Abstract
Background
C60 fullerenes and single-walled carbon nanotubes (SWCNT) are projected to be used in medicine and consumer products with potential human exposure. The hazardous effects of these particles are expected to involve oxidative stress with generation of oxidatively damaged DNA that might be the initiating event in the development of cancer.
Objective
In this study we investigated the effect of a single oral administration of C60 fullerenes and SWCNT.
Methods
We measured the level of oxidative damage to DNA as the premutagenic 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) in the colon mucosa, liver, and lung of rats after intragastric administration of pristine C60 fullerenes or SWCNT (0.064 or 0.64 mg/kg body weight) suspended in saline solution or corn oil. We investigated the regulation of DNA repair systems toward 8-oxodG in liver and lung tissue.
Results
Both doses of SWCNT increased the levels of 8-oxodG in liver and lung. Administration of C60 fullerenes increased the hepatic level of 8-oxodG, whereas only the high dose generated 8-oxodG in the lung. We detected no effects on 8-oxodG in colon mucosa. Suspension of particles in saline solution or corn oil yielded a similar extent of genotoxicity, whereas corn oil per se generated more genotoxicity than the particles. Although there was increased mRNA expression of 8-oxoguanine DNA glycosylase in the liver of C60 fullerene-treated rats, we found no significant increase in repair activity.
Conclusions
Oral exposure to low doses of C60 fullerenes and SWCNT is associated with elevated levels of 8-oxodG in the liver and lung, which is likely to be caused by a direct genotoxic ability rather than an inhibition of the DNA repair system.
Keywords: cancer, DNA damage, DNA repair, nanoparticle, oxidative stress
Humans have been exposed to particulate matter during much of evolution, but technologic achievements such as combustion engines and nanotechnology have yielded unique types of particles. Engineered nanotechnologic materials are projected to be used, for instance, in electronics, cosmetics, cleaning materials, coatings, food packaging, and medicines, with increasing human exposure. In addition, consumer products containing nanomaterials will inevitably end up as waste, and subsequent processing or deposition may liberate particles to the environment, where they might accumulate along the food chain because some are highly persistent (Helland et al. 2007). Reinforced attention to the hazardous properties of engineered nanoparticles has been evoked by a report showing that a single intra peritoneal application of carbon nanotubes generated mesotheliomas in p53+/− mice (Takagi et al. 2008). These data are supported by another recent report showing that a single injection of carbon nanotubes into the peritoneal cavity of mice elicited an inflammatory reaction and granulomas at the peritoneal surface of the diaphragm (Poland et al. 2008). Similarly, pulmonary exposure to single-walled carbon nanotubes (SWCNT) produced granulomas in the lung of rodents (Lam et al. 2004; Shvedova et al. 2005; Warheit et al. 2004). It is possible that such nanotubes possess the same hazardous effects as other fibrous materials such as asbestos. On the other hand, particles such as C60 fullerenes might be less hazardous (Nielsen et al. 2008). For example, in a recent inhalation experiment, Baker et al. (2008) found that exposure to C60 fullerenes resulted in little pulmonary toxicity.
Generation of reactive oxygen species (ROS) and oxidative stress is generally accepted to be an important mechanism of action of nanoparticles (Ayres et al. 2008; Nel et al. 2006; Oberdörster et al. 2005). Exposure to combustion particles in urban air or diesel exhaust particles (DEP) is associated with oxidative stress, generated by particles themselves or by cell-mediated inflammatory responses and increased formation of oxidatively damaged DNA (Knaapen et al. 2004; Risom et al. 2005). 8-Oxo-7,8-dihydro-2′-deoxyguanosine-(8-oxodG) is considered to be an important oxidatively generated DNA lesion in carcinogenesis because a) it is mutagenic; b) mammalian cells have a highly versatile repair system for its removal; and c) the level of 8-oxodG is elevated in several types of tumor tissue (Evans et al. 2004). In addition, high urinary excretion of 8-oxodG is associated with increased risk of lung cancer among nonsmokers (Loft et al. 2006; Loft and Møller 2006). The relevance of 8-oxodG is further strengthened by observations that this lesion is elevated in animal tissues and human blood cells upon exposure to urban air pollution, diesel exhaust, or DEP (Møller et al. 2008). In particular, a single oral dose of DEP was associated with increased levels of 8-oxodG in liver, lung, and colon tissue (Danielsen et al. 2008b). Effect modification by the DNA repair system is likely to occur by continuous exposure. For example, ingestion of DEP in the diet for 3 weeks led to an up-regulation of the DNA repair system and unaltered levels of 8-oxodG in the liver and colon mucosa cells (Dybdahl et al. 2003), whereas measurements of lung tissue indicated unaltered regulation of the DNA repair system and elevated levels of oxidatively damaged DNA (Müller et al. 2004).
The aim of the present study was to investigate the effect of a single oral administration of C60 fullerenes and SWCNT. C60 fullerenes consist of 60 carbon atoms arranged in an aromatic soccer-ball structure with a nanosized diameter. SWCNT consists of two dimensions < 100 nm, whereas the axial dimension is much larger. Both C60 fullerenes and SWCNT are hydrophobic and hence difficult to suspend in saline solution, but they suspend more easily in oils. Consequently, rats received the particles in either saline solution or corn oil by oral gavage. We then assessed the level of 8-oxodG, a highly validated biomarker [European Standards Committee on Oxidative DNA Damage (ESCODD) et al. 2005]. We investigated alterations in the regulation of DNA repair by mRNA levels of 8-oxoguanine DNA glycosylase (OGG1), nei endonuclease VIII-like 1 (E. coli) (NEIL1), mutY homolog (E. coli) (MUTYH), and nudix (nucleoside diphosphate linked moiety X)-type motif 1 (NUDT1), which are involved in the removal of 8-oxodG in DNA and the nucleotide pool and may be up-regulated by oxidative stress (Hah et al. 2007; Risom et al. 2003a, 2007). We assessed the expression of heme oxygenase 1 (HO1) mRNA as a general marker of oxidative stress elicited by exposure to particulate matter (Ayres et al. 2008).
Materials and Methods
Particle exposure of animals
We obtained 84 female Fisher 344 rats from Taconic (Ry, Denmark). Animals were acclimatized for at least 1 week before entering the experiments. The rats were housed in a temperature- controlled (22–24°C) and moisture-controlled (40–70%) room with a 12-hr light/12-hr dark cycle. All animals had free access to tap water and Standard Altromin no. 1314 rat chow (Altromin, Lage, Germany) during the acclimation and housing/treatment periods. Rats were sacrificed at 9 weeks of age. Animals were treated humanely and with regard for alleviation of suffering. All animal procedures followed the guidelines for the care and handling of laboratory animals established by the Danish government, and the Animal Experiment Inspectorate, Ministry of Justice, approved the study (no. 2006/561-1161).
The dry powder of C60 fullerenes was described by the manufacturer to be a 99.9% pure preparation with a primary particle size of 0.7 nm (Sigma-Aldrich, Brøndby, Denmark). The dry powder of SWCNT was described by the manufacturer to have a primary particle size of 0.9–1.7 nm and a fiber length < 1 μm (Thomas Swan and Co Ltd, Consett, UK). We suspended the particles in either saline or corn oil (Sigma-Aldrich) by sonication at 70 W and 42 kHz (Branson 1510, VWR–Bie & Berntsen A/S, Herlev, Denmark) in a 5-day period for 10 hr each day and again 30 min before administration. For C60 fullerene and SWCNT, the gas exchange surface areas were < 20 m2/g and 731 ± 2 m2/g and the average pore sizes were 0 and 15 nm respectively (Jacobsen et al. 2008b). We used dynamic light scattering, as described by Jacobsen et al. (2008b), to measure the particle size in suspensions at the same concentration as administered to the rats by oral gavage. In general, it was difficult to determine the presence of nanoparticles in both types of solutions by dynamic light scattering because the solutions contain agglomerates with larger particle sizes. The particle sizes of C60 fullerenes in the saline solution were 407 nm in the low dose and 621 and 5,117 nm in the high dose. In saline solution, the particle sizes of SWCNT were 195, 797, and 5,457 nm (low dose); the particle size in the highest dose could not be determined by dynamic light scattering. The particles were easier to suspend in corn oil than in saline. We obtained size modes of the C60 fullerenes in corn oil by dynamic light scattering as follows: 234 nm (low dose); 40, 713, and 3,124 nm (high dose). The SWCNT had size modes as follows: 34 and 178 nm (low dose) and 1,015 nm (high dose). The SWCNT contained transition metals (2% iron and traces of cobalt, nickel, and manganese) and polycyclic aromatic hydrocarbons [PAHs; 417 ng/g of the U.S. Environmental Protection Agency (EPA) priority PAH compounds (U.S. EPA 1991)], whereas neither transition metals nor PAH could be detected in C60 fullerenes.
The rats received a single intragastric dose of the particle preparations by oral gavage (0.064 and 0.64 mg/kg body weight suspended in saline or corn oil; n = 8), saline solution (control; n = 10), or corn oil (control; n = 10). Each rat received 200 μL fluid. The rats were sacrificed by cervical dislocation 24 hr after intragastric administration. The liver, lung, and colon tissues were snap-frozen in liquid nitrogen and stored at −80°C. We investigated effects in these organs because they are along the likely local and systemic exposure route and we have data from similar experiments on DEP as Standard Reference Material 2975 (SRM2975; National Institute of Standards and Technology, Gaithersburg, MD, USA), which was previously reported to be associated with increased levels of DNA damage 24 hr after a single intragastric application (Danielsen et al. 2008b). For all experiments, rats from each group (0, 0.064, and 0.64 mg particles/kg body weight) were treated the same day.
ROS-generating ability
The ability of the C60 fullerenes, SWCNT, SRM2975, and Printex 90 carbon black (Degussa-Hüls, Frankfurt, Germany) to generate ROS in aqueous solution was determined by oxidation of 2′,7′-dichloro dihydrofluorescin (Molecular Probes, Portland, OR, USA). The particle suspensions were prepared in Hank’s balanced saline solution and sonicated immediately before incubation, as described previously by Jacobsen et al. (2008b). The oxidation product (2′,7′-dichlorofluorescein) was determined by fluorescence spectrometry with excitation at 490 nm and emission at 520 nm on a fluorescence spectrophotometer (Victor Wallac-2 1420; PerkinElmer, Hvidovre, Denmark).
Oxidatively damaged DNA
We obtained suspensions of colon epithelial cells by scraping off the cells on the luminal side of the colon with a glass slide in ice-cold Merchant buffer, as described previously by Dybdahl et al. (2003). The DNA was extracted from colon mucosa cells, liver, and lung tissue according to the procedure recommended by ESCODD et al. (2005). We isolated nuclei in buffer containing deferoxamine mesylate (Sigma-Aldrich) to prevent spurious oxidation; this was followed by lysis, RNase, and proteinase treatment (Sigma-Aldrich). We extracted DNA in buffer containing 40 mM Tris, 20 mM Na2EDTA, 7.6 M NaI, and 0.3 mM deferoxamine mesylate, pH 8.0 (Sigma-Aldrich), 2-propanol, and ethanol. We digested DNA extracts to nucleosides using nuclease P1 and alkaline phosphatase (Merck, Darmstadt, Germany) and measured 8-oxodG and dG by HPLC with electrochemical and ultraviolet detection, respectively.
mRNA expression of HO1, MUTYH, NEIL1, NUDT1, and OGG1
We purified total RNA from liver and lung tissue using TRIzol Reagent (Invitrogen, San Diego, CA, USA) and DNase treatment according to the manufacturer’s protocol (SV Total RNA isolation kit; Promega, Madison, WI, USA). For cDNA synthesis, 100–200 ng RNA was used in a reaction volume of 20 μL using GeneAmp RNA PCR (polymerase chain reaction) Kit (Applied Biosystems, Nærum, Denmark) as recommended by the manufacturer.
We analyzed mRNA levels of the following genes: HO1 [GeneID 24451 (National Center for Biotechnology Information 2008)], MUTYH (GeneID 170841), NEIL1 (GeneID 367090), NUDT1 (GeneID 117260), OGG1 (GeneID 81528) on a Taqman ABI7900 (Applied Biosystems) as described by Risom et al. (2003b). We used Taqman probes and primers MUTYH (Rn00591196), NEIL1 (Rn01422330), NUDT1 (Rn00589097), and Taqman 18S probe (Euk 18S rRNA FAM/MGB probemix) from Applied Biosystems. All probes and primers span exon junctions and are cDNA specific. We used oligonucleotides of HO1 and OGG1 as follows (final concentrations are shown in parentheses):
RnHO1 forward primer (900 nM), 10F: 5′-CCA CAG CTC GAC AGC ATG T-3′; reverse primer (900 nM), 159R: 5′-GGA GGC CAT CAC CAG CTT AAA-3′; Taqman probe (250 nM), 135T: 5′6-FAM-TTC CCT GGA CAC CTG ACC CTT CTG A-TAMRA-3′
RnOGG1 forward primer (900 nM), 401F: 5′-TGG CTC AGA AAT TCC AAG GTG T-3′; reverse primer (900 nM) 609R, 5′-TAC TTC TGG ACC AGC CAG GG-3′; Taqman probe (250 nM), 468T: 5′6-FAM-CTG TTC TTC CAA CAA CAA CAT TGC TCG C-TAMRA-3′.
For the PCR reactions, we mixed 4 μL of the cDNA preparation with master mix and water to a final volume of 228 μL. Aliquots were mixed with probes and primers to a final volume of 36 μL; and then transferred to three wells at 10 μL/well. For the PCR reaction, we used the following protocol: activation of the polymerase, 95°C for 20 sec; followed by 45 cycles of 95°C for 1 sec; and finally 60°C for 20 sec. We measured the expressions of reference (18S) and target genes in separate tubes. The expression levels are reported as target mRNA normalized to 18S, calculated by the comparative cycle threshold (Ct) method 2−Δ Ct. The SD of the triplicates was < 0.5 (based on Ct values). Each run included a standard (for verification of the PCR efficiency) and negative (no template) and positive controls.
OGG1 repair activity
We obtained extracts of liver tissue by using a plunger to force the tissue through a sieve in one end of a stainless steel cylinder (0.5 cm in diameter, mesh size 0.4 mm) while the cylinder was submerged in 2 mL ice-cold buffer (45 mM HEPES, 0.4 M KCl, 1 mM EDTA, 0.1 mM DTT, 10% glycerol, pH 7.8), as described previously (Folkmann et al. 2007). The protein concentrations of the liver tissue extracts were measured using the Coomassie Plus – The Better Bradford Assay Reagent as recommended by the manufacturer (Pierce, Rockford, IL, USA) using bovine serum albumin as standard. We diluted liver tissue extracts to a final protein concentration of 3 mg/mL.
We analyzed OGG1 repair activity as the number of incisions in substrate nuclei (Guarnieri et al. 2008). We prepared substrate nuclei containing 8-oxodG by treating THP-1 human monocyte cells (American Type Culture Collection, Manassas, VA, USA) with 1 μM Ro19-8022 in phosphate-buffered saline and irradiating them with white light for 4 min. The Ro19-8022 photosensitizer was a gift from F. Hoffmann-La Roche Ltd (Basel, Switzerland). Substrate nuclei were embedded in low-melting-point gel (0.75%; Sigma-Aldrich), applied onto GelBond films (Cambrex; Medinova Scientific A/S, Hellerup, Denmark), and lysed in ice-cold lysis solution (2.5 M NaCl, 0.1 mM Na2EDTA, 10 mM Tris, 1% Triton X-100, pH 10) for 1 hr at 4°C. We applied liver tissue extracts onto the gels and incubated them for 20 min at 37°C in a humid box. Negative controls (buffer) and positive controls (formamidopyrimidine DNA glycosylase; gift from A. Collins, University of Oslo, Oslo, Norway) were incubated for 45 min.
The GelBonds were subsequently treated in ice-cold alkaline solution (300 mM NaOH, 1 mM Na2EDTA, pH > 13.0) for 40 min and electrophoresed 20 min in the same solution at 25 V (0.83 V/cm) and 300 mA. The nuclei were visualized using fluorescence microscope (Olympus, Ballerup, Denmark) at 40× magnification after staining with the YOYO-1 fluorescent dye (Molecular Probes). We scored the level of DNA damage according to five classes of damage of 100 randomly selected nuclei from each sample; we examined two slides for each sample. The samples were coded before scoring. We determined the repair activity of the tissue extracts as the difference in score (arbitrary units) between parallel gels incubated with extract and control solution, respectively.
Statistics
We analyzed the results statistically by two-factor analysis of variance (ANOVA) using the dose of particles and vehicle as categorical variables. We found no interactions between the categorical factors; thus, the p-values correspond to single -factor effects. We tested the homogeneity of variance by Levene’s test. To achieve homogeneity of variance, we log10-transformed all results, except those for 8-oxodG in the lung. Statistically significant effects are reported as the percent increase with either 95% confidence intervals (CIs) or p-values of post hoc Fisher least statistical difference tests. The statistical analysis was carried out using Statistica for Windows Version 5.5 (StatSoft, Inc., Tulsa, OK, USA).
Results
ROS-generating ability
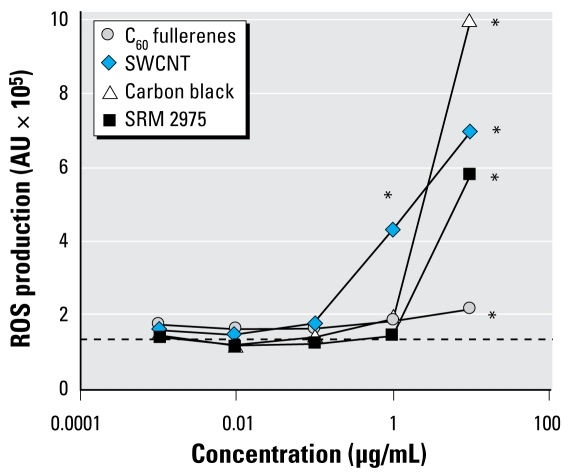
Figure 1 shows ROS generation of particles in aqueous solution. The ROS generation was 3.3-fold (95% CI, 2.1–4.5) and 5.5-fold (95% CI, 4.2–6.6) in incubations containing SWCNT at 1 and 10 μg/mL, respectively. The C60 fullerenes increased ROS production at the highest dose by 1.6-fold (95% CI, 1.5–1.9). SRM2975 and carbon black increased the level of ROS production by 4.4-fold (95% CI, 4.3–4.5) and 7.6-fold (95% CI, 7.4–7.9), respectively, at 10 μg/mL (Figure 1).
Figure 1.
ROS production [in arbitrary units (AU)] by C60 fullerenes, SWCNT, Printex 90 carbon black, and SRM2975 in Hank’s balanced saline solution detected as 2′,7′-dichlorofluorescein. Each point represents the mean ROS production of duplicate replicates in two independent experiments; the dotted line represents incubations without particles (quadruplicate in two independent experiments).
*p < 0.05 compared with incubations without particles.
Oxidatively damaged DNA
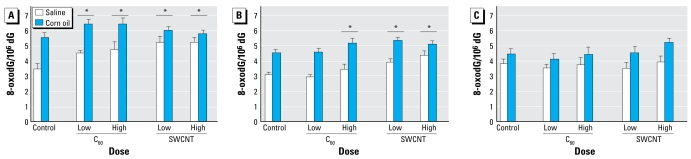
Figure 2 shows the level of 8-oxodG in colon, liver, and lung tissue of rats administered C60 fullerenes and SWCNT dispersed in either saline or corn oil. We observed significant single-factor effects of corn oil, which was associated with 25% (95% CI, 12–40), 30% (95% CI, 20–40), and 38% (95% CI, 29–47) higher level of 8-oxodG in the colon, liver, and lung, respectively.
Figure 2.
Level of 8-oxodG in liver (A), lung (B), and colon (C) tissue 24 hr after oral exposure to a single dose of C60 fullerenes or SWCNT suspended in saline or corn oil. Values shown are mean ± SE of 8 treated animals and 10 control animals.
*p < 0.05 compared with the control group (single-factor effect of particle exposure, ANOVA).
We found no interactions between type of vehicle and particle exposure, indicating that particles in corn oil generated the same level of DNA damage as particles in saline. The generation of 8-oxodG is based on data from both types of vehicles (corresponding to estimates of single-factor effects). In the liver, the exposure to C60 fullerenes was associated with 17% (95% CI, 4–34) and 25% (95% CI, 11–41) increases of 8-oxodG in groups treated with the low dose and high dose of particles, respectively. Exposure to SWCNT increased the level of 8-oxodG in the liver by 22% (95% CI, 8–38) and 20% (95% CI, 7–36) in the rats given the low dose and high dose, respectively. There were increased levels of 8-oxodG in the lung after exposure to SWCNT at both the low [21% (95% CI, 9–33)] and high doses [23% (95% CI, 11–35)], whereas only the high dose of C60 fullerenes was associated with significantly elevated levels of 8-oxodG in the lung [18% (95% CI, 4–31)]. Oral exposure to particles was not associated with increased levels of 8-oxodG in colon mucosa tissue.
mRNA expression of HO1, MUTYH, NEIL1, NUDT1, and OGG1
Table 1 outlines the gene expression of OGG1, HO1, NEIL1, MUTYH, and NUDT1 mRNA in liver and lung tissue. The type of vehicle did not affect the level of gene transcription (p > 0.05, single-factor effect in ANOVA). The low and high doses of C60 fullerenes increased the gene expression of OGG1 by 1.30-fold (95% CI, 0.9–1.9) and 1.80-fold (95% CI, 1.2–2.6), respectively, in the liver, whereas a similar effect of SWCNT did not reach statistical significance. In contrast, we found no significant effects in the expression of HO1, NEIL1, MUTYH, and NUDT1 mRNA in liver and lung tissue. Colon tissue was not analyzed because there was unaltered level of 8-oxodG and limited amounts of material were available.
Table 1.
mRNA expression levels of genes involved in the removal of oxidized DNA base lesions in the liver and lung 24 hr after oral administration of C60 fullerenes or SWCNT suspended in either saline or corn oil.
| Particle dose in saline (mg/kg body weight)
|
Particle dose in corn oil (mg/kg body weight)
|
|||||
|---|---|---|---|---|---|---|
| Tissue | 0 | 0.064 | 0.64 | 0 | 0.064 | 0.64 |
| C60 fullerenes | ||||||
| Lung | ||||||
| HO1 | 10.4 ± 1.70 | 6.30 ± 0.76 | 6.26 ± 0.96 | 5.79 ± 0.58 | 8.63 ± 2.41 | 7.66 ± 1.50 |
| MUTYH | 6.26 ± 1.23 | 2.97 ± 0.56 | 4.75 ± 0.95 | 5.65 ± 1.50 | 7.71 ± 1.67 | 8.94 ± 3.46 |
| NEIL1 | 3.16 ± 0.49 | 2.26 ± 0.51 | 2.01 ± 0.33 | 2.94 ± 0.862 | 2.97 ± 0.87 | 3.53 ± 1.01 |
| NUDT1 | 0.34 ± 0.04 | 0.27 ± 0.02 | 0.40 ± 0.09 | 1.26 ± 0.75 | 1.37 ± 0.49 | 1.29 ± 0.97 |
| OGG1 | 0.55 ± 0.07 | 0.34 ± 0.06 | 0.38 ± 0.05 | 0.49 ± 0.07 | 0.57 ± 0.11 | 0.57 ± 0.10 |
| Liver | ||||||
| HO1 | 1.05 ± 0.12 | 1.55 ± 0.28 | 1.32 ± 0.18 | 1.47 ± 0.28 | 1.49 ± 0.37 | 1.27 ± 0.22 |
| MUTYH | 0.36 ± 0.07 | 0.45 ± 0.09 | 0.69 ± 0.30 | 0.35 ± 0.04 | 0.34 ± 0.05 | 0.40 ± 0.06 |
| NEIL1 | 0.86 ± 0.16 | 1.08 ± 0.25 | 1.57 ± 0.74 | 0.72 ± 0.10 | 0.58 ± 0.13 | 0.70 ± 0.08 |
| NUDT1 | 0.25 ± 0.07 | 0.35 ± 0.1 | 0.47 ± 0.18 | 0.26 ± 0.09 | 0.18 ± 0.03 | 0.21 ± 0.07 |
| OGG1 | 0.12 ± 0.02 | 0.14 ± 0.02 | 0.25 ± 0.07* | 0.10 ± 0.02 | 0.14 ± 0.03 | 0.16 ± 0.03* |
| SWCNT | ||||||
| Lung | ||||||
| HO1 | 10.4 ± 1.70 | 5.03 ± 1.04 | 14.1 ± 7.81 | 5.79 ± 0.58 | 7.15 ± 1.73 | 8.14 ± 2.79 |
| MUTYH | 6.26 ± 1.23 | 3.48 ± 1.07 | 9.80 ± 3.54 | 5.65 ± 1.50 | 8.65 ± 3.23 | 3.63 ± 0.68 |
| NEIL1 | 3.16 ± 0.49 | 2.03 ± 0.56 | 2.80 ± 0.75 | 2.94 ± 0.862 | 4.88 ± 1.45 | 2.24 ± 0.53 |
| NUDT1 | 0.34 ± 0.04 | 0.32 ± 0.08 | 1.78 ± 0.81 | 1.26 ± 0.75 | 2.29 ± 1.15 | 0.47 ± 0.12 |
| OGG1 | 0.55 ± 0.07 | 0.34 ± 0.03 | 1.17 ± 0.59 | 0.49 ± 0.07 | 0.54 ± 0.10 | 0.45 ± 0.06 |
| Liver | ||||||
| HO1 | 1.05 ± 0.12 | 1.56 ± 0.23 | 1.65 ± 0.19 | 1.47 ± 0.28 | 1.05 ± 0.14 | 1.30 ± 0.22 |
| MUTYH | 0.36 ± 0.07 | 0.36 ± 0.05 | 0.51 ± 0.09 | 0.35 ± 0.04 | 0.37 ± 0.05 | 0.30 ± 0.06 |
| NEIL1 | 0.86 ± 0.16 | 0.88 ± 0.15 | 0.83 ± 0.17 | 0.72 ± 0.10 | 0.69 ± 0.11 | 0.57 ± 0.09 |
| NUDT1 | 0.25 ± 0.07 | 0.22 ± 0.03 | 0.48 ± 0.20 | 0.26 ± 0.09 | 0.37 ± 0.12 | 0.26 ± 0.05 |
| OGG1 | 0.12 ± 0.02 | 0.15 ± 0.03 | 0.16 ± 0.04 | 0.10 ± 0.02 | 0.15 ± 0.04 | 0.17 ± 0.04 |
The mRNA expression is relative to the expression of 18S per 106. Values are mean ± SE.
p < 0.05 (single-factor effect of the particle exposure, ANOVA).
OGG1 repair activity
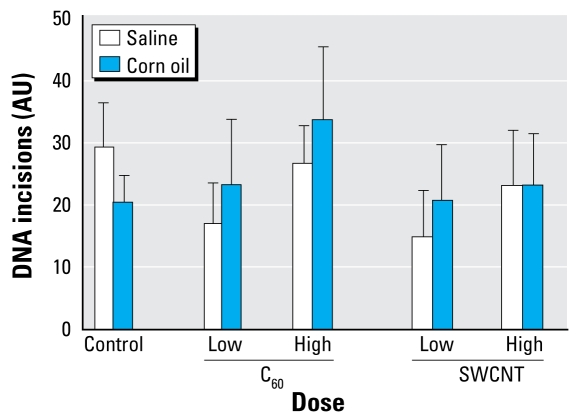
Based on the observation that the gene expression of OGG1 was increased in the liver after oral exposure to particles, we also measured the OGG1 repair activity of liver extracts (Figure 3). Neither the exposure to particles nor the vehicle were associated with a significantly altered level of OGG1 repair activity, although a dose-related effect of C60 fullerenes is suggested (p = 0.20, ANOVA).
Figure 3.
OGG1 repair activity in the liver 24 hr after exposure to a single dose of C60 fullerenes or SWCNT dissolved in saline or corn oil. The activity is indicated as the number of repair incisions in arbitrary units (AU) of substrate nuclei treated with Ro19-8022 and white light, which generate oxidative damage to the DNA. Values shown are mean ± SE for 8 treated animals and 10 control animals).
Discussion
In this study we found increased levels of oxidatively damaged DNA in liver and lung tissue 24 hr after oral administration of C60 fullerenes and SWCNT in saline or corn oil. There were virtually no alterations of the repair activity of oxidized base lesions in the same tissues, indicating that the level of DNA damage is not underestimated as a consequence of increased repair.
The levels of 8-oxodG were approximately 20% increased in the liver and lung tissue after the exposure to C60 fullerenes and SWCNT, whereas the vehicle did not affect the particle-generated genotoxicity. The effect of C60 fullerenes on OGG1 mRNA expression in the liver and 8-oxodG in the lung showed clear dose–response relationships, whereas other dose–response relationships were more flat. Possible agglomeration of particles affecting uptake and effects might have influenced the dose relationships. This could also be relevant for the lack of genotoxicity in colon mucosa cells. These cells have high turnover, which could dilute any possibly transient DNA damage, whereas liver and lung cells have a low proliferation rate, allowing damage to accumulate. We have previously shown that rats given the same oral dose of DEP as SRM2975 had approximately 50% elevated level of 8-oxodG in colon mucosa cells, liver, and lung (Danielsen et al. 2008b). Thus, our results indicate that C60 fullerenes and SWCNT are genotoxic in rats, but the effect is lower than that observed for SRM2975. The C60 fullerenes and SWCNT were administered in rather low doses because we wanted to use the SRM2975 preparation as benchmark particles. In comparison, a recent study with intravenous administration of 150 mg pegylated SWCNT per mouse argued that concentrations between 10- and 100-fold lower were sufficient in biomedical applications (Schipper et al. 2008). Other recent studies on pulmonary toxicity of SWCNT have used doses in the range of 20–40 μg/mouse (corresponding to about 1–2 mg/kg body weight) by pharyngeal aspiration (Han et al. 2008; Shvedova et al. 2008). The doses we used in the present study can thus be considered as being in the lower range of exposures.
To the best of our knowledge, our data are the first to demonstrate that C60 fullerenes and SWCNT generate oxidatively damaged DNA in rodent organs. Other reports have shown that C60 fullerenes and SWCNT exposure of cells in culture is associated with elevated levels of DNA damage measured by the comet assay (Dhawan et al. 2006; Kisin et al. 2007; Pacurari et al. 2008). These types of lesions represent general genotoxicity rather than oxidatively damaged DNA, which can be assessed by a modified version of the comet assay including digestion with DNA glycosylase or endonuclease enzymes (Møller 2006). We used this approach in a cell culture experiment and demonstrated that both C60 fullerenes and SWCNT generated oxidatively damaged DNA (Jacobsen et al. 2008b). The oxidizing effect of C60 fullerenes on biomolecules in cell culture settings could be due to generation of singlet oxygen induced by photosensitization (Kamat et al. 2000; Yamakoshi et al. 2003). Oxidations of DNA in internal organs probably arise from mixed ROS generation, as supported by increased mortality after C60 fullerene exposure in zebrafish in the dark and by enhanced mortality resulting from coexposure of C60 fullerenes with hydrogen peroxide or glutathione depletion (Usenko et al. 2008). Hydroxyl radicals appear to be the important type of ROS generated by SWCNT in cell cultures (Manna et al. 2005; Pacurari et al. 2008). The level of transition metals is not likely to explain the differences in genotoxicity between the particles used in the present study and SRM2975, because the latter has very low levels, whereas the SWCNT used here contained 2% iron, as well as traces of other metals, and the C60 fullerenes had undetectable levels. Moreover, SWCNT at low concentrations produced higher levels of ROS in cell-free systems than did SRM2975 and C60 fullerenes, whereas SRM2975 appeared to induce more guanine oxidation than the engineered particles in cell culture (Danielsen et al. 2008a; Jacobsen et al. 2008a, 2008b). The SRM2975 preparation contains substantial amounts of PAHs, which show low levels in SWCNT and are undetectable in C60 fullerenes. The content of PAHs could explain the higher DEP-induced level of 8-oxodG possibly through generation of oxidative stress by the aldo-ketoreductase metabolic pathway, as recently shown in cell culture (Park et al. 2008). Nevertheless, particles such as carbon black with very low PAH and metal levels induced the highest levels of ROS in both cell-free systems and in cell culture with resulting DNA oxidation and mutagenicity (Jacobsen et al. 2007), indicating that particles as such have the ability to induce oxidative stress. Accordingly, ROS generation in an acellular in vitro system does not appear to predict the ability of particles to oxidatively damage DNA in cell culture or in vivo.
The notion that C60 fullerenes and SWCNT induce less oxidative stress compared with DEP is supported by the unaltered regulation of HO1, which in our previous study was up-regulated by exposure to SRM2975 (Danielsen et al. 2008b). In the present experiments, we observed unaltered expression levels of NEIL1, MUTYH, and NUDT1, whereas the increased expression of OGG1 mRNA in the liver of rats exposed to C60 fullerenes was not associated with higher repair activity, which might be due to the fact that longer exposure times are required to observe increased repair activity. Indirect evidence of this effect modification comes from studies of inhalation exposure to DEP where repeated exposures on 4 consecutive days were associated with up-regulation of OGG1 and unaltered 8-oxodG in pulmonary tissue (Risom et al. 2003a), whereas an identical exposure scenario yielded higher levels of 8-oxodG in OGG1 knockout mice (Risom et al. 2007). Although increased expression levels of OGG1 might be considered beneficial to the cells, it should be emphasized that in human beings, with the variant form of OGG1 (Ser326Cys) genotype, high expression levels of OGG1 mRNA in leukocytes is associated with increased risk of lung cancer (Hatt et al. 2008).
Although our results strongly indicate that oral exposure to C60 fullerenes and SWCNT is associated with increased generation of oxidized DNA, it is still unresolved how application of particulate matter in the gut can oxidize biomolecules in internal organs. A somewhat naive notion suggests that the epithelial lining of the gut is designed to absorb substances and thus may allow passage of particulate matter. In fact, we suspended the particles in either corn oil or saline solution because of the notion that their hydrophobic nature would let them follow the regular passage of lipids in the gut. Because there was no difference in genotoxicity between the particles suspended in corn oil and saline solution, we believe that orally administered particles will distribute in the chyle of the intestinal juice, despite the fact that they may reach the gastrointestinal tract as agglomerates, as indicated by aqueous particle suspension characterization. The results suggest that the particles are absorbed from the gastrointestinal tract to blood circulation and secondary organs. However, at present the extent of translocation of particulate matter across epithelial barriers is a highly controversial issue. Studies of the pulmonary translocation of model particles to systemic circulation and secondary organs indicate only minute passage (Kreyling et al. 2002; Mills et al. 2006; Möller et al. 2008; Wiebert et al. 2006). On the other hand, whole-body inhalation exposure to ultrafine carbon particles have suggested some deposition in the liver, which has been speculated to originate from gastro intestinal exposure and uptake from the gut (Oberdörster et al. 2002). In addition, the uptake of C60 fullerenes and polystyrene latex microspheres from the gastrointestinal tract into blood circulation has been estimated in the range of 1% of the applied dose (Carr et al. 1996; Yamago et al. 1995). However, it is interesting to note that in drug delivery research, there is acceptance that particulate matter can be absorbed from the gastrointestinal tract, and this feature of nanomaterials is being used as an approach of altering the pharmacokinetic behavior of drugs (Florence 2004).
We observed an effect of corn oil in all three organs. This was not part of our a priori hypothesis. We chose the volume of corn oil to be identical to that of the saline solution (200 μL), which would allow a reasonable volume for the suspension in the aqueous solution. It should be emphasized that corn oil is rich in polyunsaturated fatty acids and was sonicated, which might produce genotoxic compounds. In addition, rat studies indicated that a diet rich in corn oil increased 8-oxodG excretion in urine (Loft et al. 1998) as well as the levels of 5-hydroxymethyl-2′-deoxyuridine, another marker of oxidatively damaged DNA in blood and mammary gland tissue (Djuric et al. 2001). A dyslipidemic state, as found in apoE knockout mice, also appears to include age-dependent accumulated oxidized DNA base lesions in the liver (Folkmann et al. 2007).
In conclusion, the data obtained from the present study indicate that C60 fullerenes and SWCNT generated oxidatively damaged DNA in liver and lung cells by a gastro intestinal route. The genotoxic effect resulting from exposure to C60 fullerenes and SWCNT was smaller than that from DEP exposure; however, it may be a cause for concern in humans.
Footnotes
This study was supported by grants from the Research Centre for Environmental Health, the Danish Research Councils, and the European Union (grant FP6-012912, NEST, Particle Risk) and ECNIS (Environmental Cancer Risk, Nutrition and Individual Susceptibility) a network of excellence operating within the European Union Sixth Framework Program, Priority 5: Food Quality and Safety (contract 513943).
References
- Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, et al. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential – a workshop report and consensus statement. Inhal Toxicol. 2008;20:75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- Baker GL, Gupta A, Clark ML, Valenzuela BR, Staska LM, Harbo SJ, et al. Inhalation toxicity and lung toxicokinetics of C60 fullerene nanoparticles and microparticles. Toxicol Sci. 2008;101:122–131. doi: 10.1093/toxsci/kfm243. [DOI] [PubMed] [Google Scholar]
- Carr KE, Hazzard RA, Reid S, Hodges GM. The effect of size on uptake of orally administered latex microparticles in the small intestine and transport to mesenteric lymph nodes. Pharm Res. 1996;13:1205–1209. doi: 10.1023/a:1016064320334. [DOI] [PubMed] [Google Scholar]
- Danielsen PH, Loft S, Møller P. DNA damage and cytotoxicity in type II lung epithelial (A549) cell cultures after exposure to diesel exhaust and urban street particles. Part Fibre Toxicol. 2008a;5:6. doi: 10.1186/1743-8977-5-6. [Online 8 April 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen PH, Risom L, Wallin H, Autrup H, Vogel U, Loft S, et al. DNA damage in rats after a single oral exposure to diesel exhaust particles. Mutat Res. 2008b;637:49–55. doi: 10.1016/j.mrfmmm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Dhawan A, Taurozzi JS, Pandey AK, Shan W, Miller SM, Hashsham SA, et al. Stable colloidal dispersions of C60 fullerenes in water: evidence for genotoxicity. Environ Sci Technol. 2006;40:7394–7401. doi: 10.1021/es0609708. [DOI] [PubMed] [Google Scholar]
- Djuric Z, Lewis SM, Lu MH, Mayhugh M, Tang N, Hart RW. Effect of varying dietary fat levels on rat growth and oxidative DNA damage. Nutr Cancer. 2001;39:214–219. doi: 10.1207/S15327914nc392_9. [DOI] [PubMed] [Google Scholar]
- Dybdahl M, Risom L, Møller P, Autrup H, Wallin H, Vogel U, et al. DNA adduct formation and oxidative stress in colon and liver of Big Blue rats after dietary exposure to diesel particles. Carcinogenesis. 2003;24:1759–1766. doi: 10.1093/carcin/bgg147. [DOI] [PubMed] [Google Scholar]
- ESCODD (European Standards Committee on Oxidative DNA damage) Gedik CM, Collins A. Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation trial. FASEB J. 2005;19:82–84. doi: 10.1096/fj.04-1767fje. [DOI] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Florence AT. Issues in oral nanoparticle drug carrier uptake and targeting. J Drug Target. 2004;12:65–70. doi: 10.1080/10611860410001693706. [DOI] [PubMed] [Google Scholar]
- Folkmann JK, Loft S, Møller P. Oxidatively damaged DNA in aging dyslipidemic ApoE−/− and wild-type mice. Mutagenesis. 2007;22:105–110. doi: 10.1093/mutage/gel059. [DOI] [PubMed] [Google Scholar]
- Guarnieri S, Loft S, Riso P, Porrini M, Risom L, Poulsen HE, et al. DNA repair phenotype and dietary antioxidant supplementation. Br J Nutr. 2008;99:1018–1024. doi: 10.1017/S0007114507842796. [DOI] [PubMed] [Google Scholar]
- Hah SS, Mundt JM, Kim HM, Sumbad RA, Turteltaub KW, Henderson PT. Measurement of 7,8-dihydro-8-oxo-2′-deoxyguanosine metabolism in MCF-7 cells at low concentrations using accelerator mass spectrometry. Proc Natl Acad Sci USA. 2007;104:11203–11208. doi: 10.1073/pnas.0701733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SG, Andrews R, Gairola CG, Bhalla DK. Acute pulmonary effects of combined exposure to carbon nanotubes and ozone in mice. Inhal Toxicol. 2008;20:391–398. doi: 10.1080/08958370801904014. [DOI] [PubMed] [Google Scholar]
- Hatt L, Loft S, Risom L, Møller P, Sørensen M, Raaschou-Nielsen O, et al. OGG1 expression and OGG1 Ser326Cys polymorphism and risk of lung cancer in a prospective study. Mutat Res. 2008;639:45–54. doi: 10.1016/j.mrfmmm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Helland A, Wick P, Koehler A, Schmid K, Som C. Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ Health Perspect. 2007;115:1125–1131. doi: 10.1289/ehp.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen NR, Møller P, Cohn CA, Loft S, Vogel U, Wallin H. Diesel exhaust particles are mutagenic in FE1-MutaMouse lung epithelial cells. Mutat Res. 2008a;641:54–57. doi: 10.1016/j.mrfmmm.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Jacobsen NR, Pojana G, White P, Møller P, Cohn CA, Korsholm KS, et al. Genotoxicity, cytotoxicity and reactive oxygen species induced by single-walled carbon nanotubes and C60 fullerenes in the FE1-Muta Mouse lung epithelial cells. Environ Mol Mutagen. 2008b;49:476–487. doi: 10.1002/em.20406. [DOI] [PubMed] [Google Scholar]
- Jacobsen NR, Saber AT, White P, Møller P, Pojana G, Vogel U, et al. Increased mutant frequency by carbon black, but not quartz, in the lacZ and cII transgenes of muta mouse lung epithelial cells. Environ Mol Mutagen. 2007;48:451–461. doi: 10.1002/em.20300. [DOI] [PubMed] [Google Scholar]
- Kamat JP, Devasagayam TP, Priyadarsini KI, Mohan H. Reactive oxygen species mediated membrane damage induced by fullerene derivatives and its possible biological implications. Toxicology. 2000;155:55–61. doi: 10.1016/s0300-483x(00)00277-8. [DOI] [PubMed] [Google Scholar]
- Kisin ER, Murray AR, Keane MJ, Shi XC, Schwegler-Berry D, Gorelik O, et al. Single-walled carbon nanotubes: geno- and cytotoxic effects in lung fibroblast V79 cells. J Toxicol Environ Health A. 2007;70:2071–2079. doi: 10.1080/15287390701601251. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: Mechanisms. Int J Cancer. 2004;109:799–809. doi: 10.1002/ijc.11708. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- Loft S, Møller P. Oxidative DNA damage and human cancer: need for cohort studies. Antioxid Redox Signal. 2006;8:1021–1031. doi: 10.1089/ars.2006.8.1021. [DOI] [PubMed] [Google Scholar]
- Loft S, Svoboda P, Kasai H, Tjonneland A, Vogel U, Møller P, et al. Prospective study of 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis. 2006;27:1245–1250. doi: 10.1093/carcin/bgi313. [DOI] [PubMed] [Google Scholar]
- Loft S, Thorling EB, Poulsen HE. High fat diet induced oxidative DNA damage estimated by 8-oxo-7,8-dihydro-2-deoxy-guanosine excretion in rats. Free Radic Res. 1998;29:595–600. doi: 10.1080/10715769800300641. [DOI] [PubMed] [Google Scholar]
- Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-κB in human keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NL, Amin N, Robinson SD, Anand A, Davies J, Patel D, et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am J Respir Crit Care Med. 2006;173:426–431. doi: 10.1164/rccm.200506-865OC. [DOI] [PubMed] [Google Scholar]
- Møller P. The alkaline comet assay: towards validation in biomonitoring of DNA damaging exposures. Basic Clin Pharmacol Toxicol. 2006;98:336–345. doi: 10.1111/j.1742-7843.2006.pto_167.x. [DOI] [PubMed] [Google Scholar]
- Møller P, Folkmann JK, Forchhammer L, Brauner EV, Danielsen PH, Risom L, et al. Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett. 2008;266:84–97. doi: 10.1016/j.canlet.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Möller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, et al. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med. 2008;177:426–432. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- Müller AK, Farombi EO, Møller P, Autrup HN, Vogel U, Wallin H, et al. DNA damage in lung after oral exposure to diesel exhaust particles in Big Blue rats. Mutat Res. 2004;550:123–132. doi: 10.1016/j.mrfmmm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. Entrez Gene. 2008. [[accessed 6 March 2009]]. Available: http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene.
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Nielsen GD, Roursgaard M, Jensen KA, Poulsen SS, Larsen ST. In vivo biology and toxicology of fullerenes and their derivatives. Basic Clin Pharmacol Toxicol. 2008;103:197–208. doi: 10.1111/j.1742-7843.2008.00266.x. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, et al. Raw single-walled carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-κB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116:1211–1217. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Mangal D, Tacka KA, Quinn AM, Harvey RG, Blair IA, et al. Evidence for the aldo-keto reductase pathway of polycyclic aromatic trans-dihydrodiol activation in human lung A549 cells. Proc Natl Acad Sci USA. 2008;105:6846–6851. doi: 10.1073/pnas.0802776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotech. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Risom L, Dybdahl M, Bornholdt J, Vogel U, Wallin H, Møller P, et al. Oxidative DNA damage and defence gene expression in the mouse lung after short-term exposure to diesel exhaust particles by inhalation. Carcinogenesis. 2003a;24:1847–1852. doi: 10.1093/carcin/bgg144. [DOI] [PubMed] [Google Scholar]
- Risom L, Dybdahl M, Møller P, Wallin H, Haug T, Vogel U, et al. Repeated inhalations of diesel exhaust particles and oxidatively damaged DNA in young oxoguanine DNA glycosylase (OGG1) deficient mice. Free Radic Res. 2007;41:172–181. doi: 10.1080/10715760601024122. [DOI] [PubMed] [Google Scholar]
- Risom L, Møller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592:119–137. doi: 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Risom L, Møller P, Vogel U, Kristjansen PE, Loft S. X-ray-induced oxidative stress: DNA damage and gene expression of HO-1, ERCC1 and OGG1 in mouse lung. Free Radic Res. 2003b;37:957–966. doi: 10.1080/1071576031000150788. [DOI] [PubMed] [Google Scholar]
- Schipper ML, Nakayama-Ratchford N, Davis C, Kam NWS, Chu P, Liu Z, et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotech. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Murray AR, Kommineni C, Castranova V, Fadeel B, et al. Increased accumulation of neutrophils and decreased fibrosis in the lung of NADPH oxidase-deficient C57BL/6 mice exposed to carbon nanotubes. Toxicol Appl Pharmacol. 2008;231:235–240. doi: 10.1016/j.taap.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, et al. Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33:105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol. 2008;229:44–55. doi: 10.1016/j.taap.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency) Method 610—Polynuclear Aromatic Hydrocarbons Appendix A: Methods for Organic Chemical Analysis of Municipal and Industrial Wastewater. 1991. [[accessed 31 March 2009]]. Available: http://www.epa.gov/waterscience/methods/method/organics/610.pdf.
- Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- Wiebert P, Sanchez-Crespo A, Falk R, Philipson K, Lundin A, Larsson S, et al. No significant translocation of inhaled 35-nm carbon particles to the circulation in humans. Inhal Toxicol. 2006;18:741–747. doi: 10.1080/08958370600748455. [DOI] [PubMed] [Google Scholar]
- Yamago S, Tokuyama H, Nakamura E, Kikuchi K, Kananishi S, Sueki K, et al. In vivo biological behavior of a water-miscible fullerene: 14C labeling, absorption, distribution, excretion and acute toxicity. Chem Biol. 1995;2:385–389. doi: 10.1016/1074-5521(95)90219-8. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, et al. Active oxygen species generated from photo-excited fullerene (C60) as potential medicines: O2−• versus 1O2. J Am Chem Soc. 2003;125:12803–12809. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]