Abstract
The development of sub-disciplines within cognitive neuroscience follows common sense categories such as language, audition, action, memory, emotion and perception among others. There are also well-established research programmes into temporal perception, spatial perception and mathematical cognition that also reflect the subjective impression of how experience is constructed. There is of course no reason why the brain should respect these common sense, text book divisions and, here, we discuss the contention that generalized magnitude processing is a more accurate conceptual description of how the brain deals with information about time, space, number and other dimensions. The roots of the case for linking magnitudes are based on the use to which magnitude information is put (action), the way in which we learn about magnitudes (ontogeny), shared properties and locations of magnitude processing neurons, the effects of brain lesions and behavioural interference studies. Here, we assess this idea in the context of a theory of magnitude, which proposed common processing mechanisms of time, space, number and other dimensions.
Keywords: parietal cortex, time, space, number, magnitude
1. Introduction
There is a great deal of evidence to suggest that quantities that can be approximated are ubiquitous throughout different species, different cultures and in all phases of human life. The functional organization of the brain is a product of evolution and development and therefore carries clues as to why the brain is organized as it is. A main feature of our magnitude perception is similar to other dimensions, i.e. they obey Weber's law across species, developmental stages and tasks. In one sense, this is unremarkable—sensory systems take ratios—but the extent to which magnitude sensitivity is similar is a good guide to whether similar mechanisms are being used. Thus, the performance of infants on number, time and area tasks (Brannon et al. 2006; Halberda et al. 2006; van Marle & Wynn 2006), of pigeons and rats on time and numerosity tasks (Church & Meck 1984; Brannon & Roitman 2003), of monkeys (Hauser et al. 2000; Cantlon & Brannon 2006) and apes (Rumbaugh et al. 1987; Boysen et al. 1995; Beran & Rumbaugh 2001; Murofushi 2002; Beran 2007) on temporal and number tasks is strongly suggestive of shared mechanisms across species and development. Manipulating magnitudes is also a feature of our language reasoning (Kemmerer 2005) in other domains such as our mental representation of maps and graphs (Feeney et al. 2004).
Some time ago, Walsh (2003a,b) proposed in a theory of magnitude (ATOM) that commonalities between time, space, number, size, speed and other magnitudes were to be found in the parietal cortex because of the need to learn about the environment through motor interactions and therefore to encode these variables for action. The hypothesis has so far successfully predicted a role for visual motion areas in time perception (Nishida & Johnston 2002; Bueti et al. 2008a,b), the temporal properties of spatially defined neurons in the parietal cortex (Leon & Shadlen 2003; Walsh 2003a,b), the effects of transcranial magnetic stimulation (TMS) over the parietal cortex (see below), co-activations in the parietal cortex (see below) and the effects of dual task and action studies involving time, size, space number and action (e.g. Brozzoli et al. 2008; Conson et al. 2008; Herrera et al. 2008). The ATOM theory also remains the explanatory account of why temporal processing may share mapping metrics with space, number, size, speed and action and also why numerical processing should originate in the parietal lobes. Here, we review recent advances in exploring the extent to which different magnitude dimensions may access common neuronal sources with particular reference to the parietal lobes.
2. Behavioural interactions between magnitudes
A good test of common resources being shared across magnitudes is to look for examples of behavioural interactions between them, and there are now several elegant and inventive accounts of interactions between time, space and number. One prediction from ATOM is that there should be some monotonic mapping of quantities: bigger, faster, brighter, further in one domain should correlate with bigger, faster, brighter, further in another. This kind of intuitive ‘more A–more B’ mapping has been noted in developmental contexts and described in some detail by Stavy & Tirosh (2000) who gave many examples of such mapping and reinterpreted several classical findings from developmental psychology (also cf. Kaufmann & Nuerk 2006; Rousselle & Noel 2007, 2008). Stavy and Tirosh suggested that children will often base magnitude judgements on irrelevant dimensions. One of their studies demonstrating this showed children two trains running along a track. The children were given all the information necessary to know that the trains ran at the same rate. When the trains differed in size, however, the subjects stated that the larger train was faster. In this case, size is affecting a judgement (speed) in which time is implicit, but children make the same class of error when making explicit temporal judgements. Levin (1977, 1979, 1982) asked children in kindergarten to judge which of two lights was presented for the longest time. The lights differed in brightness and size and the children consistently judged the larger or brighter stimuli to have persisted for more time.
These intuitive mappings are not limited to children. Xuan et al. (2007), for example, asked adult subjects to make duration judgements on stimuli that varied in non-temporal attributes such as size, luminance and numerosity and observed that temporal estimation was influenced by these non-temporal factors—increasing magnitude led subjects to make longer temporal estimations. Adults, similar to children, are influenced by irrelevant magnitude information about size and luminance when making temporal judgements. Oliveri et al. (2008) have shown that this is also true for numbers and time. Subjects were asked to make ‘longer/shorter’ duration judgements on stimuli presented as digits compared with a standard digit 5 and found that lower digits were significantly underestimated and larger digits overestimated in duration. Similar mappings have been observed by others. Dormal et al. (2006) adapted the Stroop paradigm to duration and numerosity judgements of stimuli consisting of flashing dots. Numeracy of the dots interfered with temporal duration judgements (but not vice versa, see §3). Similarly, Roitman et al. (2007a,b) required subjects to make few/short or many/long judgements on stimuli varying in duration or number and found that subjects showed similar encoding functions and reported that sensitivity for numerical differences was finer than that for temporal differences (see §3).
3. What is time for? What is space for? Why do we have numbers? (If you were Darwin for a day …)
In constructing ATOM (Walsh 2003a,b), one of the central questions was why the parietal cortex was organized as it was. Why did parietal cortex damage lead to reports of deficits in temporal, spatial and numerical perception? Indeed, what was a smart thing such as number, one of the capacities with which humans flatter themselves, doing in the ‘zombie stream’ of the visuomotor system? (Milner & Goodale 2006). The first part of the answer given to this question was based on considering why we have representations of time and space in the first place. Temporal and spatial information are of course necessary for action: for reaching, throwing, pointing and grasping, and the objects at which we direct these actions are frequently moving. Space and time can easily be segregated in psychological experiments by immobilizing subjects' heads, eye movements, restricting their actions and presenting stimuli briefly, but in everyday activity, space and time are rarely segregated—there is no such thing as getting to the right place at the wrong time: if you throw, point, reach or attempt to grasp a moving target, you need to estimate space and time accurately. In other words, space and time are coupled metrics for action and it would be very surprising if they were not in close proximity in the brain and close to the areas required for performing sensory-motor transformations for action, i.e. in the parietal lobes.
The second part of the answer is evolutionary. Imagine you are Darwin for a day and you are charged with granting a species the ability to count. Where would be the most efficient place in the brain for this discrete numerical system? The parietal cortex is already equipped with an analogue system for action that computes ‘more than–less than’, ‘faster–slower’, ‘nearer–farther’, ‘bigger–smaller’, and it is on these abilities that discrete numerical abilities hitched an evolutionary ride. Something of the ontogeny of this is seen in the primacy children's use of using ‘amount of stuff’ rather than discrete quantity to make judgements (Halberda et al. 2006; Hurewitz et al. 2006).
A consequence of this is that magnitude information from different sources would interfere with each other. So far (§2), the examples are of interactions between time and other magnitudes but there should also be interactions between other magnitudes.
4. Behavioural interactions between magnitudes
We have seen that time interacts with other magnitudes, but it is an important component of the ATOM view that other magnitudes are seen to interact in similar ways and one of its counter-intuitive predictions is that numerical information should influence action. A number–action link is not as well established as the number–space link, of which there are many examples. Fias et al. (2001) carried out a series of experiments in which subjects performed a judgement on a stimulus attribute that was more (orientation) or less (colour and shape) associated with parietal cortex processes. The stimuli were presented along with digits that were irrelevant to the task. The orientation judgements, but not the colour or shape judgements, were influenced by the irrelevant number. A less intuitive interference study, but an important one in the context of our suggestion that discrete number evolved on the back of an analogue quantity system necessary for computing the metrics of action, by Andres et al. (2004) required subjects to perform either a grip opening or closing movement to digit stimuli. Closure was initiated more quickly for small digits and opening more quickly for large digits. Andres et al. (2008) later established that as the hand neared the object, the interaction between digit magnitude and grip aperture decreased and therefore concluded that magnitude influences action at the planning or programming stage of grasping movements (see also Ishihara et al. 2008; Badets et al. 2007). Both Lindemann et al. (2007) and Moretto & di Pellegrino (2008) found evidence that mere exposure to magnitude information automatically primes grasping actions. Subjects were presented with numerical stimuli to which they made grip responses according to the semantic (parity) or surface (colour) properties of the stimulus. Although the value of the digit was irrelevant, lower numerical values facilitated precision grip responses (associated with grasping smaller objects) and larger numerical values facilitated power grip responses associated with grasping larger objects. Lindemann et al. (2007) additionally found that larger numbers were associated with a larger initial power group. These studies show that magnitude information influences the selection of action type, but at least one study (Fischer & Miller 2008) suggested that the influence of magnitude information does not extend to the dynamics of action such as force (see also Taylor-Cooke et al. 2006). One other study places the origin of interactions between space and number earlier in the chain of processing. Stoianov et al. (2008) conducted a spatial–numerical priming experiment in which they assessed forward and backward priming with verbal responses. They observed greater effects when the spatial prime followed a number target both for number comparisons and parity judgements, and concluded that the effects could not be ascribed to spatial–numerical response codes. It is a hypothesis and experiment that deserves further exploration.
One of the predictions of ATOM was that time perception should change as a function of the distance of the events being judged. Spatial judgements are affected as a function of being made in ‘near space’ or ‘far space’ (e.g. Halligan & Marshall 1991). Another way of conceptualizing near and far space is as being in or outside of ‘action space’. If magnitude systems originate in the need to compute space, time and size for action, they should behave differently towards stimuli that are within or out with action space. Zach & Brugger (2008) tested this by requiring subjects to make duration estimates of clock movement imagined at two distances. Subjects reported time to run faster for the near clock than for the far clock. There is a possibility, however, that this experiment tested the relationship between size and time rather than distance and time.
There are several other interference studies relevant to the common cortical processing of magnitudes: number can influence attentional orientation (Fischer et al. 2003; Salillas et al. 2008); temporal judgements are susceptible to spatial–numerical association of response code (SNARC)-like effects, consistent with a generalized spatial quantity association of response code (SQUARC) effect predicted in Walsh (2003a,b), Ishihara et al. (2008) and Muller & Schwarz (2008).
5. Why do we have these intuitive mappings?
So far, we have addressed our case that there is a common magnitude system and stated how it is associated with action, but why would we continue to employ common metrics in situations where action is not required? Stavy & Tirosh (2000) detailed many examples of intuitive rules that lead one to make quantitative errors in the absence of any action (although many of them require an understanding of action). More A–more B is one of these rules. We could argue that there is magnitude interference in experiments because of latent action components or some kind of spill over from the magnitude system, but another reason is that they may provide useful heuristics for things that are statistically true about the physical world: faster things do often get further, Usain Bolt, the world's fastest man, does usually have the longest legs in the field, bigger things do usually weigh more.
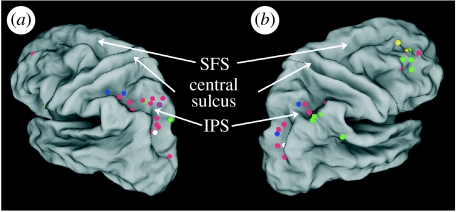
Thinking about why we have these intuitive mappings may also help to explain why all magnitudes are not created equal. An oversimplistic view of a generalized magnitude system might expect all interference effects to be symmetrical that temporal cues, number, space, luminance and action cues would all impinge on each other. This is clearly not the case. Brown (1997), for example, found that number interfered with time but not vice versa, and Dormal & Pesenti (2007) for example, found that in a modified Stroop paradigm, spatial cues interfered with number processing but number did not interfere with spatial processing. Hurewitz et al. (2006) suggested a possible hierarchy of magnitudes from continuous to discrete variables following their finding that amount of stuff interfered with numerosity judgements more than numerosity interfered with ‘stuff’ (a technical term the literature should embrace). Whether these findings are evidence of constant asymmetries or are task dependent remains to be established (cf. Göbel et al. 2004). From the point of view of cortical loci, it is clear that some activation sites for time, space and number overlap and a few do not (figure 1). This should not be surprising: the architecture activated in any given experiment is highly dependent on the task and one should therefore not expect a single locus to account for all instances of magnitude processing (Cohen Kadosh & Walsh in press). Perhaps the best example of the logic is to be found in the attention literature: whereas some studies report that saccade generation directs attention, others clearly show that visual selection functions can be segregated both in time and space. That they can be segregated under some experimental conditions does not of course mean that they are not part of an integrated system for searching the visual environment (cf. Juan et al. 2004, 2008; Schall 2004; Awh et al. 2006). The importance of motor experience is best exemplified in a recent study of the SNARC effect in early blind subjects (Castronovo & Seron 2007).
Figure 1.
(a) Left and (b) right hemisphere views of the human visual cortex showing activation sites reported in some functional magnetic resonance imaging studies of time (green dots), space (yellow dots), number (red dots) and size (blue dots) perceptions. These plots are based on Maquet et al. (1996), Schubotz et al. (2000), Rao et al. (2001), Macar et al. (2002), Ferrandez et al. (2003), Lewis & Miall (2003), Coull et al. (2004), Pouthas et al. (2005) and Bueti et al. (2008a,b). Note the spread of numerical activations along the length of the intraparietal sulcus (IPS) in areas also associated with space, time, size and luminance (white dots). SFS, superior frontal sulcus.
6. When temporal is not time
Events occur in time, some rapidly, some slowly and there is a trivial sense in which all behaviour is temporal. There are some cases, however, in which the time course of events is irrelevant to how the brain processes time. Two classes of this are present in the literature. One of these is the confusion between a stream of events and temporal processing. If a stream of events occurs too quickly for a subject to individuate events, then one can conclude that the system under investigation does not work that quickly, but cannot state anything about how that system processes information about time. This confusion occurs in studies that have used a version of the attentional blink paradigm or presented a sequence of events in which the subjects are required to identify and individuate stimuli. One of the current authors made this error in a study of visual search (see Coull et al. 2003; also Shapiro et al. 2002). The second class of error is to present stimuli in a temporal sequence but not to require the subjects to make any temporal judgements. This has occurred when temporal versions of the Posner paradigm have been used to test what has been called temporal attention. In the first of these experiments (Coull & Nobre 1998), subjects were presented with stimuli that cued a short time or a long time response (300 versus 1500 ms). As in the spatial Posner paradigm, the cues were incorrect some of the time. However, when cued to expect the long interval, the task is no longer one of temporal prediction because the subject knows that a cue to action will appear in due course, i.e. the task becomes one of simple response preparations to an external stimulus. This is evident in the behavioural data that fail to show a cueing effect for long intervals and may account for the anterior left hemisphere parietal activity (most probably associated with movement preparation; e.g. Schluter et al. 2001) reported in that study and preparation-related differences in a later study (Miniussi et al. 1999).
7. The role of the parietal cortex in magnitude processing
The most important region of the cortex associated with temporal processing is the parietal cortex. Regions within the parietal lobe are consistently associated with temporal processing in neuropsychological, brain imaging, single-unit recording and TMS studies. Here, we review some of the core findings that address the issue of common cortical metrics of time and other magnitudes.
(a) Neuropsychological findings
The range of neuropsychological case studies of time perception is reviewed by Koch et al. (2009). The relationship between parietal damage and temporal deficits has long been recognized (cf. Critchley 1953; Harrington et al. 1998; Battelli et al. 2007, 2008). Critchley in his seminal book on the parietal lobes wrote: ‘…most interesting and complicated of all are those spatial disorders which also involve the conception of time … one must distinguish between a primitive time sense, and a gnosic time-conception (by which is meant an understanding of chronological order)…’. Neuropsychological studies have clearly shown that the right parietal lobe, and in particular the intense pulsed light (IPL), might play an important role in discriminating events that are displaced in time. Patients with IPL lesions demonstrate deficits in visual event discrimination in both visual fields and that often coexist with other visuospatial deficits only in the field contralateral to the lesion (see Battelli et al. 2007, 2008 for reviews). Battelli has also suggested that bilateral parietal damage may be necessary for a timing deficit and might distinguish purely visual timing deficits from spatial ones. Other studies support Battelli's view and report deficits similar in nature to the visual timing of events, and bilateral timing deficits have been reported for visual search tasks requiring spatio-temporal segmentation (e.g. Van Vleet & Robertson 2006). The regions of damage that lead to temporal deficits also frequently cause spatial, numerical and velocity perception deficits (e.g. Cipolotti et al. 1991; Basso et al. 1996; Battelli et al. 2003; Becchio & Bertone 2006; Danckert et al. 2007, see also Cavezian et al. 2007; Zamarian et al. 2007). From the point of view of ATOM, neuropsychological patient studies can be indicative of overlapping mechanisms, but the types of lesion that patients usually suffer are too large to allow structural conclusions to be drawn.
(b) Brain imaging studies
Brain imaging studies consistently show that time, space, number and other magnitudes such as size and brightness activate overlapping regions in the parietal cortex. Kaufmann et al. (2008) have recently performed an important comparison of adults and children making non-symbolic numerical decisions and spatial decisions. Their data reveal evidence of the role of the action system in learning about magnitudes and of overlapping representations in adults. Adult subjects' brain activations showed overlapping space and number regions in the posterior superior parietal lobule, whereas children showed relatively more activation in the supramarginal gyrus, the lateral anterior intraparietal sulcus (IPS) and the precentral gyrus. Kaufmann et al. (2005) have also compared activations for number and size using a Stroop-like paradigm in which subjects viewed pairs of digits that varied in numerical value and/or size and required to make a judgement on either the value or the size. Differences as a function of congruity were not observed in the IPS, only in the prefrontal and anterior cingulated cortex, suggesting that IPS regions concerned with number and size overlapped. Cohen Kadosh et al. (2007a–c, 2008a,b) have carried out similar analyses on number–luminance and number–size pairings. In their number–size experiment (Cohen Kadosh et al. 2007a–c), they observed motor activity associated with incongruous trials and concluded that the locus of interference is late in the processing stream—at the point of response initiation (cf. Andres et al. 2008 for similar conclusions based on behavioural experiments). They also observed that interaction between the stimulus attributes occurred only in high load conditions. This is an important caveat not usually addressed in Stroop-like studies of magnitude. In their size–number studies, they also show that interference between the two variables is reflected in parietal cortex activity (see also Pinel et al. 2004; Cohen Kadosh et al. 2005; Liu et al. 2006; Zhou et al. 2006). Studies of the parietal cortex and time perception consistently report a network of areas that include regions of the parietal cortex as well as the prefrontal cortex, supplementary motor area, cerebellum and basal ganglia (e.g. Macar et al. 2002; Coull et al. 2004; Lewis & Miall 2006a,b). A sophisticated approach to parsing time functions is taken by Lewis & Miall who divide temporal functions into cognitive and non-cognitive domains in which the dorsolateral prefrontal cortex is important to both cognitive timing and working memory and the parietal cortex is associated with more automatic temporal processing.
There is also evidence of a role for the IPS in auditory magnitude processing (von Kriegstein et al. 2007), an area that is not addressed in ATOM.
(c) Single-unit studies
Neurons in regions of the parietal cortex in monkeys have been shown to be selective for the aspects of magnitude. Onoe et al. (2001) led the way with a positron emission tomography study of monkeys performing temporal duration discriminations. They observed duration-related activity in the dorsolateral prefrontal cortex and inferior parietal cortex, and suggested that the parietal activation may be due to relaying duration estimations to be matched with a stored standard. Presciently, they also suggested that ‘temporal information in these regions may be coded in neurons with multiplex properties and/or in cell assemblies with overlapping connections in the same region’. Subsequently, Leon & Shadlen (2003) observed temporally related activity in the monkey posterior parietal cortex, but it is important to note that these putative temporal neurons were de facto spatial neurons predefined by their spatial response fields (see Walsh 2003a,b). Janssen & Shadlen (2005) have also recorded activity in the lateral intraparietal cortex consistent with predicting when an event will occur. These spatio-temporal neurons overlap (indeed may be the same multiplexed neurons) with neurons displaying responses to numerical information. Sawamura et al. (2002) recorded number-selective neurons in the intraparietal sulcus and superior parietal lobule (see also Sawamura et al. 2006). These neurons recorded in the lateral intraparietal sulcus are strong candidates for the locus of a population with generalized magnitude properties because their responses are often dependent on spatial information. Neurons in the ventral intraparietal region, however, also have some interesting magnitude features. Nieder's laboratory, for example, has shown that neurons responsive to numerical information are also sensitive to non-numerical task information (Nieder et al. 2006; see also Calabrese 2007). And while some reports have concentrated on number-specific responses (Nieder & Miller 2003; Nieder 2004, 2005; Roitman et al. 2007a,b), it is also clear that neurons in the posterior parietal cortex encode different forms of quantity information (Tudusciuc & Nieder 2007). The posterior parietal cortex is not the only area that shows magnitude-selective response—following Onoe et al., neurons responsive to elapsed time or number have been found in the dorsolateral prefrontal cortex (Nieder et al. 2002; Genovesio et al. 2006).
(d) Transcranial magnetic stimulation studies
If different magnitudes rely on the parietal cortex, then interfering with parietal regions should yield deficits on tasks related to time, space, number and other magnitudes. The first study of TMS effects of number processing (Göbel et al. 2001) showed that temporary interference with the right angular gyrus disrupted the spatial representation of the number line. Later studies have shown that TMS over the right parietal cortex, in particular the IPS, can also prevent the automatic processing of magnitude information (Cohen Kadosh et al. 2007a–d). TMS over the right IPS, however, has less effect on automatic number processing and only an effect limited to the right side of space on the mental number line (Göbel et al. 2006a,b). Dormal et al. (2008) applied off-line repetitive TMS to the right and left parietal cortex and observed an effect on a number comparison task only over the right IPS. Other studies, however, have obtained temporal processing effects following right parietal stimulation (Alexander et al. 2005). The strong links between space and number and time and number have also been explored with TMS. Knops et al. (2006) delivered repetitive TMS over the right posterior parietal lobe and observed neglect-like symptoms on a line bisection task, i.e. subjects' perceived midpoint of a numerical interval shifted to the right as a consequence of TMS. Oliveri et al. (2004) also observed a correction of bias to the left following right IPS TMS (see Bjoertomt et al. 2002 for a spatial analogue of the numerical effect). The effects on any numerical, spatial or temporal task are strongly affected by task demands (Göbel et al. 2004). For example, when numerical responses are associated with finger counting, it is the left parietal cortex that is susceptible to TMS interference (Rusconi et al. 2005).
8. ATOM revisited
We have seen several lines of recent research that have tested the theoretical position that time, space, number and other magnitude processing originate in a common system for magnitudes. Before reviewing where ATOM stands now, it is worth recapping its major points and predictions. The mental representations of time, space, size, number and other magnitudes are largely studied within separate literatures, but the neuropsychological evidence from patients strongly suggested some commonality in the locus of lesions causing deficits in these domains. Based on some TMS studies, behavioural data and reinterpretations of imaging and single-unit studies, it was suggested that different magnitudes originated from a single developmental algorithm for more than–less than distinctions of any kind of stuff in the external world. The development of magnitude processing proceeds by interactions with the environment and is therefore closely linked with the motor reaching, grasping and manipulating of objects. It was further suggested that the emergence of our ability to manipulate discrete quantities evolved from our abilities with continuous quantities (see §3). Among the predictions made were that different magnitudes should show interference and priming effects; that other brain areas associated with magnitude processing (such as V5 for motion processing) should also display some evidence of involvement in other magnitudes (in the case of V5, time; see Bueti et al. 2008a,b); and that the SNARC effect, in which small number judgements are associated with response codes in left space and large numbers with response codes in right space, should prove to be a SQUARC effect in which any spatially or action-coded magnitude will yield a relationship between magnitude and space (cf. Notebaert et al. 2006). All of these predictions have been confirmed to date.
One issue that may be developed more fully in ATOM is that of hemispheric asymmetries (see Woods et al. 2006; Niemeier et al. 2007). Owing to the association of numerical mapping with space, there is an emphasis on right hemisphere functions, but it is clear that explicit numerical operations and motor action selection and preparation rely on left parietal cortex mechanisms. It is also clear that if learning about magnitudes in the world is first achieved through interacting with them as an infant (‘can I lift that; can I get over that; can I gather these; can I get all this in my mouth’?), there will be a close relationship between the right hemisphere registration of continuous quantities and left hemisphere mechanisms involved in action (cf. Andres et al. and Lindemann et al. for example above).
As noted in Walsh (2003a,b) (figure 1), the parietal cortex, although it may be considered the ‘primary magnitude cortex’, is only one locus of magnitude processing—there is a magnitude system not a single magnitude area. Accordingly, magnitude processing also overlaps in the prefrontal cortex both in monkeys (Nieder & Miller 2004; Diester & Nieder 2007) and humans (Burbaud et al. 1995; Rickard et al. 2000; Pochon et al. 2001; Rao et al. 2001; Ferrandez et al. 2003; Kansaku et al. 2007; Vallesi et al. 2008; see Lewis & Miall 2006a,b for a framework).
With any system that is distributed between visual areas, it will be possible to construct dissociations. In the visual system, for example, one can study colour, form or motion and show task-specific dissociations between them. When we see, of course, we see coloured forms moving and therefore need to know both how these specializations evolve and develop and how they form coherent percepts. In the magnitude domain, we can concentrate on size, distance, time or number and we therefore need to understand the evolution and development of these specialization; but, as in vision, when we interact with the world we do so in time and space, so much so that dissociations between them probably tell us more about the strange things we can make people do in experiments rather than how the brain operates in the real world (it is out there; cf. Glasauer et al. 2007): there is no such thing as getting to the right place at the wrong time—shaking hands, kissing, catching, throwing, playing an instrument, gathering kindling or paying by cash all require spatio-temporal coordination. Behaviour may be spatial or temporal in a laboratory, but in the real world they originate in the same coordinate system applied to all magnitudes.
Two questions remain about the view presented in this paper: what are the specific operations underlying magnitude representation and what does the generalized magnitude view mean for models of timing such as internal clock mechanisms? The first question is easier. ATOM has value because it has limits; not all aspects of space, time and number are suggested to lie within a common origin and there is no necessary prediction that ATOM should extend to episodic memory, planning, mathematical operations or allocentric spatial tasks such as navigation. The proposal is that we learn about space and time through action and associations between space, time and magnitudes relevant for action (such as size, speed and, under some conditions, luminance and contrast) will be made through action. When we later learn about number, the neurons with capacity to represent quantity are those that have information about the continuous variables learned about motorically. Thus, the neuronal scaling mechanisms used for dimensions with action-relevant magnitude information will be co-opted in development for the scaling of number. Psychophysically, this has been shown to be the case (Burr & Ross 2008); the relevant neurons are found, as predicted, in the parietal cortex and dual-task experiments show interference between number and action (see above). The single-unit recording literature erroneously emphasizes neurons specialized for time (cf. Walsh 2003a,b), space or number, but we suggest that this is a consequence of the immature state of the field. Similar overemphasis of specializations in the visual system led to concentrating on such things as ‘the colour area’, ‘the form area’ or ‘the motion area’, but it is now clear that many neurons at every level of visual analysis are double or even triple duty for form, motion and chromatic content. Given the similarity of rules observed by sensory processing within and between modalities (Shamma 2001; Sur & Leamey 2001), we predict that similar multi-duty neurons will be reported in the magnitude scaling system in due course. The second question cannot yet be answered: the basis for ATOM is not constrained by any proposed mechanisms of timekeeping and is consistent with clock or non-clock explanations.
Footnotes
One contribution of 14 to a Theme Issue ‘The experience of time: neural mechanisms and the interplay of emotion, cognition and embodiment’.
References
- Alexander I., Cowey A., Walsh V. The parietal cortex in time perception: back to Critchley, the zeitraffer phenomenon. Cogn. Neuropsychol. 2005;22:306–315. doi: 10.1080/02643290442000356. doi:10.1080/02643290442000356 [DOI] [PubMed] [Google Scholar]
- Andres M., Davare M., Pesenti M., Olivier E., Seron X. Number magnitude and grip aperture interaction. NeuroReport. 2004;15:2773–2777. [PubMed] [Google Scholar]
- Andres M., Ostry D.J., Nicol F., Paus T. Time course of number magnitude interference during grasping. Cortex. 2008;44:414–419. doi: 10.1016/j.cortex.2007.08.007. doi:10.1016/j.cortex.2007.08.007 [DOI] [PubMed] [Google Scholar]
- Awh E., Armstrong K.M., Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn. Neurosci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. doi:10.1016/j.tics.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Badets A., Andres M., Di Luca S., Pesenti M. Number magnitude potentiates action judgements. Exp. Brain Res. 2007;180:525–534. doi: 10.1007/s00221-007-0870-y. doi:10.1007/s00221-007-0870-y [DOI] [PubMed] [Google Scholar]
- Basso G., Nichelli P., Frassinetti F., di Pellegrino G. Time perception in a neglected space. Neuroreport. 1996;7:2111–2114. doi: 10.1097/00001756-199609020-00009. doi:10.1097/00001756-199609020-00009 [DOI] [PubMed] [Google Scholar]
- Battelli L., Cavanagh P., Martini P., Barton J.J. Bilateral deficits of transient visual attention in right parietal patients. Brain. 2003;126:2164–2174. doi: 10.1093/brain/awg221. doi:10.1093/brain/awg221 [DOI] [PubMed] [Google Scholar]
- Battelli L., Pascual-Leone A., Cavanagh P. The ‘when’ pathway of the right parietal lobe. Trends Cogn. Sci. 2007;11:204–210. doi: 10.1016/j.tics.2007.03.001. doi:10.1016/j.tics.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli L., Walsh V., Pascual-Leone A., Cavanagh P. The ‘when’ parietal pathway explored by lesion studies. Curr. Opin. Neurobiol. 2008;18:120–126. doi: 10.1016/j.conb.2008.08.004. doi:10.1016/j.conb.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchio C., Bertone C. Time and neglect: abnormal temporal dynamics in unilateral spatial neglect. Neuropsychologia. 2006;44:2775–2782. doi: 10.1016/j.neuropsychologia.2006.06.011. doi:10.1016/j.neuropsychologia.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Beran M.J. Rhesus monkeys (Macaca mulatta) enumerate large and small sequentially presented sets of items using analog numerical representations. J. Exp. Psychol. Anim. Behav. Process. 2007;33:42–54. doi: 10.1037/0097-7403.33.1.42. doi:10.1007/s100710100098 [DOI] [PubMed] [Google Scholar]
- Beran M.J., Rumbaugh D.M. ‘Constructive’ enumeration by chimpanzees (Pan troglodytes) on a computerized task. Anim. Cogn. 2001;4:81–89. doi:10.1037/0097-7403.33.1.42 [Google Scholar]
- Bjoertomt O., Cowey A., Walsh V. Spatial neglect in near and far space investigated by repetitive transcranial magnetic stimulation. Brain. 2002;125:2012–2022. doi: 10.1093/brain/awf211. doi:10.1093/brain/awf211 [DOI] [PubMed] [Google Scholar]
- Boysen S.T., Berntson G.G., Shreyer T.A., Hannan M.B. Indicating acts during counting by a chimpanzee (Pan troglodytes) J. Comp. Psychol. 1995;109:47–51. doi: 10.1037/0735-7036.109.1.47. doi:10.1037/0735-7036.109.1.47 [DOI] [PubMed] [Google Scholar]
- Brannon E.M., Roitman J.D. Nonverbal representations of time and number in animals and human infants. In: Meck W.H., editor. Functional and neural mechanisms of interval timing. CRC Press; Boca Raton, FL: 2003. pp. 143–182. [Google Scholar]
- Brannon E.M., Lutz D., Cordes S. The development of area discrimination and its implications for number representation in infancy. Dev. Sci. 2006;9:59–64. doi: 10.1111/j.1467-7687.2006.00530.x. doi:10.1111/j.1467-7687.2006.00530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.W. Attentional resources in timing: interference effects in concurrent temporal and non temporal working memory tasks. Percept. Psychophys. 1997;5:1118–1140. doi: 10.3758/bf03205526. [DOI] [PubMed] [Google Scholar]
- Brozzoli C., Ishihara M., Göbel S.M., Salemme R., Rossetti Y., Farne A. Touch perception reveals the dominance of spatial over digital representation of numbers. Proc. Natl Acad. Sci. USA. 2008;14:5644–5648. doi: 10.1073/pnas.0708414105. doi:10.1073/pnas.0708414105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueti D., Walsh V., Frith C., Rees G. Different brain circuits underlie time processing for action and perception. J. Cogn. Neurosci. 2008a;20:204–214. doi: 10.1162/jocn.2008.20017. doi:10.1162/jocn.2008.20017 [DOI] [PubMed] [Google Scholar]
- Bueti D., Bahrami B., Walsh V. Sensory and association cortex in time perception. J. Cogn. Neurosci. 2008b;20:1054–1062. doi: 10.1162/jocn.2008.20060. doi:10.1162/jocn.2008.20060 [DOI] [PubMed] [Google Scholar]
- Burbaud P., Degreze P., Lafon P., Franconi J.M., Bouligand B., Bioulac B., Caille J.M., Allard M. Lateralization of prefrontal activation during internal mental calculation: a functional magnetic resonance imaging study. J. Neurophysiol. 1995;74:2194–2200. doi: 10.1152/jn.1995.74.5.2194. [DOI] [PubMed] [Google Scholar]
- Burr D.C., Ross J. A visual sense of number. Curr. Biol. 2008;18:425–428. doi: 10.1016/j.cub.2008.02.052. doi:10.1016/j.cub.2008.02.052 [DOI] [PubMed] [Google Scholar]
- Calabrese R.L. Motor networks: shifting coalitions. Curr. Biol. 2007;17:R139–R141. doi: 10.1016/j.cub.2006.12.007. doi:10.1016/j.cub.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Cantlon J.F., Brannon E.M. Shared system for ordering small and large numbers in monkeys and humans. Psychol. Sci. 2006;17:402–407. doi: 10.1111/j.1467-9280.2006.01719.x. doi:10.1111/j.1467-9280.2006.01719.x [DOI] [PubMed] [Google Scholar]
- Castronovo J., Seron X. Semantic numerical representation in blind subjects: the role of vision in the spatial format of the mental number line. Q. J. Exp. Psychol. 2007;60:101–119. doi: 10.1080/17470210600598635. doi:10.1080/17470210600598635 [DOI] [PubMed] [Google Scholar]
- Cavezian C., Rossetti Y., Danckert J., d'Amato T., Dalery J., Saoud M. Exaggerated leftward bias in the mental number line of patients with schizophrenia. Brain Cogn. 2007;63:85–90. doi: 10.1016/j.bandc.2006.07.007. doi:10.1016/j.bandc.2006.07.007 [DOI] [PubMed] [Google Scholar]
- Church R.M., Meck W.H. The numerical attribute of stimuli. In: Roitblat H.L., Beaver T.G., Terrace H.S., editors. Animal cognition. Erlbaum; Hillsdale, NJ: 1984. pp. 445–464. [Google Scholar]
- Cipolotti L., Butterworth B., Denes G. A specific deficit for numbers in a case of dense acalculia. Brain. 1991;114:2619–2637. doi: 10.1093/brain/114.6.2619. doi:10.1093/brain/114.6.2619 [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh, R. & Walsh, V. In press. Numerical representation in the parietal lobes: abstract or not abstract? Behav. Brain Sci. [DOI] [PubMed]
- Cohen Kadosh R., et al. Are numbers special? The comparison systems of the human brain investigated by fMRI. Neuropsychologia. 2005;43:1238–1248. doi: 10.1016/j.neuropsychologia.2004.12.017. doi:10.1016/j.neuropsychologia.2004.12.017 [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R., Cohen Kadosh K., Schuhmann T., Kaas A., Goebel R., Henik A., Sack A.T. Virtual dyscalculia induced by parietal-lobe TMS impairs automatic magnitude processing. Curr. Biol. 2007a;17:689–693. doi: 10.1016/j.cub.2007.02.056. doi:10.1016/j.cub.2007.02.056 [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R., Cohen Kadosh K., Linden D.E.J., Gevers W., Berger A., Henik A. The brain locus of interaction between number and size: a combined functional magnetic resonance imaging and event-related potential study. J. Cogn. Neurosci. 2007b;19:957–970. doi: 10.1162/jocn.2007.19.6.957. doi:10.1162/jocn.2007.19.6.957 [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R., Henik A., Walsh V. Small is bright and big is dark in synaesthesia. Curr. Biol. 2007c;17:R834–R835. doi: 10.1016/j.cub.2007.07.048. doi:10.1016/j.cub.2007.07.048 [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R., Cohen Kadosh K., Henik A. The neuronal correlate of bidirectional synesthesia: A combined event-related potential and functional magnetic resonance imaging study. J. Cogn. Neurosci. 2007d;19:2050–2059. doi: 10.1162/jocn.2007.19.12.2050. doi:10.1162/jocn.2007.19.12.2050 [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R., Lammertyn J., Izard V. Are numbers special? An overview of chronometric, neuroimaging, developmental and comparative studies of magnitude representation. Prog. Neurobiol. 2008a;84:132–147. doi: 10.1016/j.pneurobio.2007.11.001. doi:10.1016/j.pneurobio.2007.11.001 [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R., Cohen Kadosh K., Henik A. When brightness counts: the neuronal correlate of numerical-luminance interference. Cereb. Cortex. 2008b;18:337–343. doi: 10.1093/cercor/bhm058. doi:10.1093/cercor/bhm058 [DOI] [PubMed] [Google Scholar]
- Conson M., Cinque F., Barbarulo A.M., Trojano L. A common processing system for duration, order and spatial information: evidence from a time estimation task. Exp. Brain Res. 2008;187:267–274. doi: 10.1007/s00221-008-1300-5. doi:10.1007/S00221-008-1300-5 [DOI] [PubMed] [Google Scholar]
- Coull J.T., Nobre A.C. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J. Neurosci. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J.T., Walsh V., Frith C.D., Nobre A.C. Distinct neural substrates for visual search amongst spatial and temporal distractors. Cogn. Brain Res. 2003;17:358–367. doi: 10.1016/s0926-6410(03)00138-1. doi:10.1016/S0926-6410(03)00138-1 [DOI] [PubMed] [Google Scholar]
- Coull J.T., Vidal F., Nazarian B., Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–1508. doi: 10.1126/science.1091573. doi:10.1126/science.1091573 [DOI] [PubMed] [Google Scholar]
- Critchley M. Hafner Press; New York, NY: 1953. The parietal lobes. [Google Scholar]
- Danckert J., Ferber S., Pun C., Broderick C., Striemer C., Rock S., Stewart D. Neglected time: impaired temporal perception of multisecond intervals in unilateral neglect. J. Cogn. Neurosci. 2007;19:1706–1720. doi: 10.1162/jocn.2007.19.10.1706. doi:10.1162/jocn.2007.19.10.1706 [DOI] [PubMed] [Google Scholar]
- Diester I., Nieder A. Semantic associations between signs and numerical categories in the prefrontal cortex. PLoS Biol. 2007;5:e294. doi: 10.1371/journal.pbio.0050294. doi:10.1371/journal.pbio.0050294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormal V., Pesenti M. Numerosity-length interference—a stroop experiment. Exp. Psychol. 2007;54:289–297. doi: 10.1027/1618-3169.54.4.289. doi:10.1027/1618-3169.54.4.289 [DOI] [PubMed] [Google Scholar]
- Dormal V., Seron X., Pesenti M. Numerosity-duration interference: a stroop experiment. Acta Psychol. 2006;121:109–124. doi: 10.1016/j.actpsy.2005.06.003. doi:10.1016/j.actpsy.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Dormal V., Andres M., Pesenti M. Dissociation of numerosity and duration processing in the left intraparietal sulcus: a transcranial magnetic stimulation study. Cortex. 2008;44:462–469. doi: 10.1016/j.cortex.2007.08.011. doi:10.1016/j.cortex.2007.08.011 [DOI] [PubMed] [Google Scholar]
- Feeney A., Scrafton S., Duckworth A., Handley S.J. The story of some: everyday pragmatic inference by children and adults. Can. J. Exp. Psychol. 2004;58:121–132. doi: 10.1037/h0085792. doi:10.1037/h0085792 [DOI] [PubMed] [Google Scholar]
- Ferrandez A.M., Hugueville L., Lehéricy S., Poline J.B., Marsault C., Pouthas V. Basal ganglia and supplementary motor area subtend duration perception: an fMRI study. NeuroImage. 2003;19:1532–1544. doi: 10.1016/s1053-8119(03)00159-9. doi:10.1016/S1053-8119(03)00159-9 [DOI] [PubMed] [Google Scholar]
- Fias W., Lauwereyns J., Lammertyn J. Irrelevant digits affect feature-based attention depending on the overlap of neural circuits. Cogn. Brain Res. 2001;12:415–423. doi: 10.1016/s0926-6410(01)00078-7. doi:10.1016/S0926-6410(01)00078-7 [DOI] [PubMed] [Google Scholar]
- Fischer R., Miller J. Does the semantic activation of quantity representations influence motor parameters? Exp. Brain Res. 2008;189:379–391. doi: 10.1007/s00221-008-1434-5. doi:10.1007/s00221-008-1434-5 [DOI] [PubMed] [Google Scholar]
- Fischer M.H., Castel A.D., Dodd M.D., Pratt J. Perceiving numbers causes spatial shifts of attention. Nat. Neurosci. 2003;6:555–556. doi: 10.1038/nn1066. doi:10.1038/nn1066 [DOI] [PubMed] [Google Scholar]
- Genovesio A., Tsujimoto S., Wise S.P. Neuronal activity related to elapsed time in prefrontal cortex. J. Neurophysiol. 2006;95:3281–3285. doi: 10.1152/jn.01011.2005. doi:10.1152/jn.01011.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasauer S., Schneider E., Grasso R., Ivanenko Y.P. Space–time relativity in self-motion reproduction. J. Neurophsyiol. 2007;97:451–461. doi: 10.1152/jn.01243.2005. doi:10.1152/jn.01243.2005 [DOI] [PubMed] [Google Scholar]
- Göbel S.M., Walsh V., Rushworth M. The mental number line: a TMS study. NeuroImage. 2001;14:1278–1289. doi: 10.1006/nimg.2001.0927. doi:10.1006/nimg.2001.0927 [DOI] [PubMed] [Google Scholar]
- Göbel S.M., Johansen-Berg H., Behrens T., Rushworth M.F. Response-selection-related parietal activation during number comparison. J. Cogn. Neurosci. 2004;16:1536–1551. doi: 10.1162/0898929042568442. doi:10.1162/0898929042568442 [DOI] [PubMed] [Google Scholar]
- Göbel S.M., Calabria M., Farne A., Rossetti Y. Parietal rTMS distorts the mental number line: simulating ‘spatial’ neglect in healthy subjects. Neuropsychologia. 2006a;44:860–868. doi: 10.1016/j.neuropsychologia.2005.09.007. doi:10.1016/j.neuropsychologia.2005.09.007 [DOI] [PubMed] [Google Scholar]
- Göbel S.M., Rushworth M.F.S., Walsh V. Inferior parietal rTMS affects performance in an addition task. Cortex. 2006b;42:774–781. doi: 10.1016/s0010-9452(08)70416-7. doi:10.1016/S0010-9452(08)70416-7 [DOI] [PubMed] [Google Scholar]
- Halberda J., Sires S.F., Feigenson L. Multiple spatially overlapping sets can be enumerated in parallel. Psychol. Sci. 2006;17:572–576. doi: 10.1111/j.1467-9280.2006.01746.x. doi:10.1111/j.1467-9280.2006.01746.x [DOI] [PubMed] [Google Scholar]
- Halligan P.W., Marshall J.C. Left neglect for near but not far space in man. Nature. 1991;350:498–500. doi: 10.1038/350498a0. doi:10.1038/350498a0 [DOI] [PubMed] [Google Scholar]
- Harrington D.L., Haaland K.Y., Knight R.T. Cortical networks underlying mechanisms of time perception. J. Neurosci. 1998;18:1085–1095. doi: 10.1523/JNEUROSCI.18-03-01085.1998. doi:10.1038/nn0901-860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M.D., Carey S., Hauser L.B. Spontaneous number representation in semi-free-ranging rhesus monkeys. Proc. R. Soc. Lond. B. 2000;267:829–833. doi: 10.1098/rspb.2000.1078. doi:10.1098/rspb.2000.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A., Macizo P., Semenza C. The role of working memory in the association between number magnitude and space. Acta Psychol. 2008;128:225–237. doi: 10.1016/j.actpsy.2008.01.002. doi:10.1016/j.actpsy.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Hurewitz F., Gelman R., Schnitzer B. Sometimes area counts more than number. Proc. Natl Acad. Sci. USA. 2006;103:19599–19604. doi: 10.1073/pnas.0609485103. doi:10.1073/pnas.0609485103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M., Keller P.E., Rossetti Y., Prinz W. Horizontal spatial representations of time: evidence for the STEARC effect. Cortex. 2008;44:454–461. doi: 10.1016/j.cortex.2007.08.010. doi:10.1016/j.cortex.2007.08.010 [DOI] [PubMed] [Google Scholar]
- Janssen P., Shadlen M.N. A representation of the hazard rate of elapsed time in macaque area LIP. Nat. Neurosci. 2005;8:234–241. doi: 10.1038/nn1386. doi:10.1038/nn1386 [DOI] [PubMed] [Google Scholar]
- Juan C.-H., Shorter-Jacobi S.M., Schall J.D. Dissociation of spatial attention and saccade preparation. Proc. Natl Acad. Sci. USA. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. doi:10.1073/pnas.0403507101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan C.-H., Muggleton N.G., Tzeng O.J., Hung D.L., Cowey A., Walsh V. Segregation of visual selection and saccades in human frontal eye fields. Cereb. Cortex. 2008;18:2410–2415. doi: 10.1093/cercor/bhn001. doi:10.1093/cercor/bhn001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansaku K., Carver B., Johnson A., Matsuda K., Sadato N., Hallett M. The role of the human ventral premotor cortex in counting successive stimuli. Exp. Brain Res. 2007;178:339–350. doi: 10.1007/s00221-006-0736-8. doi:10.1007/s00221-006-0736-8 [DOI] [PubMed] [Google Scholar]
- Kaufmann L., Nuerk H.C. Interference effects in a numerical stroop paradigm in 9- to 12-year-old children with ADHD-C. Child Neuropsychology. 2006;12:223–243. doi: 10.1080/09297040500477483. doi:10.1080/09297040500477483 [DOI] [PubMed] [Google Scholar]
- Kaufmann L., Koppelstaetter F., Delazer M., Siedentopf C., Rhomberg P., Golaszewski S., Felber S., Ischebeck A. Neural correlates of distance and congruity effects in a numerical Stroop task: an event-related fMRI study. Neuroimage. 2005;25:888–898. doi: 10.1016/j.neuroimage.2004.12.041. doi:10.1016/j.neuroimage.2004.12.041 [DOI] [PubMed] [Google Scholar]
- Kaufmann L., Vogel S.E., Wood G., Kremser C., Schocke M., Zimmerhackl L.B., Koten J.W. A developmental fMRI study of nonsymbolic numerical and spatial processing. Cortex. 2008;44:376. doi: 10.1016/j.cortex.2007.08.003. doi:10.1016/j.cortex.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Kemmerer D. The spatial and temporal meanings of English prepositions can be independently impaired. Neuropsychologia. 2005;43:797–806. doi: 10.1016/j.neuropsychologia.2004.06.025. doi:10.1016/j.neuropsychologia.2004.06.025 [DOI] [PubMed] [Google Scholar]
- Knops A., Nuerk H.C., Sparing R., Foltys H., Willmes K. On the functional role of human parietal cortex in number processing: how gender mediates the impact of a ‘virtual lesion’ induced by rTMS. Neuropsychologia. 2006;44:2270–2283. doi: 10.1016/j.neuropsychologia.2006.05.011. doi:10.1016/j.neuropsychologia.2006.05.011 [DOI] [PubMed] [Google Scholar]
- Koch G., Oliveri M., Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Phil. Trans. R. Soc. B. 2009;364:1907–1918. doi: 10.1098/rstb.2009.0018. doi:10.1098/rstb.2009.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M.I., Shadlen M.N. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. doi:10.1016/S0896-6273(03)00185-5 [DOI] [PubMed] [Google Scholar]
- Levin I. The development of time concepts in young children. Reasoning about duration. Child Dev. 1977;48:435–444. doi:10.2307/1128636 [PubMed] [Google Scholar]
- Levin I. Interference of time related and unrelated cues with duration comparisons of young children: analysis of piaget's formulation of the relation of time and speed. Child Dev. 1979;50:469–477. doi:10.2307/1129425 [PubMed] [Google Scholar]
- Levin I. The nature and development of time concepts in children. The effects of interfering cues. In: Friedman W.J., editor. The developmental psychology of time. Academic Press; New York, NY: 1982. pp. 47–85. [Google Scholar]
- Lewis P.A., Miall R.C. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia. 2003;41:1583–1592. doi: 10.1016/s0028-3932(03)00118-0. doi:10.1016/S0028-3932(03)00118-0 [DOI] [PubMed] [Google Scholar]
- Lewis P.A., Miall R.C. A right hemispheric prefrontal system for cognitive time measurement. Behav. Process. 2006a;71:226–234. doi: 10.1016/j.beproc.2005.12.009. doi:10.1016/j.beproc.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Lewis P.A., Miall R.C. Remembering the time: a continuous clock. Trends Cogn. Sci. 2006b;10:401–406. doi: 10.1016/j.tics.2006.07.006. doi:10.1016/j.tics.2006.07.006 [DOI] [PubMed] [Google Scholar]
- Lindemann O., Abolafia J.A., Girardi G., Bekkering H. Getting a grip on numbers: numerical magnitude priming in object grasping. J. Exp. Psychol. Hum. Percept. Perform. 2007;33:1400–1409. doi: 10.1037/0096-1523.33.6.1400. doi:10.1037/0096-1523.33.6.1400 [DOI] [PubMed] [Google Scholar]
- Liu X., Wang H., Corbly C.R., Zhang J., Joseph J.E. The involvement of the inferior parietal cortex in the numerical Stroop effect and the distance effect in a two-digit number comparison task. J. Cogn Neurosci. 2006;18:1518–1530. doi: 10.1162/jocn.2006.18.9.1518. doi:10.1162/jocn.2006.18.9.1518 [DOI] [PubMed] [Google Scholar]
- Macar F., Lejeune H., Bonnet M., Ferrara A., Pouthas V., Vidal F., Maquet P. Activation of the supplementary motor area and of attentional networks during temporal processing. Exp. Brain Res. 2002;142:475–485. doi: 10.1007/s00221-001-0953-0. doi:10.1007/s00221-001-0953-0 [DOI] [PubMed] [Google Scholar]
- Maquet P., et al. Brain activation induced by estimation of duration: a PET study. Neuroimage. 1996;3:119–126. doi: 10.1006/nimg.1996.0014. doi:10.1006/nimg.1996.0014 [DOI] [PubMed] [Google Scholar]
- Milner D., Goodale M. Oxford University Press; Oxford, UK: 2006. The visual brain in action. [Google Scholar]
- Miniussi C., Wilding E., Coull J.T., Nobre A.C. Orienting attention in time: modulation of brain potentials. Brain. 1999;122:1507–1518. doi: 10.1093/brain/122.8.1507. doi:10.1093/brain/122.8.1507 [DOI] [PubMed] [Google Scholar]
- Moretto G., di Pellegrino G. Grasping numbers. Exp. Brain Res. 2008;188:505–515. doi: 10.1007/s00221-008-1386-9. doi:10.1007/s00221-008-1386-9 [DOI] [PubMed] [Google Scholar]
- Muller D., Schwarz W. ‘1-2-3’: is there a temporal number line? Evidence from a serial comparison task. Exp. Psychol. 2008;55:143–150. doi: 10.1027/1618-3169.55.3.143. doi:10.1027/1618-3169.55.3.143 [DOI] [PubMed] [Google Scholar]
- Murofushi K. Numerical matching behavior by a Chimpanzee (Pan troglodytes): subitizing and analogue magnitude estimation. Jpn Psychol. Res. 2002;39:140–153. doi:10.1111/1468-5884.00050 [Google Scholar]
- Nieder A. The number domain—can we count on parietal cortex? Neuron. 2004;44:407–409. doi: 10.1016/j.neuron.2004.10.020. doi:10.1016/j.neuron.2004.10.020 [DOI] [PubMed] [Google Scholar]
- Nieder A. Counting on neurons: the neurobiology of numerical competence. Nat. Rev. Neurosci. 2005;6:177–190. doi: 10.1038/nrn1626. doi:10.1038/nrn1626 [DOI] [PubMed] [Google Scholar]
- Nieder A., Miller E.K. Coding of cognitive magnitude: compressed scaling of numerical information in the primate prefrontal cortex. Neuron. 2003;37:149–157. doi: 10.1016/s0896-6273(02)01144-3. doi:10.1016/S0896-6273(02)01144-3 [DOI] [PubMed] [Google Scholar]
- Nieder A., Miller E.K. A parieto-frontal network for visual numerical information in the monkey. Proc. Natl Acad. Sci. USA. 2004;101:7457–7462. doi: 10.1073/pnas.0402239101. doi:10.1073/pnas.0402239101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder A., Freedman D.J., Miller E.K. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297:1708–1711. doi: 10.1126/science.1072493. doi:10.1126/science.1072493 [DOI] [PubMed] [Google Scholar]
- Nieder A., Diester I., Tuduscius O. Temporal and spatial enumeration processes in the primate parietal cortex. Science. 2006;313:1431–1435. doi: 10.1126/science.1130308. doi:10.1126/science.1130308 [DOI] [PubMed] [Google Scholar]
- Niemeier M., Stojanoski B., Greco A.L. Influences of time and spatial frequency on the perceptual bias: evidence for competition between hemispheres. Neuropsychologia. 2007;45:1029–1040. doi: 10.1016/j.neuropsychologia.2006.09.006. doi:10.1016/j.neuropsychologia.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Nishida S., Johnston A. Marker correspondence, not processing latency, determines temporal binding of visual attributes. Curr. Biol. 2002;12:359–368. doi: 10.1016/s0960-9822(02)00698-x. doi:10.1016/S0960-9822(02)00698-X [DOI] [PubMed] [Google Scholar]
- Notebaert W., Gevers W., Verguts T., Fias W. Shared spatial representations for numbers and space: the reversal of the SNARC and the Simon effects. J. Exp. Psychol. Hum. Percept. Perform. 2006;32:1197–1207. doi: 10.1037/0096-1523.32.5.1197. doi:10.1037/0096-1523.32.5.1197 [DOI] [PubMed] [Google Scholar]
- Oliveri M., Rausei V., Koch G., Torriero S., Turriziani P., Caltagirone C. Overestimation of numerical distances in the left side of space. Neurology. 2004;63:2139–2141. doi: 10.1212/01.wnl.0000145975.58478.6d. [DOI] [PubMed] [Google Scholar]
- Oliveri M., Vicario C.M., Salerno S., Koch G., Turriziani P., Mangano R., Chillemi G., Caltagirone C. Perceiving numbers alters time perception. Neurosci. Lett. 2008;438:308–311. doi: 10.1016/j.neulet.2008.04.051. doi:10.1016/j.neulet.2008.04.051 [DOI] [PubMed] [Google Scholar]
- Onoe H., Komori M., Onoe K., Takechi H., Tsukada H., Watanabe Y. Cortical networks recruited for time perception: a monkey positron emission tomography (PET) study. Neuroimage. 2001;13:37–45. doi: 10.1006/nimg.2000.0670. doi:10.1006/nimg.2000.0670 [DOI] [PubMed] [Google Scholar]
- Pinel P., Piazza M., Le Bihan D., Dehaene S. Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron. 2004;41:983–993. doi: 10.1016/s0896-6273(04)00107-2. doi:10.1016/S0896-6273(04)00107-2 [DOI] [PubMed] [Google Scholar]
- Pochon J.-P., Levy R., Poline J.-P., Crozier S., Lehéricy S., Pillon B., Deweer B., Le Bihan D., Dubois B. The role of dorsolateral prefrontal cortex in the preparation of forthcoming actions: an fMRI study. Cereb. Cortex. 2001;11:260–266. doi: 10.1093/cercor/11.3.260. doi:10.1093/cercor/11.3.260 [DOI] [PubMed] [Google Scholar]
- Pouthas V., et al. Neural network involved in time perception: an fMRI study comparing long and short interval estimation. Hum. Brain Mapp. 2005;25:433–441. doi: 10.1002/hbm.20126. doi:10.1002/hbm.20126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.M., Mayer A.R., Harrington D.L. The evolution of brain activation during temporal processing. Nat. Neurosci. 2001;4:317–323. doi: 10.1038/85191. doi:10.1038/85191 [DOI] [PubMed] [Google Scholar]
- Rickard T.C., Romero S.G., Basso G., Wharton C., Flitman S., Grafman J. The calculating brain: an fMRI study. Neuropsychologia. 2000;38:325–335. doi: 10.1016/s0028-3932(99)00068-8. doi:10.1016/S0028-3932(99)00068-8 [DOI] [PubMed] [Google Scholar]
- Roitman J.D., Brannon E.N., Andrews J.R., Platt M.L. Nonverbal representation of time and number in adults. Acta Psychol. 2007a;124:296–318. doi: 10.1016/j.actpsy.2006.03.008. doi:10.1016/j.actpsy.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Roitman J.D., E M., Brannon E.M., Platt M. Monotonic coding of numerosity in macaque lateral intraparietal area. PLoS Biol. 2007b;5:e208. doi: 10.1371/journal.pbio.0050208. doi:10.1371/journal.pbio.0050208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle L., Noel M.P. Basic numerical skills in children with mathematics learning disabilities: a comparison of symbolic vs non-symbolic number magnitude processing. Cognition. 2007;102:361–395. doi: 10.1016/j.cognition.2006.01.005. doi:10.1016/j.cognition.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Rousselle L., Noel M.P. The development of automatic numerosity processing in preschoolers: evidence for numerosity-perceptual interference. Dev. Psychol. 2008;44:544–560. doi: 10.1037/0012-1649.44.2.544. doi:10.1037/0012-1649.44.2.544 [DOI] [PubMed] [Google Scholar]
- Rumbaugh D.M., Savage-Rumbaugh S., Hegel M.T. Summation in the chimpanzee (Pan troglodytes) J. Exp. Psychol. Anim. Behav. Process. 1987;13:107–115. doi:10.1037/0097-7403.13.2.107 [PubMed] [Google Scholar]
- Rusconi E., Walsh V., Butterworth B. Dexterity with numbers: rTMS over left angular gyrus disrupts finger gnosis and number processing. Neuropsychologia. 2005;43:1609–1624. doi: 10.1016/j.neuropsychologia.2005.01.009. doi:10.1016/j.neuropsychologia.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Salillas E., El Yagoubi R., Semenza C. Sensory and cognitive processes of shifts of spatial attention induced by numbers: an ERP study. Cortex. 2008;44:406–413. doi: 10.1016/j.cortex.2007.08.006. doi:10.1016/j.cortex.2007.08.006 [DOI] [PubMed] [Google Scholar]
- Sawamura H., Shima K., Tanji J. Numerical representation for action in the parietal cortex of the monkey. Nature. 2002;415:918–922. doi: 10.1038/415918a. doi:10.1038/415918a [DOI] [PubMed] [Google Scholar]
- Sawamura H., Orban G.A., Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the fMRI adaptation paradigm. Neuron. 2006;49:307–318. doi: 10.1016/j.neuron.2005.11.028. doi:10.1016/j.neuron.2005.11.028 [DOI] [PubMed] [Google Scholar]
- Schall J.D. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. doi:10.1016/j.visres.2003.10.025 [DOI] [PubMed] [Google Scholar]
- Schluter N., Krams M., Rushworth M.F.S., Passingham R.E. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. doi:10.1016/S0028-3932(00)00105-6 [DOI] [PubMed] [Google Scholar]
- Schubotz R.I., Friederici A.D., Von Cramon D.Y. Time perception and motor timing: a common cortical and subcortical basis revealed by fMRI. Neuroimage. 2000;11:1–12. doi: 10.1006/nimg.1999.0514. doi:10.1006/nimg.1999.0514 [DOI] [PubMed] [Google Scholar]
- Shamma S. On the role of space and time in auditory processing. Trends Cogn. Sci. 2001;5:340–348. doi: 10.1016/s1364-6613(00)01704-6. doi:10.1016/S1364-6613(00)01704-6 [DOI] [PubMed] [Google Scholar]
- Shapiro K., Hillstrom A., Husain M. Control of visuotemporal attention by inferior parietal and superior temporal cortex. Curr. Biol. 2002;12:1320–1325. doi: 10.1016/s0960-9822(02)01040-0. doi:10.1016/S0960-9822(02)01040-0 [DOI] [PubMed] [Google Scholar]
- Stavy R., Tirosh D. Teachers College Press, Columbia University; New York, London, UK: 2000. How students (mis-) understand science, mathematics: intuitive rules. [Google Scholar]
- Stoianov I., Kramer P., Umilta C., Zorzi M. Visuospatial priming of the mental number line. Cognition. 2008;106:770–779. doi: 10.1016/j.cognition.2007.04.013. doi:10.1016/j.cognition.2007.04.013 [DOI] [PubMed] [Google Scholar]
- Sur M., Leamey C.A. Development, plasticity of cortical areas and networks. Nat. Rev. Neurosci. 2001;2:251–262. doi: 10.1038/35067562. doi:10.1038/35067562 [DOI] [PubMed] [Google Scholar]
- Taylor-Cooke P.A., Ricci R., Baños J.H., Zhou X., Woods A.J., Mennemeier M.S. Perception of motor strength and stimulus magnitude are correlated in stroke patients. Neurology. 2006;66:1444–1456. doi: 10.1212/01.wnl.0000210489.92317.15. doi:10.1212/01.wnl.0000210489.92317.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudusciuc O., Nieder A. Neuronal population coding of continuous and discrete quantity in the primate posterior parietal cortex. Proc. Natl Acad. Sci. USA. 2007;104:14513–14518. doi: 10.1073/pnas.0705495104. doi:10.1073/pnas.0705495104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallesi A., Binns M.A., Shallice T. An effect of spatial–temporal association of response codes: understanding the cognitive representations of time. Cognition. 2008;107:501–527. doi: 10.1016/j.cognition.2007.10.011. doi:10.1016/j.cognition.2007.10.011 [DOI] [PubMed] [Google Scholar]
- van Marle K., Wynn K. Six-month-old infants use analog magnitudes to represent duration. Dev. Sci. 2006;9:F41–F49. doi: 10.1111/j.1467-7687.2006.00508.x. doi:10.1111/j.1467-7687.2006.00508.x [DOI] [PubMed] [Google Scholar]
- Van Vleet T.M., Robertson L.C. Cross-modal interactions in time and space: auditory influence on visual attention in hemispatial neglect. J. Cogn. Neurosci. 2006;18:1368–1379. doi: 10.1162/jocn.2006.18.8.1368. doi:10.1162/jocn.2006.18.8.1368 [DOI] [PubMed] [Google Scholar]
- von Kriegstein K., Smith D.R.R., Patterson R.D., Ives D.T., Griffiths T.D. Neural representation of auditory size in the human voice and in sounds from other resonant sources. Curr. Biol. 2007;17:1123–1128. doi: 10.1016/j.cub.2007.05.061. doi:10.1016/j.cub.2007.05.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V. A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn. Sci. 2003a;7:483–488. doi: 10.1016/j.tics.2003.09.002. doi:10.1016/j.tics.2003.09.002 [DOI] [PubMed] [Google Scholar]
- Walsh V. Time: the back-door of perception. Trends Cogn. Sci. 2003b;7:335–338. doi: 10.1016/s1364-6613(03)00166-9. doi:10.1016/S1364-6613(03)00166-9 [DOI] [PubMed] [Google Scholar]
- Woods A.J., Mermemeier M., Garcia-Rill E., Meythaler J., Mark V.W., Jewel G.R., Murphy H. Bias in magnitude estimation following left hemisphere injury. Neuropsychologia. 2006;44:1406–1412. doi: 10.1016/j.neuropsychologia.2005.12.006. doi:10.1016/j.neuropsychologia.2005.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan B., Zhang D., He S., Chen X.C. Larger stimuli are judged to last longer. J. Vis. 2007;7:1–5. doi: 10.1167/7.10.2. doi:10.1167/7.10.2 [DOI] [PubMed] [Google Scholar]
- Zach P., Brugger P. Subjective time in near and far representational space. Cogn. Behav. Neurol. 2008;21:8–13. doi: 10.1097/WNN.0b013e31815f237c. doi:10.1097/WNN.0b013e31815f237c [DOI] [PubMed] [Google Scholar]
- Zamarian L., Egger C., Delazer M. The mental representation of ordered sequences in visual neglect. Cortex. 2007;43:542–550. doi: 10.1016/s0010-9452(08)70248-x. doi:10.1016/S0010-9452(08)70248-X [DOI] [PubMed] [Google Scholar]
- Zhou X., et al. Neural substrates for forward and backward recitation of numbers and the alphabet: a close examination of the role of intraparietal sulcus and perisylvian areas. Brain Res. 2006;1099:109–120. doi: 10.1016/j.brainres.2006.01.133. doi:10.1016/j.brainres.2006.01.133 [DOI] [PubMed] [Google Scholar]