Abstract
Endogenous opioid systems are implicated in the actions of ethanol. For example, μ-opioid receptor (MOR) knockout (KO) mice self-administer less alcohol than the genetically intact counterpart wild-type (WT) mice (Roberts et al., 2000). MOR KO mice also exhibit less anxiety-like behavior than WT mice (Filliol et al., 2000). To investigate the neurobiological mechanisms underlying these behaviors, we examined the effect of ethanol in brain slices from MOR KO and WT mice using sharp-electrode and whole-cell patch recording techniques. We focused our study in the central nucleus of the amygdala (CeA) because it is implicated in alcohol drinking behavior and stress behavior. We found that the amplitudes of evoked inhibitory postsynaptic currents (IPSCs) or inhibitory postsynaptic potentials (IPSPs) were significantly greater in MOR KO mice than WT mice. In addition, the baseline frequencies of spontaneous and miniature GABAA receptor-mediated inhibitory postsynaptic currents were significantly greater in CeA neurons from MOR KO than WT mice. However, ethanol enhancements of evoked IPSP and IPSC amplitudes and the frequency of miniature IPSCs were comparable between WT and MOR KO mice. Baseline spontaneous and miniature excitatory postsynaptic currents (EPSCs) and ethanol effects on EPSCs were not significantly different between MOR KO and WT mice. Based on knowledge of CeA circuitry and projections, we hypothesize that the role of MOR- and GABA receptor-mediated mechanisms in CeA underlying reinforcing effects of ethanol operate independently, possibly through pathway-specific responses within CeA.
The endogenous opioid peptide system is implicated in ethanol reinforcement and dependence. Behavioral studies show that block of endogenous opioid action using naltrexone, a nonselective opioid antagonist, diminishes ethanol consumption (Herz, 1997), and this antagonist is currently used in the treatment of alcohol dependence in humans (Froehlich, 1996). However, the cellular mechanism underlying the interaction between endogenous opioids and ethanol for the reinforcing effects of ethanol is not yet clearly understood. Pharmacological studies using subtype-specific antagonists show that the key element in opioid peptide systems for the positive reinforcing effects of ethanol is the MOR (Ciccocioppo et al., 2002; Mhatre and Holloway, 2003). Genetically engineered mice without MOR consistently show decreased ethanol consumption compared with WT mice (Roberts et al., 2000). In addition, MOR KO mice show less anxiety-like behavior than WT mice (Filliol et al., 2000), suggesting an interaction between anxiety and ethanol drinking. In contrast, transgenic mice lacking the δ-opioid receptor drink significantly more than WT controls (Roberts et al., 2001).
The central amygdala (CeA), a structure mediating emotional behaviors such as anxiety and fear, is implicated as a critical brain region in stress-related drinking, given that the anxiolytic effects of ethanol motivate drinking as self-medication (Sher et al., 2007). In addition, recent studies suggested that the CeA, as part of the extended amygdala, plays a critical role in the positive motivational effect of ethanol (Koob et al., 1998; McBride, 2002). Lesions of the CeA reduce voluntary alcohol consumption and anxiety (Möller et al., 1997). GABAA receptor antagonists, when injected into specific regions such as the nucleus accumbens and CeA, significantly decrease ethanol consumption, with the CeA being the most sensitive brain region (Hyytiä and Koob, 1995; Foster et al., 2004). In addition, the involvement of endogenous opioids in the motivational effect of ethanol is shown by the block of the reinforcing effects of ethanol with local injection into CeA of opioid receptor antagonists (Heyser et al., 1999; Foster et al., 2004).
The CeA is one of the brain regions showing the highest immunoreactive density of the endogenous opioid peptide enkephalin (Wilson et al., 2002), localized in the soma of GABAergic neurons, the most abundant neuron type in the CeA (Veinante et al., 1997). In several brain regions, acute ethanol releases endogenous opioids that, in turn, mediate some ethanol effects such as reinforcement and anxiolysis (for review, see Oswald and Wand, 2004). Consistent with this finding, acute administration of ethanol increases c-fos expression (suggestive of increased neuronal activity), specifically in the enkephalin-containing GABAergic neurons in CeA (Morales et al., 1998). Moderate levels of MORs are detected in CeA of both rat and mouse using immunohistochemical and autoradiography studies (Moskowitz and Goodman, 1984; Wilson et al., 2002; Chieng et al., 2006). The activation of MOR in the CeA decreases glutamatergic excitatory and GABAergic inhibitory neurotransmission in the CeA through a presynaptic action (Finnegan et al., 2005; Zhu and Pan, 2005). In addition, a postsynaptic inhibitory action of MOR in CeA neurons has been reported (Chieng et al., 2006).
Ethanol also modulates glutamatergic excitatory and GABAergic inhibitory synaptic responses in CeA (Roberto et al., 2003, 2004; Nie et al., 2004; Zhu et al., 2007). Ethanol enhances GABAergic inhibitory neurotransmission in the CeA through both pre- and postsynaptic mechanisms while decreasing NMDA receptor-mediated glutamatergic excitatory neurotransmission postsynaptically. The GABAergic system in CeA has been shown to be involved in the motivational effect of ethanol because local injection of a GABAA receptor antagonist into CeA decreases ethanol self-administration in rats (Hyytiä and Koob, 1995). In addition, local injection of a GABAA receptor agonist (i.e., muscimol) into CeA also reduced ethanol self-administration in dependent, but not nondependent, rats (Roberts et al., 1996). Therefore, the interaction between ethanol and opioid systems on GABAergic neurotransmission in CeA may be involved in ethanol drinking behavior and motivational effects of ethanol. Our previous work demonstrated that ethanol more effectively enhanced GABAergic transmission in CeA of mice lacking the δ-opioid receptor (Kang-Park et al., 2007), suggesting a mechanism underlying the increased ethanol consumption in these mice (Roberts et al., 2001). In the current study, we have now examined the interaction of ethanol with the μ-opioid/GABAA systems in CeA using MOR KO mice.
Materials and Methods
Generation of Knockout Mice. The methods for generation of the knockout mice were as described in detail by Roberts et al. (2000). In brief, gene inactivation was obtained by disruption of the first coding exon of the μ-opioid receptor gene in 129/SV embryonic stem cells. Germline transmission occurred from the breeding of chimeric males with C57BL/6Orl females. After mice were genotyped, those showing germline transmission were used as founder animals to produce the F1 animals used in these experiments. We used male homozygous MOR KO and WT littermate mice (shipped from Scripps Research Institute, La Jolla, CA) in these experiments. The genetic background of these mice was a hybrid C57BL/6Orl × 129/SV strain. We housed mice one to three per cage in a temperature-controlled room in which the lights were on a 12-h light/dark cycle with lights off at 10:00 AM.
In Vitro Single-Cell Recordings. We used 4- to 5-month-old MOR KO and genetically intact WT littermate mice in this study. In some control experiments, we used C57/BL6 mice (4–6 weeks old). Brains were rapidly removed from animals under halothane anesthesia and immersed in ice-cold oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (ACSF), containing 126 mM NaCl, 3.3 mM KCl, 1.23 mM NaH2PO4, 25 mM NaHCO3, 2 mM CaCl2, 0.9 mM MgSO4, 10 mM glucose; for ACSF used only during the dissection, 2 mM CaCl2 was replaced with 0.5 mM CaCl2. We cut coronal slices (300 μm, between bregma -1.0 to ∼-1.9 mm) using a Vibratome slicer (model 752; Campden Instruments Ltd., Leicester, UK) and incubated them in ACSF continuously bubbled with 95% O2-5% CO2 at room temperature.
After a minimal 1-h incubation, we transferred slices to the recording chamber (volume, 0.5 ml) in which oxygenated ACSF was superfused over submerged slices at approximately 3 to 4 ml/min. Single-cell recordings were performed using either sharp electrodes or whole-cell patch recordings. We pulled recording electrodes from borosilicate glass capillary tubing (World Precision Instruments, Inc., Sarasota, FL) using a Flaming-Brown horizontal microelectrode puller (model P-97; Sutter Instrument Company, Novato, CA). For sharp-electrode recording, electrodes were filled with 3 M KCl (tip resistance, 50–80 MΩ). For whole-cell patch recordings, we filled pipettes (input resistance, 2–5 MΩ) with the following recording solution: 130 mM cesium gluconate, 7 mM CsCl, 10 mM HEPES, 2 mM MgCl2, 4 mM Mg-ATP, 0.3 mM Tris-GTP, and 8 mM QX314, pH 7.25 (285 mOsM). In whole-cell patch recording, we viewed individual cells with an upright Axioskop fixed-stage microscope (Zeiss Axioskop; Carl Zeiss Inc., Thornwood, NY) equipped with a water immersion objective (40×, 0.75 numerical aperture), IR-filtered light, differential interference contrast optics, and a Hitachi CCD camera (Tokyo, Japan). Liquid junction potentials were not compensated.
We recorded from single cells in the medial ventral part of CEA. For sharp-electrode recordings, we recorded inhibitory synaptic responses at resting potential at 32°C in current-clamp mode. For whole-cell patch recordings, cells were voltage-clamped at -70 mV, and recordings were performed at room temperature. For both sharp-electrode and patch recordings, we isolated inhibitory postsynaptic potentials (IPSPs) or currents (IPSCs) in the presence of ionotropic glutamate receptor antagonists, d-2-amino-5-phosphonovalerate (AP-5; 50 μM) and 6,7-dinitroquinoxaline-2,3-dione (20 μM). Non-NMDA excitatory postsynaptic currents (EPSCs) were isolated in the presence of the NMDA glutamate receptor antagonist AP-5 (50 μM) and the GABAA receptor antagonist bicuculline (20 μM). For the sharp-electrode recordings, we also blocked GABAB receptors with CGP 55845A. We recorded spontaneous IPSCs and EPSCs either with or without tetrodotoxin (TTX; 1 μM) in the bath; spontaneous IPSCs and EPSCs without TTX are referred to as sIPSCs and sEPSCs and spontaneous IPSCs and EPSCs with TTX as mIPSCs and mEPSCs (“miniature” IPSCs and EPSCs). We monitored series resistance (15–30 MΩ) online throughout the experiment using a digital oscilloscope (Nicolet model 410; Thermo Fisher Scientific, Waltham, MA) and rejected cells if resistance changed by >20%. Series resistance compensation was not used.
Each drug, [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO), naloxonazine, and ethanol, was applied through bath perfusion for 10 to 12 min after recording 10-min baseline responses, then washed out for 15 to 30 min while recordings were continued. We used 40 or 44 mM ethanol because it has been shown to produce a maximal effect on IPSCs (Roberto et al., 2003) and is a clinically relevant concentration. We diluted ethanol in gassed ACSF directly from sealed stock solutions of reagent-grade 95% ethanol immediately before administration to avoid loss of ethanol by evaporation.
Data Acquisition and Analysis. We recorded evoked synaptic responses using an Axopatch 200B headstage (Molecular Devices, Sunnyvale, CA), filtered at 2 KHz (-3 dB), and digitized them at 10 KHz using Clampex (Axon Instruments). The amplitudes of evoked IPSPs or IPSCs were quantified for analysis using Clampfit (Axon Instruments). For paired-pulse ratio (PPR), we measured the secondary IPSP amplitude over the primary amplitude. The amplitude of the second IPSP was quantified relative to its baseline, which was measured right before the second stimulation. PPR is inversely related to the neurotransmitter release such that a decrease in the PPR of IPSPs is related to an increase in the GABA release (Dobrunz and Stevens, 1997). We digitized spontaneous synaptic responses (sIPSCs or sEPSCs) at 5 KHz and 6 s/sweep using WINWCP (Strathclyde electrophysiology software, Whole Cell program, courtesy of Dr. John Dempster). We recorded spontaneous synaptic responses for 2 to 3 min in each condition including baseline, in the presence of drug (DAMGO, naloxonazine, or ethanol), and after washout. We analyzed spontaneous synaptic events using Minianalysis (Synaptosoft, Decatur, GA) using a 5-pA minimal cutoff and re-examined all events manually for data acceptance. We plotted results as cumulative amplitude and frequency histograms and tested drug effects using the Komologrov-Smirnov statistical method. We evaluated drug effects using a paired Student's t test and compared results between groups using an independent Student's t test. In some cases, the results between groups at different stimulus intensities were evaluated using two-way analysis of variance (ANOVA). P < 0.05 was accepted as indicating statistical significance. We used SPSS (SPSS Inc., Chicago, IL) for all statistical analyses and Origin (OriginLab Corp., Northampton, MA) for plotting all figures. Results in the text and figures are presented as the mean ± S.E.M.
Drugs. Drugs used were AP-5, bicuculline methiodide, 6,7-dinitroquinoxaline-2,3-dione, DAMGO, naloxonazine, CGP 55845, and TTX. We purchased all drugs from Sigma-Aldrich (St. Louis, MO), except we obtained AP-5 from Acros Organics (Fairlawn, NJ).
Results
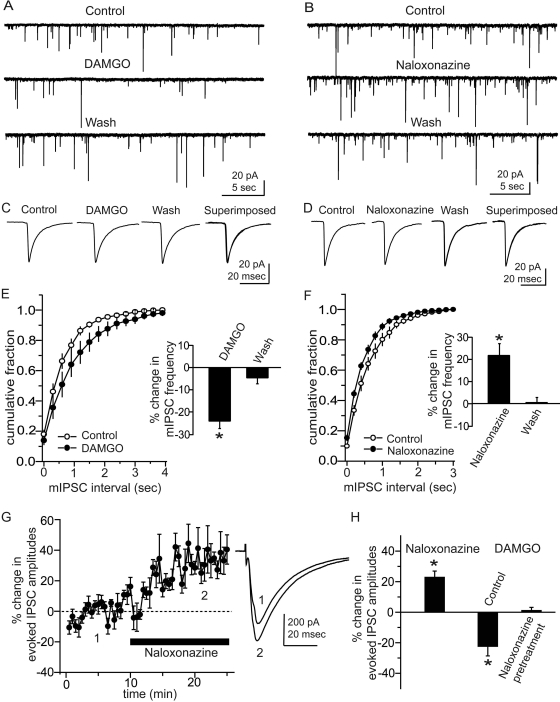
To confirm the reported effects of MOR activation in CeA (see Introduction), we first examined the actions of DAMGO, an MOR-specific agonist, on inhibitory neurotransmission in CeA neurons from control WT mice. In CeA neurons, DAMGO (1 μM) significantly decreased the mean frequency of mIPSCs from 1.4 ± 0.6 to 1.1 ± 0.6 Hz (n = 5, p < 0.01; Fig. 1, A and E), without significantly affecting the amplitudes (21.1 ± 6.1 to 22.0 ± 6.6 pA, n = 5; Fig. 1C), suggesting a presynaptic action in reducing vesicular GABA release in CeA. To characterize possible tonic MOR activation, we examined the MOR antagonist naloxonazine on mIPSCs in CeA of control WT mice. Naloxonazine (1 μM) significantly increased mean mIPSC frequency by 21.9 ± 4.8% (from 1.3 ± 0.1 to 1.6 ± 0.1 Hz; n = 6, p < 0.01) in slices from control WT mice (Fig. 1, B and F). The Komologrov-Smirnov test showed a significant increase in the frequency of mIPSCs in four of six neurons from these mice. In contrast, naloxonazine did not affect the mean amplitude of mIPSCs, from 36.1 ± 4.8 to 35.7 ± 3.2 pA (n = 6) in slices from WT mice (Fig. 1D). These results suggest the presence of tonic activation of presynaptic MORs in WT mice CeA. We also tested the effect of naloxonazine (1 μM) on the evoked IPSCs in CeA from control mice to confirm the tonic effect of endogenous opioids mediated by MOR. Naloxonazine enhanced the mean amplitude of evoked IPSCs by 22.7 ± 4.1% (p < 0.01) in seven of 13 neurons tested in CeA from control mice (Fig. 1G). Although DAMGO alone (1 μM) significantly decreased the mean amplitude of evoked IPSCs by 21.7 ± 5.4% (n = 5, p = 0.02) in CeA neurons from control mice, after pretreatment with naloxonazine (1 μM), DAMGO had no significant effect on the mean amplitude of evoked IPSCS (n = 5, p = 0.73; Fig. 1H). These results suggest that the naloxonazine effect is mediated specifically by MOR.
Fig. 1.
Effects of MOR activation on IPSCs in mice CeA neurons. A, representative current traces of mIPSCs before (Control), during application of DAMGO (1 μM) and after washout (Wash). B, representative current traces with the MOR-specific antagonist naloxonazine (1 μM). C and D, traces of averaged mIPSCs. There were no differences in the amplitude of mIPSCs with either drug. E and F, averaged cumulative fraction plots of mIPSC frequency. DAMGO shifts the mIPSC cumulative frequency plot to the right (E), whereas naloxonazine shifts the mIPSC frequency plot to the left without changes in amplitude (F), suggesting a tonic effect of MOR activation on vesicular GABA release. G, effect of naloxonazine (1 μM) on evoked IPSCs in mouse CeA neurons. Time course of mean IPSC amplitude changes, with representative traces taken before and during 1 μM naloxonazine at the corresponding numbered time points shown on the left. H, effect of DAMGO and naloxonazine on mean IPSC amplitudes compared with baseline. The DAMGO effect was abolished when slices were pretreated with naloxonazine; *, p < 0.05.
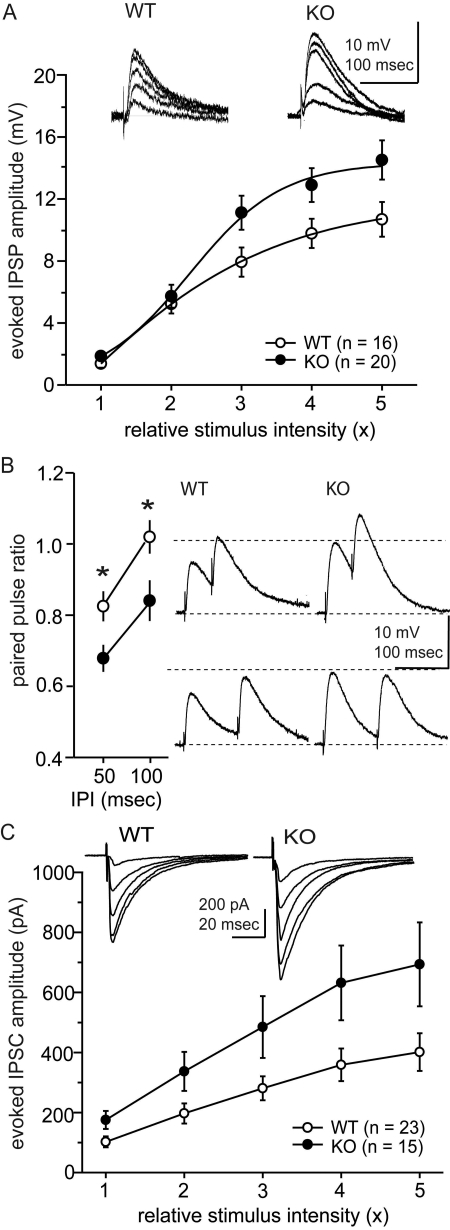
To further characterize MOR effects on inhibitory neurotransmission, we examined evoked inhibitory neurotransmission in CeA neurons from WT and MOR KO mice. Using sharp-tip electrodes, we recorded IPSPs at the resting membrane potential (RMP) of each neuron. Under this condition, the baseline membrane properties (RMP and input resistance) were not significantly different between CeA neurons from WT and MOR KO mice. The mean RMPs were -78.9 ± 1.7 (n = 17) and -79.8 mV ± 1.0 (n = 24) for WT and MOR KO mice, respectively. Mean input resistances were 84.3 ± 8.3 MΩ for WT (n = 16) and 101.9 ± 5.5 MΩ for MOR KO mice (n = 22). We examined the IPSPs at five different stimulus intensities from threshold intensities evoking IPSPs to the intensity producing maximal responses (Fig. 2A), and we found a significant difference in the IPSP amplitudes between WT and MOR KO mice [two-way ANOVA, F(1,34) = 5.9, p = 0.02].
Fig. 2.
Baseline activities of evoked GABAergic synaptic responses in CeA from MOR KO and WT mice. Inhibitory synaptic responses were examined in current-clamp (A and B) and voltage-clamp (C) modes. The IPSPs and IPSCs were evoked at five different stimulus intensities. Open circles, WT mice; closed circles, MOR KO mice. Insets, representative traces. For equivalent stimulus intensities, amplitudes of evoked IPSPs and IPSCs from MOR KO mice were greater than WT mice. B, paired-pulse ratios of IPSPs were evoked at interpulse intervals of 50 and 100 ms. For both intervals, the paired-pulse ratios were less in MOR KO mice than in WT mice, suggesting that the difference in the magnitude of IPSP amplitudes could be because of presynaptic effects resulting in enhanced GABA release in the CeA of MOR KO mice; *, p < 0.05.
We also examined the paired-pulse ratio of IPSPs because changes in this ratio reflect presynaptic effects (Dobrunz and Stevens, 1997). We evoked IPSPs in pairs with interpulse intervals of either 50 or 100 ms at a stimulus intensity eliciting 50 to 60% maximal primary IPSP. The ratios of the amplitudes of the second IPSP to the first IPSP were significantly smaller in MOR KO compared with WT mice (Fig. 2B). The paired-pulse ratios at 50-ms intervals were 0.82 ± 0.04 (n = 15) and 0.67 ± 0.04 (n = 17) for WT and MOR KO mice, respectively (p = 0.01), whereas at 100-ms intervals, they were 1.02 ± 0.05 (n = 15) and 0.84 ± 0.06 (n = 16) for WT and MOR KO mice, respectively (p = 0.02), suggesting that the greater amplitudes of IPSPs in MOR KO mice were because of greater presynaptic release at GABA synapses in MOR KO mice compared with WT mice (Fig. 2B). We re-examined the input/output responses of CeA inhibitory neurotransmission from WT and MOR KO mice with whole-cell patch recordings in voltage-clamp mode at -70 mV, measuring the amplitudes of IPSCs at five different stimulus intensities (Fig. 2C). The mean amplitudes of evoked IPSCs were significantly greater in CeA neurons of MOR KO mice than in WT mice [two-way ANOVA, F(1,36) = 4.8, p = 0.03; Fig. 2C].
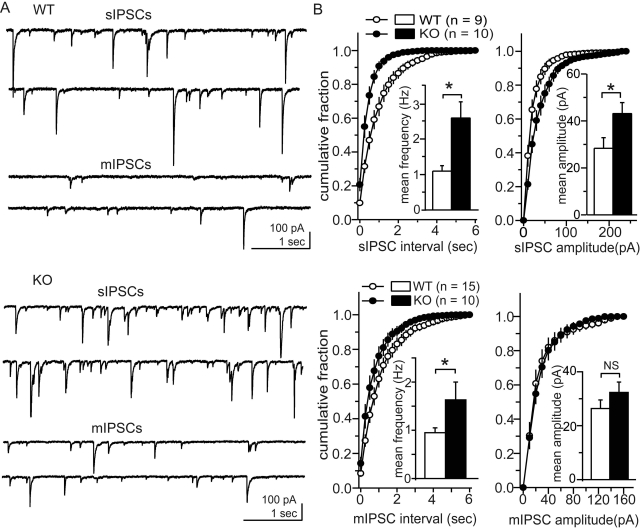
We compared baseline spontaneous IPSCs in CeA neurons from WT and MOR KO mice. Action potential-dependent sIPSCs and action potential-independent mIPSCs were examined in the absence and presence of TTX, respectively. The baseline frequencies of both sIPSCs and mIPSCs were significantly greater in MOR KO mice compared with WT mice (p = 0.03 and 0.048, respectively; Fig. 3). The mean frequencies of sIPSCs and mIPSCs in WT mice were 1.1 ± 0.1 (n = 9) and 0.9 ± 0.1 (n = 15) Hz, respectively, whereas those from MOR KO mice were 2.6 ± 0.4 (n = 10) and 1.6 ± 0.4 (n = 10) Hz, respectively. Although the mean amplitudes of sIPSCs were significantly greater (p = 0.03) in the CeA of MOR KO mice than WT mice, mean mIPSC amplitudes were not significantly (p = 0.25) different between WT mice and MOR KO mice; mean sIPSC amplitudes were 28.3 ± 4.5 (n = 9) and 43.1 ± 4.7 (n = 10) pA in WT and MOR KO mice, respectively, and mean mIPSC amplitudes were 26.4 ± 3.2 (n = 15) and 32.3 ± 3.9 pA (n = 10) in WT and MOR KO mice, respectively. The greater frequencies of mIPSCs supports the likelihood of greater presynaptic activity of the CeA GABAergic system in MOR KO mice compared with WT mice, consistent with the smaller paired-pulse ratios of evoked IPSPs observed in MOR KO mice.
Fig. 3.
Baseline activities of spontaneous IPSCs in CeA neurons from MOR KO and WT mice. A, representative traces of spontaneous IPSCs from WT (top) and MOR KO (bottom) mice. Top two traces of spontaneous IPSCs are in the absence of TTX (sIPSCs), and bottom two traces are in the presence of TTX (mIPSCs). B, averaged cumulative fraction plots of frequency (left) and amplitudes (right) of sISPCs (top) and mIPSCs (bottom). Insets, mean frequency and amplitudes, respectively, from WT mice (open columns) and MOR KO mice (closed columns). MOR KO mice show greater baseline frequency of both sIPSCs and mIPSCs and greater baseline amplitude of sIPSCs; *, p < 0.05. Amplitude of mIPSCs was not significantly different (NS).
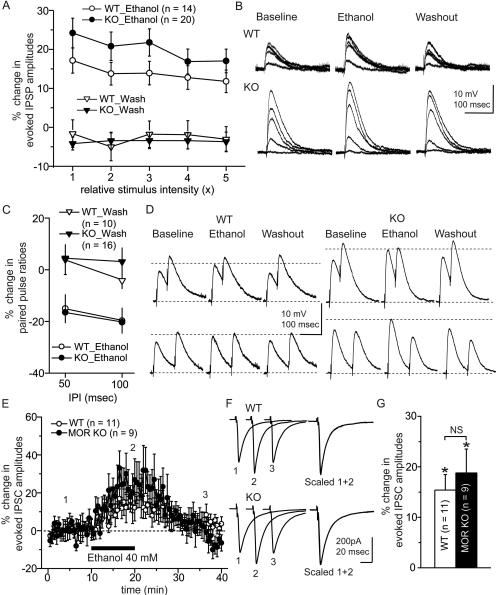
We next studied the interaction between MORs and ethanol on the GABAergic system, first by examining ethanol effects on evoked IPSPs and IPSCs in CeA neurons from WT and MOR KO mice. Using sharp recording pipettes, ethanol (44 mM) superfusion significantly increased the amplitude of evoked IPSPs in CeA neurons from both WT (n = 14) and MOR KO (n = 20) mice at all five stimulus intensities (Fig. 4B). Although responses of CeA neurons from MOR KO mice tended to show greater ethanol effects than WT mice, this was not statistically significant by two-way ANOVA over all stimulus intensities [F(1,32) = 2.23, p = 0.14]. However, ethanol effects were significantly different with different stimulus intensities [Greenhouse-Geiser, F(2.97,95.1) = 3.2, p = 0.03]. Ethanol also decreased the paired-pulse ratios of evoked IPSPs from both WT and MOR KO mice (Fig. 4, C and D), suggesting an action at presynaptic sites. In WT mice (n = 10), ethanol decreased the paired-pulse ratios by 15.0 ± 5.5% (p = 0.02) and 19.7 ± 5.0% (p < 0.01) at 50- and 100-ms interpulse intervals, respectively. In MOR KO mice (n = 16), ethanol decreased the paired-pulse ratio by 16.5 ± 4.2% (p = 0.02) and 20.3 ± 3.6% (p = 0.02), although this effect was not significantly different from WT mice. Because baseline amplitudes of evoked IPSPs were different between WT and MOR KO mice, and ethanol effects varied over different stimulus intensities, we examined ethanol effects between WT and MOR KO mice at comparable IPSC amplitudes using whole-cell patch mode. In these experiments, we evoked IPSCs at 30 to 50% maximal responses based on input/output curves. The mean baseline amplitudes of evoked IPSCs were 225.5 ± 35.0 and 258.9 ± 54.2 pA for WT (n = 11) and MOR KO (n = 9) mice, respectively. Ethanol (40 mM) significantly increased the mean amplitude of evoked IPSCs in CeA by 15.4 ± 3.1% (p = 0.03) and 18.8 ± 4.7% (p = 0.04) in slices from WT and MOR KO mice, respectively (Fig. 4, E–G), although there was no significant difference in the magnitude of ethanol effects between WT and MOR KO mice.
Fig. 4.
Ethanol effects on evoked GABAergic synaptic responses in CeA neurons from MOR KO and WT mice: effects on IPSPs in current-clamp mode (A and B) and on IPSCs in voltage-clamp mode (C). A, ethanol effects on IPSPs were not significantly different (by ANOVA) between WT and MOR KO mice over five different stimulus intensities. B, representative traces before (baseline), during ethanol treatment (ethanol), and after ethanol washout (washout). C, ethanol effects on paired-pulse ratios of IPSPs evoked at a stimulus intensity giving a 50 to 60% maximal response for the primary IPSP, with interpulse intervals of 50 and 100 ms. Ethanol decreased the paired-pulse ratio at both intervals, suggesting presynaptic effects (increased GABA release). However, the magnitude of changes in the paired-pulse ratio was not significantly different between WT and MOR KO mice. D, representative traces before (baseline), during ethanol treatment, and after ethanol washout. E, ethanol effects on evoked IPSCs in whole-cell patch showing time course of responses in CeA from WT and MOR KO mice. F, representative IPSC traces before (1), during ethanol treatment (2), and after ethanol washout (3). Scaled traces demonstrate no change in the kinetics of the IPSCs. G, mean percentage change in the amplitude of evoked IPSCs in the presence of ethanol. Ethanol increased the amplitudes of evoked IPSCs in CeA neurons from WT and MOR KO mice without a significant difference in the magnitude of ethanol effects between WT and MOR KO mice; *, p < 0.05.
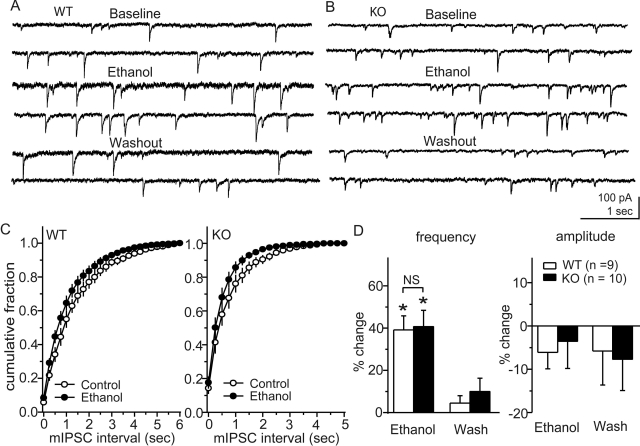
We next examined effects of ethanol on mIPSCs in CeA neurons from WT and MOR KO mice (Fig. 5). Ethanol (40 mM) increased the mean frequency of mIPSCs from 0.7 ± 0.1 to 0.9 ± 0.1 Hz in WT mice (n = 9, p < 0.01), with little change in the amplitude. Ethanol also increased the mean frequency of mIPSCs from 1.6 ± 0.4 to 2.1 ± 0.4 Hz in MOR KO mice (n = 10, p < 0.01), without significant changes in mean amplitude. There was no difference in the magnitude of ethanol effects on the mean mIPSC frequencies between WT and MOR KO mice. However, because the baseline frequency was greater in slices from MOR KO mice, the net enhancement in MOR KO mice (0.5 ± 0.07 Hz, n = 10) was significantly greater (p < 0.01) than that in WT mice (0.24 ± 0.03 Hz, n = 9).
Fig. 5.
Ethanol effects on mIPSCs in CeA neurons from MOR KO and WT mice. A and B, representative mIPSCs before and during ethanol treatment and after washout of ethanol in CeA neurons from WT (A) and MOR KO (B) mice. C, cumulative plot of mIPSC frequencies before and during ethanol treatment from WT (left) and MOR KO (right) mice. In both groups, ethanol moved the cumulative frequency plot to the left by a comparable relative magnitude. D, averaged frequency and amplitudes, respectively, from WT mice (open columns) and MOR KO mice (closed columns). Ethanol increased the frequency of mIPSCs without significant changes in amplitude in both groups, suggesting increased vesicular GABA release in both cases; *, p < 0.05.
We also examined the effect of ethanol on evoked IPSCs in the presence of naloxonazine in control mice. Ethanol (40 mM) significantly increased the mean amplitude of evoked IPSCs in CeA by 17.5 ± 3.1% (n = 11, p < 0.01) in the presence of naloxonazine (1 μM) from control mice (results not shown). This effect was comparable with the ethanol effect in the absence of naloxonazine (1 μM) (18.6 ± 5.1% enhancement, n = 8) in control mice. When we compared the ethanol effect on IPSCs in the presence of naloxonazine from control mice to the ethanol effect on IPSCs from MOR KO mice, there was no significant difference. This suggests that the lack of further facilitation of IPSCs by ethanol in the MOR KO mice is not because of compensatory developmental changes.
Because MOR activation is reported to modulate excitatory neurotransmission (Zhu and Pan, 2005), we also examined the baseline spontaneous EPSCs in CeA from MOR KO and WT mice (results not shown). There were no significant differences in either sEPSC frequency (p = 0.19) or amplitude (p = 0.27) between WT (n = 18) and MOR KO mice (n = 16). In addition, there were no significant differences in mEPSC frequency (p = 0.19) or amplitude (p = 0.18) between WT (n = 9) and MOR KO (n = 9) mice; mean mEPSC frequencies were 4.5 ± 0.6 and 3.3 ± 0.7 Hz for WT (n = 9) and MOR mice (n = 9), respectively, whereas mean mEPSC amplitudes were 14.5 ± 1.4 and 12.0 ± 0.9 pA for WT (n = 9) and MOR (n = 9) mice, respectively. Ethanol decreased the frequency and amplitude of mEPSCs in CeA, consistent with reports by Roberto et al. (2004) and Zhu et al. (2007). There were no differences in ethanol effects on mEPSC frequency (p = 0.70) or amplitude (p = 0.77) between MOR (n = 7) and WT (n = 6) mice. Ethanol (40 mM) decreased the mean frequency of mEPSCs by 27.1 ± 6.7% in WT mice (n = 6, p < 0.01) and by 24.3 ± 3.6% in MOR KO mice (n = 7, p < 0.01). Ethanol decreased the mean amplitude of mEPSCs by 12.3 ± 3.7% (n = 6, p = 0.04) in WT mice and by 10.7 ± 3.4% (n = 7, p = 0.02) in MOR KO mice. In a separate set of experiments using control mice, we examined whether MORs in the excitatory terminal are tonically activated by measuring the effect of naloxonazine on the frequency and amplitude of mEPSC (n = 7). The baseline mEPSC frequency was comparable in the absence (2.8 ± 0.4 Hz) and in the presence (2.7 ± 1.0 Hz) of naloxonazine (1 μM) (p = 0.19). Likewise, the mean amplitude of mEPSCs in the presence of naloxonazine (30.6 ± 2.3 pA) was not significantly different in the absence of naloxonazine (31.6 ± 2.3 pA) (p = 0.43).
Discussion
A major finding in this study is that CeA neurons of MOR KO mice display greater baseline GABAergic neurotransmission compared with those of WT mice. It has been shown previously that MOR activation decreases GABAergic transmission in CeA (Finnegan et al., 2005). We observed an increase in IPSC frequency after application of the MOR antagonist naloxonazine, suggesting tonic activation of MOR in CeA of WT mice. Therefore, the greater baseline GABAergic neurotransmission observed in MOR KO mice could be attributed to the lack of tonic inhibition on the GABAergic neurotransmission mediated through MOR. Tonic activation of opioid receptors has been suggested by studies showing that opioid receptor antagonists increase c-fos expression (a marker for increased neuronal activity) in CeA (Gestreau et al., 2000). The possibility of tonic inhibition of GABAergic neurotransmission is further supported by the finding that there is little or no disinhibitory effect of locally injected GABAA receptor antagonists into CeA (Sanders and Shekhar, 1995; Veinante and Freund-Mercier, 1998).
Our results showing MOR modulation of mIPSCs and paired-pulse ratios indicate presynaptic inhibition of GABAergic neurotransmission mediated by MOR. However, some CeA neurons are also directly inhibited by MOR agonists (Chieng et al., 2006). In this study, we recorded from neurons located in the medial subdivision of the CeA, which projects to major output regions of amygdala. Neurons of the medial division of CeA receive intrinsic inhibitory inputs from the lateral and capsular subdivisions of CeA (Cassell et al., 1999). Some of these intrinsic neurons might be under direct opioid-mediated inhibition. Therefore, disinhibition of these (presumed) interneurons could also contribute to increased GABAergic neurotransmission within CeA in MOR KO mice.
We also confirmed that acutely applied ethanol enhances GABAergic neurotransmission in CeA, and the relative magnitude of the enhancement was comparable between CeA neurons from MOR KO and WT mice. Ethanol effects on neurotransmission are mediated through multiple modulatory systems. Ethanol not only activates the endogenous opioid-containing neurons in CeA (Morales et al., 1998) but also increases GABAergic neurotransmission in CeA through corticotropin-releasing factor acting on presynaptic GABA terminals (Nie et al., 2004). Thus, the positively modulating effect of corticotropin-releasing factor on GABAergic neurotransmission may be counterbalanced by MOR-mediated negative presynaptic modulation in WT mice, but not in MOR KO mice, possibly resulting in greater enhancement of CeA GABAergic neurotransmission in MOR KO than WT mice. However, we did not observe such enhancement of ethanol effects on GABAergic neurotransmission in MOR KO mice. Because acute pharmacological antagonism of MORs also showed ethanol enhancement of GABAergic transmission comparable with the control condition, this is not an adaptive change because of loss of MORs during developmental maturation. This could be attributed to a “ceiling” effect that would limit the enhancing action of ethanol on GABAergic responses because of the significantly enhanced baseline GABAergic activity in the absence of MORs or MOR blockade.
Lack of MOR effects on ethanol stimulation of presynaptic GABA release in CeA leads us to hypothesize that modulation of the GABAergic system in CeA may not be the mechanism through which MORs modulate ethanol drinking behaviors. Both MOR- and GABA-mediated mechanisms may contribute independently to the reinforcing effect of ethanol, based on the observations that local injection of either GABA antagonists or opioid antagonists into CeA decrease voluntary ethanol consumption (Hyytiä and Koob, 1995; Heyser et al., 1999; Foster et al., 2004). One possibility is that the direct postsynaptic inhibitory effect of MOR on the CeA projection neurons could provide such a mechanism, independent of GABA release (Chieng et al., 2006).
The CeA, as a part of the “extended amygdala,” is connected to the mesolimbic reward circuit through a continuum of neurons that extend into the bed nucleus of the stria terminalis and through the basal forebrain to the accumbens shell (Alheid and Heimer, 1988). The CeA may modulate the mesolimbic reward circuit through a GABAergic projection to the ventral tegmental area (VTA) (Wallace et al., 1992; Fudge and Haber, 2000). The CeA contains a high proportion of GABAergic interneurons and GABAergic projection neurons (Veinante et al., 1997) with local GABAergic interneurons innervating projection neurons, leading to feed-forward inhibition (Cassell et al., 1999). Therefore, depending on the type of GABAergic neurons activated, the overall output of CeA might be inhibitory (activation of GABAergic projection neurons) or excitatory (activation of GABAergic interneurons that inhibit the GABAergic output) in CeA. In addition, CeA neurons show high heterogeneity in terms of connectivity and peptide content (Pitkänen, 2000). Thus, cell-specific modulation can lead to diverse functional results, and ethanol actions seem to be specific to particular subsets of CeA GABAergic neurons (Morales et al., 1998; Nie et al., 2004). GABA receptor antagonists injected locally into CeA could block ethanol consumption through blocking ethanol-stimulated GABA release onto these populations of neurons, whereas their effects on GABAergic activity in CeA may be minimal in the absence of ethanol (Sanders and Shekhar, 1995; Veinante and Freund-Mercier, 1998).
Inhibition of GABAergic neurotransmission could also mediate motivational effects of ethanol through the disinhibition of projection neurons in a pathway-specific manner. It has been suggested that GABAergic afferents from the CeA to the VTA may inhibit the extensive GABAergic interneuron network in the VTA, disinhibiting the VTA dopaminergic neurons (Wallace et al., 1992). MOR-mediated tonic inhibition of GABAergic neurotransmission, revealed in this study with MOR KO mice or acute MOR antagonism, could lower the threshold for such a reward pathway. In that context, greater baseline GABAergic neurotransmission observed in MOR KO mice could increase the threshold for motivational effects of ethanol in MOR KO mice, causing less voluntary ethanol consumption. On the other hand, considering the lack of correlation between the endogenous opioid terminals and MOR distribution patterns in CeA (“receptor mismatch”; Jacobsen et al., 2006), the lack of MOR modulation on ethanol stimulation of GABA transmission might be because of the lack of acutely released endogenous opioids reaching the vicinity of the MORs during ethanol application. If MORs are located away from endogenous opioid terminals, the MORs could be activated by endogenous opioids through volume transmission or spillover. In such cases, MOR activation requires greater release of endogenous opioids, possibly including extrinsic inputs to CeA such as arcuate nucleus or the bed nucleus of stria terminalis. Therefore, even though we did not observe an effect of MORs on ethanol-stimulated GABA release in slice preparations, MORs might modulate the ethanol effect on GABA release in CeA in the intact in vivo condition and, thus, play a role in behavioral motivational effects of ethanol.
We previously reported that, in contrast to MOR KO mice, DOR KO mice showed no difference in the baseline IPSC frequency but enhanced ethanol stimulation of presynaptic GABA release compared with the WT mice (Kang-Park et al., 2007). These results suggest that the modulation of GABAergic neurotransmission through opioids differs depending on opioid receptor subtypes in CeA, such that MORs may be more involved in control of tonic GABAergic neurotransmission, whereas DORs may play a role in mediating phasic GABAergic neurotransmission induced by acute ethanol. The difference in the acute ethanol effect between MOR and DOR could be because of their different localization. For example, despite the receptor mismatch (Jacobsen et al., 2006), MORs are located on cell bodies and diffusely on terminals (Wilson et al., 2002). In contrast, DORs are shown preferentially located on GABAergic axon terminals (Wilson et al., 2002), making DORs more suitable for moment-to-moment control of GABA release; for example, through acutely released endogenous opioids during ethanol application. In contrast, why the tonic effect on GABA transmission was observed only in MOR but not in DOR KO mice is presently unclear. One possibility is that lack of tonic effect through DORs could be because of a relatively small number of functional DORs, particularly if DORs are predominantly localized intracellularly in CeA as shown in other brain regions (Hack et al., 2005).
It has been reported that glutamatergic EPSCs in CeA are inhibited by MOR activation and by ethanol and that this inhibition of excitatory neurotransmission in CeA mediates the reinforcing effect of ethanol (Roberto et al., 2004; Zhu and Pan, 2005; Zhu et al., 2007). In the present study, we found that that baseline excitatory synaptic responses and their modulation by ethanol were unchanged in CeA from MOR KO mice. The lack of opioid tone on glutamatergic transmission contrasts with the presence of opioid tone on GABAergic transmission. However, similar selective enhancement of baseline activity of GABAergic but not glutamatergic neurotransmission was observed in dopaminergic neurons in VTA from MOR KO mice (Mathon et al., 2005). It remains unclear as to why MOR-mediated opioid tone appears to selectively affect GABAergic transmission in these regions.
In conclusion, baseline GABAergic neurotransmission in CeA is increased in MOR KO mice compared with WT mice, whereas ethanol effects on GABA release are similar in CeA of both MOR KO and WT mice. Based on these results, we hypothesize that MORs mediate ethanol reinforcement through pathway-specific mechanisms in CeA, possibly independently of ethanol-induced GABA release.
This study was supported by the National Institute on Alcohol Abuse and Alcoholism Integrative Neuroscience Initiative on Alcoholism [Grant UO1 AA013498]; by the National Institute on Drug Abuse [Grant R01 DA03665]; and by VA Merit Review [Grant 01160/0012].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.140749.
ABBREVIATIONS: WT, wild type; MOR, μ-opioid receptor; KO, knockout; CeA, central nucleus of the amygdala; NMDA, N-methyl-d-aspartate; ACSF, artificial cerebrospinal fluid; IPSP, inhibitory postsynaptic potential; IPSC, inhibitory postsynaptic current; EPSC, excitatory postsynaptic current; AP-5, d-2-amino-5-phosphonovalerate; CGP 55845, 3-N[1-(S)-(3,4-dichlorophenyl)ethyl]amino-2-(S)-hydroxypropyl-p-benzyl-phosphinic acid; TTX, tetrodotoxin; sIPSC, spontaneous IPSC; sEPSC, spontaneous EPSC; mIPSC, miniature IPSC; mEPSC, miniature EPSC; DAMGO, [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin; naloxonazine, bis-[5-a-4,5-epoxy-3,14-dihydroxy-17-(2-propenyl)-morph inan-6-ylidene] hydrazine dihydrochloride; PPR, paired-pulse ratio; ANOVA, analysis of variance; RMP, resting membrane potential; VTA, ventral tegmental area; DOR, δ-opioid receptor; QX 314, N-(2,6-dimethylphenylcarbamoylmethyl)triethylammonium bromide.
References
- Alheid GF, Heimer L (1988) New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of the substantia innominata. Neuroscience 27 1-39. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, and Shi C (1999) The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci 877 217-241. [DOI] [PubMed] [Google Scholar]
- Chieng BC, Christie MJ, and Osborne PB (2006) Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol 497 910-927. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, and Weiss F (2002) Effect of selective blockade of mu or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27 391-399. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE and Stevens CF (1997) Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18 995-1008. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, et al. (2000) Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25 195-200. [DOI] [PubMed] [Google Scholar]
- Finnegan TF, Chen SR, and Pan HL (2005) Effect of the mu-opioid on excitatory and inhibitory synaptic inputs to periaquaductal gray-projecting neurons in the amygdala. J Pharmacol Exp Ther 312 441-448. [DOI] [PubMed] [Google Scholar]
- Foster KL, McKay PF, Seyoum R, Milbourne D, Yin W, Sarma PV, Cook JM, and June HL (2004) GABAA and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology 29 269-284. [DOI] [PubMed] [Google Scholar]
- Froehlich JC (1996) The neurobiology of ethanol-opioid interactions in ethanol reinforcement. Alcohol Clin Exp Res 20: 181A-186A. [DOI] [PubMed] [Google Scholar]
- Fudge JL and Haber SN (2000) The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience 97 479-494. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Le Guen S, and Besson JM (2000) Is there tonic activity in the endogenous opioid systems? A c-fos study in the rat central nervous system after intravenous injection of naloxone or naloxone-methiodide. J Comp Neurol 427 285-301. [DOI] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, and Christie MJ (2005) Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci 25 3192-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A (1997) Endogenous opioid systems and alcohol addiction. Psychopharmacology 129 99-111. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Roberts AJ, Schulteis G, and Koob GF (1999) Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin Exp Res 23 1468-1476. [PubMed] [Google Scholar]
- Hyytiä P and Koob GF (1995) GABA-A receptor antagonism in the extended amygdala decreases ethanol self-administration. Eur J Pharmacol 283 151-159. [DOI] [PubMed] [Google Scholar]
- Jacobsen KX, Höistad M, Staines WA, and Fuxe K (2006) The distribution of dopamine D1 receptor and mu-opioid receptor 1 receptor immunoreactivities in the amygdala and interstitial nucleus of the posterior limb of the anterior commissure: relationships to tyrosine hydroxylase and opioid terminal systems. Neuroscience 141 2007-2018. [DOI] [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Siggins GR, and Moore SD (2007) Presynaptic delta opioid receptors regulate ethanol actions in central amygdala. J Pharmacol Exp Ther 320 917-925. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytiä P, Merlo-Pich E, and Weiss F (1998) Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res 22 3-9. [PubMed] [Google Scholar]
- Mathon DS, Lesscher HM, Gerrits MA, Kamal A, Pintar JE, Schuller AG, Spruijt BM, Burbach JP, Smidt MP, van Ree JM, et al. (2005) Increased GABAergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice. Neuroscience 130 359-367. [DOI] [PubMed] [Google Scholar]
- McBride WJ (2002) Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav 71 509-515. [DOI] [PubMed] [Google Scholar]
- Mhatre M and Holloway F (2003) Micro1-opioid antagonist naloxonazine alters ethanol discrimination and consumption. Alcohol 29 109-116. [DOI] [PubMed] [Google Scholar]
- Möller C, Wiklund L, Sommer W, Thorsell A, and Heilig M (1997) Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res 760 94-101. [DOI] [PubMed] [Google Scholar]
- Morales M, Criado JR, Sanna PP, Henriksen SJ, and Bloom FE (1998) Acute ethanol induces c-fos immunoreactivity in GABAergic neurons of the central nucleus of the amygdala. Brain Res 798 333-336. [DOI] [PubMed] [Google Scholar]
- Moskowitz AS and Goodman RR (1984) Light microscopic autoradiographic localization of mu- and delta-opioid binding sites in the mouse central nervous system. J Neurosci 4 1331-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, and Siggins GR (2004) CRF1 receptors are involved in the ethanol enhancement of GABAergic transmission in the central amygdala. Science 303 1512-1514. [DOI] [PubMed] [Google Scholar]
- Oswald LM and Wand GS (2004) Opioids and alcoholism. Physiol Behav 81 339-358. [DOI] [PubMed] [Google Scholar]
- Pitkänen A (2000) Connectivity of the rat amygdaloid complex, in The Amygdala: A Functional Analysis (Aggleton JP ed), pp 31-115, Oxford University Press, New York.
- Roberto M, Madamba SG, Moore SD, Tallent MK, and Siggins GR (2003) Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Nat Acad Sci U S A 100 2053-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, and Siggins GR (2004) Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci 24 1594-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, and Koob GF (1996) Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res 20 1289-1298. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Gold LS, Polis I, McDonald JS, Filliol D, Kieffer BL, Koob GF (2001) Increased ethanol self-administration in delta opioid receptor knockout mice. Alcohol Clin Exp Res 25 1249-1256. [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, and Gold LH (2000) Mu-opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther 293 1002-1008. [PubMed] [Google Scholar]
- Sanders SK and Shekhar A (1995) Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav 52 701-706. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Peuser K, Erickson DJ, and Wood MD (2007) Stress-response-dampening effects of alcohol: attention as a mediator and moderator. J Abnorm Psychol 116 362-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P and Freund-Mercier MJ (1998) Intrinsic and extrinsic connections of the rat central extended amygdala: an in vivo electrophysiological study of the central amygdaloid nucleus. Brain Res 794 188-198. [DOI] [PubMed] [Google Scholar]
- Veinante P, Stoeckel ME, and Freund-Mercier MJ (1997) GABA- and peptide-immunoreactivities co-localize in the rat central extended amygdala. Neuroreport 8 2985-2989. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, and Gray TS (1992) Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic and adrenergic cell groups in the rat. Brain Res Bull 28 447-454. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Mascagni F, and McDonald AJ (2002) Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci Lett 328 160-164. [DOI] [PubMed] [Google Scholar]
- Zhu W, Bie B, and Pan ZZ (2007) Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci 27 289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W and Pan ZZ (2005) mu-Opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience 133 97-103. [DOI] [PubMed] [Google Scholar]