Abstract
The use of trovafloxacin (TVX), a fluoroquinolone antibiotic, was severely restricted because of an association of TVX therapy with idiosyncratic hepatotoxicity in patients. The mechanisms underlying idiosyncratic toxicity are unknown; however, one hypothesis is that an inflammatory stress can render an individual sensitive to the drug. Previously, we reported that treatment of mice with TVX and lipopolysaccharide (LPS) induced tumor necrosis factor (TNF) α-dependent liver injury, whereas TVX or LPS treatment alone was nontoxic. The goal of this study was to elucidate the role of TNFα in TVX/LPS-induced liver injury. TNF receptor (TNFR) 1 p55-/- and TNFR2 (p75-/-) mice were protected from hepatotoxicity caused by TVX/LPS coexposure, suggesting that TVX/LPS-induced liver injury requires both TNF receptors. TNFα inhibition using etanercept significantly reduced the TVX/LPS-induced increases in the plasma concentrations of several cytokines around the time of onset of liver injury. However, despite the reduction in chemokines, etanercept treatment did not affect the TVX/LPS-induced hepatic accumulation of neutrophils. In addition, etanercept treatment attenuated TVX/LPS induction of plasminogen activator inhibitor-1, and this was associated with a reduction in hepatic fibrin deposition. Mice treated with TVX and a nontoxic dose of TNFα also developed liver injury. In summary, TNFα acts through p55 and p75 receptors to precipitate an innocuous inflammatory cascade. TVX enhances this cascade, converting it into one that results in hepatocellular injury.
The leading cause of acute liver failure in the United States is drug-induced liver injury, which represents a problem for both public health and the pharmaceutical industry (Ostapowicz et al., 2002). Drug-induced liver injury is the most common reason for restrictive regulatory actions by the United States Food and Drug Administration or pharmaceutical companies. Idiosyncratic adverse drug reactions (IADRs) are an important subset of untoward reactions and are an increasing reason for postmarket regulatory actions. Trovafloxacin (TVX), a fluoroquinolone antibiotic, is one example of a drug for which use was restricted severely because of IADRs. TVX was approved for use in the United States in 1997, and by 1999, its use was associated with 152 cases of serious hepatic events. Of these, 14 resulted in acute liver failure, five patients required liver transplants, and four died (Bertino and Fish, 2000). The mechanism by which TVX causes hepatotoxicity that is not seen with other quinolones is unknown.
One hypothesis regarding the cause of IADRs is that inflammatory stress alters the toxicity threshold of an individual, rendering a normally therapeutic dose of a drug toxic (Ganey et al., 2004). In this regard, it is interesting that human clinical studies of TVX hepatotoxicity revealed the presence of inflammatory cells in liver biopsies (Chen et al., 2000). In rats and mice, nontoxic doses of TVX and bacterial lipopolysaccharide (LPS) synergized to cause acute liver injury (Waring et al., 2006; Shaw et al., 2007). In this animal model, TVX pretreatment enhanced the LPS-induced peak in plasma tumor necrosis factor (TNF) α concentration. In addition, TNFα neutralization completely protected mice from TVX/LPS-induced liver injury (Shaw et al., 2007).
TNFα is a pleiotropic cytokine that stimulates a number of cellular responses, including proliferation, production of inflammatory mediators, up-regulation of adhesion molecules, and programmed cell death. Large amounts of TNFα are produced in response to several microbial products, including LPS. TNFα is a key mediator of inflammatory responses, which can result in both tissue damage and host defense. The main cellular source of TNFα is macrophages, but several other cell types produce TNFα, including mast cells, hepatic stellate cells, endothelial cells, fibroblasts, and neuronal cells (Wajant et al., 2003). TNFα plays a critical role in several models of liver injury caused by viral hepatitis, ischemia/reperfusion, or hepatotoxic doses of LPS (Colletti et al., 1990; Shimizu et al., 2005; Raftery et al., 2007).
The biological effects of TNFα are elicited via two high-affinity cell surface receptors, TNF receptor 1 (p55) and TNF receptor 2 (p75) (Locksley et al., 2001). The two TNF receptors are structurally similar but functionally different. The p55 receptor provides the key mode of TNFα signaling in most cell types. The cells of the lymphoid system are the exception, in which signaling through the p75 receptor plays a major role. The intracellular domains of p55 and p75 are the main difference between the two receptors. The intracellular portion of the p55 receptor contains a death domain, which couples the activation of receptor to caspase activation and cell death (Tartaglia et al., 1993a). The p75 receptor lacks the death domain. The activation of either receptor leads to intracellular signaling cascades, including mitogen-activated protein kinase activation and nuclear factor (NF)-κB activation. Ligand activation of the receptors is another functional difference. Membrane-bound TNFα has the ability to activate both p55 and p75 receptors (Wajant et al., 2003), whereas soluble TNFα activates only the p55 receptor and is the dominant signal for p55 activation (Grell et al., 1998).
The role of each receptor has been evaluated in several models of liver injury. The p55 receptor has been studied more extensively and is important in hepatotoxicity caused by LPS, acetaminophen, or carbon tetrachloride (Peschon et al., 1998; Morio et al., 2001; Ishida et al., 2004). In contrast, critical roles for both receptors have been shown only in a few models of hepatotoxicity, such as that induced by concanavalin A, Pseudomonas aeruginosa exotoxin A, or adenovirus (Küsters et al., 1997; Hayder et al., 1999; Schümann et al., 2000). The study presented here was designed to determine the importance of each receptor in TVX/LPS-induced liver injury and to evaluate the influence of TNFα on other proinflammatory factors in this IADR model.
Materials and Methods
Materials. Unless otherwise noted, all reagents were purchased from Sigma-Aldrich (St. Louis, MO). The LPS (lot 075K4038) used for all studies was derived from Escherichia coli serotype O55:B5 and had an activity of 3.3 × 106 endotoxin units/mg as determined using a colorimetric, kinetic Limulus amebocyte lysate assay purchased from Cambrex (kit no. 50-650U; East Rutherford, NJ). Recombinant murine TNFα was purchased from R&D Systems (Minneapolis, MN). Trovafloxacin was synthesized by Cayman Chemical (Ann Arbor, MI).
Animals. All animals received humane care, and all studies complied with Michigan State University guidelines. Male, 9 to 11-week-old, C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were used for all experiments. p55-/-, p75-/-, and C57/Bl6 wild-type controls were purchased from The Jackson Laboratory. They were allowed to acclimate for 1 week in a 12-h light/dark cycle. Animals were given continual access to bottled spring water and fed a standard chow (Rodent Chow/Tek8640; Harlan Teklad, Madison, WI).
Study Design and Sample Collection. Dosing protocols were the same as a previous study in which coexposure of mice to TVX and LPS caused hepatotoxicity (Shaw et al., 2007). Mice were fasted for 12 h before treatment. TVX (150 mg/kg) or its saline vehicle was administered to mice by oral gavage. The dose of TVX was chosen because it was nonhepatotoxic and synergized with LPS to cause robust liver injury without significant mortality at 15 h (Shaw et al., 2007). LPS (2 × 106 EU/kg), TNFα (50 μg/kg), or saline vehicle was given intraperitoneally 3 h later. The time of LPS or TNFα administration is designated as time 0 throughout. Food was returned immediately after LPS or TNFα administration. In the etanercept studies, etanercept (8 mg/kg i.p.) was given 1 h before LPS administration. Mice were anesthetized with sodium pentobarbital (50 mg/kg i.p.) at designated times and killed by exsanguination. The left lateral liver lobe was fixed in 10% neutral buffered formalin and blocked in paraffin.
ALT Activity and Histopathology Assessment. Plasma ALT activity was measured spectrophotometrically using Infinity ALT reagent purchased from Thermo Fisher Scientific (Waltham, MA). Paraffin-embedded liver sections were cut 5 μm thick and stained with hematoxylin and eosin. Eight high-power fields were scored for necrosis. The scoring scale was set from 0 to 5, with the following criteria: 0, no necrosis; 1, mild; 2, mild to moderate; 3, moderate; 4, moderate to severe; and 5, severe. For each liver, the eight scores were averaged, and this average was considered a replicate.
Plasma Cytokine Measurements. The plasma concentrations of interferon (IFN) γ; interleukin (IL)-6, IL-10, and IL-1β; monocyte chemoattractant protein (MCP)-1; vascular endothelial growth factor (VEGF); macrophage inflammatory protein (MIP)-2; keratinocyte chemoattractant (KC); and MIP-1α were measured using bead-plex kits purchased from Bio-Rad (Hercules, CA) and measured using a Bio-Plex 200 system (Bio-Rad).
Hepatic Neutrophil Accumulation. Neutrophil (PMN) immunohistochemistry was performed on 5 μm thick, paraffin-embedded liver sections as described previously (Yee et al., 2003). PMNs were stained using a rabbit anti-PMN Ig isolated from the serum of rabbits immunized with rat PMNs (Hewett et al., 1992). Hepatic PMN accumulation was quantified by counting the number of PMNs in six to 10 randomly selected, high-power fields (400×) for each liver.
Hemostatic System Measurements. Plasma thrombin/antithrombin III (TAT) dimers were measured using the Enzygnost TAT enzyme-linked immunosorbent assay kit purchased from Dade Behring, Inc. (Deerfield, IL). Active PAI-1 plasma concentration was measured using an enzyme-linked immunosorbent assay kit purchased from Molecular Innovations (Southfield, MI). Hepatic fibrin immunohistochemistry and estimation of deposition were done following the protocol described previously with a slight modification (Copple et al., 2002), i.e., artifactual fibrin staining seen within vessel lumens in all treatment groups was removed from quantification calculations. Vehicle controls were used to establish the threshold fluorescence. Morphometric data are expressed as the fraction of pixels for which the fluorescence exceeded the threshold.
Statistical Analyses. All bar graph results are presented as mean ± S.E.M. A one- or two-way analysis of variance was used as appropriate after data normalization. All multiple pairwise comparisons were done using Tukey's test. The criterion for significance was p < 0.05. Histopathological scores were compared using analysis of variance on ranks.
Results
p55-/- and p75-/- Mice Are Protected from TVX/LPS-Induced Liver Injury. To determine the contribution of each TNF receptor to TVX/LPS-induced liver injury, p55-/- or p75-/- mice were treated with TVX/LPS as described under Materials and Methods. TVX/LPS coexposure caused significant liver injury at 15 h in control (wild-type) mice. Both p55-/- mice and p75-/- mice were resistant to TVX/LPS-induced liver injury (Fig. 1). p75-/- mice were completely protected from TVX/LPS-induced liver injury and had significantly reduced plasma ALT activity compared with control and p55-/- mice (Fig. 1). Histopathologic examination of livers corroborated this result, inasmuch as lesions of hepatocellular necrosis were decreased in p55-/- mice and p75-/- mice compared with wild-type mice (Fig. 1; Table 1). All of the wild-type mice treated with TVX/LPS developed at least moderate hepatocellular necrosis. In contrast, none of the lesions in the p55-/- or p75-/- mice treated with TVX/LPS progressed beyond mild necrosis (Table 1).
Fig. 1.
The role of TNF receptors in TVX/LPS-induced liver injury. Wild-type, p55-/-, and p75-/- mice were treated with TVX 3 h before LPS as described under Materials and Methods. Mice were killed 15 h after LPS, and plasma ALT activity was measured. n = 5–8 animals/group. *, significantly different from wild-type group; #, significantly different from p55-/- group. Photomicrographs were taken of livers from representative mice from each group. Wild-type mice treated with TVX/LPS had severe necrosis, whereas necrosis in p55-/- and p75-/- mice was mild or nonexistent, respectively.
TABLE 1.
Scoring of histopathology of livers from wild-type, p55–/–, and p75–/– mice cotreated with TVX/LPS Wild-type, p55–/–, and p75–/– mice were treated with TVX and LPS as described under Materials and Methods. They were sacrificed 15 h after LPS. Liver sections were cut 5 μm thick and stained with hematoxylin and eosin, and resultant slides were scored for necrosis. The scoring scale was set from 0 to 5 with the following criteria: 0, no necrosis; 1, mild; 2, mild to moderate; 3, moderate; 4, moderate to severe; and 5, severe. Each replicate represents the mean of eight randomly chosen fields scored per liver. n = 5 animals/group.
|
Mouse Strain
|
Necrosis Score
|
|
|---|---|---|
| Mean | Range | |
| Wild type | 4.3 | 1.7–4.7 |
| p55–/– | 0.5* | 0.3–1.1 |
| p75–/– | 0.4* | 0.0–1.0 |
Significantly different from wild-type group
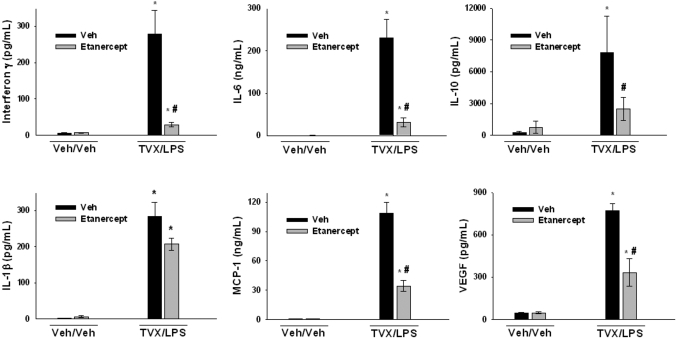
TNFα Neutralization Attenuates TVX/LPS Induction of Inflammatory Cytokines and Chemokines. In a previous study, treatment with etanercept, which is a mimic of the soluble p75 receptor, reduced TVX/LPS-induced increase in plasma TNFα concentration and protected mice from TVX/LPS-induced liver injury (Shaw et al., 2007). TVX/LPS-treated mice were dosed with etanercept to determine the effects of TNFα on the induction of proinflammatory cytokines and chemokines at 4.5 h, a time near the onset of liver injury (Shaw et al., 2008). TNFα inhibition attenuated the TVX/LPS-mediated induction of IFNγ, IL-6, IL-10, MCP-1, and VEGF (Fig. 2). The increase in IL-1β plasma concentration after TVX/LPS treatment was not changed by etanercept treatment (Fig. 2). TNFα neutralization significantly reduced the TVX/LPS induction of chemokines MIP-2, KC, and MIP-1α (Fig. 3).
Fig. 2.
Effect of TNFα inhibition on TVX/LPS-induced increases in plasma cytokines. Mice were treated with vehicles or with TVX/LPS in addition to etanercept or its vehicle as described under Materials and Methods. Mice were sacrificed 4.5 h after LPS administration. Plasma concentrations of IFNγ, IL-6, IL-10, IL-1β, MCP-1, and VEGF were measured as described under Materials and Methods. n = 4–6 animals/group. *, significantly different from respective Veh/Veh group. #, significantly different from TVX/LPS/Veh group.
Fig. 3.
Effect of TNFα inhibition on TVX/LPS-induced increases in plasma chemokines. Mice were treated with vehicles or with TVX/LPS in addition to etanercept or its vehicle as described under Materials and Methods. Mice were sacrificed 4.5 h after LPS administration. Plasma concentrations of MIP-2, KC, and MIP-1α were measured as described under Materials and Methods. n = 4–6 animals/group. *, significantly different from respective Veh/Veh group. #, significantly different from TVX/LPS/Veh group.
TVX/LPS-Induced Hepatic Neutrophil Accumulation Is Independent of TNFα. Previous results pointed to a role for PMNs in the pathogenesis of TVX/LPS-induced liver injury (Waring et al., 2006; Shaw et al., 2008). Despite causing a reduction in chemokines (Fig. 3), etanercept treatment did not reduce hepatic neutrophil accumulation induced by TVX/LPS coexposure. In fact, etanercept treatment slightly increased neutrophil accumulation (Fig. 4).
Fig. 4.
Effect of TNFα inhibition on TVX/LPS-induced hepatic PMN accumulation. Mice were treated with vehicles or with TVX/LPS in addition to etanercept or its vehicle as described under Materials and Methods. Mice were sacrificed 4.5 h after LPS administration. Paraffin-embedded livers were stained for neutrophils, and the number of neutrophils was quantified as described under Materials and Methods. HPF, high-power field. n = 4–6 animals/group. *, significantly different from respective Veh/Veh group; #, significantly different from TVX/LPS/Veh group.
TNFα Neutralization Attenuates TVX/LPS-Induced Hemostatic System Activation. The coagulation system plays an important role in TVX/LPS-induced pathogenesis (Fullerton et al., 2008). To determine whether TNFα plays a role in TVX/LPS-induced coagulation system activation, TVX/LPS-treated mice were treated with etanercept and killed at 4.5 h. The dose of etanercept markedly reduced the TVX/LPS-induced release of TNFα in this model (Shaw et al., 2007). Plasma TAT dimers, measured as a biomarker of coagulation system activation, were significantly increased in TVX/LPS-treated mice (Fig. 5A). Etanercept treatment caused a trend toward reduction in plasma TAT dimers, but this difference was not statistically significant. The plasma concentration of active PAI-1, an inhibitor of the fibrinolytic system, was increased by TVX/LPS coexposure (Fig. 5B). Etanercept significantly reduced the TVX/LPS induction of plasma active PAI-1 (Fig. 5B). Fibrin deposition in tissue occurs if the rate of coagulation system activation exceeds the rate of fibrinolysis. TVX/LPS coexposure caused a significant increase in sinusoidal fibrin deposition in the liver at 4.5 h, which was significantly reduced by etanercept treatment (Fig. 5C).
Fig. 5.
Effect of TNFα inhibition on hemostatic system dysregulation mediated by TVX/LPS coexposure. Mice were treated with vehicles or with TVX/LPS in addition to etanercept or its vehicle as described under Materials and Methods. They were sacrificed 4.5 h after LPS administration. Plasma concentrations of TAT dimers (A) and active PAI-1 (B) were measured as described under Materials and Methods. C, hepatic fibrin was stained immunohistochemically and quantified as described under Materials and Methods. n = 4–6 animals/group. *, significantly different from respective Veh/Veh group; #, significantly different from TVX/LPS/Veh group.
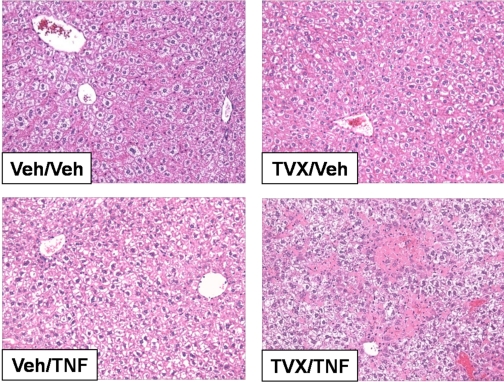
TVX and TNFα Coexposure Causes Hepatotoxicity. TVX/LPS-induced liver injury is dependent on TNFα (Shaw et al., 2007), but to determine whether TNFα alone could interact with TVX, mice were treated with TVX and recombinant murine TNFα as described under Materials and Methods. They were killed 15 h after TNFα treatment, the time of maximal plasma ALT activity in TVX/LPS-treated mice. TVX or TNFα treatment alone did not cause an increase in plasma ALT activity (Fig. 6). However, TVX/TNFα coexposure increased plasma ALT activity. Histopathological evaluation of liver sections corroborated the lack of injury from TVX or TNFα alone (Fig. 7). In contrast, TVX/TNFα coexposure caused hepatocellular necrotic and apoptotic lesions primarily in centrilobular and midzonal regions of liver lobules, and these lesions extended to periportal regions in some severely affected mice.
Fig. 6.
TVX/TNFα coexposure-induced liver injury. Mice were treated with TVX 3 h before recombinant murine TNFα as described under Materials and Methods. Mice were killed 15 h after TNFα administration, and plasma ALT activity was measured. n = 4–5 animals/group. *, significantly different from TVX/Veh group; #, significantly different from Veh/TNFα group.
Fig. 7.
Histopathology of TVX/TNFα-induced liver injury. Mice were treated with TVX 3 h before recombinant murine TNFα as described under Materials and Methods. Mice were killed 15 h after TNFα administration, and photomicrographs were taken of representative livers.
Discussion
Previously, we reported that a nontoxic dose of TVX interacts with a nontoxic dose of LPS to cause TNFα-dependent liver injury in mice (Shaw et al., 2007). The critical role of TNFα in TVX/LPS-induced liver injury was based upon TNFα neutralization; however, the role of each TNF receptor was not studied. Activation of the p55 receptor results in two main signals: NF-κB activation and activation of caspases leading to apoptosis (Varghese et al., 2001). Inasmuch as ligand binding of the p55 receptor can result in NF-κB activation and cell death, it is not surprising that the p55 receptor is critical to several models of liver injury (Küsters et al., 1997; Peschon et al., 1998; Hayder et al., 1999; Schümann et al., 2000; Morio et al., 2001; Ishida et al., 2004). Similar to other models dependent on TNFα and p55, TVX/LPS-induced liver injury was significantly attenuated in p55-/- mice. It is possible that p55-/- mice have reduced liver injury because of decreased plasma TNFα concentrations since TNFα induction by LPS was found to be attenuated in p55-/- mice (Peschon et al., 1998). However, p55 was not involved in the induction of TNFα by galactosamine/LPS coexposure (Nowak et al., 2000).
The function of the p75 receptor is less understood compared with the p55 receptor. Similar to p55, activation of the p75 receptor causes NF-κB activation, but it does not result in caspase activation (Dopp et al., 2002). The critical role of the p75 receptor in hepatotoxicity is unclear: it is not involved in some models of liver injury that are dependent on TNFα (Peschon et al., 1998; Nowak et al., 2000) but is involved in others (Küsters et al., 1997; Hayder et al., 1999; Schümann et al., 2000). Indeed, p75-/- mice were completely protected from TVX/LPS-induced liver injury and had significantly reduced hepatocellular injury compared with p55-/- mice.
Why p75-/- mice are completely protected from TVX/LPS-induced hepatotoxicity is unclear. It is possible that the p75 receptor is playing two roles in the progression of TVX/LPS-induced hepatotoxicity. It has been suggested that the p75 receptor acts to bind TNFα and transfer it to the p55 receptor, resulting in p55 activation at lower concentrations of TNFα (Tartaglia et al., 1993b). Therefore, without the p75 receptor present, the threshold for TNFα-dependent liver injury might be higher than the concentration that is achieved. Additionally, the p75 receptor can cooperate with the p55 receptor to enhance necrotic cell death in response to TNFα (Pelagi et al., 2000). To account for the minor hepatotoxicity seen in p55-/- mice, p75 activation must also cause minor hepatocellular injury independent of p55 after TVX/LPS coexposure. Therefore, it is possible that combined activation of p55 and p75 synergize to cause extensive hepatocellular necrosis, which is unseen when either receptor is absent. In addition, the p75 receptor is involved in the LPS-induced production of TNFα (Peschon et al., 1998). It is possible that the complete protection in p75-/- mice resulted from a combination of these mechanisms, including a reduction in LPS-induced TNFα production.
TNFα was involved in the TVX/LPS-mediated increases in plasma concentrations of several inflammatory cytokines: IFNγ, IL-6, MCP-1, VEGF, MIP-2, KC, and MIP-1α. IL-1β concentrations were unchanged by etanercept treatment at 4.5 h; however, IL-1β peaks 2 h after LPS administration, and we are unable rule out the possibility that etanercept reduced it at an earlier time (Givalois et al., 1994). The attenuation of IL-6 and MIP-2 after TNFα neutralization was also seen in another model of drug/LPS coexposure-induced hepatotoxicity (Tukov et al., 2007). The reduction of such a large number of cytokines might be because of a decrease in TNFα-driven NF-κB activation mediated through p55 and p75 receptor activation (Dopp et al., 2002).
Several of the cytokines that required TNFα for their release have chemotactic properties. Therefore, we measured PMN accumulation in livers of these mice. The hepatic accumulation of PMNs induced by TVX/LPS was not decreased by TNFα inhibition. Thus, it is likely that the TVX/LPS-induced hepatic PMN accumulation is mediated by selectins and other adhesion molecules as seen in endotoxemia or by TNFα-independent sinusoidal contraction (Kamochi et al., 1999). It is possible that TNFα is not involved in PMN accumulation but is needed for PMN activation since TNFα can promote neutrophil activation in vitro (Dri et al., 1999). If TNFα enhances PMN activation and degranulation, it might explain the slight increase in hepatic PMN accumulation in TVX/LPS/etanercept-treated mice because when TNFα is present, the accumulated PMNs might be activated, degranulate, and undergo clearance from the tissue.
In addition to being critical to TVX/LPS up-regulation of cytokines, it is possible that TNFα is involved in the progression of liver injury by enhancing hemostasis. TVX/LPS coexposure caused fibrin deposition in liver sinusoids, and treatment with the anticoagulant heparin significantly reduced TVX/LPS-induced liver injury (Fullerton et al., 2008). TNFα has the potential to interact with the hemostatic system in several ways. It can induce tissue factor, which activates the coagulation system, but it also increases PAI-1 expression, which could depress fibrinolysis (Takeshita et al., 2006; Tukov et al., 2007). Indeed, TVX/LPS-induced increases in active PAI-1 and hepatic fibrin deposition were TNFα-dependent, whereas coagulation system activation showed a trend but was not significantly reduced after TNFα inhibition. The results suggest that if tissue factor induction occurs during TVX/LPS coexposure, it is TNFα-independent, but that a slight reduction in coagulation system activation by etanercept along with a more pronounced reduction in active PAI-1 was able to prevent hepatic fibrin deposition.
From the cytokine studies, TNFα appears to be a proximal mediator in a cascade of inflammatory events synergistically induced by TVX/LPS coexposure. These include the release of cytokines and production of PAI-1 and hepatic fibrin deposition. Several of these factors are known to be involved in TVX/LPS-induced liver injury; however, how they interplay with one another in the pathogenesis is currently being investigated.
Based on the importance of TNFα in TVX/LPS-induced liver injury, we examined whether TVX can interact with a dose of TNFα to induce similar hepatocellular damage. Indeed, TVX treatment before a nonhepatotoxic dose of recombinant murine TNFα resulted in significant liver injury. Other studies have shown that TNFα by itself does not cause liver injury in mice but can when administered with galactosamine or a DNA synthesis inhibitor (Schwabe and Brenner, 2006; Shen and Pervaiz, 2006). It is unclear from these results whether TVX sensitized mice to TNFα-induced liver injury or vice versa. Topoisomerase inhibitors render hepatocytes sensitive to cell death induced by TNFα (Hentze et al., 2004). In accordance, it is possible that TVX renders hepatocytes sensitive to cell death induced by TNFα by affecting eukaryotic topoisomerases and inhibiting protein synthesis. Consistent with this hypothesis, the hepatocellular lesions in TVX/TNFα-treated mice appear similar to those seen after galactosamine/TNFα coexposure, suggesting commonalities in mechanisms (Gezginci and Bolkent, 2007). Recently, CYP2E1 induction by pyrazole was shown to sensitize mice to TNFα-induced liver injury (Wu and Cederbaum, 2008). It is unlikely that TVX/TNFα-induced liver injury is related to an effect on CYP2E1 activity by TVX because TVX treatment alone did not have any effect on CYP2E1 expression (Shaw et al., 2008). However, to exclude this possibility, CYP2E1 activity would need to be measured after TVX exposure. It is also possible that TVX treatment reduced TNFα clearance and that the prolonged presence of TNFα resulted in cell death. This is consistent with the prolonged presence of TNFα in the plasma of TVX/LPS-treated mice compared with LPS treatment alone (Shaw et al., 2007). TNFα inactivation and clearance are mediated by soluble forms of the two receptors. It is possible that TVX reduces the cleavage or expression of these receptors, in turn reducing TNFα clearance. Further studies are required to understand better the mechanism by which TVX and TNFα interact to cause hepatocellular damage.
In summary, TVX/LPS-induced liver injury depended on the presence of both TNF receptors, p55 and p75. The p75 receptor may play an even more important role than the p55 receptor in the progression of TVX/LPS-induced hepatotoxicity. At the onset of liver injury, the TVX/LPS coexposure-related increase in several cytokines, active PAI-1, and hepatic fibrin was TNFα-dependent. However, despite the observation that the induction of chemokines was TNFα-dependent, hepatic PMN accumulation was independent of TNFα. The critical role of TNFα in TVX/LPS-induced liver injury was likely through up-regulation of cytokines and activation of the hemostatic system. The observation that the liver injury could be reproduced by substituting TNFα administration for LPS supports the critical importance of this cytokine in the pathogenesis.
This study was supported by National Institutes of Health [Grant DK061315] and by National Institute of Environmental Health Sciences [Training Grant GM075685].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.143792.
ABBREVIATIONS: IADR, idiosyncratic adverse drug reaction; TVX, trovafloxacin; LPS, lipopolysaccharide; TNF, tumor necrosis factor; p55, TNF receptor 1; p75, TNF receptor 2; NF, nuclear factor; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; VEGF, vascular endothelial growth factor; MIP, macrophage inflammatory protein; KC, keratinocyte chemoattractant; PMN, neutrophil; TAT, thrombin/antithrombin III; PAI, plasminogen activator inhibitor; Veh, vehicle.
References
- Bertino J Jr and Fish D (2000) The safety profile of the fluoroquinolones. Clin Ther 22 798-817; discussion 797. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Bloch KJ, and Maclean JA (2000) Acute eosinophilic hepatitis from trovafloxacin. N Engl J Med 342 359-360. [DOI] [PubMed] [Google Scholar]
- Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, and Campbell DA Jr (1990) Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest 85 1936-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copple BL, Banes A, Ganey PE, and Roth RA (2002) Endothelial cell injury and fibrin deposition in rat liver after monocrotaline exposure. Toxicol Sci 65 309-318. [DOI] [PubMed] [Google Scholar]
- Dopp JM, Sarafian TA, Spinella FM, Kahn MA, Shau H, and de Vellis J (2002) Expression of the p75 TNF receptor is linked to TNF-induced NFkappaB translocation and oxyradical neutralization in glial cells. Neurochem Res 27 1535-1542. [DOI] [PubMed] [Google Scholar]
- Dri P, Haas E, Cramer R, Menegazzi R, Gasparini C, Martinelli R, Scheurich P, and Patriarca P (1999) Role of the 75-kDa TNF receptor in TNF-induced activation of neutrophil respiratory burst. J Immunol 162 460-466. [PubMed] [Google Scholar]
- Fullerton AM, Shaw PJ, Ganey PE, and Roth RA (2008) Hemostatic system activation contributes to hepatotoxicity in mice treated with trovafloxacin and lipopolysaccharide. Toxicologist 102 622. [Google Scholar]
- Ganey PE, Luyendyk JP, Maddox JF, and Roth RA (2004) Adverse hepatic drug reactions: inflammatory episodes as consequence and contributor. Chem Biol Interact 150 35-51. [DOI] [PubMed] [Google Scholar]
- Gezginci S and Bolkent S (2007) The effect of Z-FA.FMK on d-galactosamine/TNF-alpha-induced liver injury in mice. Cell Biochem Funct 25 277-286. [DOI] [PubMed] [Google Scholar]
- Givalois L, Dornand J, Mekaouche M, Solier MD, Bristow AF, Ixart G, Siaud P, Assenmacher I, and Barbanel G (1994) Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol 267 R164-R170. [DOI] [PubMed] [Google Scholar]
- Grell M, Wajant H, Zimmermann G, and Scheurich P (1998) The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A 95 570-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayder H, Blanden RV, Körner H, Riminton DS, Sedgwick JD, and Müllbacher A (1999) Adenovirus-induced liver pathology is mediated through TNF receptors I and II but is independent of TNF or lymphotoxin. J Immunol 163 1516-1520. [PubMed] [Google Scholar]
- Hentze H, Latta M, Künstle G, Dhakshinamoorthy S, Ng PY, Porter AG, and Wendel A (2004) Topoisomerase inhibitor camptothecin sensitizes mouse hepatocytes in vitro and in vivo to TNF-mediated apoptosis. Hepatology 39 1311-1320. [DOI] [PubMed] [Google Scholar]
- Hewett JA, Schultze AE, VanCise S, and Roth RA (1992) Neutrophil depletion protects against liver injury from bacterial endotoxin. Lab Invest 66 347-361. [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Tsuneyama K, Lu P, Takayasu T, and Mukaida N (2004) The pathogenic roles of tumor necrosis factor receptor p55 in acetaminophen-induced liver injury in mice. J Leukoc Biol 75 59-67. [DOI] [PubMed] [Google Scholar]
- Kamochi M, Kamochi F, Kim YB, Sawh S, Sanders JM, Sarembock I, Green S, Young JS, Ley K, Fu SM, et al. (1999) P-selectin and ICAM-1 mediate endotoxin-induced neutrophil recruitment and injury to the lung and liver. Am J Physiol 277: L310-L319. [DOI] [PubMed] [Google Scholar]
- Küsters S, Tiegs G, Alexopoulou L, Pasparakis M, Douni E, Künstle G, Bluethmann H, Wendel A, Pfizenmaier K, Kollias G, et al. (1997) In vivo evidence for a functional role of both tumor necrosis factor (TNF) receptors and transmembrane TNF in experimental hepatitis. Eur J Immunol 27 2870-2875. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, and Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104 487-501. [DOI] [PubMed] [Google Scholar]
- Morio LA, Chiu H, Sprowles KA, Zhou P, Heck DE, Gordon MK, and Laskin DL (2001) Distinct roles of tumor necrosis factor-alpha and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol 172 44-51. [DOI] [PubMed] [Google Scholar]
- Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, MacKay SL, Edwards CK 3rd, and Moldawer LL (2000) LPS-induced liver injury in d-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol 278 R1202-R1209. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, et al. (2002) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 137 947-954. [DOI] [PubMed] [Google Scholar]
- Pelagi M, Curnis F, Colombo B, Rovere P, Sacchi A, Manfredi AA, and Corti A (2000) Caspase inhibition reveals functional cooperation between p55- and p75-TNF receptors in cell necrosis. Eur Cytokine Netw 11 580-588. [PubMed] [Google Scholar]
- Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, and Mohler KM (1998) TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol 160 943-952. [PubMed] [Google Scholar]
- Raftery G, Griffiths B, Kay L, and Kane D (2007) Chronic viral hepatitis and TNF-alpha blockade. Rheumatology (Oxford) 46 1381-1382. [DOI] [PubMed] [Google Scholar]
- Schümann J, Bluethmann H, and Tiegs G (2000) Synergism of Pseudomonas aeruginosa exotoxin A with endotoxin, superantigen, or TNF results in T. Immunol Lett 74 165-172. [DOI] [PubMed] [Google Scholar]
- Schwabe RF and Brenner DA (2006) Mechanisms of liver injury: I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290 G583-G589. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Ditewig AC, Waring JF, Liguori MJ, Blomme EA, Ganey PE, and Roth RA (2008) Coexposure of mice to trovafloxacin and lipopolysaccharide, a model of idiosyncratic hepatotoxicity, results in a unique gene expression profile and interferon gamma-dependent liver injury. Toxicol Sci doi: 10.1093/toxsci/kfn205. [DOI] [PMC free article] [PubMed]
- Shaw PJ, Hopfensperger MJ, Ganey PE, and Roth RA (2007) Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol Sci 100 259-266. [DOI] [PubMed] [Google Scholar]
- Shen HM and Pervaiz S (2006) TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J 20 1589-1598. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Yamada Y, Okuno M, Ohnishi H, Osawa Y, Seishima M, and Moriwaki H (2005) Liver injury induced by lipopolysaccharide is mediated by TNFR-1 but not by TNFR-2 or Fas in mice. Hepatol Res 31 136-142. [DOI] [PubMed] [Google Scholar]
- Takeshita Y, Takamura T, Hamaguchi E, Shimizu A, Ota T, Sakurai M, and Kaneko S (2006) Tumor necrosis factor-alpha-induced production of plasminogen activator inhibitor 1 and its regulation by pioglitazone and cerivastatin in a nonmalignant human hepatocyte cell line. Metabolism 55 1464-1472. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Ayres TM, Wong GH, and Goeddel DV (1993a) A novel domain within the 55 kD TNF receptor signals cell death. Cell 74 845-853. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Pennica D, and Goeddel DV (1993b) Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem 268 18542-18548. [PubMed] [Google Scholar]
- Tukov FF, Luyendyk JP, Ganey PE, and Roth RA (2007) The role of tumor necrosis factor alpha in lipopolysaccharide/ranitidine-induced inflammatory liver injury. Toxicol Sci 100 267-280. [DOI] [PubMed] [Google Scholar]
- Varghese J, Chattopadhaya S, and Sarin A (2001) Inhibition of p38 kinase reveals a TNF-alpha-mediated, caspase-dependent, apoptotic death pathway in a human myelomonocyte cell line. J Immunol 166 6570-6577. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, and Scheurich P (2003) Tumor necrosis factor signaling. Cell Death Differ 10 45-65. [DOI] [PubMed] [Google Scholar]
- Waring JF, Liguori MJ, Luyendyk JP, Maddox JF, Ganey PE, Stachlewitz RF, North C, Blomme EA, and Roth RA (2006) Microarray analysis of lipopolysaccharide potentiation of trovafloxacin-induced liver injury in rats suggests a role for proinflammatory chemokines and neutrophils. J Pharmacol Exp Ther 316 1080-1087. [DOI] [PubMed] [Google Scholar]
- Wu D and Cederbaum A (2008) Cytochrome P4502E1 sensitizes to tumor necrosis factor alpha-induced liver injury through activation of mitogen-activated protein kinases in mice. Hepatology 47 1005-1017. [DOI] [PubMed] [Google Scholar]
- Yee SB, Hanumegowda UM, Hotchkiss JA, Ganey PE, and Roth RA (2003) Role of neutrophils in the synergistic liver injury from monocrotaline and bacterial lipopolysaccharide exposure. Toxicol Sci 72 43-56. [DOI] [PubMed] [Google Scholar]