Abstract
Involuntary choreiform movements are a clinical hallmark of Huntington's disease. Studies in clinically affected patients suggest a shift of motor activations to parietal cortices in response to progressive neurodegeneration. Here, we studied pre-symptomatic gene carriers to examine the compensatory mechanisms that underlie the phenomenon of retained motor function in the presence of degenerative change. Fifteen pre-symptomatic gene carriers and 12 matched controls performed button presses paced by a metronome at either 0.5 or 2 Hz with four fingers of the right hand whilst being scanned with functional magnetic resonance imaging. Subjects pressed buttons either in the order of a previously learnt 10-item finger sequence, from left to right, or kept still. Error rates ranged from 2% to 7% in the pre-symptomatic gene carriers and from 0.5% to 4% in controls, depending on the condition. No significant difference in task performance was found between groups for any of the conditions. Activations in the supplementary motor area (SMA) and superior parietal lobe differed with gene status. Compared with healthy controls, gene carriers showed greater activations of left caudal SMA with all movement conditions. Activations correlated with increasing speed of movement were greater the closer the gene carriers were to estimated clinical diagnosis, defined by the onset of unequivocal motor signs. Activations associated with increased movement complexity (i.e. with the pre-learnt 10-item sequence) decreased in the rostral SMA with nearing diagnostic onset. The left superior parietal lobe showed reduced activation with increased movement complexity in gene carriers compared with controls, and in the right superior parietal lobe showed greater activations with all but the most demanding movements. We identified a complex pattern of motor compensation in pre-symptomatic gene carriers. The results show that preclinical compensation goes beyond a simple shift of activity from premotor to parietal regions involving multiple compensatory mechanisms in executive and cognitive motor areas. Critically, the pattern of motor compensation is flexible depending on the actual task demands on motor control.
Keywords: pre-symptomatic Huntington's disease, fMRI, motor control
Introduction
Huntington's disease is an autosomal dominant inherited neurodegenerative disorder caused by an expansion of CAG repeats in the gene encoding huntingtin (Huntington's Disease Collaborative Research Group, 1993). Affected patients show progressive motor and cognitive dysfunction with psychiatric problems (for a recent review see Walker, 2007). Generalized chorea, the clinical hallmark of Huntington's disease has been the focus of a number of functional neuroimaging studies (Bartenstein et al., 1997; Weeks et al., 1997; Gavazzi et al., 2007). A first study used positron emission tomography (PET) to examine changes in regional cerebral blood flow when mild or moderately affected patients performed auditory paced unilateral finger opposition movements (Bartenstein et al., 1997). In that study, Huntington's disease patients showed reduced task related activation in the striatum, rostral supplementary motor area (pre-SMA) and contralateral primary sensorimotor cortex (M1) compared with healthy controls, while parietal and posterior cingulate areas were relatively overactive (Bartenstein et al., 1997). The authors suggested a shift of cortical motor control towards more posterior and parietal brain regions to counterbalance dysfunctional pre- and primary motor areas.
Conflicting results come from a more recent functional MRI (fMRI) study, which examined clinically affected Huntington's disease patients while performing a finger tapping task (Gavazzi et al., 2007). Although the authors found reduced activation in M1 during tapping, there was a concurrent increase in activation in caudal supplementary motor area (SMA), which seems to contradict a shift of activation away from pre-motor areas. More divergent data are to be found in another PET study in which Huntington's disease patients with chorea and bradykinesia performed freely chosen joystick movements (Weeks et al., 1997). Here, reduced activation in pre-motor areas was combined with a reduction of activity in parietal areas. A straightforward interpretation of these apparently conflicting results in affected Huntington's disease patients is precluded either because of between-group differences in performance, or because performance during functional imaging was not recorded.
The availability of a sensitive and specific genetic test for Huntington's disease allows preclinical diagnosis, many years before the onset of unequivocal motor signs. Years to clinical onset can be estimated based on age and number of CAG repeats (Langbehn et al., 2004). Morphometric MRI studies in these pre-symptomatic mutation carriers show degeneration, primarily affecting the striatum but also including cortical areas (Thieben et al., 2002; Rosas et al., 2005, 2006). Despite those structural changes, behavioural studies in pre-symptomatic mutation carriers have demonstrated abnormalities only on more demanding motor tasks (see Farrow et al., 2006 for an overview). This may be attributed to an effective recruitment of compensatory mechanisms that help to maintain routine motor functions in the context of slowly progressive neurodegeneration. Indeed, functional imaging studies of working memory (Wolf et al., 2007) or motor sequence learning (Feigin et al., 2006) suggest a compensatory reorganisation in the cortex. A deeper understanding of such compensatory processes could prove critical for the understanding of early and pre-symptomatic stages of degeneration in Huntington's disease, which may also have relevance for other basal ganglia disorders.
While the PET study by Feigin and colleagues (2006) studied neuronal activations in pre-symptomatic mutation carriers during trial and error learning of a motor sequence based on feedback, we chose an experimental motor set up that taps into executive (speed of movement) and cognitive (movement complexity) aspects of motor control (Lehericy et al., 2006). Based on previous work in affected Huntington's disease patients with a similar task (Bartenstein et al., 1997), we hypothesized that distinct parietal areas will show compensatory increases in activation to keep motor performance within the normal range. In the light of the apparently conflicting fMRI results on motor compensation in clinically affected Huntington's disease patients, we predicted that the regional expression of compensatory activity in premotor and parietal areas will critically depend on task difficulty and on which aspects of motor control are probed by the experimental motor task.
Material and Methods
Participants
We examined a group of 15 pre-symptomatic gene carriers (7 F, 8 M; mean age: 36.9 years; range: 26–49) and a group of 12 age- and sex-matched controls (4 F, 8 M; mean age: 36.5 years; range: 23–60). All participants were right-handed. A neurologist experienced in Huntington's disease assessed subjects with the Unified Huntington's Disease Rating Scale (UHDRS) to confirm pre-symptomatic status (i.e. no unequivocal Huntington's disease motor signs) prior to inclusion. Pre-symptomatic gene carriers were selected to cover a wide range of years to estimated onset based on age and the number of CAG repeats (Langbehn et al., 2004) to allow meaningful correlations. Refer Table 1 for full demographic and clinical details. The local Ethics Committee approved the study and all participants gave written informed consent according to the declaration of Helsinki.
Table 1.
Demographic details reported with median and range
| Controls | Pre-symptomatic Huntington's disease | |
|---|---|---|
| Number of subjects | 12 | 15 |
| Female/Male | 4/8 | 7/8 |
| Age | 32.5 (23:60) | 37 (26:54) |
| Number CAG repeats | NA | 42 (39:47) |
| Motor score | NA | 2 (0:17) |
| Year to 60% probability of diagnostic onset | NA | 12.51 (6.3:35.4) |
Experimental procedure
Following up on previous work in Huntington's disease (Bartenstein et al., 1997), subjects performed auditory paced sequential finger movements with the second to fifth fingers of their right dominant hand during fMRI. An MRI-compatible hand mould was crafted in-house, allowing participants to comfortably press all four buttons while simultaneously restricting excessive movement. The tips of the four fingers are placed beside each other on top of four buttons while the thumb rests beside them. The experimental paradigm varied the executive (speed) and cognitive (complexity) demands of the task. Participants performed sequential finger movements at a rate of 0.5 or 2 Hz (referred to as ‘slow’ and ‘fast’ sequences). Each movement was paced by a metronome click presented via headphones. Participants performed two types of sequence. The first was regular and consisted of unidirectional sequential button presses with the index, middle, ring and little fingers (i.e. 1–2–3–4–1–2–3–4 referred to as the ‘simple’ sequence). The other sequence was irregular but without immediate repetitions (e.g. 1–3–4–2–3–4–2–1–3–4; referred to as the ‘complex’ sequence). A new complex sequence was generated for each participant. The simple sequence remained unchanged as performing it in reverse order is of itself less natural and more difficult.
Participants were trained on the simple and complex sequence in a testing room prior to their fMRI session. They were told to take as much time as necessary to learn it. Once they felt comfortable, they were asked to repeat it from memory simultaneously with click sounds at 0.5 and 2 Hz. Before scanning, subjects were required to perform the complex sequence, error-free, eight times from memory.
There were six types of block of 20s each (simple-slow, simple-fast, complex-slow and complex-fast sequences; rest-slow and rest-fast listening to metronome clicks but without movement). The different types of block were presented in a pseudo-randomized order. At the beginning of each block, instructions appeared with numbers counting down from 3 to 0 over 3 s to help subjects predict onset and prepare for each task. The set up was such that subjects performed the tasks without sight of their hands.
MRI scanning
Scanning was performed on a 1.5T MRI system (Sonata; Siemens, Erlangen, Germany). Scanning parameters included: TR 3600 ms; TE 50 ms; FOV 192 mm; distance factor (the edge-to-edge gap as a function of slice width) 50%; flip angle 90. We used 40 slices of 3 mm to cover the whole cerebrum. Total scanning time for the fMRI task was around 15 min per subject. A T1-weighted MDEFT sequence was acquired to exclude structural abnormalities (Deichmann et al., 2004).
Statistical analysis of behavioural data
We primarily focused on sequence errors, which were detected by two criteria. Single omitted or wrongly added button presses were counted as one mistake. In sections with more complex errors only sequences of three or more buttons in the appropriate order were counted as correct. We also explored timing inaccuracy and its standard deviation (SD) as additional measures of performance. This criterion was defined by the time between a button press and closest click. Since auditory cues were regular, this measure reflects the ability to anticipate the next click rather than reaction time. A positive sign signified a button was pressed after a click and vice versa. The Mann–Whitney test was employed to compare performance between groups, separately for each condition. Spearman's correlation coefficient was used to test for a correlation between the estimated years to clinical onset and the SD of timing inaccuracy, for comparison with previous work (Hinton et al., 2007).
Image data analysis
Pre-processing and statistical analysis of the fMRI data were carried out using SPM5 software (www.fil.ion.ucl.ac.uk/spm/). Before smoothing with an 8 mm full-width at half-maximum Gaussian kernel, volumes were realigned and spatially normalized to a standard echo-planar-imaging template in MNI space. A first-level analysis based on the general linear model (Friston et al., 1995) was performed for each subject individually. Task-related changes in fMRI signal were estimated at each voxel by modelling each block separately for each of the six conditions after convolving with a haemodynamic response function. The instruction screen between blocks was modelled as a separate regressor. Blocks during which subjects performed the wrong condition (e.g. the simple instead of the complex condition) were excluded by modelling them as separate regressors. Those blocks were also excluded from performance analysis as these errors do not represent problems with learning or sequence execution. Button presses during the rest condition and errors on simple or complex sequences were modelled as single events. Six regressors obtained at the realignment step were included to account for head movements (translations in three planes and rotations along three axes). The resulting set of voxel values for each contrast constituted a statistical parametric map, which then entered second level analysis. For group inferences, we performed a random-effects analysis treating subjects as a random variable. To characterize effects of increased speed or complexity in controls or pre-symptomatic gene carriers we used one-sample t-tests, entering the respective parametric images from the first level analysis.
To test for differences with gene status we used a three-way factorial design with two within-subject factors (COMPLEXITY: simple and complex; SPEED: 0.5 and 2 Hz) and GROUP (pre-symptomatic gene carriers and controls) as a between-subject factor. Age was included as a covariate. Since we were interested in the cortical motor system, we defined spheres of 1 cm radius as regions of interests (ROIs) defined by activations described previously in healthy controls for regions shown to be involved in this task (Lehericy et al., 2006). These spheres were centered on peak activations in left M1, pre-SMA, caudal SMA, dorsal premotor cortex (PMd) and superior parietal lobe (SPL), found from activations of the control group. Refer Table 2 for coordinates of these voxels. Although regional activations due to SPEED and COMPLEXITY in a given anatomical region may be localized very close to each other (Table 2), two separate ROIs were created. Choosing one over another could otherwise have biased the sensitivity of detecting differences in the neuronal processing of speed and complexity between groups. Correction for multiple comparisons within each ROI was done using a family-wise error correction as implemented in SPM5 with a critical P-value of 0.05 at the voxel level. P-values for regions outside these pre-determined ROIs are reported within and corrected for a mask generated from the main effect of the task (i.e. combination of the two movement conditions at slow and fast speed conditions versus rest) at an uncorrected threshold of P < 0.05 (see supplementary material for resulting mask). This mask was preferred over a correction across the whole brain to improve sensitivity outside predefined ROIs. Labelling of premotor regions was done using maps of anatomical probabilities available from version 1.5 of the anatomy toolbox (Eickhoff et al., 2005).
Table 2.
Imaging results from control group
| Side | x | y | z | T | P | |
|---|---|---|---|---|---|---|
| Fast > slow | ||||||
| Caudal SMA | L | −6 | −10 | 54 | 5.14 | <0.001 |
| Primary motor cortex | L | −40 | −18 | 60 | 8.70 | <0.001 |
| Dorsal premotor cortex | L | −18 | −8 | 54 | 6.45 | <0.001 |
| Dorsal premotor cortex | R | 24 | −10 | 60 | 9.90 | <0.001 |
| Superior parietal cortex | L | −24 | −58 | 56 | 9.74 | <0.001 |
| Superior parietal cortex | R | 26 | −58 | 60 | 10.5 | <0.001 |
| Complex > simple | ||||||
| Pre-SMA | 0 | 6 | 54 | 5.74 | <0.001 | |
| Dorsal premotor cortex | L | −24 | 0 | 54 | 7.11 | <0.001 |
| Dorsal premotor cortex | R | 26 | −6 | 52 | 6.64 | <0.001 |
| Superior parietal cortex | L | −22 | −68 | 58 | 6.39 | <0.001 |
| Superior parietal cortex | R | 22 | −66 | 60 | 7.36 | <0.001 |
Areas showing greater activations are reported with uncorrected P-values and co-ordinates in MNI space. Also, see Fig. 1. Reported voxels were used to define regions of interest.
Correlational analysis
We tested for correlations between the estimated years to clinical onset and cerebral activations with COMPLEXITY; SPEED and the main effect of task in pre-symptomatic gene carrier. Based on previous work (Bartenstein et al., 1997) we expected increasing activations with approaching clinical onset in parietal areas and the reverse in premotor regions. We included age as a regressor of no interest to partial out non-specific age effects. The same ROIs and statistical thresholds were applied as outlined above.
Results
Behaviour
Training took around 10–20 min and resulted in low error rates for all four tasks (Table 3). Blocks in which subjects performed the wrong condition were excluded from behavioural and imaging data analysis. This was equally frequent for both groups and affected 3.5% of all blocks, often at the first occurrence of a new condition. No significant performance difference between groups was found for any of the tasks (Table 3). No correlations between error rate and estimated years to onset were found. The SD of timing inaccuracy increased with approaching clinical onset when pre-symptomatic gene carriers performed simple-slow (r = −0.72; P = 0.002), simple-fast (r = −0.63; P = 0.01) and complex-fast (r = −0.58; P = 0.024) tasks, while this effect only reached trend significance for the simple-slow condition (r = −0.50; P = 0.06). Scatter plots and correlations with the imaging data are provided in a supplementary section.
Table 3.
Performance in each of the four motor conditions is given with median and range
| Controls | Pre-symptomatic Huntington's disease | Significance | |
|---|---|---|---|
| Error complex, slow (%) | 2 (0:6) | 4 (0:11) | 0.072 |
| Error complex, fast (%) | 4 (0:16) | 7 (0:26) | 0.25 |
| Error simple, slow (%) | 2 (0:10) | 2 (0:8) | 0.56 |
| Error simple, fast (%) | 0.5 (0:3) | 2 (0:9) | 0.082 |
| Cue-response interval complex, slow (ms) | 64 (−36:363) | 69 (−188:261) | 0.31 |
| Cue-response interval complex, fast (ms) | −16 (−54:74) | −2 (−66:20) | 0.46 |
| Cue-response interval simple, slow (ms) | 161 (−38:337) | 108 (−57:286) | 0.35 |
| Cue-response interval simple, fast (ms) | −22 (−59:79) | −16 (−71:13) | 0.75 |
| SD cue-response interval complex, slow (ms) | 238 (158:480) | 257 (127:512) | 0.76 |
| SD cue-response interval complex, fast (ms) | 67 (60:190) | 89 (46:136) | 0.17 |
| SD cue-response interval simple, slow (ms) | 195 (124:552) | 249 (132:556) | 0.58 |
| SD cue-response interval simple, fast (ms) | 64 (54:131) | 70 (41:127) | 0.55 |
Whitney test is used to compare groups.
Imaging results
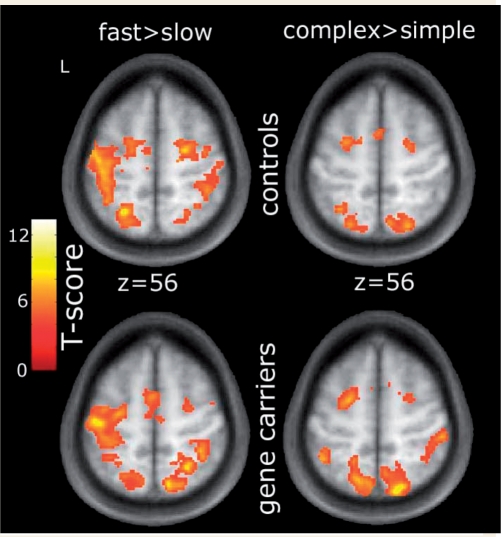
Task related BOLD signal changes in the control group were very similar to those reported (Lehericy et al., 2006). Increased pace of sequential movements resulted in greater activations in M1, caudal PMd, caudal SMA and superior parietal lobe. Pre-SMA, rostral PMd as well as SPL displayed an increase in activation with complexity (Fig. 1 top row and Table 2). A similar pattern of task related activations were present in the pre-symptomatic gene carrier group (Fig. 1, bottom row).
Figure 1.
Pattern of activations for the main effect of SPEED and COMPLEXITY for controls (top row) and pre-symptomatic gene carriers (bottom row) are overlaid on the averaged T1-images from all subjects (P < 0.001; uncorrected; Voxel extent = 100).
Primary and premotor areas
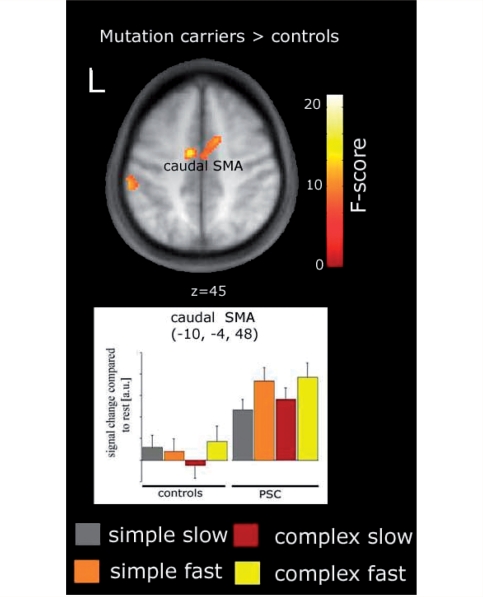
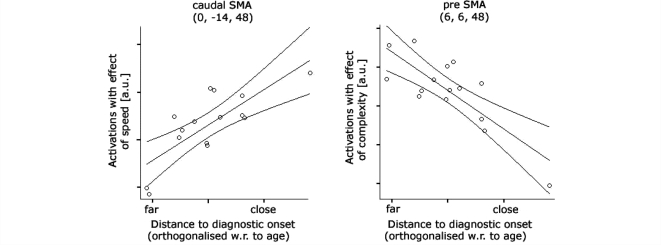
Across all movement conditions (main effect of GROUP), pre-symptomatic gene carriers showed greater activation in caudal SMA (at x, y, z = −10, −4, 48; F = 16.03, Z = 3.67, P = 0.012) (Fig. 2), uncorrelated with estimated years to onset. In accordance with its executive role in motor control, task-related activation of this region was modulated by the rate of sequential movements but not by its complexity (bar plot in Fig. 2). A more posterior region, still within the caudal SMA (at x, y, z = 2, −12, 48; T = 5.5; P = 0.05), showed increasing activations with increasing SPEED as pre-symptomatic gene carriers approach clinical onset (Fig. 3; left panel). While no interaction between the effect of either complexity or rate was observed in the caudal SMA, a rostral region located in the pre-SMA (x, y, z = 6, 6, 48) showed a linear decrease in activation with increasing sequence complexity when gene carriers were closer to estimated time of clinical onset (T = 5.08; P = 0.031; Fig. 3; right panel). No differences in the ROIs around M1 and PMd were found.
Figure 2.
Areas displaying differential activations with group membership. The plot below depicts condition specific activations compared with rest for indicated voxels in arbitrary units (a.u.). Error bars represent one SEM activations are displayed at P < 0.01; uncorrected (Voxel extent = 50).
Figure 3.
Linear changes in activity in the caudal SMA and pre-SMA with the estimated years to diagnostic onset of Huntington's disease. Scatter plots illustrate peak correlations between estimated years to diagnostic onset and neuronal activations after controlling for linear age-effects. The fitted regression line is shown with 95% mean prediction interval.
Superior parietal cortex
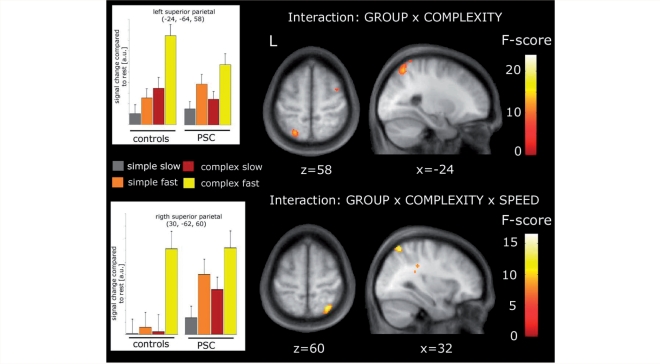
In the left SPL, the increase in task-related activation with sequence complexity was attenuated in pre-symptomatic gene carriers compared with controls, resulting in a significant interaction between complexity and group (x, y, z = −24, –64, 58; F = 13.6, Z = 3.37, P = 0.031; Fig. 4; top row). A post hoc test revealed that this effect does not correlate with the estimated years to clinical onset.
Figure 4.
Regions in the right and left SPL displaying differential activations with indicated interactions. Activations are displayed at an uncorrected P-value of p < 0.01 (Voxel extent = 50). Plots on the left depict condition specific activations compared with rest as in Fig. 2.
In the right SPL, healthy controls showed a selective activation only with the most difficult task, which required fast and complex movements. In contrast, the gene carrier group displayed activity in right SPL with all movement conditions (bar plots in Fig. 4; bottom row). These between-group differences in the activity profile were reflected in a three-way interaction between SPEED, COMPLEXITY and GROUP (x, y, z = 32, −62, 60; F = 13.24, P = 0.036; Fig. 4, bottom row). No correlation of this interaction with estimated years to clinical onset was found. Supplementary table S1 summarizes all imaging results.
Discussion
We employed a sequential finger movement task that was designed to probe executive (speed) and cognitive (sequence complexity) aspects of manual motor control to tap into the preclinical motor reorganization in Huntington's disease. We identified distinct regions in the mesial premotor cortex and superior parietal cortex showing enhanced task related activity in gene carriers compared with controls, including the caudal SMA as well as the right and left SPL. Critically, the activity profile in these regions differed from each other in the amount of extra recruitment during the various task conditions. Compared with healthy controls, pre-symptomatic gene carriers showed greater activations of left caudal SMA with all movement conditions. The left SPL showed a shift in the weighing of neuronal activity, with a relative increase in activation during simple finger sequences but a relative reduction during complex sequences. In contrast, the right SPL showed greater activations with all but the most demanding movements. Our results show that the motor system in Huntington disease recruits premotor and parietal areas in a highly flexible manner depending on which motor functions are demanded for task execution.
We discuss the results in four sections. In the first section, we focus on the behavioural results; in the second part, we describe regional changes in task related activation in the pre-symptomatic gene carrier group. The third section compares the present results to changes in motor activation associated with other genetic mutations that can cause parkinsonism or dystonia. We end the discussion with some methodological considerations.
Task performance
It is important to stress that the changes in task related neuronal activity with group status were present in the absence of behavioural differences. This also confirms previous work showing that routine motor behaviour remains largely unaffected in pre-symptomatic gene carriers (Giordani et al., 1995; Campodonico et al., 1996). As performance was matched between gene carrierss and controls, differential activation between them reflects a primary effect of disease and/or of resultant compensation. The variability of timing error increased with approaching clinical onset, mirroring previous work (Hinton et al., 2007). As shown in the supplementary material, no significant correlation between this variability and neuronal activations was found in the gene carrier group.
Regional changes in movement related neuronal activity
Primary- and premotor areas
The caudal ‘executive’ part of the SMA showed increased movement related activity in pre-symptomatic gene carriers regardless of the pace or complexity of motor sequences. This is consistent with a recent neuroimaging study in manifesting Huntington's disease patients (Gavazzi et al., 2007), but at variance with the general notion that movement related activity shifts from premotor to parietal areas in Huntington's disease (Bartenstein et al., 1997).
In the light of preserved task performance, we propose that this greater activation effectively compensated for the effect of Huntington's disease-associated neurodegeneration in primary motor areas. This interpretation is supported in two ways. First, caudal SMA is functionally and anatomically well connected to M1 (Picard and Strick, 2001; Johansen-Berg et al., 2004). Second, a repetitive transcranial magnetic stimulation (rTMS) study on healthy subjects has identified the caudal SMA as a region that potentially compensates for disruption of M1 function (Lee et al., 2003). In that study, rTMS was used to generate a temporary lesion in left M1 in healthy subjects. Such focal perturbation of M1 function resulted in an increase in coupling between caudal SMA and M1 activity. The location of caudal SMA closely matches that found in our study [at x, y, z = −10, −4, 48] compared to x, y, z = −12, −4, 56 (Lee et al., 2003). When performing a similar analysis of functional coupling in a post hoc analysis of our data, stronger positive coupling between caudal SMA and ipsilateral M1 was found in pre-symptomatic gene carriers compared with controls (see supplementary data).
Previous functional imaging studies show impaired activation of M1 (Bartenstein et al., 1997; Gavazzi et al., 2007). This abnormal activation of M1 is difficult to interpret as task performance in these studies was either not matched between groups or not recorded during imaging. We find normal activation of M1, but a structural MRI study has previously revealed reduced M1 cortical thickness suggesting regional atrophy is present in the pre-symptomatic stage (Rosas et al., 2005). Our subjects were on average 6 years younger than those in the study by Rosas and colleagues but had the same number of CAG repeats. In addition, it is possible that the greater coherence between M1 and SMA may be responsible for a normal level of movement related M1 activity in the pre-symptomatic gene carrier group. The caudal SMA showed a gradual increase in activation with faster movements as gene carriers approached clinical onset. This correlation suggests that in the asymptomatic stage of Huntington's disease the compensatory role of caudal SMA may increase with progressive neurodegeneration.
The pre-SMA represents a more cognitive motor area than the caudal SMA (Picard and Strick, 2001). In agreement with previous neuroimaging studies (Boecker et al., 1999; Lehericy et al., 2006), we found increased activation of the pre-SMA with the complex motor sequence relative to the simpler one. Correlational analyses of fMRI data revealed a monotonic attenuation of the effect of COMPLEXITY on task related activity in pre-SMA with approaching clinical onset (Fig. 3). The attenuated neuronal activity in the pre-SMA in the context of complex movements most likely indicates a progressive dysfunction of the pre-SMA during the preclinical stage of Huntington's disease. This assumption is supported by a neuroimaging study in clinically affected Huntington's disease-patients that reported lesser activations in pre-SMA with complex sequential movements (Bartenstein et al., 1997).
Superior parietal lobule
Within the parietal lobule, mutation carriers also showed differential activations compared with controls. Plots of task specific activations for the left SPL (Fig. 4) show a significant interaction resulting from relatively higher activations in both simple tasks and the reverse in complex movements, when pre-symptomatic gene carriers are compared with controls. Of note, a different pattern is observed in the right SPL and indicates compensatory hyper-activation with the three simplest tasks. Ceiling effects could explain equal recruitment between groups with the fast-complex task.
There is also some evidence that the neurodegenerative process may directly affect SPL. A prospective PET study found a reduction of parietal resting state metabolism in gene carriers followed over a 16 months period (Ciarmiello et al., 2006) while structural MRI showed bilateral cortical thinning in BA7 (Rosas et al., 2005). It is conceivable that increasing local damage limits the ability of SPL to effectively compensate for motor dysfunction in more advanced stages of symptomatic Huntington's disease.
A differential role of the left and right parietal cortex in motor control has been suggested (Rushworth et al., 2001). Figure 1 indicates that our task resulted in a relatively symmetrical activation of the parietal cortex even though the two hemispheres may have distinct functional contributions. Our task was not designed to disentangle side specific contributions of SPL to the motor system (e.g. spatial attention versus motor attention) so that a specific differential disease effect cannot be deduced from our results. It is, however, noteworthy that morphometric MRI reveales a leftward bias for Huntington's disease-associated degeneration (Muhlau et al., 2007; Klöppel et al., 2008). This could explain why activations with complex movements are lesser in pre-symptomatic gene carriers than in controls on the left, while they are of equal magnitude on the right.
In left SPL, gene carriers displayed greater activations with the simple motor sequence, whereas controls showed equal or greater activations of the same region with complex motor sequences (bar plots in Fig. 4). This pattern suggests that the compensatory mechanisms that are observed in gene carriers depend very much on the demands of the task. A more differentiated picture of preclinical compensation in Huntington's disease has also emerged from a recent study on working memory in pre-symptomatic gene carriers (Wolf et al., 2007). Here again, compensatory mechanisms varied with different levels of working memory load. While our data disagrees with a simple shift of activations to parietal areas suggested in previous work in clinically manifesting Huntington's disease patients, other results of our study are in accordance with those reported by Bartenstein and colleagues (1997). In concordance with their study, gene carriers tended to have greater SPL activations bilaterally with the simple sequence condition compared with controls (Fig. 4). Yet, this increased activation failed to reach significance in the pre-symptomatic stage under study here.
Other basal ganglia disorders
A complex picture of motor compensation has also emerged from related research in pre-symptomatic carriers of other basal ganglia disorders (Ghilardi et al., 2003; Buhmann et al., 2005; Carbon et al., 2008; van Nuenen et al., 2009). These studies have shown partly overlapping patterns of hyper-activation that have been interpreted in the context of functional compensation.
Familial, early-onset primary torsion dystonia is often caused by an autosomal dominant genetic mutation in the DYT1 gene with reduced penetrance of 25–30%. Asymptomatic DYT1-carriers show deficient activations in dorso-lateral premotor cortex and primary motor cortex which are associated with hyper-activation in the cerebellum with sequence learning (Ghilardi et al., 2003; Carbon et al., 2008). Non-manifesting subjects with a mutation in the recessively inherited PARKIN gene have mild striatal dopaminergic dysfunction, often considered a model for pre-manifesting Parkinson's disease. Compensatory hyper-activation in these asymptomatic carriers performing internally selected movements was found in motor areas more rostral than those identified in the present work. They include PMd and rostral cingulate cortex (Buhmann et al., 2005; van Nuenen et al., 2009). These differences in regional patterns may be explained by the fact that the tasks drew on different components of manual motor control. It is also important to keep in mind that only the carriers of the Huntington's disease mutation will later develop the disease and therefore show a truly pre-manifesting pattern of compensatory changes. To improve our understanding of the range of compensatory mechanisms, future work could directly compare pre-symptomatic subjects of more than one basal ganglia disorder.
Methodological considerations
The activity patterns observed in healthy controls confirmed that our experimental design probed executive and cognitive aspects of motor control. There were increased activations in motor executive areas with increasing pace of the motor sequence, while secondary motor areas such as pre-SMA, rostral parts of PMd and SPL showed greater activation with more complex movement patterns. These results are in line with those reported previously (Lehericy et al., 2006) and the known involvement of these areas in different aspects of motor control (Picard and Strick, 2001; Rushworth et al., 2001).
Our study has its focus on cortical regions but interactions with well-known changes in the basal ganglia are very likely. Studies on Huntington's disease-specific metabolic patterns found with PET imaging indicate a combination of cortical and subcortical regions are involved (Feigin et al., 2007).
Our ROI based approach was designed to increase sensitivity for detection of between-group differences in key cortical motor areas. The pay-off is that our approach was less sensitive to compensatory changes in brain regions where we did not have a strong a priori hypothesis (Weeks et al., 1997).
Conclusion
In pre-symptomatic gene carriers, executive motor areas such as the caudal SMA as well as cognitive motor areas such as the SPL showed evidence for compensatory activation. We therefore propose that the caudal SMA plays a central role in maintaining executive aspects of motor control that increases with approaching disease onset, compensating for dysfunctions in other executive parts of the motor system (e.g. the M1). Likewise, the SPL appears to be central to the compensation of regional dysfunctions in other ‘cognitive’ motor regions (e.g. the pre-SMA).
The complex pattern of differences in neuronal processing found in this study contradicts the claim that a shift of neuronal activity from premotor to parietal regions constitutes the main feature of compensation. Instead, our data are in keeping with regional compensatory hyperactivation in an executive motor area (i.e. caudal SMA). Depending on task difficulty, the SPL exhibits compensatory functions. Studies on functional and anatomical connectivity may prove useful for advancing our current understanding of the interaction between these cortical regions and subcortical structures.
Supplementary material
Supplementary material is available at Brain online.
Funding
Wellcome Trust (075696 2/04/2 to R.S.J.F., S.J.T. and John Ashburner). Medizinische Fakultät of the University of Freiburg (to S.K.). Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Supplementary Material
Acknowledgements
The authors would like to thank Elisabeth Rounis for helpful suggestions with the design of this study and Maggie Burrows, Susie Henley, Rachel Taylor, Tom Warner and Edward Wild for their help with the recruitment. Christoph Kaller and Magnus-Sebastian Vry contributed valuable comments on the preparation of this manuscript.
Glossary
Abbreviations:
- fMRI
functional MRI
- M1
primary sensorimotor cortex
- PET
positron emission tomography
- PMd
dorsal premotor cortex
- ROIs
regions of interests
- rTMS
repetitive transcranial magnetic stimulation
- SMA
supplementary motor area
- SPL
superior parietal lobe
- UHDRS
Unified Huntington's Disease Rating Scale
References
- Bartenstein P, Weindl A, Spiegel S, Boecker H, Wenzel R, Ceballos-Baumann AO, et al. Central motor processing in Huntington's disease. A PET study. Brain. 1997;120(Pt 9):1553–67. doi: 10.1093/brain/120.9.1553. [DOI] [PubMed] [Google Scholar]
- Boecker H, Ceballos-Baumann A, Bartenstein P, Weindl A, Siebner HR, Fassbender T, et al. Sensory processing in Parkinson's and Huntington's disease: investigations with 3D H(2)(15)O-PET. Brain. 1999;122(Pt 9):1651–65. doi: 10.1093/brain/122.9.1651. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Binkofski F, Klein C, Buchel C, van Eimeren T, Erdmann C, et al. Motor reorganization in asymptomatic carriers of a single mutant Parkin allele: a human model for presymptomatic parkinsonism. Brain. 2005;128:2281–90. doi: 10.1093/brain/awh572. [DOI] [PubMed] [Google Scholar]
- Campodonico JR, Codori AM, Brandt J. Neuropsychological stability over two years in asymptomatic carriers of the Huntington's disease mutation. J Neurol Neurosurg Psychiatry. 1996;61:621–4. doi: 10.1136/jnnp.61.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Ghilardi MF, Argyelan M, Dhawan V, Bressman SB, Eidelberg D. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131:146–54. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, et al. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington's disease. J Nucl Med. 2006;47:215–22. [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–67. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Farrow M, Chua P, Churchyard A, Bradshaw JL, Chiu E, Georgiou-Karistianis N. Proximity to clinical onset influences motor and cognitive performance in presymptomatic Huntington disease gene carriers. Cogn Behav Neurol. 2006;19:208–16. doi: 10.1097/01.wnn.0000213914.64772.b6. [DOI] [PubMed] [Google Scholar]
- Feigin A, Ghilardi MF, Huang C, Ma Y, Carbon M, Guttman M, et al. Preclinical Huntington's disease: compensatory brain responses during learning. Ann Neurol. 2006;59:53–9. doi: 10.1002/ana.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, et al. Thalamic metabolism and symptom onset in preclinical Huntington's disease. Brain. 2007;130:2858–67. doi: 10.1093/brain/awm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2:157–65. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Gavazzi C, Nave RD, Petralli R, Rocca MA, Guerrini L, Tessa C, et al. Combining functional and structural brain magnetic resonance imaging in Huntington disease. J Comput Assist Tomogr. 2007;31:574–80. doi: 10.1097/01.rct.0000284390.53202.2e. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54:102–9. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- Giordani B, Berent S, Boivin MJ, Penney JB, Lehtinen S, Markel DS, et al. Longitudinal neuropsychological and genetic linkage analysis of persons at risk for Huntington's disease. Arch Neurol. 1995;52:59–64. doi: 10.1001/archneur.1995.00540250063014. [DOI] [PubMed] [Google Scholar]
- HD Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Paulsen JS, Hoffmann RG, Reynolds NC, Zimbelman JL, Rao SM. Motor timing variability increases in preclinical Huntington's disease patients as estimated onset of motor symptoms approaches. J Int Neuropsychol Soc. 2007;13:539–43. doi: 10.1017/S1355617707070671. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA. 2004;101:13335–40. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel S, Draganski B, Golding CV, Chu C, Nagy Z, Cook PA, et al. White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington's disease. Brain. 2008;131:196–204. doi: 10.1093/brain/awm275. [DOI] [PubMed] [Google Scholar]
- Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin Genet. 2004;65:267–77. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, et al. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:5308–18. doi: 10.1523/JNEUROSCI.23-12-05308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Bardinet E, Tremblay L, Van de Moortele PF, Pochon JB, Dormont D, et al. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb Cortex. 2006;16:149–61. doi: 10.1093/cercor/bhi089. [DOI] [PubMed] [Google Scholar]
- Muhlau M, Gaser C, Wohlschlager AM, Weindl A, Stadtler M, Valet M, et al. Striatal gray matter loss in Huntington's disease is leftward biased. Mov Disord. 2007;22:1169–73. doi: 10.1002/mds.21137. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–72. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65:745–7. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, et al. Diffusion tensor imaging in presymptomatic and early Huntington's disease: selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21:1317–25. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci. 2001;4:656–61. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, et al. The distribution of structural neuropathology in pre-clinical Huntington's disease. Brain. 2002;125:1815–28. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- van Nuenen BFL, Weiss MM, Bloem BR, Reetz K, van Eimeren T, Lohmann K, et al. Heterozygous carriers of a Parkin or PINK1 mutation share a common functional endophenotype. Neurology. 2009;72:1041–47. doi: 10.1212/01.wnl.0000338699.56379.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FO. Huntington's disease. Lancet. 2007;369:218–28. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Weeks RA, Ceballos-Baumann A, Piccini P, Boecker H, Harding AE, Brooks DJ. Cortical control of movement in Huntington's disease. A PET activation study. Brain. 1997;120(Pt 9):1569–78. doi: 10.1093/brain/120.9.1569. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Schonfeldt-Lecuona C, Landwehrmeyer GB, Ecker D. Dorsolateral prefrontal cortex dysfunction in presymptomatic Huntington's disease: evidence from event-related fMRI. Brain. 2007;130:2845–57. doi: 10.1093/brain/awm210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.