Abstract
β-Glucan is an immuno-stimulating agent that has been used to treat cancer and infectious disease for many years with varying and unpredictable efficacy. Recent studies have unraveled the action mode of yeast-derived β-glucan in combination with anti-tumor monoclonal antibodies (mAbs) in cancer therapy. It has demonstrated that particulate or large molecular weight soluble β-glucans are ingested and processed by macrophages. These macrophages secrete the active moiety that primes neutrophil complement receptor 3 (CR3) to kill iC3b-opsonized tumor cells. In vitro and in vivo data demonstrate that successful combination therapy requires complement activation and deposition on tumors and CR3 expression on granulocytes. Pre-clinical animal studies have demonstrated the efficacy of combined β-glucan with anti-tumor mAbs therapy in terms of tumor regression and long-term survival. Clinical trials are underway using anti-epidermal growth factor receptor mAb (Erbitux) in combination with β-glucan for metastatic colorectal cancer. This review provides a brief overview of this combination therapy in cancer and describes in detail the β-glucan composition and structure, mechanism of action, and preclinical studies in human carcinoma xenograft models. It is proposed that the addition of β-glucan will further improve the therapeutic efficacy of anti-tumor mAbs in cancer patients.
Keywords: β-glucan, anti-tumor monoclonal antibody, immunotherapy, complement, neutrophils, complement regulatory proteins
Introduction
Humanized anti-tumor monoclonal antibody (mAb) therapy has been widely used to treat cancer (Adams and Weiner, 2005). The effector mechanisms mediated by these anti-tumor mAbs are diverse and include antagonizing receptor tyrosine kinases that are vital for tumor cell proliferation and transformation (Zhang et al., 2003), directly inducing tumor cell apoptosis (Johnson and Glennie, 2003), and eliciting immunological effects such as Ab-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) (Gelderman et al., 2004). However, mAb therapy is not uniformly effective, even in patients whose tumors express a high level of tumor antigen. Most of these mAbs are used in combination with chemotherapy drugs to further their therapeutic efficacy (Sobrero et al., 2008). However, combination therapy of anti-tumor mAb with such agents also significantly increases severalty of adverse effects, thus limiting its utilization in a greater number of cancer patients.
Many efforts have been made to maximize therapeutic efficacy of anti-tumor mAb with limited adverse effect. For example, tetravalent anti-tumor mAb can increase its half-life in the circulation and augment its anti-tumor efficacy (Li et al., 2008; Meng et al., 2004). In addition, anti-tumor mAbs can be conjugated with toxin to increase tumor killing activity (Senter et al., 1989). Studies are also being carried out to augment the immunological effect of anti-tumor mAbs, such as in ADCC and/or CDC (Gelderman et al., 2004; Hinton et al., 2004; Hinton et al., 2006). Over the past decade, we have demonstrated that yeast-derived β-glucan is capable of augmenting anti-tumor mAb efficacy to treat cancer (Hong et al., 2003; Hong et al., 2004; Yan et al., 1999). Other investigators have showed that barley β-glucan has similar effects (Cheung and Modak, 2002; Cheung et al., 2002; Modak et al., 2005). Further mechanistic studies demonstrate that β-glucan in combination with complement-activating mAbs elicits complement receptor 3 (CR3)-dependent cellular cytotoxicity (CR3-DCC) (Hong et al., 2004; Li et al., 2006).
β-Glucans are biological response modifiers (BRMs) and have been used for cancer treatment for more than 40 years particularly in Asia with varying and unpredictable efficacy (Yan et al., 2005). In vitro and in vivo studies have shown that soluble, low molecular weight β-glucan binds to its receptor CR3 (CD11b/CD18, Mac-1, αMβ2-integrin) (Thornton et al., 1996; Xia and Ross, 1999). CR3, a member of the β2-intergrin family, is a multifunctional adhesion molecule in which a common β2 (CD18) subunit is non-covalently bound to the αM subunit (CD11b) (Ross, 2000). A previous study demonstrated that the ability of CR3 to bind diverse ligands is mainly contributed to a consensus-binding site within CD11b (Yakubenko et al., 2002). Ligands for the inserted (I) domain of CD11b include complement activation component iC3b, intercellular adhesion molecule-1 (ICAM-1), fibrinogen, factor X, and heparin (Diamond et al., 1995; Diamond et al., 1993). Lectin-like domain (LLD), which is located proximal to the membrane, binds microbial polysaccharides such as β1,3-linked glucose polymers (β-glucan). Dual ligation of CR3 leads to degranulation and cytotoxic effects (Li et al., 2006).
Combined therapy of β-glucan with anti-tumor mAbs has been studied in a variety of murine syngeneic tumors (Hong et al., 2003; Hong et al., 2004; Yan et al., 1999) as well as human carcinoma xenograft models (Cheung and Modak, 2002; Cheung et al., 2002; Li et al., 2007a; Modak et al., 2005; Salvador et al., 2008) to demonstrate its therapeutic efficacy. The FDA has approved its clinical investigation in Phase I/II trials. In this review, we focus on yeast-derived β-glucan and discuss its composition, mechanism of action, and preclinical animal studies.
β-Glucan sources and structure
β-Glucans are polysaccharides found as constituents in a variety of plants and microorganisms, including oat, barley, mushroom, seaweed, some bacteria, and yeast (Gawronski et al., 1999; Wasser and Weis, 1999). β-Glucans from various sources are differential in their structure, conformation, and thus biological activity. Oat and barley β-glucans are primarily linear with large regions of β(1,4) linkages; mushroom and fungus β-glucans have the β(1,3) backbone branched with short β(1,6)-linked side chains (Ensley et al., 1994; Yan et al., 2005). Accordingly, these structural differences could affect both the β-glucan extraction and the biological activity (Williams et al., 1991). A recent study further revealed that the molecular size and complexity of β-glucan, more than the enrichment or the exclusive presence of the β(1,3) or β(1,6)-linkage, affect the interaction of β-glucan with human monocytes (Nisini et al., 2007).
Herein, β-glucan refers to yeast-derived β-glucan isolated from Saccharomyces cerevisiae unless otherwise noted. Three preparations of β-glucan are discussed in detail.
Particulate β-glucan
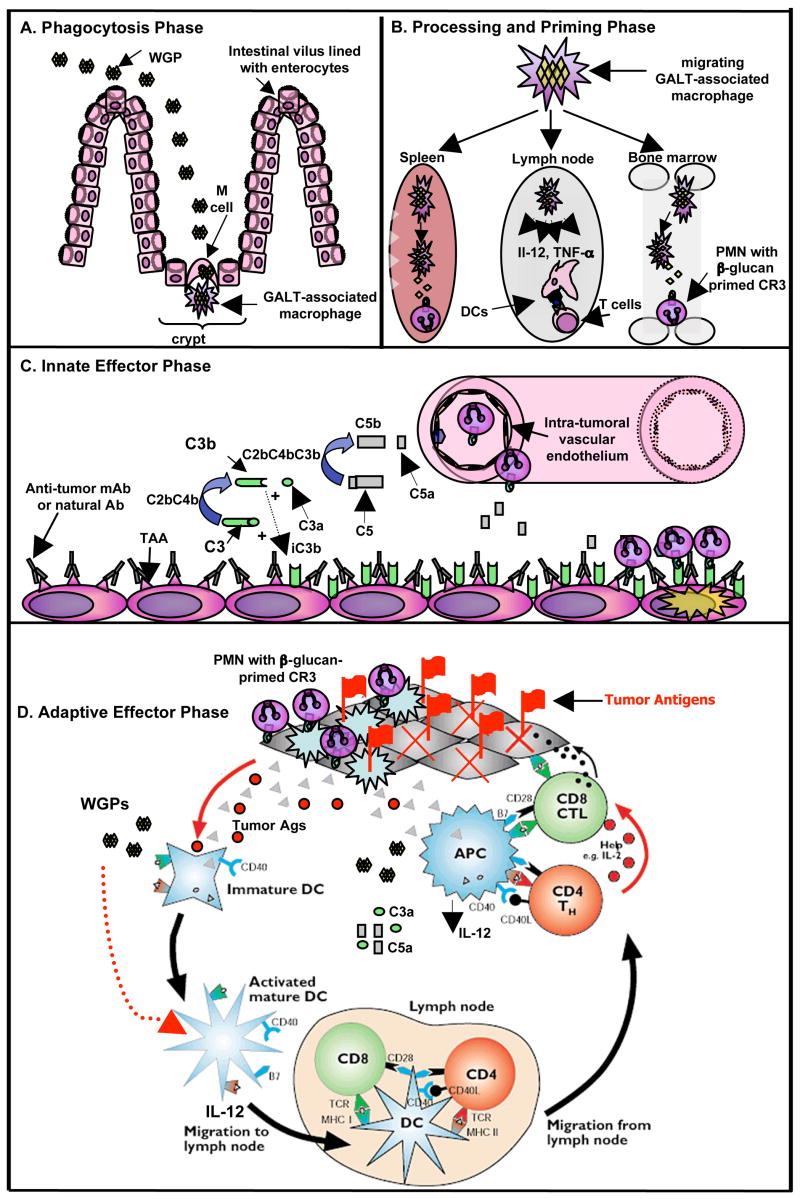
Whole glucan particles (WGPs) are a purified hollow yeast cell ‘ghost’ containing rich β-glucan sphere, generally 2–4 microns in diameter (Yan et al., 2005). Orally administered WGP β-glucans are ingested by gastrointestinal macrophages and then transported to spleen and bone marrow (Hong et al., 2004). Subsequently, small fragments are released when WGP β-glucans are digested by macrophages. The processing of WGPs by macrophages occurs presumably through an oxidative-dependent pathway since macrophages do not have glucanase. The soluble β-glucan released is the active moiety that can prime neutrophil CR3 to kill iC3b-opsonized target cells. In addition, WGP β-glucans stimulate macrophages to secrete cytokines such as tumor necrosis factor-α (TNF-α), monocyte chemotactic protein-1 (MCP-1), and interleukin-6 (IL-6) (Li et al., 2007b). These proinflammatory cytokines can potentially enhance the activation of adaptive immunity and may link the activation of both innate and adaptive immunity. A schematic model (Figure 1) is proposed to illustrate the mechanism by which orally administered WGPs are injected, demonstrating the four Phases characterized by: Phagocytosis Phase, Processing and Priming Phase, the Innate Effector Phase, and the Adaptive Effector Phase.
Figure 1. A schematic illustration of the mechanism by which orally administered WGPs are injected, demonstrating the four Phases characterized by: Phagocytosis Phase, Processing and Priming Phase, the Innate Effector Phase, and the Adaptive Effector Phase.
Briefly, the Phagocytosis Phase is characterized by the uptake of WGPs by gastrointestinal macrophages that are associated with M cells within the gut-associated lymphoid tissue (GALT) near the Peyer’s patches. The Processing and Priming Phase is characterized by the migration of macrophages from GALT to lymphoid organs, including spleen, lymph nodes, and bone marrow. WGPs also stimulate DCs for IL-12 and TNF-α production. During this time, the particulate β-glucan WGPs are digested and small soluble β-glucan is released, which primes neutrophils in a CR3-dependent manner. The Innate Effector Phase is characterized by the egress of β-glucan-primed neutrophils to the tumor in the response to C5a within the tumor milieu. Anti-tumor mAbs or natural Ab binds to tumor-associated antigens (TAA) to initiate the classical pathway of complement activation, leading to iC3b deposition on tumors. β-Glucan-primed neutrophils can engage iC3b-opsonized tumor cells for cytotoxicity. The Adaptive Effector Phase is characterized by the activation and maturation of immature DCs by WGPs. The combined WGP with antitumor mAb therapy eradicates tumors and releases tumor antigens. Immature DCs that capture tumor antigens are activated by WGPs. WGPs also stimulate DCs for IL-12 production. Maturated DCs in turn activate antigen-specific CD4 and CD8 T cells to eradicate residual tumors. PMN: polymorphonuclear leukocytes
Soluble β-glucan
Poly-(1,6)-β-D-glucopyranosyl-(1,3)-β-D-glucopyranose (PGG) β-glucan is a highly purified, water-soluble, intermediate sized (approximately150 KD) triple helix of glucose polymer with a β-(1–3)-glucan backbone containing β-(1–6)-linked β-(1–3) branches (Gawronski et al., 1999). Extensive preclinical studies have shown that PGG β-glucan substantially enhances anti-tumor mAb-mediated tumor immunotherapies (Li et al., 2007a; Li et al., 2006; Salvador et al., 2008). Soluble PGG β-glucan can be administered intravenously and is taken up by macrophages. Like WGPs, macrophages also process PGG β-glucan into small bioactive fragments to prime neutrophil CR3 for the efficient killing of the iC3b-opsonised tumor cells (Li et al., 2006). However, PGG β-glucan does not induce any proinflammatory responses while most of other glucan preparations do.
Active moiety of β-glucan
Both PGG and WGP β-glucans exert their function through macrophage digestion into a small biologically active fragment, which binds CR3 in the neutrophil and primes the effector cells for tumoricidal effect. In an ex vivo culture system, fresh isolated peritoneal macrophages can break down the WGP- or PGG β-glucan into soluble, biologically active components released into the culture medium, which can be measured using a β-1,3-glucan specific bioassay. The digested product can be detected as early as day 3 and a more complete processing of parental β-glucans requires more than 14 days. Although active moiety of β-glucan is an efficacious component that could be administered intravenously, the short-half time in vivo limits its use in combinational cancer therapy (Yan et al., 2000).
Mechanisms of action for the combined β-glucan and anti-tumor mAb immunotherapy
CR3-DCC is a critical mechanism for killing microorganisms (Ross et al., 1987). Following mAb binding to the surface antigen, the activated complement pathway leads to iC3b deposition on the microorganisms. The iC3b-opsonized microorganisms can be efficiently recognized by leukocyte CR3. However, induction of CR3-DCC requires dual occupation of CR3 to both iC3b and β-glucan, which exists in the cell walls of microorganisms. The binding of CR3 to iC3b is not sufficient for leukocytes to kill tumor cells due to the lack of β-glucan in mammalian cells. Therefore, it was hypothesized that co-administration of complement-activating anti-tumor Abs with β-glucan would prime CR3 on effector cells for a successful tumoricidal effect. Indeed, combined therapy of β-glucan with anti-tumor mAbs has achieved significant therapeutic efficacy in a variety of murine syngeneic tumors (Hong et al., 2003; Hong et al., 2004; Yan et al., 1999) as well as in human carcinoma xenograft models (Cheung and Modak, 2002; Cheung et al., 2002; Li et al., 2007a; Modak et al., 2005; Salvador et al., 2008).
The therapeutic efficacy of combined β-glucan with anti-tumor mAbs could not be achieved if C3- or CR3-deficient (C3−/−, CR3−/−) mice were used, which reiterates the importance of complement and CR3 for in vivo tumoricidal effect (Hong et al., 2003; Yan et al., 1999). Further in vivo studies demonstrated that PGG and WGP β-glucan exerts their respective effects via release of active moiety of β-glucan fragments processed by macrophages (Hong et al., 2004; Li et al., 2006). However, the uptake of PGG or WGP β-glucan by macrophages does not require CR3 as characterized by the similar percentage of PGG or WGP-binding macrophages in WT vs CR3−/− mice (Hong et al., 2004; Li et al., 2006). The CR3-independent uptake of particulate or PGG β-glucan may suggest a potential role for other β-glucan receptors, including dectin-1, in the phagocytosis phase. Dectin-1 was first reported as a dendritic cell (DC)-specific type II C-type lectin receptor expressed as a 43 kDa membrane-associated glycoprotein (Ariizumi et al., 2000). Previous studies demonstrated that dectin-1 is a receptor for particulate β-glucan such as zymosan (Brown and Gordon, 2001). Further studies indicated that a complex of toll-like receptor (TLR)-2 and/or TLR6 in collaboration with dectin-1 receptor is required for the production of proinflammatory cytokines such as tumor necrosis factor (TNF)-α in response to zymosan stimulation (Brown et al., 2003; Gantner et al., 2003). Recent studies also revealed that different sources of β-glucan may use distinct pathways to elicit their biological functions (Saijo et al., 2007; Taylor et al., 2007; LeibundGut-Landmann et al., 2007). Nevertheless, we demonstrated that the active moiety β-glucan fragment released from macrophages primes the CR3 of effector cells to elicit CR3-DCC of iC3b-opsonized tumor cells in cancer therapy.
The signaling event that mediates CR3-DCC was further characterized (Li et al., 2006). The requirement of tyrosine kinase has been revealed in an earlier study, which showed that protein tyrosine kinase inhibitors genistein or herbimycin A significantly blocked CR3 priming by β-glucan (Vetvicka et al., 1996). The specific kinase Syk activation was confirmed when mimicking CR3 dual ligation with active moiety β-glucan fragments and anti-CR3 I-domain mAbs. It was found that this dual ligation could stimulate the phosphorylation of Syk kinase. In addition, the phosphorylated Syk could be co-immunoprecipitated with the CR3 receptor (Li et al., 2006). This suggested that after dual ligation, Syk kinase is phosphorylated and recruited to the CR3 intracellular side. CR3 dual ligation also increased PI3 kinase activity. The Syk kinase inhibitor, piceatannol, could significantly block the dual ligation-mediated increase in PI3 kinase activity, indicating that PI3 kinase activation is a downstream event after Syk phosphorylation. This notion is further supported by the finding that CR3-DCC was greatly abrogated by PI3K inhibitor LY 294002 and Piceatannol, suggesting that Syk phosphorylation and the subsequent PI3K activation are the signaling events mediating CR3-DCC. However, the kinase that phosporylates Syk is still unknown. An investigation conducted recently may give us some hints. CR3 was found to move to lipid rafts and interact with Lyn kinase after dual ligation activation (Nakayama et al., 2008). LacCer-enriched-lipid rafts were found to be necessary for CR3-mediated phagocytosis of nonopsonized zymosans by human neutrophils. CD11b activation may cause cytoskeletal rearrangement resulting in the translocation of CR3 into Lyn-coupled, LacCer-enriched lipid rafts, thus allowing neutrophils to phagocytose nonopsonized zymosans. Whether and how the same mechanism applies to the killing of the iC3b-opsonized tumor cells awaits further investigation.
Pre-clinical human carcinoma xenograft models
Immunotherapy with β-glucan substantially enhances the therapeutic efficacy of anti-tumor mAb in the experimental murine breast, lung and lymphoma tumor models. To facilitate translation from preclinical models to clinical application, human carcinoma-challenged xenograft models were established in severe combined immunodeficient (SCID) mice. The human non-small cell lung carcinoma (NSCLC) cell line NCI-H23 was implanted in SCID mice to study the therapeutic efficacy of the combined therapy of PGG β-glucan with humanized anti-epidermal growth factor receptor (EGFR) mAb (cetuximab) (Li et al., 2007a). In vitro study showed that anti-EGFR mAb could bind to surface EGFR on tumors and activate mouse and human complement leading to iC3b deposition on the tumor cell surface. In this tumor model, the combined treatment of cetuximab with PGG β-glucan successfully suppressed tumor development and greatly improved long-term tumor-free survival rate. EGFR is widely expressed in the colorectal, lung, breast, pancreatic, gastrointestinal carcinomas, and melanoma, is associated with tumor progression, and indicates poor prognosis in patients. The success of the combinational therapy of PGG β-glucan with anti-EGFR Ab in the xenograft model holds great promise for the eradication of tumors and improving the long-term survival for patients whose tumors express high levels of EGFR.
In another xenograft model, a human ovarian carcinoma cell line, SKOV-3, which expresses high levels of Her-2/neu, was implanted subcutaneously in SCID mice (Li et al., 2007a). Preliminary in vitro studies showed that humanized anti-Her-2/neu Ab (trastuzumab) is able to activate mouse and human complement leading to iC3b deposition on tumor cells. However, the combination of PGG β-glucan and trastuzumab did not have a protective effect as shown in the NCI-H23 tumor model. Further investigation demonstrated that SKOV-3 cells over-express decay-accelerating factor CD55, which is a membrane-bound complement regulation protein (mCRP). Over-expressing mCRPs on tumors is one of mechanisms for tumor cells to escape immune surveillance (Fishelson et al., 2003; Macor and Tedesco, 2007). Due to a lack of effective complement activation caused by CD55 overexpression, the level of chemoattractant C5a was greatly diminished resulting in a paucity of effector neutrophils infiltrating into the tumors. Indeed, co-administration of anti-CD55 mAb with the combined trastuzumab and β-glucan therapy rescued the otherwise failed combined immunotherapy (Li et al., 2007a). This further strengthens the claim that β-glucan and mAb combined therapy kills tumors via CR3-DCC.
Another recently FDA-approved anti-vascular endothelial growth factor (VEGF) mAb (bevacizumab) was also used to test the combined therapy with PGG β-glucan in the SKOV-3 cell implanted SCID mice (Salvador et al., 2008). Bevacizumab might suppress tumor progression by blocking VEGF-mediated neovascularization, which is necessary for the solid tumor growth. VEGF receptors (VEGFR) are expressed widely in NSCL, leukemia, prostate carcinoma, and breast carcinoma (Bellamy et al., 1999; Decaussin et al., 1999; Ferrer et al., 1999; Price et al., 2001). Interestingly, VEGFR have been found on tumor cells as well as in endothelial cells. In addition, a significant fraction of VEGF has also been detected in tumor cells, possibly mediated by its heparin-binding properties or through the binding to VEGFRs. This observation makes bevacizumab a potential candidate that could work in concert with PGG β-glucan immunotherapy to treat cancer patients. This possibility was examined in the SKOV-3 carcinoma xenograft model. Anti-VEGF mAb is a humanized IgG1 Ab that could cause efficient iC3b deposition on the SKOV-3 cells. The combination of anti-VEGF with PGG β-glucan indeed induced massive neutrophil infiltration in the tumor and led to enhanced cytotoxicity both in vitro and in vivo (Salvador et al., 2008). Thus, co-administration of PGG β-glucan augments the anti-tumor effects of bevacizumab, resulting in synergistic anti-tumor effects.
Neutrophils are the effector cells for β-glucan-mediated antitumor therapy
Although β-glucan has been found to prime CR3 of macrophages, neutrophils and NK cells in vitro, neutrophils have been distinguished as the primary effector cells in the immune response elicited by combined β-glucan plus anti-tumor mAb therapy (Allendorf et al., 2005; Hong et al., 2003). This was illustrated by the observed reversal of combined β-glucan with anti-tumor mAb therapeutic effectiveness in GR-1 treated, granulocyte-depleted animals (Allendorf et al., 2005). The critical components for enlisting neutrophil involvement to the tumor site have been uncovered more recently. The potent chemoattractant of neutrophils, C5a, was found to be necessary for the infiltration of β-glucan-primed neutrophils into the tumor (Allendorf et al., 2005; Li et al., 2007a). In the absence of C5a receptor and/or BLT-1, a Leukotriene B4 receptor, tumors contained significantly less neutrophils as seen by immunohistochemistry (Allendorf et al., 2005). Leukotriene B4 is capable of perpetuating C5a-initiated neutrophil influx into the tumor.
The mechanism by which neutrophils kill cancer cells needs further evaluation. Activated neutrophils can phagocytose pathogens, release microbiocidal enzymes, activate the perforin and granzyme B pathway, and increase the assembly and activity of the NADPH oxidase complex contained within these cells (Di Carlo et al., 2001). A recent study suggested that neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL) plays a critical role in neutrophil-mediated tumor killing (Koga et al., 2004). In Candida albicans studies, β-glucan of the yeast cell wall was found to be critical for effective neutrophil reaction to the fungi. The blastoconidia, or cellular form of C. albicans is phagocytosed by neutrophils once CR3 binds β-glucan on the yeast surface. However, on the larger hyphae, β-glucan recognition does not result in phagosytosis because of the large size of this form of C. albicans. When the hyphae predominates, β-glucan is bound by neutrophils and respiratory burst follows (Lavigne et al., 2006).
Phagocytosis by neutrophils as well as the generation of reactive oxygen species (ROS) appears to be critical functions when neutrophils are faced with pathogens and even tumors, but other mechanisms of cell-mediated killing should be explored also. When neutrophils are involved in Ab-dependent killing of cells, the production of ROS has been observed during this process. However, neutrophils were markedly different when collected from patient donors with Chronic Granulomatous Disease, a genetic disease in which one of the four required NADPH oxidase complex proteins responsible for the mobilization of electrons transferred in the production of superoxide anions has a mutation, resulting in non-functional NADPH oxidase. These neutrophils were capable of killing malignant lymphoma cells despite lacking the respiratory burst response (Horner et al., 2007). Additionally, in animal tumor models, neutrophil production of ROS proved necessary for killing cancerous cells, but when human neutrophils and human tumor cell lines were examined, tumor cell killing via ADCC was independent of respiratory burst/NADPH oxidase activity (Kushner and Cheung, 1991). Although cell-to-cell contact was observed between neutrophils and tumor cells, inhibition of perforin did not result in the reduction of tumoricidal activity by neutrophils. However, in close contact between cells, membrane lipid exchange by both cells to each other resulted and was followed by apoptosis of the cancer cells by Ab-dependent, granulocyte-mediated cytotoxicity (Horner et al., 2007). These interesting results indicate a mechanism of neutrophil-mediated cytotoxicity of cancer cells independent of perforin or the production of ROS, but relevant to and dependent on Ab therapy, which may be the mechanism utilized during β-glucan and anti-tumor mAb combinational treatment.
Another potential therapeutic benefit of β-glucan treatment
Thus far, neutrophils have proven to be efficient tumor cell killing innate effector cells when primed with β-glucan and administered in conjunction with anti-tumor mAbs. The addition of PGG β-glucan to mAb cancer treatment adds therapeutic advantages without adverse side effects to the patients. Studies are showing that not only may β-glucan aid in tumor targeting and destruction for eradication, but after chemotherapeutic treatment or radiation therapy, β-glucan can hasten bone marrow hematopoietic cell recovery (Cramer et al., 2006). After chemo- or radiation therapy, iC3b deposition is observed on bone marrow stromal cells and works along with β-glucan to promote proliferation and expansion of CR3+ hematopoietic progenitor/stem cells (HPSCs). In addition, PGG β-glucan was shown in both in vivo and ex vivo models to enhance murine and primate myelopoiesis (Patchen et al., 1998b; Patchen et al., 1998a; Cramer et al., 2008). During PGG β-glucan-mediated HPSC mobilization, there was an absence of proinflammatory cytokine induction observed, contrary to other treatments such as granulocyte-colony stimulating factor (G-CSF) or granulocyte macrophage (GM)-CSF which could benefit cancer patients (Cramer et al., 2008).
Current and future challenges
β-Glucan has demonstrated its synergistic effect with currently clinically available anti-tumor mAbs in tumor therapy. The novel mechanism mediated by this combination therapy via innate effector neutrophil CR3-DCC would not interfere with other killing mechanisms elicited by anti-tumor mAb itself. Any anti-tumor mAb that is capable of activating complement could be used in combination with β-glucan for tumor therapy. In addition to current FDA-approved anti-tumor Abs, there are also more and more anti-tumor mAbs that have been developed or are being developed to treat cancer, such as anti-CEA Ab in colorectal cancer (Stein et al., 2005) and anti-carbonic anhydrase IX Ab in clear cell renal cell carcinoma (Davis et al., 2007). Increases in the number of anti-tumor Abs offer more opportunities to design versatile combinations with β-glucan therapy. Even with tumor vaccines that generate non-protective Ab responses and fail in clinical trial (Foon et al., 1995; Karanikas et al., 1997; Nicholson et al., 2004), as long as the Abs could bind to tumors resulting in complement activation, it is still possible for the vaccines to be used in conjunction with β-glucan to elicit CR3-DCC protective anti-tumor immune responses. For those tumor vaccines that elicit potent cytotoxic T lymphocyte (CTL) responses as well as humoral responses (Boscardin et al., 2006), combined therapy with β-glucan could add another protective layer of innate anti-tumor immunity to the adaptive anti-tumor immunity these tumor vaccines generate. Although the primary effectors of β-glucan-mediated tumor therapy are triggering innate effector neutrophils, it does not necessarily exclude the possibility that it is involved in the anti-tumor adaptive immunity. For example, particulate β-glucan WGP treatment has been reported to promote Th1 responses (Baran et al., 2007). Therefore, β-glucan-mediated therapy could link both innate and adaptive anti-tumor immunity.
There are several potential challenges for combined β-glucan with anti-tumor mAb therapy. A successful combination therapy requires complement activation and iC3b deposition and release of chemoattractant C5a, which in turn recruits effector neutrophils within tumor tissues. In some tumors, e.g., SKOV-3 ovarian tumors, immune surveillance can be evaded through impairment of complement-mediated cytotoxicity by overexpressing mCRPs (Bjorge et al., 1997). Therefore, This factor must be considered when β-glucan combined anti-tumor mAb therapy is used. Addition of the anti-mCRP mAb may help to rescue what may otherwise be a failure of tumor therapy. The need to develop other efficient targets to minimize the mCRP expression is urgent. These strategies may include small interfering RNAs or anti-sense oligos to knockdown mCRPs (Buettner et al., 2007; Loberg et al., 2006), utilization of chemotherapeutic drugs or cytokines to downregulate mCRPs (Di Gaetano et al., 2001), and a new, recently proposed approach for suppressing the expression of membrane-bound complement regulator (mCR) genes (Donev et al., 2006). In addition to this, stable tumor-associated antigen (TAA) expression also holds a key for successful combined therapy since complement activation and iC3b deposition on tumors are required. As observed with other immunotherapy approaches, a hallmark of cancer is genomic instability and tumors are heterogeneous populations of cells with potentially mixed populations of TAA expression (Ko et al., 2003). Thus, any single therapeutic strategy is a selection pressure for a tumor; sensitive cells will be effectively killed, but resistant variants will be selected for continued propagation. Therefore, the most successful anti-cancer treatments rely on a combination of many strategies. To that end, β-glucan can be used with anti-tumor cocktail mAbs that bind to multi-targets of TAA (Spiridon et al., 2002) or with cytokines such as interferon (IFN)-γ, which can increase the expression of TAA (Dutta et al., 2003). However, caution is needed because cocktail anti-tumor mAbs may also increase unwanted adverse effects. Nevertheless, the combined β-glucan with anti-tumor mAb therapy has demonstrated promising results in pre-clinical studies. The initial clinical study using anti-EGFR mAb in combination with PGG β-glucan for metastatic colorectal cancer conducted in Asia showed promising and exciting results. More clinical investigations are underway to truly test its efficacy for cancer treatment.
Acknowledgments
This research was supported by grants from the National Institutes of Health R01 CA86412 and the Kentucky Lung Cancer Research Board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams GP, et al. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- Allendorf DJ, et al. C5a-Mediated Leukotriene B4-Amplified Neutrophil Chemotaxis Is Essential in Tumor Immunotherapy Facilitated by Anti-Tumor Monoclonal Antibody and {beta}-Glucan. J Immunol. 2005;174:7050–7056. doi: 10.4049/jimmunol.174.11.7050. [DOI] [PubMed] [Google Scholar]
- Ariizumi K, et al. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–20167. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- Baran J, et al. Oral beta-glucan adjuvant therapy converts nonprotective Th2 response to protective Th1 cell-mediated immune response in mammary tumor-bearing mice. Folia Histochem Cytobiol. 2007;45:107–114. [PubMed] [Google Scholar]
- Bellamy WT, et al. Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res. 1999;59:728–733. [PubMed] [Google Scholar]
- Bjorge L, et al. Complement-regulatory proteins in ovarian malignancies. Int J Cancer. 1997;70:14–25. doi: 10.1002/(sici)1097-0215(19970106)70:1<14::aid-ijc3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Boscardin SB, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, et al. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- Brown GD, et al. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R, et al. Activated signal transducers and activators of transcription 3 signaling induces CD46 expression and protects human cancer cells from complement-dependent cytotoxicity. Mol Cancer Res. 2007;5:823–832. doi: 10.1158/1541-7786.MCR-06-0352. [DOI] [PubMed] [Google Scholar]
- Cheung NK, et al. Oral (1-->3),(1-->4)-beta-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin Cancer Res. 2002;8:1217–1223. [PubMed] [Google Scholar]
- Cheung NK, et al. Orally administered beta-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol Immunother. 2002;51:557–564. doi: 10.1007/s00262-002-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer DE, et al. {beta}-Glucan enhances complement-mediated hematopoietic recovery after bone marrow injury. Blood. 2006;107:835–840. doi: 10.1182/blood-2005-07-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer DE, et al. Mobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9. Stem Cells. 2008;26:1231–1240. doi: 10.1634/stemcells.2007-0712. [DOI] [PubMed] [Google Scholar]
- Davis ID, et al. A phase I multiple dose, dose escalation study of cG250 monoclonal antibody in patients with advanced renal cell carcinoma. Cancer Immun. 2007;7:13. [PMC free article] [PubMed] [Google Scholar]
- Decaussin M, et al. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): correlation with angiogenesis and survival. J Pathol. 1999;188:369–377. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Di Carlo E, et al. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- Di Gaetano N, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br J Haematol. 2001;114:800–809. doi: 10.1046/j.1365-2141.2001.03014.x. [DOI] [PubMed] [Google Scholar]
- Diamond MS, et al. Heparin is an adhesive ligand for the leukocyte integrin Mac-1 (CD11b/CD1) J Cell Biol. 1995;130:1473–1482. doi: 10.1083/jcb.130.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, et al. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donev RM, et al. p53 regulates cellular resistance to complement lysis through enhanced expression of CD59. Cancer Res. 2006;66:2451–2458. doi: 10.1158/0008-5472.CAN-05-3191. [DOI] [PubMed] [Google Scholar]
- Dutta T, et al. Robust ability of IFN-gamma to upregulate class II MHC antigen expression in tumor bearing rat brains. J Neurooncol. 2003;64:31–44. doi: 10.1007/BF02700018. [DOI] [PubMed] [Google Scholar]
- Ensley HE, et al. NMR spectral analysis of a water-insoluble (1-->3)-beta-D-glucan isolated from Saccharomyces cerevisiae. Carbohydr Res. 1994;258:307–311. doi: 10.1016/0008-6215(94)84098-9. [DOI] [PubMed] [Google Scholar]
- Ferrer FA, et al. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54:567–572. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- Fishelson Z, et al. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–123. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Foon KA, et al. Immune response to the carcinoembryonic antigen in patients treated with an anti-idiotype antibody vaccine. J Clin Invest. 1995;96:334–342. doi: 10.1172/JCI118039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, et al. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawronski M, et al. Microfibrillar structure of PGG-glucan in aqueous solution as triple-helix aggregates by small angle x-ray scattering. Biopolymers. 1999;50:569–578. doi: 10.1002/(SICI)1097-0282(199911)50:6<569::AID-BIP1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Gelderman KA, et al. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004;25:158–164. doi: 10.1016/j.it.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Hinton PR, et al. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem. 2004;279:6213–6216. doi: 10.1074/jbc.C300470200. [DOI] [PubMed] [Google Scholar]
- Hinton PR, et al. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176:346–356. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- Hong F, et al. Beta-glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res. 2003;63:9023–9031. [PubMed] [Google Scholar]
- Hong F, et al. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- Horner H, et al. Intimate cell conjugate formation and exchange of membrane lipids precede apoptosis induction in target cells during antibody-dependent, granulocyte-mediated cytotoxicity. J Immunol. 2007;179:337–345. doi: 10.4049/jimmunol.179.1.337. [DOI] [PubMed] [Google Scholar]
- Johnson P, et al. The mechanisms of action of rituximab in the elimination of tumor cells. Semin Oncol. 2003;30:3–8. doi: 10.1053/sonc.2003.50025. [DOI] [PubMed] [Google Scholar]
- Karanikas V, et al. Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest. 1997;100:2783–2792. doi: 10.1172/JCI119825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EC, et al. Immunotherapy of malignant diseases. Challenges and strategies. Int Arch Allergy Immunol. 2003;132:294–309. doi: 10.1159/000074897. [DOI] [PubMed] [Google Scholar]
- Koga Y, et al. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res. 2004;64:1037–1043. doi: 10.1158/0008-5472.can-03-1808. [DOI] [PubMed] [Google Scholar]
- Kushner BH, et al. Clinically effective monoclonal antibody 3F8 mediates nonoxidative lysis of human neuroectodermal tumor cells by polymorphonuclear leukocytes. Cancer Res. 1991;51:4865–4870. [PubMed] [Google Scholar]
- Lavigne LM, et al. Beta-glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J Immunol. 2006;177:8667–8675. doi: 10.4049/jimmunol.177.12.8667. [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Li B, et al. Combined yeast {beta}-glucan and antitumor monoclonal antibody therapy requires C5a-mediated neutrophil chemotaxis via regulation of decay-accelerating factor CD55. Cancer Res. 2007a;67:7421–7430. doi: 10.1158/0008-5472.CAN-07-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, et al. Yeast beta-glucan amplifies phagocyte killing of iC3b-opsonized tumor cells via complement receptor 3-Syk-phosphatidylinositol 3-kinase pathway. J Immunol. 2006;177:1661–1669. doi: 10.4049/jimmunol.177.3.1661. [DOI] [PubMed] [Google Scholar]
- Li B, et al. Yeast glucan particles activate murine resident macrophages to secrete proinflammatory cytokines via MyD88- and Syk kinase-dependent pathways. Clin Immunol. 2007b;124:170–181. doi: 10.1016/j.clim.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, et al. Development of novel tetravalent anti-CD20 antibodies with potent antitumor activity. Cancer Res. 2008;68:2400–2408. doi: 10.1158/0008-5472.CAN-07-6663. [DOI] [PubMed] [Google Scholar]
- Loberg RD, et al. Inhibition of decay-accelerating factor (CD55) attenuates prostate cancer growth and survival in vivo. Neoplasia. 2006;8:69–78. doi: 10.1593/neo.05679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macor P, et al. Complement as effector system in cancer immunotherapy. Immunol Lett. 2007;111:6–13. doi: 10.1016/j.imlet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Meng R, et al. The evaluation of recombinant, chimeric, tetravalent antihuman CD22 antibodies. Clin Cancer Res. 2004;10:1274–1281. doi: 10.1158/1078-0432.ccr-1154-03. [DOI] [PubMed] [Google Scholar]
- Modak S, et al. Rituximab therapy of lymphoma is enhanced by orally administered (1-->3),(1-->4)-D-beta-glucan. Leuk Res. 2005;29:679–683. doi: 10.1016/j.leukres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Nakayama H, et al. Lyn-coupled LacCer-enriched lipid rafts are required for CD11b/CD18-mediated neutrophil phagocytosis of nonopsonized microorganisms. J Leukoc Biol. 2008;83:728–741. doi: 10.1189/jlb.0707478. [DOI] [PubMed] [Google Scholar]
- Nicholson S, et al. A phase I trial of idiotypic vaccination with HMFG1 in ovarian cancer. Cancer Immunol Immunother. 2004;53:809–816. doi: 10.1007/s00262-004-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisini R, et al. beta-Glucan of Candida albicans cell wall causes the subversion of human monocyte differentiation into dendritic cells. J Leukoc Biol. 2007;82:1136–1142. doi: 10.1189/jlb.0307160. [DOI] [PubMed] [Google Scholar]
- Patchen ML, et al. Mobilization of peripheral blood progenitor cells by Betafectin PGG-Glucan alone and in combination with granulocyte colony-stimulating factor. Stem Cells. 1998a;16:208–217. doi: 10.1002/stem.160208. [DOI] [PubMed] [Google Scholar]
- Patchen ML, et al. In vitro and in vivo hematopoietic activities of Betafectin PGG-glucan. Exp Hematol. 1998b;26:1247–1254. [PubMed] [Google Scholar]
- Price DJ, et al. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ. 2001;12:129–135. [PubMed] [Google Scholar]
- Ross GD. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit Rev Immunol. 2000;20:197–222. [PubMed] [Google Scholar]
- Ross GD, et al. Specificity of membrane complement receptor type three (CR3) for beta-glucans. Complement. 1987;4:61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- Saijo S, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- Salvador C, et al. Yeast-Derived {beta}-Glucan Augments the Therapeutic Efficacy Mediated by Anti-Vascular Endothelial Growth Factor Monoclonal Antibody in Human Carcinoma Xenograft Models. Clin Cancer Res. 2008;14:1239–1247. doi: 10.1158/1078-0432.CCR-07-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senter PD, et al. Enhancement of the in vitro and in vivo antitumor activities of phosphorylated mitomycin C and etoposide derivatives by monoclonal antibody-alkaline phosphatase conjugates. Cancer Res. 1989;49:5789–5792. [PubMed] [Google Scholar]
- Sobrero AF, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- Spiridon CI, et al. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res. 2002;8:1720–1730. [PubMed] [Google Scholar]
- Stein R, et al. Advantage of a residualizing iodine radiolabel in the therapy of a colon cancer xenograft targeted with an anticarcinoembryonic antigen monoclonal antibody. Clin Cancer Res. 2005;11:2727–2734. doi: 10.1158/1078-0432.CCR-04-2100. [DOI] [PubMed] [Google Scholar]
- Taylor PR, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton BP, et al. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- Vetvicka V, et al. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser SP, et al. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Crit Rev Immunol. 1999;19:65–96. [PubMed] [Google Scholar]
- Williams DL, et al. Development, physicochemical characterization and preclinical efficacy evaluation of a water soluble glucan sulfate derived from Saccharomyces cerevisiae. Immunopharmacology. 1991;22:139–155. doi: 10.1016/0162-3109(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Xia Y, et al. Generation of recombinant fragments of CD11b expressing the functional beta-glucan-binding lectin site of CR3 (CD11b/CD18) J Immunol. 1999;162:7285–7293. [PubMed] [Google Scholar]
- Yakubenko VP, et al. A molecular basis for integrin alphaMbeta 2 ligand binding promiscuity. J Biol Chem. 2002;277:48635–48642. doi: 10.1074/jbc.M208877200. [DOI] [PubMed] [Google Scholar]
- Yan J, et al. Yeast whole glucan particle (WGP) beta-glucan in conjunction with antitumour monoclonal antibodies to treat cancer. Expert Opin Biol Ther. 2005;5:691–702. doi: 10.1517/14712598.5.5.691. [DOI] [PubMed] [Google Scholar]
- Yan J, et al. Beta-glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18) J Immunol. 1999;163:3045–3052. [PubMed] [Google Scholar]
- Yan J, et al. Critical role of Kupffer cell CR3 (CD11b/CD18) in the clearance of IgM-opsonized erythrocytes or soluble beta-glucan. Immunopharmacology. 2000;46:39–54. doi: 10.1016/s0162-3109(99)00157-5. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. Therapeutic monoclonal antibodies for the ErbB family of receptor tyrosine kinases. Cancer Biol Ther. 2003;2:S122–126. [PubMed] [Google Scholar]