, fishes, turtles, birds, rodents, primates as well as human infants and adults (reviewed by Paule et al. 1999; Buhusi & Meck 2005). Here, we review behavioural and neurobiological data in support of an alternative view that emphasizes the role of attentional processing in time perception. This alternative view—the time-sharing paradigm—is based on the simple observation that durations seem shorter when one does not pay attention to the timing process (e.g. when one is distracted by events unrelated to timing), and seem longer when one actively times a particular event (e.g. cooking an egg). This attentional perspective has a long and distinguished history in the field of human timing (e.g. Casini et al. 1992; Grondin & Macar 1992; Casini & Macar 1997; Fortin 2003; Pouthas & Perbal 2004), but has only recently been accepted as a mainstream hypothesis in the animal timing literature (Buhusi 2003; Buhusi & Meck 2006a,b). While the animal timing literature traditionally focuses on the stopwatch paradigm and assumes that alterations in durations reflect properties of the stopwatch (see Buhusi (2003) for a discussion), the human timing literature traditionally explores the time-sharing paradigm, with an emphasis on the role of attention in time estimation in dual-task protocols (Brown 1997, 1998, 2008; Fortin 2003; Macar & Vidal 2009).
The ‘time flies when one has fun’ phenomenon has been rigorously explored in human timing using dual-task paradigms in which human participants estimate durations while performing in parallel a second task. The typical result is as expected; participants estimate durations as being shorter when they perform in parallel a second—perceptual, mental arithmetic or motor-tracking—task (e.g. Hicks et al. 1976, 1977; Zakay et al. 1983; Brown 1985, 1995, 1997; Zakay 1989; Macar et al. 1994). These results have been interpreted to suggest a limited pool of attentional or memory resources, shared by cognitive processes (including the stopwatch). The sharing of attentional and/or memory resources between the internal stopwatch and other mental processes is referred to as the resource allocation, or time-sharing, hypothesis (e.g. Thomas & Weaver 1975; Zakay 1989, 2000; Block & Zakay 1996; Fortin & Massé 2000; Fortin et al. 2005; Fortin & Tremblay 2006; Champagne & Fortin 2008). Briefly, manipulations that increase the resources allocated to processing other events (than the timed event) result in the internal clock loosing resources, thus diminishing its ability to time accurately.
2. Neurobiological evidence for the time-sharing hypothesis
The time-sharing hypothesis is supported both by neuroimaging studies in human participants and electrophysiological recordings, lesions and pharmacological studies in lower animals, suggesting that timekeeping is accomplished by a functional circuit involving fronto-striatal circuits (Meck 1988, 1996, 2006a,b; MacDonald & Meck 2004; Matell & Meck 2004). Importantly, both humans and lower animal timing studies strongly implicate brain regions thought to be involved in attentional and working memory processing. For example, neuroimaging studies in human participants (reviewed by Meck & Malapani 2004; Buhusi & Meck 2005; Meck et al. 2008) suggest that interval timing is accomplished by a functional circuit comprising the right middle frontal gyrus, left cingulate, supplementary motor area, middle temporal gyrus, right supramarginal gyrus, bilateral insula, bilateral caudate, bilateral putamen, bilateral globus pallidus and bilateral thalamus (Coull et al. 2004; Harrington et al. 2004; Stevens et al. 2007). Notable among these circuits are the dorsolateral prefrontal and parietal regions linked to attention and working memory function (Fletcher & Henson 2001), and medial frontal regions involved in maintaining relevant sensory information (Picard & Strick 2001). Activity in these regions was shown to increase when longer intervals were timed (Stevens et al. 2007) compatible with these regions being involved in short-term storage of temporal information. Importantly, the brain circuits involved in timekeeping can be dissociated from those involved in other cognitive processes by asking participants to pay attention to either the timing or sensory features (e.g. colour) of the event (Coull et al. 2004). The abundance of studies that report activity in dorsolateral prefrontal, medial frontal and parietal regions in interval timing tasks (reviewed by Meck et al. 2008) suggest that attention and working memory processing is necessary for timekeeping, in accord with the time-sharing hypothesis. Situations that disengage these regions from the timekeeping circuits are likely to result in a shortening of perceived durations, in as much as we regularly experience when we are having fun.
The idea that attentional and memory resources are crucial to interval timing also receives direct support from studies examining neuronal firing in the striatum and prefrontal cortex in timing tasks in lower animals. For example, when rats are probabilistically rewarded at multiple target durations, distinct subsets of striatal neurons are activated for the different durations (Matell et al. 2003; Matell & Meck 2004). However, striatal neurons code multiple durations only if the prefrontal cortex is intact. Lesions of the agranular frontal cortex or the nucleus basalis magnocellularis (Olton et al. 1988) impair rats' ability to time two stimuli simultaneously, but not the ability to time each stimulus sequentially (Meck & MacDonald 2007). For example, in rats trained to time two signals of different durations (Pang et al. 2001), most neurons in the agranular frontal cortex respond only to the compound stimulus, and fewer neurons respond to only one stimulus, suggesting that agranular cortex is important for divided attention, i.e. for shifting attention between multiple stimuli and their associated responses and/or for the dynamic allocation of attention in time (Pang & McAuley 2003; Coull 2004; Nobre et al. 2007).
Moreover, work in rats has also shown that both the frontal and temporal systems are involved in the reference and working memory of the duration of an event, but in complementary ways. A double dissociation resulted from lesions of the frontal cortex and the nucleus basalis magnocellularis, on the one hand, and the hippocampus, fimbria fornix and the medial septal area, on the other. Lesions in the frontal system produce a lengthening of perceived durations, while lesions in the hippocampal system produce a shortening of perceived durations (for review see Meck 1988, 2002; Gibbon et al. 1997; Buhusi & Meck 2005). Dissociation was also found in the effects of these lesions on working memory for the duration of a retention interval interpolated during that stimulus. Fimbria-fornix lesions produce a complete amnesia for the memory of the duration of the event prior to a retention interval, while lesions in the frontal system had no effect (Meck et al. 1984, 1987; Olton et al. 1987).
Finally, two lines of research suggest that time accumulation and working memory for temporal information can be dissociated pharmacologically, and that they depend on the dopamine and serotonin systems in the cortex and striatum. For example, administration of the indirect dopamine agonist methamphetamine shortens estimated durations, and impairs maintenance of temporal information in working memory (Buhusi & Meck 2002). In turn, the dopamine D2 receptor blocker haloperidol lengthens estimated durations but facilitates the maintenance of temporal information in working memory (Buhusi & Meck 2002). However, the effect of amphetamine to shorten estimated durations was blocked by depletion of serotonin, suggesting that an intact serotonergic system is required for interval timings (Body et al. in press). Indeed, specific stimulation of either 5-HT1A and 5-HT2A receptors was found to shorten estimated durations in qualitatively similar ways, an effect antagonized by specific blockade of these receptors (Asgari et al. 2006). Interestingly, clozapine—a drug acting both on the dopamine and serotonin systems—shortens estimated durations (MacDonald & Meck 2005; Buhusi & Meck 2007) and also facilitates the maintenance of temporal information in working memory (Buhusi & Meck 2007). Clozapine is reported to have differential effects on dopamine levels in the frontal cortex and striatum; it serves as a dopamine receptor antagonist in the mesolimbic dopaminergic system, and an indirect dopamine agonist in the frontal cortex by its activation of the serotonin 5-HT2A receptors on dopamine neurons (Rollema et al. 1997; Ichikawa et al. 2001; Chou et al. 2003; Meltzer et al. 2003). Thus, this pattern of pharmacological results is consistent with the hypothesis that the effect of clozapine on time estimation is due to an increase in dopamine neurotransmission in frontal cortex, but not in the dorsal striatum (Body et al. 2006). Together, these data suggest that drug effects on time accumulation and attention to time rely on the interaction between the dopaminergic and serotonergic activation in frontal cortex and striatum.
3. The locus of interaction between the timekeeper and resource allocation
Time-sharing hypothesis is largely unconcerned with the specifics of the internal clock. The same logic relative to resources applies irrespective of whether the internal clock is based on a gated accumulation of pulses from a putative pacemaker (Church 1984; Gibbon et al. 1984; Zakay 2000), on the detection of coincidental activation of multiple neural cortical oscillators by dorsal striatum (reviewed by Matell & Meck 2004; Buhusi & Meck 2005; Meck et al. 2008), on temporal integration of signals (Leon & Shadlen 2003), on the dynamics of state-dependent networks (Karmarkar & Buonomano 2007) or on the firing rate of ensembles of neurons (Lebedev et al. 2008). Irrespective of the specific details of the timing mechanism, should the protocol at hand require attentional or memory resources, and should these resources be diverted to other non-timing tasks, estimated durations would be shorter. Therefore, to explore the question of the locus of interaction between timekeeping and attention, we turn to a specific implementation of the timekeeper—the stopwatch—and to work in lower animals—as it better reflects the multitude of possible solutions that need to be evaluated.
While usually explored in human participants performing in dual-task paradigms, this prediction can also be explored in lower animals performing simpler paradigms to evaluate the possible locus of interaction between the timer and resource allocation. Indeed, dual-task performance in animals has been sporadically examined (e.g. Olton et al. 1988; Block & Zakay 1996; Lejeune 1998, 1999; Zakay 2000; Kladopoulos et al. 2004). Typical animal protocols use single-task paradigms. Contrary to the general wisdom that simple, single-task paradigms do not involve time sharing, because all resources are dedicated to timing, here we suggest that such interactions are present in all interval-timing paradigms. A case in point is the peak-interval (PI) procedure with gaps (Church 1978; Roberts 1981; Meck et al. 1984).
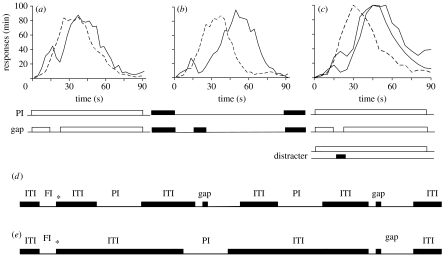
In the standard version of the PI procedure, subjects are trained to time a specified target duration, say 30 s (figure 1a). In reinforced trials, the to-be-timed signal is turned on, and the first response after the given criterion interval (30 s) is reinforced and turns the to-be-timed signal off. Reinforced trials are randomly intermixed with unreinforced PI trials. In PI trials, the to-be-timed signal is presented for approximately three times the criterion interval (say 90 s), but responses are not reinforced. In well-trained subjects, the mean response rate in PI trials increases after the onset of the to-be-timed signal, reaches a peak approximately the target duration and gradually declines afterwards (Catania 1970; Church 1978). Observed changes in the distribution of responses in PI trials following behavioural (e.g. Church 1978; Roberts & Church 1978) and neurobiological (e.g. Meck et al. 1984; Buhusi & Meck 2002, 2007) manipulations are used to address the functioning of the components involved in duration discrimination, and to differentiate the timekeeper from the attentional mechanism.
Figure 1.
Peak-interval (PI) procedure with gaps and distracters. (a) Standard PI procedure with gaps. (b) Reversed PI procedure with gaps. (c) Standard PI procedure with gaps and distracters (dashed curve, PI; black curve, gap; grey curve, distracter). (d) Reversed PI procedure with gaps, using short ITI. (e) Reversed PI procedure with gaps, using long ITI. The asterisk denotes the delivery of reinforcement. Data for (a–c) are adapted from Buhusi & Meck (2000, 2006a).
When unpredictable infrequent gaps interrupt the to-be-timed signal, subjects behave as if they are able to run, stop or reset their clocks on command (Church 1978; Roberts & Church 1978; Buhusi 2003). For example, data showing that rats' response functions after the gap are delayed relative to PI trials by an amount approximately equal to the duration of the gap (see figure 1a) was taken to suggest that rodents retain in working memory the pre-gap interval and resume timing after the gap where they left off before the gap, using a stop mode (e.g. Church 1978; Roberts & Church 1978). By contrast, the finding that pigeons tend to delay their response function, after the gap for a duration that is approximately the sum of the gap and pre-gap intervals, has been taken to suggest that birds restart the entire timing process after the gap, using a reset mode (Roberts et al. 1989; Cabeza de Vaca et al. 1994; Bateson & Kacelnik 1998; Brodbeck et al. 1998).
Interestingly, the reset mode was also observed when rats time the absence of a light stimulus (figure 1b) in a so-called reversed PI procedure with gaps (Buhusi & Meck 2000). When an illuminated gap is presented during the dark to-be-timed signal, the response function is delayed considerably, as if rats reset their timer after the gap (figure 1b). To address data from the standard and reversed PI procedure with gaps and distracters, several hypotheses with roots in the animal learning literature have been proposed: the attentional switch hypothesis (Gibbon et al. 1984; Meck 1984), the instructional ambiguity hypothesis (Sherburne et al. 1998; Kaiser et al. 2002), the passive memory decay hypothesis (Cabeza de Vaca et al. 1994) and the time-sharing hypothesis (Buhusi 2003). Each of these hypotheses assumes a different mechanism and a different locus of action for the effect of gaps.
(a) The attentional switch hypothesis
This hypothesis assumes that a putative attentional switch is closed in the presence of the to-be-timed signal and is open in the absence of the signal (Church 1978; Roberts & Church 1978; Roberts 1981; Gibbon et al. 1984; Meck et al. 1984; Penney et al. 2000), and interprets the stop behaviour in rats as evidence for this attentional switch. Therefore, in the simplest case, this hypothesis predicts the use of a stop rule irrespective of any manipulations of the procedure. While this hypothesis does seem to fit much of the rat data better (reviewed by Buhusi 2003), it cannot easily account for data showing that pigeons tend to reset. We tested this hypothesis in a reversed gap procedure by keeping all temporal parameters of the PI procedure constant, and changing only the content of the trial events. When rats time the presence of a visual stimulus, a standard (dark) gap prompts rats to stop timing as illustrated in figure 1a, but when they time the absence of a visual stimulus, a reversed (illuminated) gap, rats reset their clocks as illustrated in figure 1b (Buhusi & Meck 2000). This result indicates that rats have a flexible timing mechanism, and that non-temporal parameters of the procedure are critical for the observed results.
(b) The instructional ambiguity hypothesis
Originally proposed to address timing data from pigeons, this hypothesis assumes that the stop/reset mechanism of interval timing is activated when subjects are presented with ambiguous, ITI-like events, such as the gap (Sherburne et al. 1998; Kaiser et al. 2002). This can be easily seen in figure 1d,e, where the trials of a reversed PI procedure with gaps are presented in sequence, separated by the (illuminated) intertrial interval (ITI). Figure 1 shows that in the sequence of events the gap is similar to the ITI: both are illuminated and both separate dark events. Considering that a session comprises tens of such trials, it is easy to understand how the illuminated events are ambiguous; they can be either gaps or ITIs. Because the subject reset their timer after each trial, it is possible that the more similar the gap is to the ITI, the more it will prompt subjects to reset.
Indeed, the ambiguity hypothesis predicts that manipulations that render the gap similar to the ITI result in subjects resetting their clock, while manipulations that render the gap dissimilar from the ITI result in the subjects ignoring the gap, and continuing timing through it (run mode). These predictions were confirmed experimentally both in pigeons and rats (Buhusi & Meck 2002; Kaiser et al. 2002). We further tested this prediction by manipulating the perceived salience of the to-be-timed signal while having both the gap and the ITI dark (identical). Although the instructional ambiguity hypothesis predicts a clock reset under these conditions, rats' responses to the gaps varied depending on other manipulated variables (e.g. signal modality, signal intensity and gap-signal contrast) likely to have affected the salience (discriminability) of the gap from the to-be-timed signal, rather than the discriminability of the gap from (the otherwise identical) ITI. We found that albino rats (which are typically used in timing experiments despite having lower visual sensitivity compared to rats with pigmented eyes) typically stop timing after a gap in a visual signal and reset after a gap in an auditory signal (Buhusi et al. 2005). Similarly, despite the gap and the ITI being similar (dark and silent), rats stop after a gap in a low-intensity signal and reset after a gap in a high-intensity signal (Buhusi et al. 2005). Although the similarity between the gap and the ITI plays a role in the gap procedure, it cannot explain the relatively complex pattern of results in this procedure.
(c) The passive memory decay hypothesis
To account for the flexible use of the run/stop/reset rules employed by rats and pigeons, Cabeza de Vaca et al. (1994) proposed that subjective time—stored in working memory—decays passively (at a fixed rate) during the gap. A parametric study in pigeons that manipulated gap duration and its position within the to-be-time signal (Cabeza de Vaca et al. 1994) found that the rightward shift in peak time was greater than what could be attributed to the duration of the gap alone, and that this additional factor (attributed to decay) increased with gap duration. This decay was minimal for short gaps, but quite dramatic for longer gaps, thus accounting for both the stop and reset timing modes. Importantly, Cabeza de Vaca et al. (1994) showed that an exponential decay function quantitatively fits the data observed in their study, thus providing a parsimonious account of timing data in the gap procedure. This hypothesis predicts that the effect of a gap should depend solely on the absolute gap duration, and that manipulations of non-temporal features of the gap procedure should be relatively ineffective, a result that runs contrary to evidence showing that the effect of a gap is affected by the contrast in intensity between the gap and the timed signal (Buhusi & Meck 2000; Buhusi et al. 2002, 2005), and by the perceptual acuity of the subjects (Buhusi et al. 2005).
(d) The time-sharing hypothesis applied to the PI procedure with gaps and distracters
Buhusi (2003) applied the time-sharing hypothesis to the PI procedure with gaps by proposing that during gaps in the to-be-timed signal resources are reallocated (diverted away from timing), which results in timing delays because the clock is unable to maintain its current subjective time in working memory. While Cabeza de Vaca et al. (1994) proposed that memory decays passively (at a fixed rate) during the gap, Buhusi (2003) proposed that the memory decays due to resource reallocation in an active manner, at a rate proportional to the salience (discriminability) of the gap. This view is supported by data showing that pigeons run, stop or reset their clocks when the gap/signal contrast is manipulated (Buhusi et al. 2002, 2006), by data showing that rats run, stop or reset their clocks depending on gap content (Buhusi & Meck 2000), gap discriminability (Buhusi et al. 2005) and level of visual acuity (Buhusi et al. 2005). There are also data showing that rats normally reset their timing in PI trials upon presentation of food reward (Matell & Meck 1999; Thorpe et al. 2002; Cheng et al. 2006). This proposal bridges the human and animal timing literatures, and provides a quantitative mechanism for the time-sharing hypothesis in which reallocation of cognitive resources results in memory decay at a rate proportional to the relative salience of the interrupting event (Buhusi 2003; Buhusi et al. 2005, 2006).
The most compelling argument for time sharing during the PI procedure comes from a set of experiments originally proposed by Block & Zakay (1996, p. 184; Buhusi et al. 2006). According to the time-sharing hypothesis, memory decay is due to a process inherently (i) concurrent with interval timing (Thomas & Weaver 1975; Zakay 1989, 2000; Block & Zakay 1996), i.e. operating throughout the procedure, not only during the gap and (ii) activated whenever non-temporal components of the task engage in contextual processing, i.e. not only by a break or gap, but also by novel, distracter events as illustrated in figure 1c. Indeed, delays in timing were observed both after gaps in the to-be-timed signal, and after presenting novel distracter events during the uninterrupted presentation of the to-be-timed signal (Buhusi & Meck 2006a,b; Buhusi et al. 2006), suggesting that time sharing is a process concurrent with interval timing. These distracter results are difficult to reconcile with either the instructional ambiguity hypothesis (Sherburne et al. 1998; Kaiser et al. 2002) or the switch hypothesis (Gibbon et al. 1984) without additional modifications, because both hypotheses predict that time should continue to accumulate during the uninterrupted presentation of the to-be-timed signal even in the presence of distracters. The distracter data indicate that neither gaps nor ITI-like events are necessary for observing delays in timing. By contrast, events such as gaps/distracters may cause a reallocation of memory resources for time (Thomas & Weaver 1975; Zakay 1989, 2000; Block & Zakay 1996), which would cause the working memory for clock readings to decay (Buhusi 2003). Under these conditions, subjects should be less able to maintain an accurate representation of the pre-gap duration, thus delaying the response function.
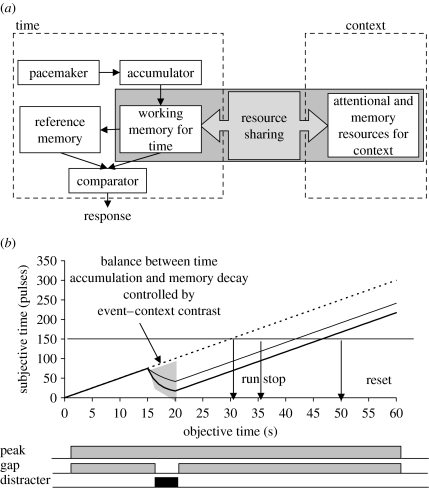
Figure 2 shows an implementation of the time-sharing hypothesis. The left side of figure 2a shows the generally accepted depiction of the internal clock (Church 1984; Gibbon et al. 1984). Time estimation is accomplished by the accumulation of pulses emitted at regular intervals by a putative pacemaker. The accumulated time pulses are temporarily stored in working memory for immediate use, and stored in long-term (reference) memory for later retrieval. When timing a previously learned duration, the currently accumulated time pulses are compared with the number of pulses stored in reference memory. The right side of figure 2a shows other cognitive processes required by processing other events (context), which also require attentional and/or memory resources. Within this framework, the locus of interference of time sharing into the timing process has been proposed to be at the level of the accumulation, either before (Thomas & Weaver 1975; Zakay 1989, 2000; Block & Zakay 1996) or after the accumulator (Buhusi 2003). For example, Zakay (2000) proposed an attentional gate by which pulses reach the accumulator. Manipulations that increase the resources allocated to processing the context result in the opening of the attentional gate, and an inability of pulses to reach the accumulator, such that the currently estimated duration is shorter. Alternatively, Buhusi (2003) proposed that working memory resources are shared between the internal clock (left side of figure 2a) and other cognitive processes (right side of figure 2a). Manipulations that increase the working memory resources allocated to processing the context result in an inability of the internal clock to maintain the accumulated time pulses in working memory, and in a working memory decay for timing (figure 2b), such that the currently estimated duration is shorter.
Figure 2.
(a) The relative time-sharing hypothesis. Attentional and/or memory resources are shared between the timer and context (other processes). (b) Lack of timing resources results in working memory decay at a rate proportional to the relative contrast between the event (gap/distracter) and context (dashed line, peak; thin solid line, peak+distracter; thick solid line, gap). The rate of memory decay increases with the non-temporal contrast (relative salience, relative intensity, etc.) and decreases with the temporal contrast (relative duration).
4. New findings: the effect of the gap depends on its temporal context
While recent research using the PI procedure with gaps concentrates on the effect of non-temporal features of events, here we evaluated whether the effect of a gap is dependent on its absolute duration or on its relative duration to the temporal context in which the gap is presented (Spetch & Rusak 1992b). For example, in a delay-matching-to-sample-duration (DMTS-D) procedure, the response chosen by pigeons when presented with a test interval depends on the relative duration of the test interval to the temporal context in which it is embedded (Spetch & Rusak 1992b), which includes (in the DMTS-D procedure) the delay between the test interval and the opportunity to make a response (Spetch & Wilkie 1983) as well as the mean of the ITI used at the time of the test (Spetch & Rusak 1992a).
In order to explore this experimentally, two groups of rats timed the same target duration with a short or a long ITI using a reversed PI procedure, i.e. the absence of a stimulus defined the to-be-timed signal (figure 1b). The typical result in such a procedure is that rats reset timing after gaps, even at very short gap durations (Buhusi & Meck 2000). Should the effect of the gap depend on its relative duration to its temporal context, and should the temporal context include (as in the SMTS-D procedure) the ITI, we expected that an increase in the duration of the ITI would decrease the resetting effect of the gap and shift responding towards the use of a stop rule.
(a) Methods
Ten four-month-old Sprague-Dawley male rats were maintained at 85 per cent of their ad libitum weight by restricting access to food (Rodent Diet 5001, PMI Nutrition International, Inc., Brentwood, MO). Rats were trained in 10 standard operant boxes (MED Associates, Inc.) housed in sound attenuating cubicles, and equipped with three response levers (two retractable and one fixed) situated on the front wall of the box. Only the left lever was used in this experiment. According to the schedule, 45 mg Noyes precision food pellets (Research Diets, Inc., New Brunswick, NJ) were delivered in a food cup situated on the front wall by a pellet dispenser. The to-be-timed signal was a 28 V 100 mA house light mounted at the centre-top of the front wall. A 66 dB background noise produced by a fan was present throughout all procedures.
Rats were first trained in a 20 s reversed fixed-interval (RFI) procedure (Buhusi & Meck 2000). On each daily session rats received 64 RFI trials during which the house light was turned off at the beginning of the trial; the first lever press occurring 20 s after the offset of the house light triggered the immediate delivery of a food pellet and turned on the house light for the duration of the random ITI. For five rats, the ITI was 15±5 s in length (short group), while for the other five rats the ITI was 120±40 s in length (long group).
After seven sessions of RFI training, rats received 15 daily sessions of reversed PI (RPI) training. During each session rats received 32 RFI trials randomly intermixed with 32 non-reinforced probe trials in which the to-be-timed signal was turned off for a duration three times longer than the RFI time (60 s), before being terminated irrespective of responding. Trials were separated by a random ITI, short or long, according to the assigned group.
In each of the next four daily sessions, rats received 32 RFI trials randomly intermixed with 32 non-reinforced test trials. Sixteen of the test trials were regular RPI trials (peak trials). During the remaining 16 test trials, the house light was turned on (reversed gap trials) 5 s from the beginning of the trial for 10 s (10@5 condition, eight trials), or 10 s from the beginning of the trial for 5 s (5@10 condition, eight trials). At the offset of the gap, the house light was turned off for a duration that matched the duration used in the RPI trials, and then terminated independently of responding for the random duration of the ITI. Trials were separated by a random ITI, short or long, as described above.
Lever presses were recorded in real time using a MED-PC software system (MED-Associates 1999). These responses were used to estimate the peak time, peak rate and precision of timing from the response functions from the two test sessions for each rat. First, the number of responses (in 4 s bins) was averaged daily over trials, to obtain a mean response rate for each rat. Further analyses were conducted on the data from an interval twice as large as the fixed interval (i.e. 40 s), starting at the onset of the signal (for data in RPI trials) or at the offset of the gap/distracter (for gap trials). The mean response rate in the interval of interest was fit using the Marquardt–Levenberg (Marquardt 1963) iterative algorithm to find the coefficients (parameters) of a Gaussian+linear equation that gave the ‘best fit’ (least-squares minimization) between the equation and the data, as described by Buhusi & Meck (2000). The iterative algorithm provided parameters a, b, c, d and t0. Parameter t0 was used as an estimate of timing accuracy (peak time of responding), a+d was used as an estimate of the peak rate of response and parameter b was used as an estimate of the precision of timing; the width of the response function was estimated to be equal to 2b. A shift in peak time was computed for each rat by subtracting the estimated t0 in RPI trials and the duration of the gap from the estimated t0 in gap trials; a null shift was taken as evidence for the stop rule, while a shift equal to the pre-gap interval was taken as evidence for the reset rule. The parameters t0, 2b, a+d and shift were submitted to statistical analyses. All statistical tests were evaluated at a significance level of 0.05.
(b) Results
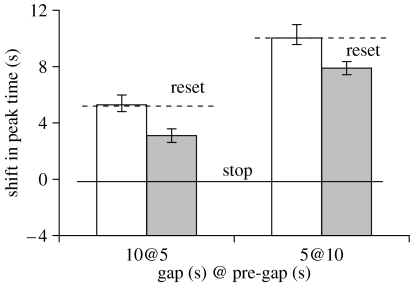
The estimated peak time in RPI trials was submitted to a one-way ANOVA that failed to reveal baseline differences between the two groups (F1,8=1.42, p=0.27). The mean peak time in RPI trials was 21.12±0.9 s, suggesting that rats acquired the timing task with a high degree of accuracy. The shift in peak time in gap trials relative to RPI trials in the two groups is shown in figure 3. A null shift is taken as evidence for the stop rule, while a shift equal to the pre-gap interval is taken as evidence for the reset rule. The results shown in figure 3 indicate that both the 5@10 and 10@5 gaps fully reset timing in the short group, but were less effective in resetting timing in the long group. These observations were supported by a mixed ANOVA performed on the estimated shift in peak time with factors group (short versus long) and gap (two conditions), which indicated reliable effects for group (F1,8=15.47, p=0.004) and gap (F1,8=36.78, p=0.0003), but no group×gap interaction (F1,8<0.01, p=0.98). Analyses indicated that for the short group, the shift in peak time was not reliably different from reset (t(4)=0.41, p=0.70 for the 10@5 gap and t(4)=0.03, p=0.97 for the 5@10 gap). By contrast, for the long group, the shift in peak time was reliably smaller than reset for both gaps (t(4)=3.96, p=0.017 for the 10@5 gap and t(4)=4.50, p=0.011 for the 5@10 gap).
Figure 3.
Temporal context of a gap includes the ITI. Observed shift in peak times in gap trials in two groups or rats trained to time a 20 s criterion interval with a 15 s ITI (white bars, short) or a 120 s ITI (grey bars, long).
No differences in response rate and width of the response functions were found between peak and gap trials for the two groups. A mixed ANOVA conducted on the response rate (parameter a+d) failed to show significant effects for the group (F1,8=1.82, p=0.21), gap (F1,8=3.5, p=0.055) or group×gap interaction (F1,8=1.12, p=0.35). The estimated rate of response was 33.89±3.85 resp min−1 in RPI trials, 37.97±4.75 resp min−1 in the 10@5 gap trials and 33.05±3.72 resp min−1 in the 5@10 gap trials. Similarly, a mixed ANOVA conducted on the width of the response function (parameter 2b) failed to show significant effects for group (F1,8=1.99, p=0.20) gap (F1,8=0.91, p=0.42) or group×gap interaction (F1,8=1.69, p=0.22). Taken together, these results support the proposal that temporal context is important, i.e. gaps with the same duration and placement within the to-be-timed signal shifted the response function less in the long-ITI group (towards stop) than in the short-ITI group (reset).
(c) Discussion: time sharing, expanded
While rats trained in a reversed PI procedure with a short ITI reset timing upon the presentation of an illuminated gap (see also Buhusi & Meck 2000), rats trained with a long ITI showed a reliably smaller shift in response function than the reset rule. These results are incompatible with the switch hypothesis and the passive memory decay hypothesis, which predict that the effect of the gap depends strictly on the duration of the gap. The switch hypothesis predicts a stop rule, and the passive memory decay hypothesis predicts equal effects in both the short- and the long-ITI groups. Neither of these predictions were observed experimentally. The present results are instead compatible with both the instructional ambiguity hypothesis and the time-sharing hypothesis. The instructional ambiguity hypothesis assumes that the insertion of a gap affects timing only in as much as it resembles the ITI, such that changes in the ITI may result in changes in the stop/reset rule.
The time-sharing hypothesis assumes that attentional or memory resources are shared throughout all trial components, including the ITI (figure 2a). Consequently, the present data suggest a logical extension of the time-sharing hypothesis: during a gap/distracter event the rate of memory decay is proportional to the salience (discriminability) of the gap and inversely proportional to the temporal context of the event, which includes the to-be-timed signal and the ITI. Thus, in the short-ITI group, the reversed gap is very salient and determines a rapid reallocation of resources away from timing, thus leading to a timing reset, while in the long-ITI group, the same reversed gap determines a slower reallocation of resources away from timing, because the salience of the gap is counterbalanced by an increased temporal context of this gap. Nevertheless, because an eightfold increase in the ITI resulted in a relatively small effect, the present results suggest that the ITI only represents a relatively small portion of this temporal context. A much larger effect, ranging from run, stop, to reset, was instead observed when manipulating the target duration of the to-be-timed signal (C. V. Buhusi & W. H. Meck, submitted), suggesting that the to-be-timed signal duration is the major factor used to define the temporal context of a gap.
Irrespective of the mechanism implicated, the present experiment suggests that the effect of a gap inserted during a to-be-timed signal depends on the duration of the ITI used during training, a result that may explain the differences in stop/reset between rats and pigeons (reviewed by Buhusi 2003). Rats tend to use a stop rule (reviewed by Roberts & Church 1978; Roberts 1981; Meck et al. 1984; Buhusi & Meck 2000; Buhusi et al. 2005), while at similar gap durations as those used with rats, pigeons tend to use a reset rule (Roberts et al. 1989; Cabeza de Vaca et al. 1994; Buhusi et al. 2006). A close analysis of the experimental design reveals that pigeon experiments frequently use a short-ITI (typically 15 s, as in our short-ITI group), while rat experiments frequently use a longer ITI (typically 1–2 min, as in our long-ITI group). The present results suggest that the difference in the temporal context in which the gap is presented in experiments using pigeons and rats might contribute (among other factors) to pigeons resetting their clocks (due to a long gap relative to the ITI) and rats stopping (due to a short gap relative to the ITI). Taken together, these data and the new findings from the present experiment suggest that the temporal context, which includes the to-be-timed signal and the ITI, contributes to the pattern of responding to the gap procedure.
5. Conclusions
We reviewed behavioural and neurobiological data in favour of the time-sharing hypothesis that attentional processing is crucial for time estimation both in humans and lower animals. This proposal was supported by data from dual-task paradigms as well as by data from a very simple behavioural protocol in which a to-be-timed signal is interrupted by an unexpected gap or distracter. These behavioural data strongly support a time-sharing model in which presentation of a gap/distracter determines resources to be taken away from the timing system, such that, left with fewer resources, the timing system fails to maintain a veridical representation of the current subjective time in working memory, resulting in a delay in timing (Zakay 1989; Fortin 2003; Buhusi et al. 2005; Buhusi & Meck 2006a,b; Droit-Volet & Meck 2007; Noulhiane et al. 2007). This time-sharing model addresses the results of manipulating gap position, duration and salience (Church 1978; Roberts & Church 1978; Roberts 1981; Meck et al. 1984; Olton et al. 1988; Lustig & Meck 2001; Buhusi & Meck 2002, 2006a,b, 2007; Buhusi et al. 2002, 2005, 2006; Buhusi 2003; Fortin 2003; Bherer et al. 2007; Fortin et al. 2009) by assuming that during the gap/distracter resources decay and/or are reallocated in proportion to the relative saliency (discriminability) of the gap to the timed signal (Buhusi et al. 2005, 2008; Buhusi & Meck 2006a,b). Here, we extended this model to include the relative duration of the gap to a temporal context that includes the to-be-timed signal duration and the ITI. We extended the qualitative description of the relative-duration hypothesis (Spetch & Rusak 1992b) by assuming that working memory decays at a rate inversely proportional to the temporal context. Our new findings provide support for this assumption: the effect of a gap was found to depend on its relative duration to the ITI.
Neurobiological data reviewed here suggest that time estimation engages circuits involved in timekeeping, such as the striatum, circuits involved in attention and working memory, such as the dorsolateral prefrontal cortex and the parietal cortex, and other circuits involved in process of reallocation of resources, possibly the temporal lobe system, as suggested by lesion studies in rats, and the agranular cortex, as suggested by neural recordings in rats. Given the complexity of the circuits engaged by time estimation, it is not surprising that pharmacological studies involve multiple neurotransmitter systems, notably the dopamine and serotonin systems. Future studies need to differentiate the role of these circuits, and to clarify the neurobiological mechanisms involved in the monitoring, maintenance and reallocation of attentional and working memory resources involved in interval timing, subjective shortening and the categorical scaling of duration (e.g. Meck 2005; Santi et al. 2007; Penney et al. 2008; Pfeuty et al. 2008; Wittmann et al. 2008; van Rooyen & Santi 2009).
Acknowledgments
This work was supported by National Institutes of Health (R01MH065561 and R01MH073057 to C.V.B.) and by a fellowship from the James McKeen Cattell Fund and CNRS, France (to W.H.M.).
Footnotes
One contribution of 14 to a Theme Issue ‘The experience of time: neural mechanisms and the interplay of emotion, cognition and embodiment’.
References
- Asgari K., Body S., Zhang Z., Fone K.C., Bradshaw C.M., Szabadi E. Effects of 5-HT1A and 5-HT2A receptor stimulation on temporal differentiation performance in the fixed-interval peak procedure. Behav. Process. 2006;71:250–257. doi: 10.1016/j.beproc.2005.06.007. doi:10.1016/j.beproc.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Bateson M., Kacelnik A. Risk-sensitive foraging: decision making in variable environments. In: Dukas R., editor. Cognitive ecology: the evolutionary ecology of information processing and decision making. Chicago University Press; Chicago, IL: 1998. pp. 297–341. [Google Scholar]
- Bherer L., Desjardins S., Fortin C. Age-related differences in timing with breaks. Psychol. Aging. 2007;22:398–403. doi: 10.1037/0882-7974.22.2.398. doi:10.1037/0882-7974.22.2.398 [DOI] [PubMed] [Google Scholar]
- Block R.A., Zakay D. Models of psychological time revisited. In: Helfrich H., editor. Time and mind. Hogrefe and Huber; Kirkland, WA: 1996. pp. 171–195. [Google Scholar]
- Body S., Asgari K., Cheung T.H., Bezzina G., Fone K.F., Glennon J.C., Bradshaw C.M., Szabadi E. Evidence that the effect of 5-HT2 receptor stimulation on temporal differentiation is not mediated by receptors in the dorsal striatum. Behav. Process. 2006;71:258–267. doi: 10.1016/j.beproc.2005.10.004. doi:10.1016/j.beproc.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Body, S., Cheung, T. H. C., Hampson, C. L., den Boon, F. S., Bezzina, G., Fone, K. F., Bradshaw, C. M. & Szabadi, E. In press. Attenuation of the effects of d-amphetamine on interval timing behaviour by central 5-hydroxytrptamine depletion. Psychopharmacology (Berl.) (doi:10.1007/s00213-008-1400-8) [DOI] [PMC free article] [PubMed]
- Brodbeck D.R., Hampton R.R., Cheng K. Timing behaviour of blackcapped chickadees (Parus atricapillus) Behav. Process. 1998;44:183–195. doi: 10.1016/s0376-6357(98)00048-5. doi:10.1016/S0376-6357(98)00048-5 [DOI] [PubMed] [Google Scholar]
- Brown S.W. Time perception and attention: the effects of prospective versus retrospective paradigms and task demands on perceived duration. Percept. Psychophys. 1985;38:115–124. doi: 10.3758/bf03198848. [DOI] [PubMed] [Google Scholar]
- Brown S.W. Time, change, and motion: the effects of stimulus movement on temporal perception. Percept. Psychophys. 1995;57:105–116. doi: 10.3758/bf03211853. [DOI] [PubMed] [Google Scholar]
- Brown S.W. Attentional resources in timing: interference effects in concurrent temporal and non-temporal working memory tasks. Percept. Psychophys. 1997;59:1118–1140. doi: 10.3758/bf03205526. [DOI] [PubMed] [Google Scholar]
- Brown S.W. Automaticity versus timesharing in timing and tracking dual-task performance. Psychol. Res. 1998;61:71–81. doi:10.1007/s004260050014 [Google Scholar]
- Brown S.W. The attenuation effect in timing: counteracting dual-task interference with time-judgment skill training. Perception. 2008;37:712–724. doi: 10.1068/p5698. doi:10.1068/p5698 [DOI] [PubMed] [Google Scholar]
- Buhusi C.V. Dopaminergic mechanisms of interval timing and attention. In: Meck W.H., editor. Functional and neural mechanisms of interval timing. CRC Press; Boca Raton, FL: 2003. pp. 317–338. [Google Scholar]
- Buhusi C.V., Meck W.H. Timing for the absence of a stimulus: the gap paradigm reversed. J. Exp. Psychol. Anim. Behav. Process. 2000;26:305–322. doi: 10.1037//0097-7403.26.3.305. doi:10.1037/0097-7403.26.3.305 [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Meck W.H. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav. Neurosci. 2002;116:291–297. doi: 10.1037//0735-7044.116.2.291. doi:10.1037/0735-7044.116.2.291 [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Meck W.H. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. doi:10.1038/nrn1764 [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Meck W.H. Interval timing with gaps and distracters: evaluation of the ambiguity, switch, and time-sharing hypotheses. J. Exp. Psychol. Anim. Behav. Process. 2006a;32:329–338. doi: 10.1037/0097-7403.32.3.329. doi:10.1037/0097-7403.32.3.329 [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Meck W.H. Time sharing in rats: a peak-interval procedure with gaps and distracters. Behav. Process. 2006b;71:107–115. doi: 10.1016/j.beproc.2005.11.017. doi:10.1016/j.beproc.2005.11.017 [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Meck W.H. Effect of clozapine on interval timing and working memory for time in the peak-interval procedure with gaps. Behav. Process. 2007;74:159–167. doi: 10.1016/j.beproc.2006.10.004. doi:10.1016/j.beproc.2006.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi C.V., Sasaki A., Meck W.H. Temporal integration as a function of signal and gap intensity in rats (Rattus norvegicus) and pigeons (Columba livia) J. Comp. Psychol. 2002;116:381–390. doi: 10.1037/0735-7036.116.4.381. doi:10.1037//0735-7036.116.4.381 [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Perera D., Meck W.H. Memory for timing visual and auditory signals in albino and pigmented rats. J. Exp. Psychol. Anim. Behav. Process. 2005;31:18–30. doi: 10.1037/0097-7403.31.1.18. doi:10.1037/0097-7403.31.1.18 [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Paskalis J.-P.G., Cerutti D.T. Time-sharing in pigeons: independent effects of gap duration, position and discriminability from the timed signal. Behav. Process. 2006;71:116–125. doi: 10.1016/j.beproc.2005.10.006. doi:10.1016/j.beproc.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Buhusi C.V., Lamoureux J.A., Meck W.H. Prenatal choline supplementation increases sensitivity to contextual processing of temporal information. Brain Res. 2008;1237:204–213. doi: 10.1016/j.brainres.2008.08.072. doi:10.1016/j.brainres.2008.08.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S., Brown B.L., Hemmes N.S. Internal clock and memory processes in animal timing. J. Exp. Psychol. Anim. Behav. Process. 1994;20:184–198. doi: 10.1037//0097-7403.20.2.184. doi:10.1037/0097-7403.20.2.184 [DOI] [PubMed] [Google Scholar]
- Casini L., Macar F. Effects of attention manipulation on perceived duration and intensity in the visual modality. Mem. Cogn. 1997;25:812–818. doi: 10.3758/bf03211325. [DOI] [PubMed] [Google Scholar]
- Casini L., Macar F., Grondin S. Time estimation and attentional sharing. In: Macar F., Pouthas V., Friedman W., editors. Time, action and cognition: towards bridging the gap. Kluwer Academic Publisher; Dordrecht, The Netherlands: 1992. pp. 177–180. [Google Scholar]
- Catania A.C. Reinforcement schedules and psychophysical judgements: a study of some temporal properties of behavior. In: Schoenfeld W.N., editor. The theory of reinforcement schedules. Appleton-Century-Crofts; New York, NY: 1970. pp. 1–42. [Google Scholar]
- Champagne J., Fortin C. Attention sharing during timing: modulation by processing demands of an expected stimulus. Percept. Psychophys. 2008;70:630–639. doi: 10.3758/pp.70.4.630. doi:10.3758/PP.70.4.630 [DOI] [PubMed] [Google Scholar]
- Cheng R.K., Meck W.H., Williams C.L. α7 nicotinic acetylcholine receptors and temporal memory: synergistic effects of combining prenatal choline and nicotine on reinforcement-induced resetting of an interval clock. Learn. Mem. 2006;13:127–134. doi: 10.1101/lm.31506. doi:10.1101/lm.31506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y.H., Halldin C., Farde L. Occupancy of 5-HT1A receptors by clozapine in the primate brain: a PET study. Psychopharmacology (Berl.) 2003;166:234–240. doi: 10.1007/s00213-002-1256-2. doi:10.1007/s00213-002-1256-2 [DOI] [PubMed] [Google Scholar]
- Church R.M. The internal clock. In: Hulse S.H., Fowler H., Honig W.K., editors. Cognitive processes in animal behavior. Erlbaum; Hillsdale, NJ: 1978. pp. 277–310. [Google Scholar]
- Church R.M. Properties of an internal clock. Ann. NY Acad. Sci. 1984;423:566–582. doi: 10.1111/j.1749-6632.1984.tb23459.x. doi:10.1111/j.1749-6632.1984.tb23459.x [DOI] [PubMed] [Google Scholar]
- Coull J.T. fMRI studies of temporal attention: allocating attention within, or toward, time. Cogn. Brain Res. 2004;21:216–226. doi: 10.1016/j.cogbrainres.2004.02.011. doi:10.1016/j.cogbrainres.2004.02.011 [DOI] [PubMed] [Google Scholar]
- Coull J.T., Vidal F., Nazarian B., Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–1508. doi: 10.1126/science.1091573. doi:10.1126/science.1091573 [DOI] [PubMed] [Google Scholar]
- Droit-Volet S., Meck W.H. How emotions colour our perception of time. Trends Cogn. Sci. 2007;11:504–513. doi: 10.1016/j.tics.2007.09.008. doi:10.1016/j.tics.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Fletcher P.C., Henson R.N.A. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. doi:10.1093/brain/124.5.849 [DOI] [PubMed] [Google Scholar]
- Fortin C. Attentional time-sharing in interval timing. In: Meck W.H., editor. Functional and neural mechanisms of interval timing. CRC Press; Boca Raton, FL: 2003. pp. 235–260. [Google Scholar]
- Fortin C., Massé N. Expecting a break in time estimation: attentional time-sharing without concurrent processing. J. Exp. Psychol. Hum. Percept. Perform. 2000;26:1788–1796. doi: 10.1037//0096-1523.26.6.1788. doi:10.1037/0096-1523.26.6.1788 [DOI] [PubMed] [Google Scholar]
- Fortin C., Tremblay S. Interrupting timing in interval production and discrimination: similarities and differences. Behav. Process. 2006;71:336–343. doi: 10.1016/j.beproc.2005.10.003. doi:10.1016/j.beproc.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Fortin C., Bédard M.C., Champagne J. Timing during interruptions in timing. J. Exp. Psychol. Hum. Percept. Perform. 2005;31:276–288. doi: 10.1037/0096-1523.31.2.276. doi:10.1037/0096-1523.31.2.276 [DOI] [PubMed] [Google Scholar]
- Fortin, C., Fairhurst, S., Malapani, C., Morin, C., Towey, J. & Meck, W. H. In press. Expectancy in multisecond peak-interval timing with gaps in humans. Atten. Percept. Psychophys. [DOI] [PubMed]
- Gibbon J., Church R.M., Meck W.H. Scalar timing in memory. Ann. NY Acad. Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. doi:10.1111/j.1749-6632.1984.tb23417.x [DOI] [PubMed] [Google Scholar]
- Gibbon J., Malapani C., Dale C.L., Gallistel C.R. Toward a neurobiology of temporal cognition: advances and challenges. Curr. Opin. Neurobiol. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. doi:10.1016/S0959-4388(97)80005-0 [DOI] [PubMed] [Google Scholar]
- Grondin S., Macar F. Dividing attention between temporal and nontemporal tasks: a performance operating characteristic-POC-analysis. In: Macar F., Pouthas V., Friedman W., editors. Time, action and cognition: towards bridging the gap. Kluwer Academic Publisher; Dordrecht, The Netherlands: 1992. pp. 119–128. [Google Scholar]
- Harrington D.L., Boyd L.A., Mayer A.R., Sheltraw D.M., Lee R.R., Huang M., Rao S.M. Neural representation of interval encoding and decision making. Cogn. Brain Res. 2004;21:193–205. doi: 10.1016/j.cogbrainres.2004.01.010. doi:10.1016/j.cogbrainres.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Hicks R.E., Miller G.W., Kinsbourne M. Prospective and retrospective judgments of time as a function of amount of information processed. Am. J. Psychol. 1976;89:719–730. doi:10.2307/1421469 [PubMed] [Google Scholar]
- Hicks R.E., Miller G.W., Gaes G., Bierman K. Concurrent processing demands and the experience of time-in-passing. Am. J. Psychol. 1977;90:413–446. doi:10.2307/1421874 [Google Scholar]
- Ichikawa J., Ishii H., Bonaccorso S., Fowler W.L., O'Laughlin I.A., Meltzer H.Y. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J. Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. doi:10.1046/j.1471-4159.2001.00154.x [DOI] [PubMed] [Google Scholar]
- Kaiser D.H., Zentall T.R., Neiman E. Timing in pigeons: effects of the similarity between intertrial interval and gap in a timing signal. J. Exp. Psychol. Anim. Behav. Process. 2002;28:416–422. doi:10.1037/0097-7403.28.4.416 [PubMed] [Google Scholar]
- Karmarkar U.R., Buonomano D.V. Timing in the absence of clocks: encoding time in neural network states. Neuron. 2007;53:427–438. doi: 10.1016/j.neuron.2007.01.006. doi:10.1016/j.neuron.2007.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kladopoulos C.N., Hemmes N.S., Brown B.L. Prospective timing under dual-task paradigms: attentional and contextual-change mechanisms. Behav. Process. 2004;67:221–233. doi: 10.1016/j.beproc.2003.12.004. doi:10.1016/j.beproc.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Lebedev M.A., O'Doherty J.E., Nicolelis M.A.L. Decoding of temporal intervals from cortical ensemble activity. J. Neurophysiol. 2008;99:166–186. doi: 10.1152/jn.00734.2007. doi:10.1152/jn.00734.2007 [DOI] [PubMed] [Google Scholar]
- Lejeune H. Switching or gating? The attentional challenge in cognitive models of psychological time. Behav. Process. 1998;44:127–145. doi: 10.1016/s0376-6357(98)00045-x. doi:10.1016/S0376-6357(98)00045-X [DOI] [PubMed] [Google Scholar]
- Lejeune H. Prospective timing, attention and the switch: A response to ‘Gating or switching? Gating is a better model of prospective timing’ by Zakay. Behav. Process. 1999;52:71–76. doi: 10.1016/s0376-6357(00)00136-4. doi:10.1016/S0376-6357(00)00136-4 [DOI] [PubMed] [Google Scholar]
- Leon M.I., Shadlen M.N. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. doi:10.1016/S0896-6273(03)00185-5 [DOI] [PubMed] [Google Scholar]
- Lustig C., Meck W.H. Paying attention to time as one gets older. Psychol. Sci. 2001;12:478–484. doi: 10.1111/1467-9280.00389. doi:10.1111/1467-9280.00389 [DOI] [PubMed] [Google Scholar]
- Macar, F. & Vidal, F. In press. Timing processes: an outline of behavioural and neural indicies partially considered in timing models. Can. J. Exp. Psychol. [DOI] [PubMed]
- Macar F., Grondin S., Casini L. Controlled attention sharing influences time estimation. Mem. Cogn. 1994;22:673–686. doi: 10.3758/bf03209252. [DOI] [PubMed] [Google Scholar]
- MacDonald C.J., Meck W.H. Systems-level integration of interval timing and reaction time. Neurosci. Biobehav. Rev. 2004;28:747–769. doi: 10.1016/j.neubiorev.2004.09.007. doi:10.1016/j.neubiorev.2004.09.007 [DOI] [PubMed] [Google Scholar]
- MacDonald C.J., Meck W.H. Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacology. 2005;182:232–244. doi: 10.1007/s00213-005-0074-8. doi:10.1007/s00213-005-0074-8 [DOI] [PubMed] [Google Scholar]
- Marquardt D.W. An algorithm for least squares estimation of parameters. J. Soc. Ind. Appl. Math. 1963;11:431–441. doi:10.1137/0111030 [Google Scholar]
- Matell M.S., Meck W.H. Reinforcement-induced within trial resetting of an internal clock. Behav. Process. 1999;45:157–171. doi: 10.1016/s0376-6357(99)00016-9. doi:10.1016/S0376-6357(99)00016-9 [DOI] [PubMed] [Google Scholar]
- Matell M.S., Meck W.H. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cogn. Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. doi:10.1006/brcg.2001.1313 [DOI] [PubMed] [Google Scholar]
- Matell M.S., Meck W.H., Nicolelis M.A. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav. Neurosci. 2003;117:760–773. doi: 10.1037/0735-7044.117.4.760. doi:10.1037/0735-7044.117.4.760 [DOI] [PubMed] [Google Scholar]
- Meck W.H. Attentional bias between modalities: effect on the internal clock, memory, and decision stages used in animal time discrimination. Ann. NY Acad. Sci. 1984;423:528–541. doi: 10.1111/j.1749-6632.1984.tb23457.x. doi:10.1111/j.1749-6632.1984.tb23457.x [DOI] [PubMed] [Google Scholar]
- Meck W.H. Hippocampal function is required for feedback control of an internal clock's criterion. Behav. Neurosci. 1988;102:54–60. doi: 10.1037//0735-7044.102.1.54. doi:10.1037//0735-7044.102.1.54 [DOI] [PubMed] [Google Scholar]
- Meck W.H. Neuropharmacology of timing and time perception. Cogn. Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. doi:10.1016/0926-6410(96)00009-2 [DOI] [PubMed] [Google Scholar]
- Meck W.H. Choline uptake in the frontal cortex is proportional to the absolute error of a temporal memory translation constant in mature and aged rats. Learn. Motiv. 2002;33:88–104. doi:10.1006/lmot.2001.1101 [Google Scholar]
- Meck W.H. Neuropsychology of timing and time perception. Brain Cogn. 2005;58:1–8. doi: 10.1016/j.bandc.2004.09.004. doi:10.1016/j.bandc.2004.09.004 [DOI] [PubMed] [Google Scholar]
- Meck W.H. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res. 2006a;1108:157–167. doi: 10.1016/j.brainres.2006.06.046. doi:10.1016/j.brainres.2006.06.046 [DOI] [PubMed] [Google Scholar]
- Meck W.H. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 2006b;1109:93–107. doi: 10.1016/j.brainres.2006.06.031. doi:10.1016/j.brainres.2006.06.031 [DOI] [PubMed] [Google Scholar]
- Meck W.H., MacDonald C.J. Amygdala inactivation reverses fear's ability to impair divided attention and make time stand still. Behav. Neurosci. 2007;121:707–720. doi: 10.1037/0735-7044.121.4.707. doi:10.1037/0735-7044.121.4.707 [DOI] [PubMed] [Google Scholar]
- Meck W.H., Malapani C. Neuroimaging of interval timing. Cogn. Brain Res. 2004;21:133–137. doi: 10.1016/j.cogbrainres.2004.07.010. doi:10.1016/j.cogbrainres.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Meck W.H., Church R.M., Olton D.S. Hippocampus, time, and memory. Behav. Neurosci. 1984;98:3–22. doi: 10.1037//0735-7044.98.1.3. doi:10.1037/0735-7044.98.1.3 [DOI] [PubMed] [Google Scholar]
- Meck W.H., Church R.M., Wenk G.L., Olton D.S. Nucleus basalis magnocellularis and medial septal area lesions differentially impair temporal memory. J. Neurosci. 1987;7:3505–3511. doi: 10.1523/JNEUROSCI.07-11-03505.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck W.H., Penney T.B., Pouthas V. Cortico-striatal representation of time in animals and humans. Curr. Opin. Neurobiol. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. doi:10.1016/j.conb.2008.08.002 [DOI] [PubMed] [Google Scholar]
- MED-Associates 1999 WMPC software, v. 1.15 [Computer software]. St Albans, VT.
- Meltzer H.Y., Li Z., Kaneda Y., Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Progr. Neurol. Psychopharmacol. Biol. Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. doi:10.1016/j.pnpbp.2003.09.010 [DOI] [PubMed] [Google Scholar]
- Nobre A.C., Correa A., Coull J.T. The hazards of time. Curr. Opin. Neurobiol. 2007;17:465–470. doi: 10.1016/j.conb.2007.07.006. doi:10.1016/j.conb.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Noulhiane M., Melia N., Samson S., Ragot R., Pouthas V. How emotional auditory stimuli modulate time perception. Emotion. 2007;7:697–704. doi: 10.1037/1528-3542.7.4.697. doi:10.1037/1528-3542.7.4.697 [DOI] [PubMed] [Google Scholar]
- Olton D.S., Meck W.H., Church R.M. Separation of hippocampal and amygdaloid involvement in temporal memory dysfunctions. Brain Res. 1987;404:180–188. doi: 10.1016/0006-8993(87)91369-2. doi:10.1016/0006-8993(87)91369-2 [DOI] [PubMed] [Google Scholar]
- Olton D.S., Wenk G.L., Church R.M., Meck W.H. Attention and the frontal cortex as examined by simultaneous temporal processing. Neuropsychologia. 1988;26:307–318. doi: 10.1016/0028-3932(88)90083-8. doi:10.1016/0028-3932(88)90083-8 [DOI] [PubMed] [Google Scholar]
- Pang K.C., McAuley J.D. Importance of frontal motor cortex in divided attention and simultaneous temporal processing. In: Meck W.H., editor. Functional and neural mechanisms of interval timing. CRC Press; Boca Raton, FL: 2003. pp. 351–369. [Google Scholar]
- Pang K.C., Yoder R.M., Olton D.S. Neurons in the lateral agranular frontal cortex have divided attention correlates in a simultaneous temporal processing task. Neuroscience. 2001;103:615–628. doi: 10.1016/s0306-4522(01)00018-5. doi:10.1016/S0306-4522(01)00018-5 [DOI] [PubMed] [Google Scholar]
- Paule M.G., Meck W.H., McMillan D.E., McClure G.Y.H., Bateson M., Popke E.J., Chelonis J.J., Hinton S.C. The use of timing behaviors in animals and humans to detect drug and/or toxicant effects. Neurotoxicol. Teratol. 1999;21:491–502. doi: 10.1016/s0892-0362(99)00015-x. doi:10.1016/S0892-0362(99)00015-X [DOI] [PubMed] [Google Scholar]
- Penney T.B., Gibbon J., Meck W.H. Differential effects of auditory and visual signals on clock speed and temporal memory. J. Exp. Psychol. Hum. Percept. Perform. 2000;26:1770–1787. doi: 10.1037//0096-1523.26.6.1770. doi:10.1037/0096-1523.26.6.1770 [DOI] [PubMed] [Google Scholar]
- Penney T.B., Gibbon J., Meck W.H. Categorical scaling of duration bisection in pigeons (Columba livia), mice (Mus musculus), and human (Homo sapiens) Psychol. Sci. 2008;19:1103–1109. doi: 10.1111/j.1467-9280.2008.02210.x. doi:10.1111/j.1467-9280.2008.02210.x [DOI] [PubMed] [Google Scholar]
- Pfeuty M., Ragot R., Pouthas V. Brain activity during interval timing depends on sensory structure. Brain Res. 2008;1024:112–117. doi: 10.1016/j.brainres.2008.01.022. doi:10.1016/j.brainres.2008.01.022 [DOI] [PubMed] [Google Scholar]
- Picard N., Strick P.L. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. doi:10.1016/S0959-4388(01)00266-5 [DOI] [PubMed] [Google Scholar]
- Pouthas V., Perbal S. Time perception depends on accurate clock mechanisms as well as unimpaired attention and memory processes. Acta Neurobiol. Exp. 2004;64:367–385. doi: 10.55782/ane-2004-1520. [DOI] [PubMed] [Google Scholar]
- Roberts S. Isolation of an internal clock. J. Exp. Psychol. Anim. Behav. Process. 1981;7:242–268. doi:10.1037/0097-7403.7.3.242 [PubMed] [Google Scholar]
- Roberts S., Church R.M. Control of an internal clock. J. Exp. Psychol. Anim. Behav. Process. 1978;4:318–337. doi:10.1037/0097-7403.4.4.318 [Google Scholar]
- Roberts W.A., Cheng K., Cohen J.S. Timing light and tone signals in pigeons. J. Exp. Psychol. Anim. Behav. Process. 1989;15:23–35. doi:10.1037/0097-7403.15.1.23 [PubMed] [Google Scholar]
- Rollema H., Lu Y., Schmidt A.W., Zorn S.H. Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Eur. J. Pharmacol. 1997;338:3–5. doi: 10.1016/s0014-2999(97)81951-6. doi:10.1016/S0014-2999(97)81951-6 [DOI] [PubMed] [Google Scholar]
- Santi A., Keough D., Gagne S., van Rooyen P. Differential effects of empty and filled intervals on duration estimation by pigeons: tests of an attention-sharing explanation. Behav. Process. 2007;74:176–186. doi: 10.1016/j.beproc.2006.08.008. doi:10.1016/j.beproc.2006.08.008 [DOI] [PubMed] [Google Scholar]
- Sherburne L.M., Zentall T.R., Kaiser D.H. Timing in pigeons: the choose-short effect may result from a confusion between delay and intertrial intervals. Psychonom. Bull. Rev. 1998;5:516–522. [Google Scholar]
- Spetch M.L., Rusak B. Temporal context effects in pigeons' memory for event duration. Learn. Motiv. 1992a;23:117–144. doi:10.1016/0023-9690(92)90013-C [Google Scholar]
- Spetch M.L., Rusak B. Time present and time past. In: Honig W.K., Fetterman J.G., editors. Cognitive aspects of stimulus control. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1992b. pp. 47–67. [Google Scholar]
- Spetch M.L., Wilkie D.M. Subjective shortening: a model of pigeons' memory for event duration. J. Exp. Psychol. Anim. Behav. Process. 1983;9:14–30. doi:10.1037/0097-7403.9.1.14 [Google Scholar]
- Stevens M.C., Kiehl K.A., Pearlson G., Calhoun V.D. Functional neural circuits for mental timekeeping. Hum. Brain Mapp. 2007;28:394–408. doi: 10.1002/hbm.20285. doi:10.1002/hbm.20285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E.A.C., Weaver W.B. Cognitive processing and time perception. Percept. Psychophys. 1975;17:363–367. [Google Scholar]
- Thorpe C.M., Petrovic V., Wilkie D.M. How rats process spatiotemporal information in the face of distraction. Behav. Process. 2002;58:79–90. doi: 10.1016/s0376-6357(02)00003-7. doi:10.1016/S0376-6357(02)00003-7 [DOI] [PubMed] [Google Scholar]
- van Rooyen P., Santi A. Pigeons' memory for time: assessment of the role of subjective shortening in the duration-comparison procedure. Learn. Behav. 2009;37:74–84. doi: 10.3758/LB.37.1.74. doi:10.3758/LB.37.1.74 [DOI] [PubMed] [Google Scholar]
- Wittmann, M., Simmons, A., Aron, J., Paulus, M. 2008 Accumulation of neural activity in the posterior insula encodes the passage of time. Natu. Precedings See http://hdl.handle.net/10101/npre.2008.2062.1 [DOI] [PMC free article] [PubMed]
- Zakay D. Subjective time and attentional resource allocation: an integrated model of time estimation. In: Levin I., Zakay D., editors. Time and human cognition: a life-span perspective. Elesevier/North-Holland; Amsterdam, The Netherlands: 1989. pp. 365–397. [Google Scholar]
- Zakay D. Gating or switching? Gating is a better model of prospective timing (a response to ‘switching or gating?’ by Lejeune) Behav. Process. 2000;52:63–69. doi: 10.1016/s0376-6357(00)00141-8. doi:10.1016/S0376-6357(00)00141-8 [DOI] [PubMed] [Google Scholar]
- Zakay D., Nitzan D., Glicksohn J. The influence of task difficulty and external tempo on subjective time estimation. Percept. Psychophys. 1983;34:451–456. doi: 10.3758/bf03203060. [DOI] [PubMed] [Google Scholar]