Abstract
Background
Perchlorate (ClO4−) is an environmental contaminant known to disrupt the thyroid axis of many terrestrial and aquatic species. ClO4− competitively inhibits iodide uptake into the thyroid at the sodium/iodide symporter and disrupts hypothalamic–pituitary–thyroid (HPT) axis homeostasis in rodents.
Objective
We evaluated the proposed mode of action for ClO4−-induced rat HPT axis perturbations using a biologically based dose–response (BBDR) model of the HPT axis coupled with a physiologically based pharmacokinetic model of ClO4−.
Methods
We configured a BBDR-HPT/ClO4− model to describe competitive inhibition of thyroidal uptake of dietary iodide by ClO4− and used it to simulate published adult rat drinking water studies. We compared model-predicted serum thyroid-stimulating hormone (TSH) and total thyroxine (TT4) concentrations with experimental observations reported in these ClO4− drinking water studies.
Results
The BBDR-HPT/ClO4− model failed to predict the ClO4−-induced onset of disturbances in the HPT axis. Using ClO4− inhibition of dietary iodide uptake into the thyroid, the model underpredicted both the rapid decrease in serum TT4 concentrations and the rise in serum TSH concentrations.
Conclusions
Assuming only competitive inhibition of thyroidal uptake of dietary iodide, BBDR-HPT/ClO4− model calculations were inconsistent with the rapid decrease in serum TT4 and the corresponding increase in serum TSH. Availability of bound iodide in the thyroid gland governed the rate of hormone secretion from the thyroid. ClO4− is translocated into the thyroid gland, where it may act directly or indirectly on thyroid hormone synthesis/secretion in the rat. The rate of decline in serum TT4 in these studies after 1 day of treatment with ClO4− appeared consistent with a reduction in thyroid hormone production/secretion. This research demonstrates the utility of a biologically based model to evaluate a proposed mode of action for ClO4− in a complex biological process.
Keywords: BBDR model, HPT axis, iodide, mode of action, PBPK model, perchlorate, rat, sodium/iodide symporter, thyroid, thyroid hormone secretion
Perchlorate (ClO4−), a water-soluble chemical distributed extensively in the environment, has caused public health concerns because of widespread exposures to populations by ingestion of ClO4−-contaminated food and water. The ClO4− anion has recently been classified as a ubiquitous environmental contaminant throughout the United States, with detectable concentrations in many drinking water supplies (Motzer 2001) and food and beverage products (e.g., milk, lettuce, grains) (El Aribi et al. 2006), and is also found as a contaminant in dietary supplements (Snyder et al. 2006). The occurrence of ClO4− in the environment is most often attributed to anthropogenic uses of ClO4− salts as oxidizers in solid rocket propellants and application of Chilean nitrate fertilizers (Motzer 2001); however, ClO4− is also formed naturally by atmospheric processes (Dasgupta et al. 2005). The primary health concern for environmental exposures to ClO4− is adverse (and irreversible) neurodevelopmental outcomes mediated by disruption of the hypothalamic–pituitary–thyroid (HPT) axis in the immature fetus or child. Adequate levels of thyroid hormones are essential for proper growth, development, reproduction, and metabolism.
ClO4− blocks thyroidal uptake of radiolabeled iodide and alters thyroid hormone homeostasis [an increase in serum thyroid-stimulating hormone (TSH) concentrations and a decline in serum thyroxine (T4) concentrations] (National Research Council 2005; U.S. Environmental Protection Agency 2002). Dietary iodide is an essential nutrient used in the formation of thyroid hormones, and if in short supply, from the blocking effect of ClO4−, an iodide-deficiency–induced decrease in thyroid hormone production is thought to occur. For humans, under clinical conditions, ClO4− clearly inhibits thyroidal uptake of radiolabeled iodide (Greer et al. 2002); however, disruption of the HPT axis by ClO4− remains to be clearly demonstrated in euthyroid adults. Interestingly, ClO4− was used to treat thyrotoxicosis (overactive thyroid gland) with large doses, ranging from 17–29 mg/kg, until several cases of fatal aplastic anemia occurred in the early 1960s. The mechanism of action for this adverse effect of ClO4− is unknown. ClO4− was also used clinically to test thyroid function, referred to as the ClO4− discharge test. For this test, radiolabeled tracer iodide is administered a few hours before dosing with 2–13 mg/kg ClO4− (Wolff 1998), causing blocking of active uptake of radiolabeled iodide for several hours. By 2 hr after dosing, the thyroid gland has sequestered radiolabeled iodide, but circulating levels of the iodide remain in the body and are available for sequestration into the thyroid gland. If the thyroid gland is defective in binding (trapping) iodide, rapid and excessive efflux of radiolabeled iodide from the thyroid is observed in the presence of ClO4−.
The primary mechanism by which ClO4− blocks thyroidal uptake of iodide is competitive inhibition at the sodium/iodide symporter (NIS) protein. The NIS protein is responsible for active translocation of both iodide and ClO4− from circulating blood to the thyroid follicle. Bidirectional passive diffusion of iodide and ClO4− also occurs between the thyroid gland and the blood supply perfusing the thyroid. When the ClO4− discharge test is administered, a possible mechanism of action for efflux of radiolabeled iodide from defective thyroid glands may be simple diffusion of radiolabeled iodide down a concentration gradient from the thyroid gland into the blood supply because the NIS “pump” that maintains an iodide gradient between the blood and the thyroid gland is shut down by ClO4−. A rapid efflux of radiolabeled iodide has been observed in cell preparations deficient in the binding protein, thyroglobulin (Kosugi et al. 1996).
Several decades ago, in vitro studies were conducted in Sprague-Dawley rat thyroid homogenates to examine the effects of antithyroid compounds on iodide peroxidase activity (Alexander 1959). For each antithyroid compound tested, thyroid homogenates were incubated with 1 μmol potassium iodide (KI) and 2 × 10−3 M antithyroid compound for 2 hr. Thiocyanate, thiouracil, and cyanide were several of the antithyroid compounds shown to inhibit incorporation of 131I into L-tyrosine, decreasing the formation of monoiodotyrosine (MIT) and diiodotyrosine (DIT). However, ClO4− did not significantly alter the synthesis of MIT and DIT. Thus, the data suggest that ClO4− does not alter organification of iodide, which is a key step in thyroid hormone synthesis (Alexander 1959). Several years later, Greer et al. (1966) provided contradicting results, demonstrating a direct effect of ClO4− on the rat thyroid gland in vitro. These authors reported that ClO4− reduced formation of MIT and DIT in rat thyroid lobes when incubated with ClO4−. They observed changes in MIT and DIT formation for ClO4− media concentrations starting at 10 mg/L, with a reported 50% effective reduction of DIT at 250 mg/L.
In addition to ClO4− acting at the NIS protein of the thyroid gland, a few other studies have demonstrated another potential mode of action (MOA) for high doses of ClO4− to disrupt the HPT axis. Using an in vitro system and very high concentrations of ClO4− (≥ 1,700 mg/L), Okabe and Hokaze (1993) reported that ClO4− displaces T4 bound to bovine serum albumin. This observation implies that free T4 (fT4) dislodged from serum proteins would then be cleared from the body more quickly, leading to a disruption of the HPT axis. In earlier in vivo studies, Yamada (1967), who was interested in this same MOA, removed the thyroid or gave methimazole [methylmercaptoimidazole (MMI)] to rats to inhibit thyroid function (and treated them with T4) and then evaluated the ClO4− dose-dependent decline in serum protein-bound iodide (PBI; primarily radiolabeled T4). The decline in serum PBI was interpreted as displacement of protein bound T4 by ClO4−. The daily intake rates of ClO4− were estimated to be 10–1,000 mg/kg/day.
Historically, pharmacokinetic or computational analyses of the HPT axis have played a prominent role in efforts to quantify and understand the complex relationships between biological action of thyroid hormones and their production, metabolism, transport, distribution, and interaction with receptors (DiStefano and Landaw 1984; Oppenheimer 1983). Recently, physiologically based pharmacokinetic (PBPK) models were developed to describe the kinetics of ClO4− and radiolabeled iodide and the interaction of ClO4− on the thyroidal uptake of radiolabeled iodide in the adult rat (Fisher et al. 2000; Merrill et al. 2003). The rodent PBPK model described transport of ClO4− into the thyroid gland by the NIS protein because high concentrations of ClO4− were measured in thyroid tissue relative to serum (Yu et al. 2002). High concentrations of ClO4− in the thyroid gland had also been observed in laboratory animals administered ClO4− (Chow et al. 1969; Chow and Woodbury 1970).
However, using an indirect electrochemical technique to infer movement of ClO4− by the NIS into thyroid cells, Riedel et al. (2001) concluded that ClO4− was not taken up into the thyroid gland because an electrical gradient was not created. Evidence for ClO4− movement into the thyroid gland was reviewed by Wolff (1998) and Clewell et al. (2004). Finally, a recent study directly measured the TSH-mediated movement of ClO4− into NIS-expressing FRTL-5 rat thyroid cells. This research provided conclusive evidence that the NIS protein did translocate ClO4− into the thyroid cells (Tran et al. 2008). At the same time, Dohan et al. (2007) concluded that ClO4− is transferred by the NIS based on indirect lines of evidence using another anion (perrhenate) that is also transported with an electroneutral stoichiometry into thyroid cells.
In the study we report here, using computational analysis, we tested the hypothesis that the primary MOA of ClO4− on the HPT axis is competitive inhibition of uptake of thyroidal iodide. We combined our recently published biologically based dose–response (BBDR) model for the HPT axis (McLanahan et al. 2008), which we calibrated to describe perturbations resulting from dietary iodide deficiency, with a simple PBPK model for ClO4−. The published ClO4− dose–response data sets for the adult rat HPT axis (Männistö et al. 1979; Yu et al. 2002) were simulated with our combined BBDR-HPT/ClO4− models. Additionally, we conducted limited in vitro experiments at relevant ClO4− plasma concentrations to evaluate the role of ClO4− in displacement of T4 from serum-binding proteins.
Materials and Methods
Laboratory experiments
Displacement of T4 from serum proteins
Radiolabeled L-[3′,5′-125I]thyroxine (125I-T4; specific activity, 1,500 μCi/μg; Perkin Elmer Life Sciences, Waltham, MA) was prepared by diluting 25 μCi (0.5 mL of 50 μCi/mL solution) with 1.0 mL 10% bovine serum albumin solution and predialyzing it against 2 mL 0.15 M phosphate buffer solution to reduce the amount of free 125I in the solution. We spiked male rat and male human serum (both obtained from Bioreclamation Inc., Hicksville, NY) with ClO4− (Sigma Aldrich, Milwaukee, WI; 0.01 mL ClO4−/mL serum) diluted in saline to give a serum concentration of 0, 1.0, 10.0, 50.0, 100, 200, or 300 μg/mL, vortexed the solution, and allowed it to stand for 30 min. We then added 0.02 mL (200,000–300,000 cpm) predialyzed 125I-T4 solution/mL serum, vortexed the solution, and allowed it to stand for 30 min. One mL of spiked serum was dialyzed across a 6,000-kDa membrane overnight against 1.0 mL 0.15 M phosphate buffer (pH 7.3) at 37°C in a gently shaking water bath. Serum and dialysate were removed from each half-cell with a Pasteur pipette precoated with carrier solution. We immediately placed 750 μL dialysate into 750 μL carrier solution (3.0 mg/mL T4 and 3.72 mg/mL NaI in 0.5 M NaOH) and vortexed it. Volume recovered was assessed in order to account for possible fluid shifts across the membrane. The dialysate/carrier solution was further processed by adding 1.5 mL magnesium precipitating solution (6.05 g/L Trizma base, 5.85 g/L NaCl, and 100 g/L MgCl2·6H2O, pH 9.274); the solution was vortexed and allowed to stand for 10 min. The precipitate was centrifuged for 5 min at 2,000 rpm and the supernatant was removed. The pellet was washed three times by resuspending it in 1.5 mL washing solution (precipitate solution with pH adjusted to 8.772), centrifuging it at 2,000 rpm for 5 min, and removing the supernatant. We assessed the tube with 750 μL serum and the pellet precipitated from 750 μL dialysate by gamma counting and compared the results with control incubations.
Model development
HPT axis
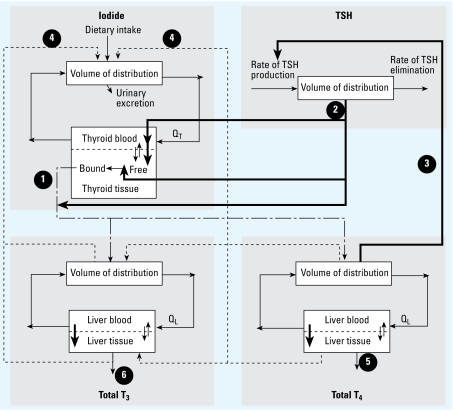
We constructed the BBDR-HPT axis model in acslXtreme, version 2.4 (AEgis Technologies, Huntsville, AL) and solved it using the Gear algorithm for stiff systems. The model for dietary iodide and the thyroid axis was used as previously described (McLanahan et al. 2008). Briefly, this BBDR-HPT axis model includes submodels for dietary iodide, TSH, and the thyroid hormones T4 and T3 (Figure 1). The submodels combine to form a simplified, quantitative description of the thyroid axis in the adult rat. Regulatory and compensatory effects of TSH were empirically described for several thyroidal processes, including TSH stimulation of a) NIS thyroidal iodide uptake, b) formation of thyroid hormone precursors, and c) thyroid hormone secretion. The TSH/T4 negative feedback loop is described using the relationship of serum total T4 (TT4) and TSH, as well as the metabolism of thyroid hormones with recycling of iodide.
Figure 1.
BBDR model structure of the HPT axis for the description of dietary iodide, TSH, TT4, and TT3. The model provides for recycling of dietary iodide released from metabolism of thyroid hormones (dashed lines), as well as the stimulation and regulation of the HPT axis by TSH. Numbers indicate several key processes: 1, loss of thyroidal-bound iodide secreted as thyroid hormones; 2, TSH stimulation of NIS iodide uptake, organification of iodide, and stimulation of thyroid hormone production; 3, T4 negative feedback on TSH production (thick solid lines); 4, formation of free iodide from serum and liver T3 and T4 metabolism; 5, phase II metabolism of T4 and excretion into feces; and 6, fecal elimination of T3. For additional details on the BBDR-HPT axis model, see McLanahan et al. (2008). Bold arrows represent active transport and double thin arrows represent passive diffusion processes. Thin solid lines represent blood flows to or from the thyroid (QT) or the liver (QL).
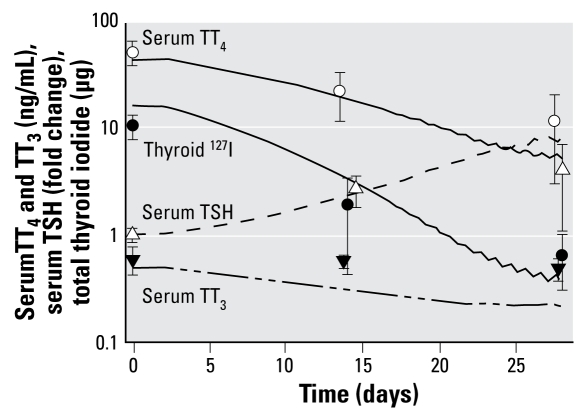
We developed the BBDR-HPT axis model (McLanahan et al. 2008) to predict perturbations in serum TT4, total T3 (TT3), TSH, and total thyroidal iodide content that resulted from a decrease in dietary iodide intake. Normal laboratory rat iodide intake is approximately 20 μg/day. Figure 2 shows a model simulation up to 28 days for Simonsen Albino rats placed on a diet containing 0.33 μg iodide per day from day 0 through day 28 (Okamura et al. 1981).
Figure 2.
BBDR-HPT axis model simulation (lines) of HPT axis alterations in serum TT4, TT3, and TSH and thyroid iodide up to 28 days after administration of a low-iodide diet (0.33 μg/day). Data (mean ± SD) are adapted from Okamura et al. (1981) and are offset at days 14 and 28 to aid in visualization of data.
Perchlorate
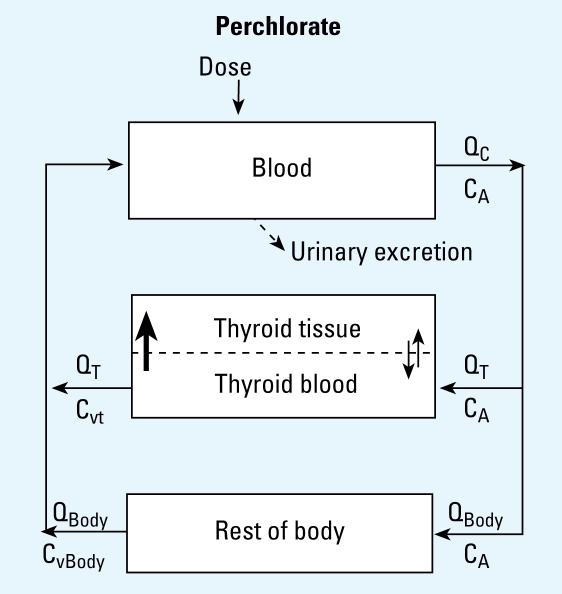
Instead of the more elaborate modeling approach implemented by Merrill et al. (2003), a simple model structure for ClO4− was constructed (Figure 3), similar to the approaches reported by Crump and Gibbs (2005) and Lorber (2008). This PBPK model consisted of three compartments: plasma, thyroid, and the rest of the body. ClO4− is rapidly absorbed after oral administration and is distributed throughout the body but excreted unchanged through the urine (Wolff 1998). Urinary excretion of ClO4− is described by first-order clearance from the plasma. The thyroid gland is described with a blood and tissue compartment using diffusion limitation and active uptake into the thyroid via the NIS. Several studies have shown that ClO4− and 36ClO4− are transported into the thyroid via the NIS, and an increased uptake has been observed in rats administered TSH and ClO4− (Anbar et al. 1959; Chow et al. 1969; Chow and Woodbury 1970; Dohan et al. 2007; Goldman and Stanbury 1973; Tran et al. 2008; Yu et al. 2002).
Figure 3.
ClO4− PBPK model structure for the adult male rat. ClO4− (intravenous or oral drinking water dose) enters the plasma (Dose), where it is distributed to tissues or excreted in urine. Thyroid is modeled as diffusion limited, with active uptake (bold arrow) into the thyroid via the NIS. The “Rest of Body” compartment is a flow-limited compartment and includes all other body tissues in which ClO4− may distribute. Double thin arrows represent passive diffusion. Abbreviations: CA, concentration of ClO4− in arterial blood; CvBody, concentration of ClO4− in venous blood leaving the rest of the body compartment; Cvt, concentration of ClO4− in venous blood leaving thyroid; QC, cardiac output; QBody, blood flow to the rest of the body; QT, blood flow to the thyroid.
BBDR-HPT axis and ClO4− PBPK model integration
We linked the PBPK model for ClO4− with the BBDR-HPT axis model (McLanahan et al. 2008) by competition between ClO4− and iodide at the NIS protein. Equations 1 and 2 describe, respectively, the competitive interaction between ClO4− and the NIS protein and TSH stimulation of NIS activity:
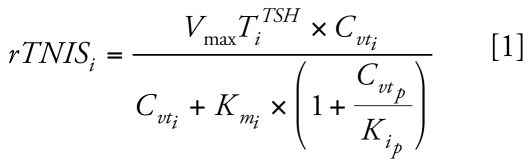 |
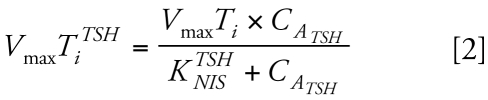 |
where rTNISi is the rate of iodide actively transported into the thyroid via NIS (nanomoles per hour), Cvti is the free concentration of iodide in thyroid blood (nanomoles per liter), Kmi is the affinity constant of iodide for the NIS (nanomoles per liter), Cvtp is the concentration of ClO4− in the thyroid blood (nanomoles per liter), Kip is the inhibition constant of ClO4− for NIS iodide transport (nanomoles per liter), Vmax Ti is the maximum rate of NIS iodide uptake (nanomoles per hour), CATSH is the serum concentration of TSH (nanomoles per liter), and KNISTSH is the concentration of TSH that gives rise to half-maximal rate of NIS transport of iodide (nanomoles per liter). We previously simultaneously optimized Vmax Ti (nanomoles per hour) and KNISTSH (nanomoles per liter) (McLanahan et al. 2008) to euthyroid, dietary-iodine–sufficient (~ 20 μg/day) rat data for serum and thyroid iodide reported by Eng et al. (1999) and McLanahan et al. (2007), respectively. The other parameter, NIS affinity constant for iodide (Kmi, nanomoles per liter) in Equation 1 was obtained from Gluzman and Niepomniszcze (1983) as reported by McLanahan et al. (2008).
We used an equation similar to Equation 1 to model iodide’s inhibition of the uptake of ClO4− via the NIS; however, the effect of iodide on transport of ClO4− into the thyroid is minimal because ClO4− has a higher affinity for the NIS protein compared with iodide (1,500 nmol ClO4−/L vs. 31,519 nmol I/L).
Model parameter values
We obtained physiologic parameters (Table 1) for tissue volumes and blood flows from previous studies (Brown et al. 1997; Everett et al. 1956; Malendowicz and Bednarek 1986; McLanahan et al. 2007, 2008). The BBDR–HPT axis model parameters had been calibrated for the euthyroid adult rat previously and used to describe iodide-deficient conditions (McLanahan et al. 2008).
Table 1.
Physiologic parameters for the adult rat perchlorate model.
| Parameter | Value | Source |
|---|---|---|
| Tissue volumes (V) | ||
| Plasma, VPl (% BW) | 4.44 | Brown et al. 1997, Everett et al. 1956 |
| Thyroid, VTc (% BW) | 0.005 | McLanahan et al. 2007 |
| Thyroid blood, VTBc (% VT) | 15.7 | Malendowicz and Bednarek 1986 |
| Rest of body, VBody (L) | BW − VT − VPl | |
| Blood flows (Q) | ||
| Cardiac output, QCcc (L/hr/kg0.75) | 14.0 | Brown et al. 1997 |
| Thyroid, QTc (% QC) | 1.6a | Brown et al. 1997 |
| Rest of body, QBody | QC − QT | |
BW, body weight; VT, volume of thyroid; QC, cardiac output; QT, blood flow to thyroid
Human value.
Table 2 shows model parameters for ClO4−. We set the affinity constant, Kip, for the inhibition of iodide uptake by ClO4− equal to Kmp, the measured affinity constant for ClO4− and the NIS. A Kmp value of 1.5 μM was used, which was reported as an inhibition affinity constant for uptake of radiolabeled iodide in thyroid cells (Kosugi et al. 1996). We calculated a ClO4− tissue:blood partition coefficient for the body compartment (minus the thyroid and plasma) by weighting the partition coefficients based on tissue volume for tissue:blood partition coefficient values reported in Merrill et al. (2003) for the adult male rat. Optimization was carried out using acslXtreme parameter estimation tool kit, using 36ClO4− kinetic data reported by Yu et al. (2002), who administered a single intravenous bolus dose of 3.3 mg/kg 36ClO4−. Optimized values were obtained for Vmax Tcp, the maximal rate of uptake of 36ClO4− into the thyroid and urinary clearance constant, and for ClUcp, a term used to describe the rate of excretion of 36ClO4− in urine. The ClUcp value was obtained by fitting both the urinary excretion of 36ClO4− and serum concentrations of 36ClO4−.
Table 2.
Compound-specific parameters.
| Parameter | Value | Source |
|---|---|---|
| Partition coefficient (dimensionless) | ||
| Body:blood, PBp | 0.416a | Merrill et al. 2003 |
| Permeability area cross-product (L/hr/kg0.75) | ||
| Iodide, thyroid blood:thyroid tissue, PATci | 1 × 10−4 | McLanahan et al. 2008 |
| ClO4−, thyroid blood:thyroid tissue, PATcp | 2.8 × 10−4 | Merrill et al. 2003 |
| Affinity constants (nmol/L) | ||
| Iodide, thyroid NIS, Kmi | 3.15 × 104 | Merrill et al. 2003, Gluzman and Niepomniszcze 1983 |
| ClO4−, thyroid NIS, Kmp | 1.5 × 103 | Kosugi et al. 1996 |
| Maximum velocities (nmol/hr/kg0.75) | ||
| Iodide, thyroid NIS, VmaxTci | 5.7 × 103 | McLanahan et al. 2008 |
| ClO4−, thyroid NIS, VmaxTcp | 1.8 × 102 | Optimized |
| Clearance (L/hr/kg0.25) | ||
| Iodide, urinary excretion, ClUci | 5 × 10−3 | McLanahan et al. 2008 |
| ClO4−, urinary excretion, ClUcp | 7.0 × 10−2 | Optimized |
Weighted based on PBPK model partition coefficients. Data from Merrill et al. (2003).
Experimental simulations
Yu et al. (2002) administered 0, 0.1, 1, 3, and 10 mg ClO4−/kg/day in drinking water to adult male Sprague-Dawley rats and determined serum TT4, fT4, TT3, TSH, and ClO4− concentrations after exposures of 1, 5, and 14 days. We did not consider the Yu et al. (2002) data for fT4 for this study because the increase in fT4 with ClO4− concentration is considered to be erroneous owing to a nondialysis method. The problem with this method has been previously discussed (Fisher et al. 2006). In addition, Männistö et al. (1979) administered ClO4− at a rate of 15 mg/kg/day in drinking water to adult male Sprague-Dawley rats and measured serum TT4, TT3, and TSH after exposure for 0, 2, 4, 6, 9, and 14 days. The BBDR-HPT/ClO4− model was configured to simulate these experiments. Ingestion of ClO4− in drinking water took place over a 12-hr period.
Results
Laboratory binding and displacement experiments
ClO4− had little or no effect on displacing 125I-T4 from serum proteins for both human and rats [see Supplemental Material, Figure 1 (http://www.ehponline.org/members/2009/0800111/suppl.pdf)] across the relevant range of ClO4− concentrations tested (1–300 μg/mL). The highest concentration tested, 300 μg/mL, is 30 times greater than the range of interest for ClO4. Thus, older studies that examined high doses of ClO4− probably caused a displacement in protein-bound T4, but for the concentration range of interest, 0.01–1.0 μg/mL (corresponding to a ClO4− dose of 0.01–10 mg/kg/day), there appears to be little displacement of protein-bound T4 by ClO4−. We found no significant differences by analysis of variance for either rat (p = 0.13) or human (p = 0.96) serum. Therefore, this MOA was not explored in the computational analysis.
PBPK model predictions
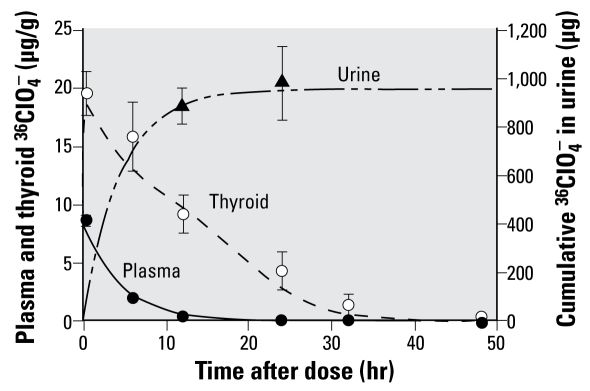
Figure 4 shows model-predicted and laboratory-observed serum and thyroid 36ClO4− concentrations and cumulative urinary excretion of 36ClO4− after a single intravenous bolus dose of 3.3 mg/kg 36ClO4−. We obtained these successful predictions of thyroidal and serum concentrations of 36ClO4− and urinary clearance using an optimized Vmax Tcp value of 1,800 nmol/hr/kg0.75 and an optimized ClUcp value of 0.007 L/hr/kg0.25 (Table 2). Simulations of ClO4− in serum and the thyroid gland were similar to previous model findings of Merrill et al. (2003) for the drinking water dose groups of 0.1, 1.0, 3.0, and 10 mg/kg/day that were originally reported by Yu et al. (2002) (data not shown).
Figure 4.
Serum and thyroid concentration and cumulative urinary excretion of 36ClO4− after a 3.3-mg/kg intravenous dose. Lines represent model predictions. Data (mean ± SD) are from Yu et al. (2002).
HPT response predictions: testing the hypothesis
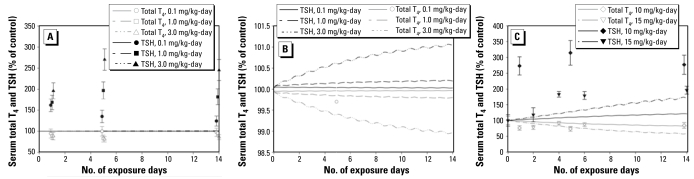
We linked the ClO4− model with the BBDR-HPT axis model that we previously calibrated to predict serum TT4, TT3, TSH, and total thyroid iodide for sufficient and insufficient dietary iodide intakes and evaluated the ability of the combined model to predict ClO4−-induced HPT axis disturbances. The hypothesis was that the BBDR-HPT/ClO4− model would predict the HPT axis disturbances based on competitive inhibition of thyroidal uptake of dietary iodide (Equation 1) for ClO4− drinking water dose rates reported by Männistö et al. (1979) (15 mg/kg/day) and Yu et al. (2002) (0.1, 1, 3, 10 mg/kg/day). In this case, the BBDR-HPT/ClO4− model failed to predict the HPT axis responses. Dose-dependent increases in serum TSH concentrations were severely underpredicted, and dose-dependent decreases in serum TT4 concentrations were also under-predicted (Figure 5A,B). The small decrease in serum TT3 in response to 10 mg/kg/day ClO4− was better predicted by the model [see Supplemental Material, Figure 2 (http://www.ehponline.org/members/2009/0800111/suppl.pdf)].
Figure 5.
(A) Model predictions (lines) of serum T4 and TSH after exposure to 0.1, 1, or 3 mg/kg/day ClO4− in drinking water. Data for TSH and T4 from Yu et al. (2002) (0.1, 1.0, 3.0 mg/kg/day). (B) A closer look at model simulations in A, expanding the y-axis. (C) Model predictions of serum T4 and TSH after exposure to 10 or 15 mg/kg/day ClO4− in drinking water. Data (mean ± SD) for T4 and TSH for 10 mg/kg/day (~ 350 g rat) from Yu et al. (2002), and for 15 mg/kg/day
(~ 200 g rat) from Männistö et al. (1979). The model does not predict the rapid changes in serum T4 and TSH observed in these studies.
Yu et al. (2002) reported dose-dependent serum TSH increases ranging from 60% for the lowest dose to 174% for the highest dose compared with controls after 1 day of ClO4− treatment. For all dose groups, serum TSH level continued to increase by day 5 of treatment, with the exception of the 0.1 mg/kg/day dose group. By day 14 of treatment, TSH concentrations were in decline relative to the peak measured TSH concentrations. Männistö et al. (1979) reported that 15 mg/kg/day caused serum TSH levels to increase by 18% on day 2 of treatment and by 96% on day 14 (Figure 5C). These measured TSH concentrations are in stark contrast to the model-predicted values (Figure 5C). The model-simulated serum TSH concentrations were predicted to increase only by 2–6% after 1 day of treatment and by 21–36% after 14 days of treatment with the highest ClO4− doses (10 and 15 mg/kg/day; Figure 5C).
Similar to the TSH findings, the dose-dependent decline in serum TT4 concentrations reported by Yu et al. (2002) ranged from 6% for the lowest dose to 24% for the highest dose after 1 day of ClO4− treatment. Consistent with the increases in serum TSH on day 5, all treatment groups, except for the 0.1 mg/kg/day ClO4− dose group, displayed a continued decline in serum TT4 concentrations. By day 14 of ClO4− treatment, TT4 levels were recovering (Figure 5A). Model predictions of serum TT4 concentrations were also in disagreement with observations. The model-predicted decreases in serum TT4 concentrations were only 2–6% by day 1 of treatment and 13–26% by day 14 for the 10- and 15-mg/kg/day ClO4− dose groups. Although the model-predicted and observed serum TT4 concentrations were in agreement for the 10-mg/kg/day group by day 14, the temporal aspect of the TT4 perturbations was not predicted (Figure 5C).
The model-predicted interaction of ClO4− (competitive inhibition) on thyroidal uptake of dietary iodide (Table 3) demonstrated the nonlinear uptake behavior of dietary iodide that is described by Michaelis-Menten kinetics for each anion. NIS-mediated thyroidal uptake of dietary iodide (micrograms per day) was predicted to be similar on days 1 and 14 (Table 3), with a slight increase on day 14 because of TSH stimulation of the NIS for the two highest ClO4− doses (10 and 15 mg/kg/day). Although thyroidal iodide stores were not reported by Yu et al. (2002) and Männistö et al. (1979), only a 6% and 12% depletion was predicted to occur after 1 day and 2 weeks of treatment of ClO4− for dose rates of 10 and 15 mg/kg/day, respectively (Table 3). The model-predicted depletion of thyroidal iodide stores was 33% and 46% by day 14 for these two doses, respectively.
Table 3.
Model predictions of daily NIS uptake of iodide into thyroid (μg/day) and total thyroid iodide stores (μg) for a 300-g adult rat, after 1 and 14 days of ClO4− exposure in drinking water at various doses.
| Dose (mg/kg/day)
|
||||||
|---|---|---|---|---|---|---|
| ClO4− exposure | 0 | 0.1 | 1 | 3 | 10 | 15 |
| 1 day | ||||||
| Average amount of iodide transported by NIS into thyroid (μg/day) | 38 | 28 | 10 | 4.6 | 1.7 | 1.2a |
| Total thyroid iodide stores (μg) | 17.0 | 17.0 | 16.9 | 16.8 | 16.0 | 14.9a |
| 14 days | ||||||
| Average amount of iodide transported by NIS into thyroid (μg/day) | 38 | 28 | 10 | 4.6 | 1.9 | 1.5 |
| Total thyroid iodide stores (μg) | 17.1 | 17.0 | 16.9 | 16.4 | 11.4 | 9.31 |
Model simulation for 2 days of exposure to 15 mg/kg/day. Data from Männistö et al. (1979).
The model-predicted percent inhibition in thyroidal uptake of dietary iodide was predicted to range from 26% for the 0.1 mg/kg ClO4− dose group to 96% and 97% inhibition for the two highest dose groups (Table 3).
Is ClO4− altering thyroid hormone secretion?
The best approach to answer this question would be to design studies focused on evaluating the status of the thyroid gland for a known intake of dietary iodide, such as measuring the ratios of MIT and DIT, and thyroid hormones in animals treated with ClO4−. Another equally important and time-intensive effort would be to develop a mechanistic-based model of the thyroid gland that would include explicit descriptions of the synthesis and secretion pathways for thyroid hormones, for which some data are still lacking. Instead, we simply imposed “non-mechanistic” conditions on the thyroid gland by limiting thyroid hormone production and asking whether the systemic concentrations of TT4 and TSH can be described by simply limiting thyroid hormone production and secretion. To account for inhibition of thyroid hormone production, we modified equation 14 from McLanahan et al. (2008), which describes the overall rate of thyroid hormone production (rTHprod, nanomoles per hour), by the addition of a proportional inhibition term (INHp), such that
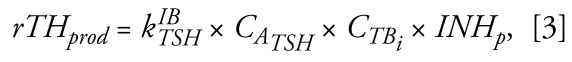 |
where kTSHIB (square liters/nanomole per hour) is a linear rate term, CATSH (nanomoles per liter) is the concentration of TSH in the serum, and CTBi (nanomoles per liter) is the bound concentration of iodide in the thyroid as thyroid hormone precursors. The production rate of T4 and T3 was described as a fraction of the total production rate, and the proportion of T4 and T3 changes as a result of depletion of thyroidal iodide stores (McLanahan et al. 2008).
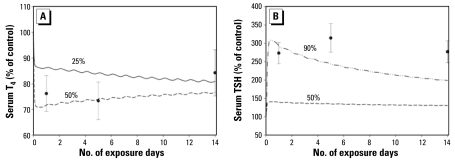
Figure 6A shows the 10-mg/kg/day dose group (Yu et al. 2002) model predictions for TT4 when a 25% (INHp = 0.75) or 50% (INHp = 0.50) inhibition of thyroid hormone synthesis is tested using the model. A 50% decrease in thyroid hormone production rate appears to describe the decreased TT4 concentrations for the first 5 days of exposure, but for serum TSH (Figure 6B) 90% inhibition of thyroid hormone synthesis did not describe the kinetic behavior for TSH. Serum TT3 was predicted fairly well without imposing inhibition of thyroid hormone production; however, a 25% decrease in thyroid hormone production provided a slightly better fit to the Yu et al. (2002) 10 mg/kg/day dose group [see Supplemental Material, Figure 2 (http://www.ehponline.org/members/2009/0800111/suppl.pdf)]. This simple simulation exercise appears to suggest that thyroid hormone production may be partially inhibited on a transient basis. However the relationship between TSH production and serum TT4 is complex and very different from dietary-iodide–induced hypothyroidism.
Figure 6.
Serum T4 model predictions (A; lines) and serum TSH model predictions (B; lines) shown as percentage of control (100%) values at a ClO4− dose of 10 mg/kg/day and varying degrees of inhibition of T4 and T3 synthesis and secretion from the thyroid (25, 50, or 90%). These model predictions also include inhibition of NIS thyroidal iodide uptake by ClO4−. Data (mean ± SD) from Yu et al. (2002).
Discussion
In this article, we report on the use of computational modeling analyses to examine the biological processes associated with disruption of an endocrine system, the HPT axis in the adult rat, by ClO4−. The BBDR-HPT/ClO4− model simulation results suggest that ClO4− administered in drinking water (or by other routes of administration) interacts with the rat thyroid gland itself, potentially altering thyroid hormone synthesis or secretion. This interaction is in addition to blocking thyroidal uptake of iodide. Current presumptions of a single MOA for ClO4− on the HPT axis do not appear tenable based on these results.
Changes in serum TSH and TT4 after only 1 day of ClO4− treatment were surprising because thyroidal iodide depletion was expected to be minimal over this time period, although 26–96% inhibition of NIS thyroidal iodide uptake was predicted by the model, which is in close agreement with Yu et al. (2002). The only reported effects of ClO4− on thyroidal iodide content we found were those of Ortiz-Caro et al. (1983), who reported a decrease in thyroidal 127I of nearly 8% in rats administered 1,000 ppm ClO4− in drinking water for 2 days and provided a low-iodide diet. A 10–15% decrease over 1 day is predicted by our model, if ClO4− caused a 100% block of thyroidal uptake of dietary iodide. In comparison, for a severely iodide-deficient diet (0.3 μg I/day), it would take 7 days to deplete thyroid stores by 43% and result in a 35% decrease in serum T4 (McLanahan et al. 2008). This degree of perturbation in the HPT axis compares with the 10 mg/kg/day ClO4− dose group after only 1 day of exposure (Yu et al. 2002). In the iodide-deficient BBDR-HPT axis model (McLanahan et al. 2008), the thyroidal iodide stores were closely related to iodide deficiency perturbations in the HPT axis. With ClO4−, it is apparent that the HPT axis is disturbed before sufficient model-predicted depletion of thyroidal iodide stores. For iodide deficiency, slow depletion of thyroidal iodide stores was governed by iodide available for thyroidal uptake and the secretion and metabolism rates of thyroid hormones.
McNabb et al. (2004a, 2004b) reported on the dose-dependent thyroidal depletion of T4 from bobwhite quail chicks ingesting ClO4−. This depletion in thyroidal T4 may have occurred because of the blocking effect of ClO4− on thyroidal uptake of iodide, ClO4− acting on the thyroid gland synthesis and secretion of T4, or a combination of both modes of action. The idea that ClO4− may affect thyroid hormone synthesis/secretion has previously been discussed (Hildebrandt and Halmi 1981; Wolff 1998). Greer et al. (1966) provided experimental evidence for a direct effect on ClO4− on the rat thyroid gland in vitro. These authors reported that ClO4− reduced formation of MIT and DIT in rat thyroid lobes when incubated with ClO4−. They observed changes in MIT and DIT formation for ClO4− media concentrations starting at 10 mg/L with a reported 50% effective reduction of DIT at 250 mg/L. By comparison, in vivo, rats ingesting 1, 3, and 10 mg/kg/day ClO4− in drinking water had measured serum ClO4− concentrations of approximately 0.3, 1.4, and 4.9 mg/L and thyroidal ClO4− concentrations of 10, 50, and 176 mg/kg, respectively (Yu et al. 2002). Although difficult to compare, the in vitro media and in vivo serum ClO4− concentrations suggest that the in vitro ClO4−-induced changes in organification may be a high-dose effect of ClO4−. Nevertheless, the data reported by Greer et al. (1966) demonstrate that interactions of ClO4− and thyroid gland processes are possible.
Interestingly, Yu et al. (2002) also reported that when rats were administered an intravenous dose of 3 mg/kg of ClO4−, serum TSH and TT4 concentrations were at control levels 8 hr postdosing, but by 12 hr serum TSH concentrations increased and serum TT4 concentrations decreased. The secretion rate of iodide (as thyroid hormones) is about 0.5–1 μg over a 12-hr period, which suggests that thyroidal iodide stores may be depleted by only 5–8% when HPT axis perturbations were observed.
Männistö et al. (1979) administered propylthiouracil (PTU), MMI, potassium perchlorate (KClO4), and KI to rats in drinking water and measured serum TSH and TT4 at several time points over 14 days of treatment. KI and KClO4 both rapidly depleted serum TT4 concentrations by day 2 of treatment. KI animals recovered by day 4 of treatment, whereas the KClO4 rats did not, and serum TT4 concentrations remained low, similar to MMI and PTU animals, over 14 days of treatment. Excess iodide is well known for this Wolff-Chaikoff effect, which is associated with a transient “shutdown” in the thyroid gland (Wolff and Chaikoff 1948). MMI and PTU interact with thyroid peroxidase in vitro, but ClO4− does not (Magnusson et al. 1984).
A recent in vitro study conducted by Tonacchera et al. (2004) in Chinese hamster ovary cells transfected with human NIS affirm that ClO4− inhibits iodide uptake in a competitive fashion, with potencies 15, 30, and 240 times greater than the related compounds thiocyanate (SCN−), iodide, and nitrate (NO3−), respectively. The relative concentrations of these monovalent anions determine the ability of NIS to transport iodide, and because SCN− and NO3− are present in human sera at greater concentrations than ClO4−, several researchers (De Groef et al. 2006; Gibbs 2006) have suggested that SCN− and NO3− are more likely than ClO4− to adversely affect (e.g., correlate with) thyroid function. However, Blount et al. (2006) reported correlations between ClO4− and serum TT4 and TSH, no correlation with nitrate, but a negative association of TT4 with thiocyanate. That a decrease in serum TT4 and increase in serum TSH correlated with ClO4− but not with the other monovalent anions suggests that ClO4− may act through another MOA in addition to simple competitive inhibition.
TSH secretion from the pituitary is controlled by thyroid-releasing hormone (TRH) from the hypothalamus, and secretion of both TSH and TRH is partially controlled by thyroid hormones derived from serum. Using a quantitative evaluation of the pulsatile nature of the HPT axis, Li et al. (1995) suggest that the feedback effects of thyroid hormones on the hypothalamus and control of TRH secretion are much smaller than effects on the pituitary for control of TSH secretion. However, if TRH secretion is high, TRH may dominate the control of TSH secretion. In the case of ClO4−, the current BBDR–HPT axis model, calibrated for iodide deficiency, would need to be calibrated to explicitly include differential control of TSH secretion by both TRH and thyroid hormones.
Hypothalamic TRH does drive pituitary TSH secretion; however, details in rats, humans, and other species are not sufficient to distinguish between hypothalamic- and pituitary-derived TSH secretion. Measuring pituitary portal blood TRH is not realistic. A few attempts in rodents, using extreme surgical techniques, have been carried out, but not in reference to perturbations of the HPT axis. This aspect of HPT axis regulation remains a data gap. Eisenberg et al. (2008) recently published a control systems simulation model of the HPT axis feedback system in humans. They also did not include the hypothalamic aspect in their feedback simulator because of a lack of information. In our BBDR-HPT axis model (McLanahan et al. 2008), the TRH aspect of TSH production is embedded or lumped with the equation that describes the relationship between serum TSH and TT4 concentrations (Equation 3). Eisenberg et al. (2008) also lumped the hypothalamic contribution to describe TSH secretion in humans.
In conclusion, our computational analysis indicates that ClO4− alters the HPT axis by blocking iodide transport and by interacting in some fashion with hormone production/release from the thyroid. The present description of synthesis and secretion of thyroid hormones (Equation 3) is simple, and if the thyroid gland itself is a target of ClO4−, as the modeling suggests, then a more complex description of the thyroid hormone synthesis and secretion process will be required. Our goal in this effort was to impose “nonmechanistic” outcomes to better understand what conditions may help explain the observed ClO4− dose response. A significant correlation of effects of ClO4− with serum thyroid hormones in humans has yet to be confirmed, so the importance of this potential MOA in humans is not known. Computational models can provide quantitative tools to evaluate possible MOAs of ClO4− on the HPT axis. This evaluation would be supported by conducting specific studies to develop a quantitative mathematical description of the MOA, along with studies that measure temporal perturbations in several HPT axis end points. Studies are needed that evaluate the molecular biology of the genes within the thyroid gland, coupled with measurement of thyroid hormone precursors and thyroid hormones using modern analytical techniques such as liquid chromatography/mass spectrometry. These studies need to be completed for known iodine intake rates along with sufficient doses and time points to construct a temporal dose–response curve after ClO4− exposure via drinking water.
Footnotes
Supplemental Material is available online at http://www.ehponline.org/members/2009/0800111/suppl.pdf
Funding was provided by a U.S. Environmental Protection Agency (EPA) Science to Achieve Results (STAR) research grant (RD83213401-0) and a U.S. EPA STAR fellowship (FP-91679301-0 to E.D.M.). Views expressed in this article are those of the authors and do not represent official opinions of the U.S. EPA.
References
- Alexander NM. Iodide peroxidase in rat thyroid and salivary glands and its inhibition by antithyroid compounds. J Biol Chem. 1959;234:1530–1533. [PubMed] [Google Scholar]
- Anbar M, Guttmann S, Lewitus Z. The mode of action of perchlorate ions on the iodine uptake of the thyroid gland. Int J Appl Radiat Iso. 1959;7:87–96. doi: 10.1016/0020-708x(59)90153-x. [DOI] [PubMed] [Google Scholar]
- Blount BC, Pirkle JL, Osterloh JD, Valentin-Blasini L, Caldwell KL. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect. 2006;114:1865–1871. doi: 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Delp M, Lindstedt S, Rhombert L, Belies R. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- Chow SY, Chang LR, Yen MS. A comparison between the uptakes of radioactive perchlorate and iodide by rat and guinea-pig thyroid glands. J Endocrinol. 1969;45:1–8. doi: 10.1677/joe.0.0450001. [DOI] [PubMed] [Google Scholar]
- Chow SY, Woodbury DM. Kinetics of distribution of radioactive perchlorate in rat and guinea-pig thyroid glands. J Endocrinol. 1970;47:207–218. doi: 10.1677/joe.0.0470207. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Merrill EA, Narayanan L, Gearhart JM, Robinson PJ. Evidence for competitive inhibition of iodide uptake by perchlorate and translocation of perchlorate into the thyroid. Int J Toxicol. 2004;23:17–23. doi: 10.1080/10915810490275044. [DOI] [PubMed] [Google Scholar]
- Crump KS, Gibbs JP. Benchmark calculations for perchlorate from three human cohorts. Environ Health Perspect. 2005;113:1001–1008. doi: 10.1289/ehp.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta PK, Martinelango PK, Jackson WA, Anderson TA, Tian K, Tock RW, et al. The origin of naturally occurring perchlorate: the role of atmospheric processes. Environ Sci Technol. 2005;39:1569–1575. doi: 10.1021/es048612x. [DOI] [PubMed] [Google Scholar]
- De Groef B, Decallonne BR, Van der Geyen S, Darras VM, Bouillon R. Perchlorate versus other environmental sodium/iodide symporter inhibitors: potential thyroid-related health effects. Eur J Endocrinol. 2006;155:17–25. doi: 10.1530/eje.1.02190. [DOI] [PubMed] [Google Scholar]
- DiStefano JJ, III, Landaw EM. Multiexponential, multi-compartmental, and noncompartmental modeling. I. Methodological limitations and physiological interpretations. Am J Physiol. 1984;246:R651–R664. doi: 10.1152/ajpregu.1984.246.5.R651. [DOI] [PubMed] [Google Scholar]
- Dohan O, Portulano C, Basquin C, Reyna-Neyra A, Amzel LM, Carrasco N. The Na+/I− symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc Natl Acad Sci USA. 2007;104:20250–20255. doi: 10.1073/pnas.0707207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg M, Samuels M, DiStefano JJ. Extensions, validation, and clinical applications of a feedback control system simulator of the hypothalamic-pituitary-thyroid axis. Thyroid. 2008;18(10):1071–1085. doi: 10.1089/thy.2007.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Aribi H, Le Blanc YJ, Antonsen S, Sakuma T. Analysis of perchlorate in foods and beverages by ion chromatography coupled with tandem mass spectrometry (IC-ESI-MS/MS) Anal Chim Acta. 2006;567:39–47. doi: 10.1016/j.aca.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Eng PH, Cardona GR, Fang SL, Previti M, Alex S, Carrasco N, et al. Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology. 1999;140:3040–3410. doi: 10.1210/endo.140.8.6893. [DOI] [PubMed] [Google Scholar]
- Everett NB, Simmons B, Lasher EP. Distribution of blood (Fe59) and plasma (I131) volumes of rats determined by liquid nitrogen freezing. Circ Res. 1956;4:419–424. doi: 10.1161/01.res.4.4.419. [DOI] [PubMed] [Google Scholar]
- Fisher JW, Campbell J, Muralidhara S, Bruckner JV, Ferguson D, Mumtaz M, et al. Effect of PCB 126 on hepatic metabolism of thyroxine and perturbations in the hypothalamic-pituitary-thyroid axis in the rat. Toxicol Sci. 2006;90:87–95. doi: 10.1093/toxsci/kfj069. [DOI] [PubMed] [Google Scholar]
- Fisher J, Todd P, Mattie D, Godfrey D, Narayanan L, Yu K. Preliminary development of a physiological model for perchlorate in the adult rat: a framework for further studies. Drug Chem Toxicol. 2000;23:243–258. doi: 10.1081/dct-100100113. [DOI] [PubMed] [Google Scholar]
- Gibbs JP. A comparative toxicological assessment of perchlorate and thiocyanate based on competitive inhibition of iodide uptake as the common mode of action. Hum Ecol Risk Assess. 2006;12:157–173. [Google Scholar]
- Gluzman BE, Niepomniszcze H. Kinetics of iodide trapping mechanism in normal and pathological human thyroid slices. Acta Endocrinol. 1983;103:34–39. doi: 10.1530/acta.0.1030034. [DOI] [PubMed] [Google Scholar]
- Goldman SJ, Stanbury JB. The metabolism of perchlorate in the rat. Endocrinology. 1973;92:1536–1538. doi: 10.1210/endo-92-5-1536. [DOI] [PubMed] [Google Scholar]
- Greer MA, Goodman G, Pleus RC, Greer SE. Health effects assessment for environmental contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ Health Perspect. 2002;110:927–937. doi: 10.1289/ehp.02110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer MA, Stott AK, Milne KA. Effects of thiocyanate, perchlorate and other anions on thyroidal iodine metabolism. Endocrinology. 1966;79:237–247. doi: 10.1210/endo-79-2-237. [DOI] [PubMed] [Google Scholar]
- Hildebrandt JD, Halmi NS. Intrathyroidally generated iodide: the role of transport in its utilization. Endocrinology. 1981;108:842–849. doi: 10.1210/endo-108-3-842. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Sasaki N, Hai N, Sugawa H, Aoki N, Shigemasa C, et al. Establishment and characterization of a Chinese hamster ovary cell line, CHO-4J, stably expressing a number of Na+/I− symporters. Biochem Biophys Res Commun. 1996;227:94–101. doi: 10.1006/bbrc.1996.1473. [DOI] [PubMed] [Google Scholar]
- Li G, Liu B, Liu Y. A dynamical model of the pulsatile release the hypothalamo-pituitary-thyroid axis. BioSystems. 1995;35:83–92. doi: 10.1016/0303-2647(94)01484-o. [DOI] [PubMed] [Google Scholar]
- Lorber M. Use of a simple pharmacokinetic model to characterize exposure to perchlorate. J Exp Sci Environ Epidemiol. 2008 doi: 10.1038/jes.2008.8. [Online 16 April 2008] [DOI] [PubMed] [Google Scholar]
- Magnusson RP, Taurog A, Dorris ML. Mechanism of iodide-dependent catalatic activity of thyroid peroxidase and lactoperoxidase. J Biol Chem. 1984;259:197–205. [PubMed] [Google Scholar]
- Malendowicz LK, Bednarek J. Sex dimorphism in the thyroid gland IV. Cytologic aspects of sex dimorphism in the rat thyroid gland. Acta Anat. 1986;127:115–118. doi: 10.1159/000146273. [DOI] [PubMed] [Google Scholar]
- Männistö PT, Ranta T, Leppäluoto J. Effects of methyl-mercaptoimidazole (MMI), propylthiouracil (PTU), potassium perchlorate (KClO4) and potassium iodide (KI) on the serum concentrations of thyrotrophin (TSH) and thyroid hormones in the rat. Acta Endocrinol. 1979;91:271–281. doi: 10.1530/acta.0.0910271. [DOI] [PubMed] [Google Scholar]
- McLanahan ED, Andersen ME, Fisher JW. A biologically based dose-response model for dietary iodide and the hypothalamic-pituitary-thyroid axis in the adult rat: evaluation of iodide deficiency. Toxicol Sci. 2008;102:241–253. doi: 10.1093/toxsci/kfm312. [DOI] [PubMed] [Google Scholar]
- McLanahan ED, Campbell JL, Jr, Ferguson DC, Harmon B, Hedge JM, Crofton KM, et al. Low-dose effects of ammonium perchlorate on the hypothalamic-pituitary-thyroid (HPT) axis of adult male rats pretreated with PCB126. Toxicol Sci. 2007;97:308–317. doi: 10.1093/toxsci/kfm063. [DOI] [PubMed] [Google Scholar]
- McNabb FM, Jang DA, Larsen CT. Does thyroid function in developing birds adapt to sustained ammonium perchlorate exposure? Toxicol Sci. 2004a;82:106–113. doi: 10.1093/toxsci/kfh247. [DOI] [PubMed] [Google Scholar]
- McNabb FM, Larsen CT, Pooler PS. Ammonium perchlorate effects on thyroid function and growth in bobwhite quail chicks. Environ Toxicol Chem. 2004b;23:997–1003. doi: 10.1897/03-362. [DOI] [PubMed] [Google Scholar]
- Merrill EA, Clewell RA, Gearhart JM, Robinson PJ, Sterner TR, Yu KO, et al. PBPK predictions of perchlorate distribution and its effect on thyroid uptake of radioiodide in the male rat. Toxicol Sci. 2003;73:256–269. doi: 10.1093/toxsci/kfg080. [DOI] [PubMed] [Google Scholar]
- Motzer WE. Perchlorate: problems, detection, and solutions. Environ Foren. 2001;2:301–311. [Google Scholar]
- National Research Council. Health Implications of Perchlorate Ingestion. Washington, DC: National Academies Press; 2005. [Google Scholar]
- Okabe N, Hokaze M. Effect of inorganic anions on the binding of thyroxine by bovine serum albumin. Chem Pharm Bull. 1993;41:430–432. doi: 10.1248/cpb.41.430. [DOI] [PubMed] [Google Scholar]
- Okamura K, Taurog A, Krulich L. Strain differences among rats in response to Remington iodine-deficient diets. Endocrinology. 1981;109:458–463. doi: 10.1210/endo-109-2-458. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH. The nuclear receptor-triiodothyronine complex: relationship to thyroid hormone distribution, metabolism, and biological action. In: Oppenheimer JH, Samuels HH, editors. Molecular Basis of Thyroid Hormone Action. New York: Academic Press; 1983. pp. 1–34. [Google Scholar]
- Ortiz-Caro J, Pastor RM, Jolin T. Effects of KClO4 in propylthiouracil-hypothyroid rats. Acta Endocrinol. 1983;103:81–87. doi: 10.1530/acta.0.1030081. [DOI] [PubMed] [Google Scholar]
- Riedel C, Levy O, Carrasco N. Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem. 2001;276:21458–21463. doi: 10.1074/jbc.M100561200. [DOI] [PubMed] [Google Scholar]
- Snyder SA, Pleus RC, Vanderford BJ, Holady JC. Perchlorate and chlorate in dietary supplements and flavor enhancing ingredients. Anal Chim Acta. 2006;567:26–32. doi: 10.1016/j.aca.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14:1012–1019. doi: 10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- Tran N, Valentin-Blasini L, Blount BC, McCuistion CG, Fenton MS, Gin E, et al. Thyroid-stimulating hormone increases active transport of perchlorate into thyroid cells. Am J Physiol Endocrinol Metab. 2008;294:E802–E806. doi: 10.1152/ajpendo.00013.2008. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Perchlorate Environmental Contamination: Toxicological Review and Risk Characterization. Washington, DC: U.S. Environmental Protection Agency; 2002. NCEA-01-0503 (External Review Draft) [Google Scholar]
- Wolff J. Perchlorate and the thyroid gland. Pharmacol Rev. 1998;50:89–105. [PubMed] [Google Scholar]
- Wolff J, Chaikoff IL. Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem. 1948;174:555–564. [PubMed] [Google Scholar]
- Yamada T. Effects of perchlorate and other anions on thyroxine metabolism in the rat. Endocrinology. 1967;81:1285–1290. doi: 10.1210/endo-81-6-1285. [DOI] [PubMed] [Google Scholar]
- Yu KO, Narayanan L, Mattie DR, Godfrey RJ, Todd PN, Sterner TR, et al. The pharmacokinetics of perchlorate and its effect on the hypothalamus-pituitary-thyroid axis in the male rat. Toxicol Appl Pharmacol. 2002;182:148–159. doi: 10.1006/taap.2002.9432. [DOI] [PubMed] [Google Scholar]