Abstract
Background
Ambient particulate matter (PM) air pollution is associated with coronary heart disease, but the pathways underlying the association remain to be elucidated.
Methods
We studied the association between PM and ischemia among 57,908 Women’s Health Initiative clinical trial participants from 1999–2003. We used the Minnesota Code criteria to identify ST-segment and T-wave abnormalities, and estimated T amplitude (microvolt) from resting, standard 12-lead electrocardiogram (ECG). We used U.S. Environmental Protection Agency’s monitor data to estimate concentrations of PM < 2.5 μm (PM2.5) at geocoded participant addresses over 6 days before the ECGs (lag0 through lag5). We excluded 2,379 women with ECG QRS duration ≥ 120 msec.
Results
Overall, 6% of the remaining 55,529 women (52–90 years of age; 83% non-Hispanic white) had ST abnormalities and 16% had T abnormalities. Lead-specific T amplitude was normally distributed (range of means from −14 to 349 μV). PM2.5 (mean ± SD) averaged over lag0–2 was 14 ± 7 μg/m3. In logistic and linear regression models adjusted for demographic, clinical, temporal, and climatic factors, a 10-μg/m3 increase in lag0–2 PM2.5 was associated with a 4% [95% confidence interval (CI), −3%, to 10%] increase in the odds of ST abnormality and a 5% (95% CI, 0% to 9%) increase in the odds of T abnormality. We observed corresponding decreases in T amplitude in all exam sites and leads except lead V1, reaching a minimum of −2 μV (95% CI, −5 to 0 μV) in lead V3.
Conclusions
Short-term PM2.5 exposure is associated with ECG evidence of myocardial ischemia among postmenopausal women. The principal manifestations include subclinical but potentially arrhythmogenic ST–T abnormalities and decreases in T amplitude.
Keywords: air pollution, cardiovascular disease, electrocardiography, myocardial ischemia, particulate matter
Studies of human populations show that ambient particulate matter (PM) air pollution is associated with coronary heart disease (CHD) morbidity and mortality. The consistent findings suggest direct associations between both short- and long-term exposure to PM and CHD incident [Brook et al. 2004; Dockery et al. 1993; Miller et al. 2007; Pope et al. 2006; U.S. Environmental Protection Agency (EPA) 2006]. However, biological mechanisms underlying the association between PM and CHD remain incompletely characterized in human populations despite calls for epidemiologic research designed to help elucidate them (Brook et al. 2004; Health Effects Institute 2002; National Research Council 2004).
Resting, supine, standard 12-lead electro-cardiography (ECG) has been recognized as a potentially valuable epidemiologic tool in this regard, yet to date much of its value remains unrealized. ECG findings characteristic of myocardial ischemia, for example, have not been adequately evaluated in this context. These measures include ST-segment depression and T-wave inversion on the ECG.
Because of their clinical value, these ECG findings have also attracted environmental, toxicologic, and epidemiologic interest. Indeed, they are thought to be relevant in studies focusing on arrhythmogenic effects of air pollution (Zareba et al. 2001) and risk factors for sudden death due to cardiac arrhythmia (Huikuri et al. 2001). The literature in this area has nevertheless focused on experimentation in animal models of coronary vasoconstriction (Campen et al. 2005; Godleski et al. 2000; Nikolov et al. 2007; Wellenius et al. 2003) or examination of typically small, single-city panels of CHD-burdened, Holter-monitored male participants during exercise (Gold et al. 2005; Henneberger et al. 2005; Lanki et al. 2006; Mills et al. 2007; Pekkanen et al. 2002; Yue et al. 2007). For this reason, epidemiologic evidence linking PM exposure to minor but prognostically important abnormalities of the ST segment and T wave in human populations at rest is quite limited.
We therefore designed the present study to investigate the association between acute exposures to ambient PM < 2.5 μm in diameter (PM2.5) and ECG measures of myocardial ischemia in a relatively large, geographically diverse, healthy female population enrolled in the Women’s Health Initiative (WHI) clinical trials.
Methods
Study design
We examined the association between PM2.5 and ischemia in the Environmental Epidemiology of Arrhythmogenesis in WHI (EEAWHI) study, an ancillary study of proarrhythmic mechanisms linking air pollution and CHD among 68,133 WHI clinical trial participants examined at 49 sites. The WHI clinical trial is a multicenter study of risk factors for prevention of common causes of mortality, morbidity, and impaired quality of life in U.S. postmenopausal women. Details of the study design, including recruitment procedures and selection and exclusion criteria, have been published elsewhere (WHI Study Group 1998). The study was approved by each study site’s institutional review board. All participants provided written informed consent.
Study population
Of the 68,133 WHI clinical trial participants at baseline, we excluded 2,082 (3.1%) with foreign, U.S. military, U.S. protectorate, Hawaiian, Alaskan, or missing addresses; 417 (0.6%) with missing or poor-quality ECGs; 2,379 (3.5%) with ECG QRS duration ≥ 120 msec; and 7,726 (11%) without an ECG recording between 1999 and 2003, the initial 5-year period in which PM2.5 data were routinely collected by the U.S. EPA Air Quality System (AQS). The present study focuses on the initial high-quality, resting, standard 12-lead ECGs recorded during this period among the remaining 55,529 participants, all of whom had addresses in the contiguous United States.
ECG methodology
Identical ECGs (MAC PC, Marquette Electronics, Inc., Milwaukee, WI) were used in all clinic sites, and resting, standard 12-lead ECGs were recorded for all participants using strictly standardized procedures. The chest electrodes were located in precise positions. All ECGs were processed in a central ECG laboratory (Epidemiological Cardiology Research Center, Wake Forest University, Winston-Salem, NC), where they were visually inspected for technical errors and inadequate quality. The ECGs were initially processed with the Dalhousie ECG program, and for the present study we reprocessed them with the 2001 version of the GE Marquette 12-SL program (GE Healthcare 2008; Prineas et al. 1982; Rautaharju et al. 1998). The Marquette measurement matrix contained several ECG measures of myocardial ischemia that we used as outcome variables in this study.
ST-segment and T-wave amplitudes (microvolt)
We defined lead-specific depth (or height) of the ST segment (ST) 60 msec after the J point and T-wave amplitude (T) relative to the isoelectric line.
ST, T, and ST–T abnormalities
We applied Minnesota Code (MC) criteria to the former interval-scale measures to identify ST abnormality (MC 4.1–4.4), T abnormality (MC 5.1–5.4), or any ST–T abnormality (MC 4.1–4.4 or MC 5.1–5.4) (Prineas et al. 1982).
QRS/T angles (degrees)
The frontal plane QRS/T angle was defined as the absolute value of the difference between the frontal plane QRS and T axes (range, −89° to 270°). When > 180°, it was adjusted to the minimal angle as follows: 360° – frontal plane QRS/T angle (Zhang et al. 2007). We defined the spatial QRS/T angle as the angle between the mean QRS and T vectors, which we calculated from quasi-orthogonal X, Y, and Z leads reconstructed from the standard ECG leads via matrix transformation (Rautaharju et al. 2006b).
Air pollutant concentrations, weather variables, and other participant characteristics
We obtained all ambient PM2.5 concentration data recorded at monitors operating in the contiguous United States during the study from the U.S. EPA AQS. The data included the longitude and latitude of each monitor. We used a semiautomated program built on ArcView GIS (version 8.3) software and its Geostatistical Analyst extension (ESRI, Inc. Redlands, CA) to produce kriging-estimated daily mean concentrations (and their SEs) at each geocoded participant and exam site address from the baseline examination through 2004 (Liao et al. 2006, 2007; Whitsel et al. 2006). The program relied on a spherical model to perform national-scale, lognormal ordinary kriging and the weighted least-squares method to estimate semivariograms, and ran and cross-validated each daily semivariogram. From the kriging-estimated, daily mean, location-specific PM2.5 concentrations, we then computed a matrix of 15 short-term pollutant exposures by averaging within overlapping 1-, 2-, and 3-day lag combinations inside a 6-day exposure window beginning 5 days before and ending on the examination date, that is, days 0–5. Associations between PM and ischemia were strongest on days 0–2 (lag0–2), the emphasis of the present report.
We obtained all meteorologic data recorded at relevant stations and times from the National Climatic Data Center (U.S. National Climatic Data Center 2007): ambient temperature (degrees centigrade), dew point (degrees centigrade), and barometric pressures (kilo pascal), as well as station longitudes, latitudes, and altitudes. Daily mean temperature, dew point, and pressure at each geocoded participant and exam site address from baseline to closeout were estimated by averaging daily means across all stations within 50 km, a distance over which their station-to-station correlations exceed 0.90 (Ito et al. 2001). Thereafter, we computed weather variables at lag0–2/lag3–5 as described above for PM2.5.
Self-reported education, medication use, health history, and a variety of other attributes were determined at each visit by standardized participant interview and examination by the WHI staff. Interim health events were identified via standardized medical record review and physician adjudication. Specifically, diabetes was defined by antidiabetic medication use or history; hypertension by antihypertensive medication use, systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or history; body mass index (BMI) as the ratio of weight (kilograms) to height (square meters); hypercholesterolemia by antihyperlipidemic medication use or history; smoking as current, former, or never; chronic lung disease by history of asthma, emphysema, or lung cancer; CHD by antianginal medication use, history of angina, or myocardial infarction and medical record review/adjudication; revascularization by history of coronary artery angioplasty, stent, or bypass and medical record review/adjudication; and congestive heart failure by cardiac glycoside/diuretic use, history, and medical record review/adjudication.
Statistical analysis methods
We inspected frequency distributions of all variables for outliers but did not identify influential values. Initially ignoring the influences of exam site, we explored the associations between ischemia and PM2.5 in unadjusted linear and logistic regression models. To control for residual confounding by exam season, day of week, time of day, health, and weather, we added temporal, demographic, clinical, and meteorologic characteristics to the models. We then conducted hierarchical analyses in two stages. In the first stage we used site-specific, adjusted regression models to estimate strength of the ischemia–PM2.5 associations. The models allowed these estimates to vary among sites. In the second stage we used random effects meta-regression of the site-specific, linear regression coefficients from the first stage to estimate overall strength of the ischemia–PM associations across sites. These weighted, iterative regression models allowed for an additive, between-study component of residual variance estimated via restricted maximum likelihood (Berkey et al. 1995). We repeated the second-stage analyses by placing uninformative priors on the second-stage parameters and Monte Carlo sampling from the conditional distribution of each unknown using standard Bayesian hierarchical regression algorithms (Robert and Casella 2004). We evaluated effect measure modification by including cross-product terms for the interaction between PM2.5 and CHD and its major risk factors at the first stage. Analyses based on logistic and linear regression models adjusting for exam site, and both the random-effects meta-analyses and Bayesian hierarchical regression analyses of the adjusted, site-specific regression coefficients produced similar results. We conducted all analyses using SAS version 9.1 (SAS Institute, Inc., Cary, NC) and MATLAB, version 7.0, software (MathWorks, Inc., Natick, MA). By convention, we report resulting estimates as odds ratios for ST, T, and ST–T abnormalities, or microvolt (μV) changes in ST-segment and T-wave amplitudes associated with 10 μg/m3 increases in PM2.5 concentrations.
Results
Of the 55,529 female participants (age range, 52–90 years), 83% were non-Hispanic white, 52% hypertensive, and 20% hypercholesterolemia (Table 1). Somewhat smaller percentages were current smokers (6%) or had diabetes (8%), CHD (11%), or chronic lung disease (10%). Six percent of the study population had ST abnormalities, and 16% had T abnormalities. Lead-specific ST and T amplitudes were normally distributed (range of means = 4, 64 μV and −14, 349 μV, respectively). Mean ± SD spatial and frontal plane QRS/T angles were 60° ± 28° and 29° ± 27°, with heart rate 66 ± 10 beats/min (Table 2). Mean PM2.5 averaged over the first 3 days and second 3 days preceding the ECG (lag0–2 and lag3–5) were equivalent, as were mean temperature, dew point, and barometric pressure (Table 3).
Table 1.
The demographic and clinical characteristics of the study population on the date of ECG examination (n = 55,529).
| Characteristic | Mean ± SD or percent |
|---|---|
| Age in years on ECG exam date | 67 ± 7 |
| Race | |
| White | 83 |
| Black | 10 |
| Other | 7 |
| Education < college | 23 |
| BMI (kg/m2) | 29 ± 6 |
| SBP (mmHg) | 126 ± 17 |
| DBP (mmHg) | 73 ± 9 |
| Hypertension | 52 |
| Hypercholesterolemia | 20 |
| Diabetes | 8 |
| Current smoking | 6 |
| CHDa | 11 |
| Chronic lung disease | 10 |
| Beta-blocker use | 13 |
| Exam season | |
| Spring | 27 |
| Summer | 26 |
| Fall | 25 |
| Winter | 22 |
| Exam day | |
| Monday–Thursday | 86 |
| Friday | 12 |
| Saturday–Sunday | 2 |
| Exam time | |
| Morning | 64 |
| Afternoon | 36 |
CHD includes congestive heart failure, coronary revascularization, or myocardial infarction by Minnesota Code or Novacode.
Table 2.
The ECG parameters of the study population on the date of ECG examination (n = 55,529).
| ECG parameter | Mean ± SD or percent |
|---|---|
| Minnesota codes | |
| MC 4a | 6 |
| MC 5b | 16 |
| MC 4 or MC 5c | 18 |
| T-wave amplitude (μV) | |
| Lead I | 202 ± 105 |
| Lead II | 241 ± 105 |
| Lead aVL | 86 ± 93 |
| Lead V1 | −14 ± 116 |
| Lead V2 | 313 ± 197 |
| Lead V3 | 349 ± 203 |
| Lead V4 | 324 ± 193 |
| Lead V5 | 284 ± 167 |
| Lead V6 | 221 ± 126 |
| ST-segment amplitude (μV) | |
| Lead I | 13 ± 21 |
| Lead II | 19 ± 25 |
| Lead aVL | 4 ± 17 |
| Lead V1 | 27 ± 27 |
| Lead V2 | 64 ± 46 |
| Lead V3 | 47 ± 41 |
| Lead V4 | 29 ± 35 |
| Lead V5 | 18 ± 29 |
| Lead V6 | 11 ± 23 |
| QRS/T angles and heart rate | |
| Heart rate (beats/min) | 66 ± 10 |
| QRS/T angle–spatial (°) | 60 ± 28 |
| QRS/T angle–frontal plane (°) | 29 ± 27 |
Any ST abnormality (MC 4.1–4.4).
Any T abnormality (MC 5.1–5.4).
Any ST–T abnormality (MC 4.1–4.4 or MC 5.1–5.4).
Table 3.
PM concentrations and weather variables for the study population on the date of ECG examination.
| Characteristic | Mean ± SD |
|---|---|
| PM2.5a | |
| Lag0 | 14.1 ± 8 |
| Lag1 | 13.8 ± 8 |
| Lag2 | 13.8 ± 8 |
| Lag3 | 13.8 ± 8 |
| Lag4 | 13.9 ± 8 |
| Lag5 | 14.1 ± 8 |
| Lag0–2b | 13.9 ± 7 |
| Weather | |
| Temperature lag0–2b | 13.9 ± 9 |
| Dew point lag0–2b | 7.6 ± 9 |
| Barometric pressure lag0–2b | 102 ± 1 |
Concentrations at geocoded participant addresses on day of exam (lag0) and preceding 5 days (lag1–5).
The mean ± SD for PM2.5 and weather variables were the same at lag3–5.
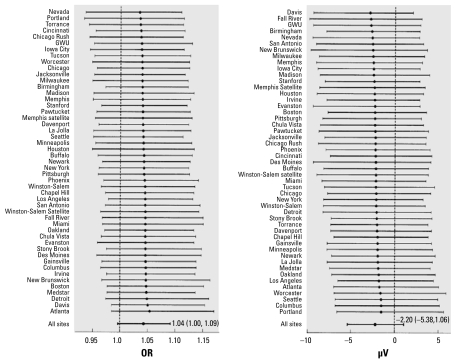
In logistic and linear regression models adjusted for demographic, clinical, temporal, and climatic factors, 10 μg/m3 increases in lag0–2 PM2.5 were associated with 4–5% increases in odds ratios (ORs) for ST, T, and ST–T abnormalities, small decreases in lead-specific ST and T amplitudes, and slight increases in the QRS/T angles and heart rate (Table 4). We observed decreases in T amplitude in all leads except V1, reaching a minimum of −2.3 μV [95% confidence interval (CI), −5.2 to 0.5 μV] in lead V3 even after controlling for its reported dependence on heart rate (Couderc et al. 2007). These observations were consistent across exam sites (Figure 1). The observed magnitude and direction of effects associated with lag3–5 PM2.5 were often weaker and less consistent [Table 4 and Supplemental Material, Tables 1 and 2 (available online at http://www.ehponline.org/members/2009/0800046/suppl.pdf)]. Findings based on unadjusted models and those controlling for randomization status as well as additional participant- and contextual- level measures of education, occupation, income, and housing were comparable.
Table 4.
Multivariable-adjusteda ORs and changes (95% CI) per 10-μg/m3 increase in PM2.5.
| ECG parameter | Lag0–2 (95% CI) | Lag3–5 (95% CI) |
|---|---|---|
| Minnesota Codes (OR) | ||
| MC 4b | 1.04 (0.97 to 1.10) | 1.04 (0.98 to 1.11) |
| MC 5c | 1.05 (1.00 to 1.09) | 1.04 (1.00 to 1.08) |
| MC 4 or MC 5d | 1.04 (1.00 to 1.09) | 1.03 (0.99 to 1.07) |
| ST-segment amplitude (μV, change) | ||
| Lead I | −0.07 (−0.36 to 0.21) | 0.18 (−0.10 to 0.46) |
| Lead II | −0.12 (−0.47 to 0.23) | 0.16 (−0.18 to 0.50) |
| Lead aVL | −0.01 (−0.25 to 0.23) | 0.11 (−0.12 to 0.34) |
| Lead V1 | −0.02 (−0.39 to 0.35) | −0.22 (−0.58 to 0.14) |
| Lead V2 | 0.07 (−0.57 to 0.70) | −0.01 (−0.61 to 0.62) |
| Lead V3 | −0.11 (−0.68 to 0.47) | −0.02 (−0.58 to 0.54) |
| Lead V4 | −0.03 (−0.51 to 0.45) | 0.24 (−0.23 to 0.71) |
| Lead V5 | −0.01 (−0.41 to 0.39) | 0.35 (−0.04 to 0.74) |
| Lead V6 | 0.02 (−0.30 to 0.33) | 0.35 ( 0.04 to 0.65) |
| T-wave amplitude (μV, change) | ||
| Lead I | −1.60 (−3.07 to −0.13) | −0.31 (−1.73 to 1.11) |
| Lead II | −0.54 (−1.99 to 0.92) | 0.71 (−0.70 to 2.13) |
| Lead aVL | −1.21 (−2.50 to 0.10) | −0.55 (−1.81 to 0.71) |
| Lead V1 | 1.45 (−0.16 to 3.06) | 0.03 (−1.53 to 1.59) |
| Lead V2 | −0.18 (−2.96 to 2.60) | 0.57 (−2.12 to 3.27) |
| Lead V3 | −2.33 (−5.15 to 0.49) | −0.13 (−2.87 to 2.60) |
| Lead V4 | −2.03 (−4.69 to 0.63) | 0.64 (−1.94 to 3.22) |
| Lead V5 | −1.92 (−4.22 to 0.38) | 0.55 (−1.69 to 2.78) |
| Lead V6 | −0.63 (−2.36 to 1.10) | 0.82 (−0.86 to 2.49) |
| QRS/T angles and heart rate (change) | ||
| QRS/T angle–spatial (°) | 0.19 (−0.21 to 0.59) | −0.20 (−0.59 to 0.19) |
| QRS/T angle–frontal plane (°) | 0.13 (−0.24 to 0.50) | 0.35 (−0.01 to 0.71) |
| Heart rate (beats/min) | 0.16 ( 0.02 to 0.30) | 0.04 (−0.10 to 0.18) |
Estimates based on logistic and linear regression models adjusted for demographic, clinical, and weather variables: age; race/ethnicity; education; exam site; BMI; current smoking status; history of CHD, diabetes, hypertension, SBP, chronic lung disease, or hypercholesterolemia; day of week; time of day; temperature; dew point; pressure and season;
Any ST abnormality (MC 4.1–4.4).
Any T abnormality (MC 5.1–5.4).
Any ST–T abnormality (MC 4.1–4.4 or MC 5.1–5.4).
Figure 1.
Multivariable-adjusted ORs for ST–T abnormality and changes in T amplitude (μV) in lead V3 (95% CI) per 10-μg/m3 increase in lag0–2 PM2.5, by exam site. We based estimates on Bayesian hierarchical regression models adjusted for demographic, clinical, and weather variables: age; race/ethnicity; education; BMI; current smoking status; history of CHD, diabetes, hypertension, SBP, chronic lung disease, or hypercholesterolemia; season; day of week; time of day; temperature; dew point; and pressure.
Effects of lag0–2 PM2.5 were slightly stronger among users versus nonusers of beta-blockers: 10% (1%, 20%) versus 3% (−1%, 8%) increases in the odds of T abnormality (p = 0.098) and −8 (−15, −1) versus −2 (−4, 1) μV decreases in T amplitude in lead V3 (p = 0.076), but in general, there was little suggestion of effect measure modification by CHD or its major risk factors.
Discussion
Judging from the U.S. EPA’s Provisional Assessment of Recent Studies on Health Effects of Particulate Matter Exposure (National Center for Environmental Assessment 2006), interest in subclinical, atherosclerotic manifestations of long-term ambient PM2.5 exposure has dramatically increased. Indeed, the July 2006 assessment included only one such study (of coronary artery calcium), yet in the interim, several others have also examined associations between PM2.5 exposures sustained over months to years and noninvasive measures of atherosclerosis, including carotid intimal-medial thickness, abdominal aortic calcification, and ankle-brachial index (Allen et al. 2009; Diez Roux et al. 2008; Hoffmann et al. 2007; Kunzli et al. 2005). The epidemiologic associations observed in these studies are not entirely consistent with one another or collectively as strong as those that may have been anticipated on the basis of recently reported associations between chronic PM exposure and cardiovascular disease (e.g., Miller et al. 2007), and the role of atherosclerotic mechanisms in the association remains uncertain.
We examined the role of acute, ischemic mechanisms in the association between PM2.5 and CHD among WHI clinical trial participants with this uncertainty in mind, using ECG—a simple, noninvasive, and underused research tool. In this epidemiologic context, however, we did not limit the examination to characteristics of clinically manifest ischemia (e.g., ≥ 0.1 mV of downsloping ST segment depression and/or deep, symmetrical, pre-cordial T-wave inversion). We opted instead to include minor abnormalities of ventricular repolarization (ST–T abnormalities, ST and T amplitudes) and depolarization (QRS/T angles) to increase sensitivity of the ECG for subclinical forms of myocardial ischemia. Using this tool, we found that a 10 μg/m3 increment in lag0–2 PM2.5 is associated with 4–5% increased odds of minor ST, T, and ST–T abnormalities, small decreases in ST and T amplitudes across most leads and exam sites, and corresponding increases in QRS/T angles.
Previous findings from the WHI clinical trials cohort include significant prospective relationships between isolated ST–T abnormalities, lead-specific ST and T amplitudes, and wide QRS/T angle at baseline, and CHD mortality, incident CHD, and congestive heart failure (Rautaharju et al. 2006b). Similar findings also have been described in population-based settings, such as the Atherosclerosis Risk in Communities study (Zhang et al. 2007), Cardiovascular Health Study (Rautaharju et al. 2006a), and Chicago Western Electric Study (Daviglus et al. 1999), among others (Engel et al. 2004; Larsen et al. 2002; Okin et al. 2005, 2007; Schouten et al. 1992). However, the clinical utility of these ECG measures remains limited.
Considering the consistent associations between PM2.5 and the ECG measures of myocardial ischemia observed in this context and the established, prospective association of these measures with CHD, the findings from the present study provide additional support for the hypothesis that subclinical myocardial ischemia has a pathophysiologic role in the adverse effects of acute exposure to PM2.5 on the heart, including predisposition to ventricular arrhythmogenesis and sudden cardiac death (Rubart and Zipes 2005). This is the first study to examine such an association in a large population of postmenopausal women. Indeed, prior studies have focused on either animal models of coronary vasoconstriction (Campen et al. 2005; Godleski et al. 2000; Nikolov et al. 2007; Wellenius et al. 2003) or typically small, single-city panels of CHD-burdened, Holter-monitored male participants during exercise (Gold et al. 2005; Henneberger et al. 2005; Lanki et al. 2006; Mills et al. 2007; Pekkanen et al. 2002; Yue et al. 2007) (Table 5). The findings described herein are nonetheless consistent with and thereby extend the findings previously described in these populations. They do not imply, however, that ischemia is the sole process by which PM2.5 exerts its adverse effects on the heart. Autonomic, thrombotic, endothelial, inflammatory, oxidative, and other mechanisms of disease have all been implicated in cardiovascular effects of PM (Chuang et al. 2007; Mills et al. 2007).
Table 5.
Human studies of association of PM and ischemia.
| Reference | No. | Location | Mean age (years) | Female (%) | Outcomes | Detection method |
|---|---|---|---|---|---|---|
| Pekkanen et al. 2002 | 45 | Helsinki, Finland | 68 | 47 | STD | E Holter |
| Henneberger et al. 2005 | 56 | Efurt, Germany | 66 | 0 | TWA | R Holter |
| Gold et al. 2005 | 24 | Boston, MA, USA | 73 | 75 | STD | E Holter |
| Lanki et al. 2006 | 45 | Helsinki, Finland | 68 | 47 | STD | E Holter |
| Yue et al. 2007 | 56 | Efurt, Germany | 66 | 0 | TWA | R Holter |
| Mills et al. 2007 | 20 | Southampton, UK | 60 | 0 | STD | E Holter |
| Zhang et al. 2008 | 55,529 | 49 exam sitesa | 67 | 100 | STT, STA, TWA | Resting ECG |
Abbreviations: E, exercise; R, rest; STA, ST-segment amplitude; STD, ST-segment depression; STT, ST-segment and T-wave abnormalities; TWA, T-wave amplitude. ECG involved resting, supine, standard 12-lead ECG.
In the contiguous United States.
This study has several limitations that may affect interpretation of its findings. First, it is an ancillary study of participants in the WHI clinical trials. Inference is therefore limited to postmenopausal women 52–90 years of age living in the contiguous 48 United States between 1999 and 2003, the initial 5-year period during which PM2.5 data were routinely collected by the U.S. EPA AQS. Because women in the WHI clinical trials were randomized to estrogen with or without progestin treatment, calcium/vitamin D supplementation, and/or dietary modification, these exposures could have affected the ECG measures examined in this setting. Because ambient PM2.5 concentrations also decreased 11% in the United States between 2000 and 2007 (U.S. EPA 2008a), observed associations between PM and ischemia could be weaker if this study were repeated today. Findings were nevertheless robust to adjustment for randomization status. Moreover, seasonally weighted annual average PM2.5 concentrations in the United States still exceed the 15 μg/m3 national ambient air quality standard at approximately 10% of trend sites (U.S. EPA 2008b).
Second, we did not measure PM2.5 in the personal breathing space of participants. Instead, it was spatially interpolated at each participant’s geocoded address, raising questions about its validity (Szpiro et al. 2007). Third, the associations described herein also may be subject to residual socioeconomic confounding because we defined diabetes and hypercholesterolemia by self-reported history of the disease and/or use of antihyperlipidemic or antidiabetic medications rather than fasting glucose or lipid concentrations, which were only available for 6% site- and race-stratified minority participant oversample.
In the face of these additional limitations, in this study we took several precautions. We carefully established the validity of the geo-codes relative to criterion standards (Whitsel et al. 2006) and the spatial interpolations using standard cross-validation statistics: the prediction error (PE = predicted minus measured pollutant concentration at each monitor site), standardized prediction error (SPE = PE divided by its estimated standard error), root mean square standardized error (RMSS = standard deviation of SPE across sites), root mean square prediction error (RMS = empirical standard error based on the mean square of the predictions), and mathematically calculated standard error (SE). Observed values of PE and SPE near 0 and RMSS near 1, and similarity of RMS and SE provide evidence of model validity (Liao et al. 2006, 2007). In addition, the study documented modest effects of geocoding and kriging error on PM–CHD associations (Crooks et al. 2008; Whitsel et al. 2006). Finally, the study demonstrated high reliability of treated diabetes and hypercholesterolemia (Langer et al. 2003), and we adjusted effect sizes for additional participant- and contextual-level measures of socioeconomic status, noting only small changes as a result of adjustment.
Conclusion
We conclude that short-term exposure to PM2.5 is associated with ECG evidence of myocardial ischemia among postmenopausal women. The principal manifestations include ST-segment and T-wave abnormalities, decreases in ST segment and T amplitude, and increases in QRS/T angles. These findings suggest that the adverse effects of PM2.5 on CHD risk among postmenopausal women may be related to its subclinical, ischemic effects on the myocardium.
Footnotes
Supplemental Material is available online at http://www.ehponline.org/members/2009/0800046/suppl.pdf
We acknowledge the contributions of WHI investigators listed in the Short List of WHI Investigators (http://www.whiscience.org/publications/WHI_investigators_shortlist.pdf).
The Environmental Epidemiology of Arrhythmogenesis in the Women’s Health Initiative (WHI) study is supported by the National Institute of Environmental Health Sciences (5-R01-ES012238); an ancillary study of the WHI program is funded by the National Heart, Lung, and Blood Institute, Department of Health and Human Services.
References
- Allen RW, Criqui MH, Diez Roux AV, Allison M, Shea S, Detrano R, et al. Fine particulate air pollution, proximity to traffic, and aortic atherosclerosis: the Multi-ethnic Study of Atherosclerosis. Epidemiology. 2009;20(2):254–264. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med. 1995;14(4):395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong YL, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Babu NS, Helms GA, Pett S, Wernly J, Mehran R, et al. Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE mice. Toxicol Sci. 2005;88:95–102. doi: 10.1093/toxsci/kfi283. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respi Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- Couderc JP, Vaglio M, Xia X, Mcnitt S, Wicker P, Sarapa N, et al. Impaired T-amplitude adaptation to heart rate characterizes I(Kr) inhibition in the congenital and acquired forms of the long QT syndrome. J Cardiovasc Electrophysiol. 2007;18:1299–1305. doi: 10.1111/j.1540-8167.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- Crooks JL, Whitsel EA, Quibrera PM, Catellier DJ, Liao D, Smith RL. The effect of ignoring interpolation error on the inferred relationship between ambient particulate matter exposure and median RR interval in post-menopausal women [Abstract] Epidemiology. 2008;19(6):S127. [Google Scholar]
- Daviglus ML, Liao Y, Greenland P, Dyer AR, Liu K, Xie X, et al. Prognosis of simple and multiple episodes of isolated nonspecific minor ST-T abnormalities: Chicago Western Electric Study. JAMA. 1999;281:530–536. doi: 10.1001/jama.281.6.530. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Auchincloss AH, Franklin TG, Raghunathan T, Barr RG, Kaufman J, et al. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:667–675. doi: 10.1093/aje/kwm359. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, III, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six US cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Engel G, Beckerman JG, Froelicher VF, Yamazaki T, Chen HA, Richardson K, et al. Electrocardiographic arrhythmia risk testing. Curr Probl Cardiol. 2004;29:365–384. doi: 10.1016/j.cpcardiol.2004.02.007. [DOI] [PubMed] [Google Scholar]
- GE Healthcare. Marquette 12SL ECG Analysis Program. 2008. [[accessed 22 October 2008]]. (416791-004_E_12SL_PhysGd.pdf) Available: http://apps.gehealthcare.com/servlet/ClientServlet?REQ=RNEW&MODALITY=Cardiology.
- Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, et al. Mechanisms of morbidity and mortality from exposure to ambient air particles. Res Rep Health Eff Inst. 2000;(91):5–88. [PubMed] [Google Scholar]
- Gold DR, Litonjua AA, Zanobetti A, Coull BA, Schwartz J, McCollum G, et al. Air pollution and ST-segment depression in elderly subjects. Environ Health Perspect. 2005;113:883–887. doi: 10.1289/ehp.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Effects Institute. Understanding the health effects of components of the particulate matter mix: progress and next steps. [[accessed 1 April 2002]];HEI Perspect. 2002 2:1–20. Available: http://pubs.healtheffects.org/getfile.php?u=244. [Google Scholar]
- Henneberger A, Zareba W, Ibald-Mulli A, Rckerl R, Cyrys J, Couderc JP, et al. Repolarization changes induced by air pollution in ischemic heart disease patients. Environ Health Perspect. 2005;113:440–446. doi: 10.1289/ehp.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Moebus S, Mohlenkamp S, Stang A, Lehmann N, Dragano N, et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345(20):1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- Ito K, Thurston GD, Ndas A, Lippmann M. Monitor-to-monitor temporal correlation of air pollution and weather variables in the North-Central U.S. J Expo Anal Environ Epidemiol. 2001;11(1):21–32. doi: 10.1038/sj.jea.7500144. [DOI] [PubMed] [Google Scholar]
- Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, et al. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9S):S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- Lanki T, de Hartog JJ, Heinrich J, Hoek G, Janssen NA, Peters A, et al. Can we identify sources of fine particles responsible for exercise-induced ischemia on days with elevated air pollution? The ULTRA study. Environ Health Perspect. 2006;114:655–660. doi: 10.1289/ehp.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CT, Dahlin J, Blackburn H, Scharling H, Appleyard M, Sigurd B, et al. Prevalence and prognosis of electrocardiographic left ventricular hypertrophy, ST segment depression and negative T-wave; the Copenhagen City Heart Study. Eur Heart J. 2002;2002;23(4):315–324. doi: 10.1053/euhj.2001.2774. [DOI] [PubMed] [Google Scholar]
- Liao D, Peuquet DJ, Duan Y, Whitsel EA, Dou J, Smith RL, et al. GIS approaches for the estimation of residential-level ambient PM concentrations. Environ Health Perspect. 2006;2006;114:1374–1380. doi: 10.1289/ehp.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Peuquet DJ, Lin H, Duan Y, Whitsel EA, Smith RL, et al. National kriging exposure estimation. Environ Health Perspect. 2007;115:A338–A339. doi: 10.1289/ehp.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson G, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, et al. Ischemic and thrombotic effected of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- National Research Council. Continuing Research Progress. Washington, DC: National Academies Press; 2004. Research Priorities for Airborne Particulate Matter: IV. [Google Scholar]
- Nikolov MC, Coull BA, Catalano PJ, Godleski JJ. An informative Bayesian structural equation model to assess source-specific health effects of air pollution. Biostatistics. 2007;8(3):609–624. doi: 10.1093/biostatistics/kxl032. [DOI] [PubMed] [Google Scholar]
- Okin PM, Roman MJ, Best LG, Lee ET, Galloway JM, Howard BV, et al. C-reactive protein and electrocardiographic ST-segment depression additively predict mortality: the Strong Heart Study. J Am Coll Cardiol. 2005;45(11):1787–1793. doi: 10.1016/j.jacc.2005.02.072. [DOI] [PubMed] [Google Scholar]
- Okin PM, Roman MJ, Lee ET, Galloway JM, Best LG, Howard BV, et al. Usefulness of quantitative assessment of electrocardiographic ST depression for predicting new-onset heart failure in American Indians (from the Strong Heart Study) Am J Cardiol. 2007;100(1):94–98. doi: 10.1016/j.amjcard.2007.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkanen J, Peters A, Hoek G, Tiittanen P, Brunekreef B, de Hartog J, et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: the Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study. Circulation. 2002;106(8):933–938. doi: 10.1161/01.cir.0000027561.41736.3c. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Muhlestein JB, Mary HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease event triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Boston, MA: John Wright PSG; 1982. [Google Scholar]
- Rautaharju PM, Ge S, Nelson JC, Marino Larsen EK, Psaty BM, Furberg CD, et al. Comparison of mortality risk for electrocardiographic abnormalities in men and women with and without coronary heart disease (from the Cardiovascular Health Study) Am J Cardiol. 2006a;97:309–315. doi: 10.1016/j.amjcard.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women. The Women’s Health Initiative. Circulation. 2006b;113:473–480. doi: 10.1161/CIRCULATIONAHA.104.496091. [DOI] [PubMed] [Google Scholar]
- Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol. 1998;31(3):157–187. [PubMed] [Google Scholar]
- Robert CP, Casella G. Monte Carlo Statistical Methods. 2nd ed. New York: Springer Verlag; 2004. [Google Scholar]
- Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten EG, Dekker JM, Pool J, Kok FJ, Simoons ML. Well shaped ST segment and risk of cardiovascular mortality. BMJ. 1992;304(6823):356–359. doi: 10.1136/bmj.304.6823.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpiro AA, Sheppard L, Sampson PD, Kim SY. Validating national kriging exposure estimation [Letter] Environ Health Perspect. 2007;115:A338. doi: 10.1289/ehp.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency) Air Trends National Trends in Particulate Matter Levels . 2008a. [[accessed 4 September 2008]]. Available: http://www.epa.gov/airtrends/pm.html.
- U.S. EPA (Environmental Protection Agency) Air and Radiation National Ambient Air Quality Standards . 2008b. [[accessed 28 March 2008]]. Available: http://www.epa.gov/air/criteria.html.
- U.S. EPA (Environmental Protection Agency) Provisional Assessment of Recent Studies on Health Effects of Particulate Matter Exposure. 2006. [[accessed 1 July 2006]]. Available: http://www.epa.gov/particles/pdfs/ord_report_20060720.pdf.
- U.S. National Climatic Data Center. Global Surface Hourly Data, 2007. 2007. [[accessed 29 March 2007]]. Available: http://hurricane.ncdc.noaa.gov/pls/plclimprod/poemain.accessrouter?datasetabbv=DS3505.
- Wellenius GA, Coull BA, Godleski JJ, Koutrakis P, Okabe K, Savage ST, et al. Inhalation of concentrated ambient air particles exacerbates myocardial ischemia in conscious dogs. Environ Health Perspect. 2003;111:402–408. doi: 10.1289/ehp.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel EA, Quibrera PM, Smith RL, Catellier DJ, Liao D, Henley AC, et al. Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov. 2006;3(1):8. doi: 10.1186/1742-5573-3-8. [Online 20 July 2006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHI (Women’s Health Initiative) Study Group. Design paper: design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Yue W, Schneider A, Stölzel M, Rückerl R, Cyrys J, Pan X, et al. Ambient source-specific particles are associated with prolonged repolarization and increased levels of inflammation in male coronary artery disease patients. Mutat Res. 2007;621(1–2):50–60. doi: 10.1016/j.mrfmmm.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Zareba W, Nomura A, Couderc JP. Cardiovascular effects of air pollution: what to measure in ECG? Environ Health Perspect. 2001;109(suppl 4):533–538. doi: 10.1289/ehp.01109s4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZM, Prineas RJ, Case D, Soliman EZ, Rautaharju PM ARIC Research Group. Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2007;100(5):844–849. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZM, Whitsel EA, Smith RL, Quibrera PM, Liao D, Anderson GL, et al. Ambient particulate matter air pollution (PM) and myocardial ischemia: the Environmental Epidemiology of Arrhythmogenesis in the Women’s Health Initiative (EEAWHI) [Astract] Circulation. 2008;117(11):e253. doi: 10.1161/CIRCULATIONAHA.107.189344. [DOI] [Google Scholar]