Abstract
Targeted reinnervation is a new neural-machine interface that has been developed to help improve the function of new-generation prosthetic limbs. Targeted reinnervation is a surgical procedure that takes the nerves that once innervated a severed limb and redirects them to proximal muscle and skin sites. The sensory afferents of the redirected nerves reinnervate the skin overlying the transfer site. This creates a sensory expression of the missing limb in the amputee's reinnervated skin. When these individuals are touched on this reinnervated skin they feel as though they are being touched on their missing limb. Targeted reinnervation takes nerves that once served the hand, a skin region of high functional importance, and redirects them to less functionally relevant skin areas adjacent to the amputation site. In an effort to better understand the sensory capacity of the reinnervated target skin following this procedure, we examined grating orientation thresholds and point localization thresholds on two amputees who had undergone the targeted reinnervation surgery. Grating orientation thresholds and point localization thresholds were also measured on the contralateral normal skin of the targeted reinnervation amputees and on analogous sites in able-bodied controls. Grating orientation thresholds for the reinnervated skin of the targeted reinnervation amputees were found to be similar to normal ranges for both the amputees’ contralateral skin and also for the control population. Point localization thresholds for these amputees were found to be lower for their reinnervated skin than for their contralateral skin. Reinnervated point localization thresholds values were also lower in comparison to homologous chest sites on the control population. Mechanisms appear to be in place to maximize re-established touch input in targeted reinnervation amputees. It seems that sound sensory function is provided to the denervated skin of the residual limb when connected to afferent pathways once serving highly functionally relevant regions of the brain. This suggests that tactile interface devices could be used to give a physiologically appropriate sense of touch to a prosthetic limb, which would likely help with better functional utilization of the prosthetic device and possibly help to more effectively integrate the device with the user's self-image.
Keywords: targeted reinnervation, sensation, grating orientation, point localization, amputee
Introduction
Currently, artificial limbs are very difficult for an amputee to control efficiently. A principal reason for this is that the prosthetic device does not provide the user with direct sensory feedback. Interestingly, one of the reasons that body-powered, cable-actuated, prosthetic limbs continue to see widespread use is that the wearer can sense the movement of the limb through the actuator cables (Stark and LeBlanc, 2004). However, when the amputee picks up or manipulates an object, the activity still must be monitored visually. Recently, work has been undertaken to address the fundamental issues of controlling a prosthetic device more effectively. Through a surgical procedure that we call ‘Targeted Reinnervation’, the residual nerves left following an amputation are surgically redirected to the skin and biomechanically non-functional muscles adjacent to the amputation site (Kuiken et al., 2004, 2007a, b; Zhou et al., 2007; O'Shaughnessy et al., 2008).
The large transferred nerves contain both motor and sensory axons. The motor axons, which still transmit the control signals that once directed the movement of the missing limb, reinnervate the purposely denervated target muscles and are utilized to control multifunction prosthetic limbs (Zhou et al., 2007). The focus of this work is on the sensory nerves, which appear to regenerate through the muscle out to the purposely denervated skin overlying the transfer site. Work by others has provided evidence that regenerating sensory fibers can often extend some distance to make terminal connections (Manger et al., 1996; Shaw et al., 1997) and are able to traverse different tissues before settling (Gutmann, 1945; Weiss and Edds, 1945). The redirected afferents establish a sensory expression of the missing limb in the skin (Kuiken et al., 2004, 2007a). When these individuals are touched at different points on this reinnervated skin it feels as though they are being touched at different locations on their missing limb. Targeted Reinnervation amputees report that the evoked sensations are very clear and are distinctly felt as occurring in the missing limb. The new sensation appears to be different than the highly organized phantom sensations that likely arise from reorganization of functional connections and representations within the central nervous system (Ramachandran et al., 1992a, b; Borsook et al., 1998; Flor et al., 1998, 2000). Instead, the new limb sensation appears to stem from the redirection of amputated peripheral nerves to the denervated target skin (Kuiken et al., 2007a). These reinnervated amputees feel pressure at near-normal levels and also feel vibration, heat, cold and pain that is projected to their missing limb (Kuiken et al., 2007a; Schultz et al., 2008).
The redirected sensation is likely communicated through the original sensory pathways of the missing limb. The large peripheral nerves transferred during targeted reinnervation surgery initially served the skin of the hand and forearm. In this study, the amputated nerves of targeted reinnervation amputees were redirected to the skin of the chest. Interesting questions arise following this procedure: What is the sensory capacity of a skin surface that previously communicated to an area of the brain with little functional relevance when it is reinnervated by afferents that communicate to an area of the brain with considerable functional importance? The skin of the chest has a much lower innervation density than that of the hand. Is sensory recovery more influenced by receptor density or by the region of the brain that is connected to the skin surface? Examination of these questions may help shed light on the way in which the body integrates and utilizes reorganized sensory input following major injury.
The goal of this study was to facilitate understanding of the redirected sensation by examining the sensory characteristics of the reinnervated skin through perceptions of objectively measured stimuli. We used grating orientation thresholds and point localization thresholds to quantitatively assess the integrity and sensory function of the skin following the targeted reinnervation nerve redirection. Grating orientation thresholds have been used extensively to study sensory function following nerve injury and sensory impairment (Van Boven and Johnson, 1994; Bara-Jimenez et al., 2000; Zeuner and Hallett, 2003; Walsh et al., 2007). These measures have been used to characterize sensation in studies ranging from plastic changes following acute deafferentation and amputation (Vega-Bermudez and Johnson, 2002; Werhahn et al., 2002) to investigations of central sensory processing (Sathian et al., 1997; Hsiao et al., 2002; Sathian and Zangaladze, 2002). Point localization thresholds are typically employed to investigate referred touch and sensory re-education following nerve injury (Bell-Krotoski et al., 1993). Point localization thresholds have also been used to assess tactile capacity and hand function related to brain injury and peripheral neuropathies (Travieso and Lederman, 2007; Valentini et al., 2008). In addition to examining the functional capacity of the reinnervated skin to provide insight into mechanisms of plasticity, we also seek to learn about the qualities of the redirected sensation and how it may be used as a touch interface for integration with a prosthetic limb. The more that is understood about the mechanisms of sensory integration involving the reinnervated skin, the better we will be able to develop tactile interfaces for returning physiologically appropriate sensory feedback from prosthetic devices.
Methods
Two individuals, one male and one female, with shoulder-level amputations and subsequent targeted reinnervation surgeries were tested for grating orientation thresholds and point localization thresholds in this study. Fourteen able-bodied (control) participants were tested for grating orientation thresholds: seven males aged 24–55 (mean 34.1 years) and seven females aged 24–61 (mean 33 years). Six control participants were tested for point localization thresholds: three males aged 26–55 (mean 37 years) and three females aged 22–36 (mean 29.7 years). Control subject ages covered the age range of the amputee subjects, and a 50/50 division between males and females was used to reflect the single male and female amputees. All experiments were performed at the Rehabilitation Institute of Chicago with the written informed consent of each subject according to protocols approved by the Institutional Review Board at Northwestern University.
Amputees and Targeted Reinnervation Surgery
Both amputees examined in this study had proximal upper limb amputation following traumatic injury. The first amputee (TR1) was a 55-year-old male who underwent a bilateral shoulder disarticulation amputation following severe electrical burns. Nine months following his amputation, TR1 underwent targeted reinnervation surgery. During this procedure, the residual muscles of the chest were denervated and the distal nerve stumps of the brachial plexus were redirected to the cut motor innervation points of the muscles. The ulnar and musculocutaneous nerves were redirected to the underside of the pectoralis minor and clavicular head of the pectoralis major respectively, where they were end-to-end anastomosed to the cut motor points and then secured to the muscle. The sternal head of the pectoralis major was divided and the median nerve was redirected to the cut motor points on the underside of the superior section whereas the radial nerve was redirected to the cut motor points on the underside of the inferior section. The subcutaneous tissue and fat between the skin and the target muscles was removed to allow the skin to directly appose the muscle surface. This was done to provide clear EMG signal transmission for better prosthesis control. The removal of tissue likely resulted in a limited denervation of skin receptors, which provided an environment receptive to reinnervation from the redirected afferents of the limb. After a period of 5 months, TR1 developed sensation on the chest that was referred to the missing limb. When TR1 was touched on the reinnervated skin he felt as though his missing limb was being touched (Kuiken et al., 2004, 2007a). The evoked limb sensation was localized to the palmar and dorsal aspects of his hand and forearm. These sensations appeared to be representative of cutaneous sensation from the four transferred limb nerves. Pressing the skin over the median nerve transfer site elicited sensations on the palm and first three digits. Pressing the skin over the ulnar nerve transfer site elicited sensations projected to the palm, forearm, digit four and digit five. Likewise, pressing on the skin over the radial and musculocutaneous transfer sites created sensations localized to the back of the hand and forearm. For a detailed description of these sensations please see (Kuiken et al., 2007a).
The second amputee (TR2) was a 25-year-old female who underwent a proximal unilateral transhumeral amputation (functional shoulder disarticulation) following a motor vehicle collision. TR2 underwent the targeted reinnervation surgery 15 months after her initial amputation. Her pectoralis major and serratus anterior muscles were denervated to provide targets for the amputated limb nerves. The ulnar nerve was redirected to a medial cut motor innervation point under the clavicular head of the pectoralis major while the musculocutaneous was redirected to a lateral cut motor point of the same muscle. The large nerves were end-to-end anastomosed to the cut motor points and then secured to the muscle. The median nerve was divided and each half was redirected, in a similar fashion as above, to the superior and inferior sections of the sternal head of the pectoralis major. The distal radial nerve was redirected to the distal long thoracic nerve innervating the distal serratus anterior muscle. Removal of subcutaneous tissue in order to create targets for regenerating sensory afferents, such as was done for TR1, was not an option for TR2 because it would have disfigured her chest. Instead, her supraclavicular cutaneous nerve was cut, resulting in an insensate area overlying the nerve transfer area. After 4 months, TR2 developed sensation from the missing limb within this area. As with TR1, when TR2 was touched on the reinnervated skin she felt as though her missing limb was being touched (Kuiken et al., 2007a, b). The evoked limb sensations were localized primarily to the palmar aspect of her hand. These sensations appeared to be expressions of cutaneous sensation from two of the transferred nerves. Pressing the skin over the median nerve transfer site elicited sensations mostly referred to the central palm and digits one and two. Pressing on other areas of the reinnervated chest elicited sensations referred to the palm and palmer sides of digits four and five. These sensations were likely associated with a neural conduit (an end-to-side anastomosis) that joined the ulnar nerve and the proximal end of the supraclavicular cutaneous nerve. For a detailed description of these sensations please see (Kuiken et al., 2007a).
Grating orientation task
Grating orientation thresholds were measured with spatial discrimination domes. The domes were constructed in-house from polyethylene plastic disks that were 4 cm thick and 12.5 cm in diameter. The surface that contacted the study participant was profiled with a 33 cm radius convexity and had equally sized square wave ridges and grooves cut at specific intervals. These domes were built to be similar in profile to commercially available JVP domes (Stoelting Co. Wood Dale, IL). The largest domes used for testing that could be found in the literature were 5 cm wide and were used by Schlereth et al. (2001) to study the hairy skin of the back of the hand. However, we found that domes of that size did not provide large enough grating widths to be resolved by the chest skin. Based on preliminary experiments that we conducted using a makeshift adjustable grating apparatus, we constructed a set of large domes to account for the low spatial resolution of the chest skin. A series of equally sized alternating grooves and ridges were cut into the surfaces of 11 domes at spacings of 20, 25, 30, 35, 40, 45, 50, 55, 60, 70 and 90 mm. The 70 and 90 mm grating width domes were 15.5 cm in diameter to maintain a circular profile. Subjects were blindfolded and seated comfortably in a quiet room. The gratings were pressed to the skin at the testing location either vertically or horizontally for 2 s. When necessary, the test site was shaved to prevent the hair from providing clues to the orientation of the grating. Although it has been demonstrated that grating orientation thresholds are relatively insensitive to differences in application pressure, care was taken to consistently press to where a slight blanching of the skin was observed (Gibson and Craig, 2006; Bleyenheuft and Thonnard, 2007) Grating orientation in each trial was random. The subjects were instructed to verbally indicate the direction of the grooves following each presentation and, in accordance with a forced-choice paradigm, they were only allowed to choose from one of the two possible directions. The subjects were not given feedback concerning correct and incorrect responses. Dome gratings were applied to the testing locations using a staircase routine designed to converge to a threshold value corresponding to 75% correct responses (Vega-Bermudez and Johnson, 2001; Grant et al., 2006). The staircase routine called for an increase in grating width following an incorrect response and a decrease in width following two consecutive correct responses (Wetherill and Levitt, 1965). The wider the grating width, the easier it is to determine orientation. Testing covered 16 reversals. The first four reversals were discarded and the grating widths for the final 12 reversals were averaged to calculate the threshold value. Each site on each subject was tested a total of three separate times. The approximate standard deviation of each threshold value was estimated by calculating the standard deviation of the average of each consecutive pair of reversal points (one peak and one valley) (Wetherill and Levitt, 1965).
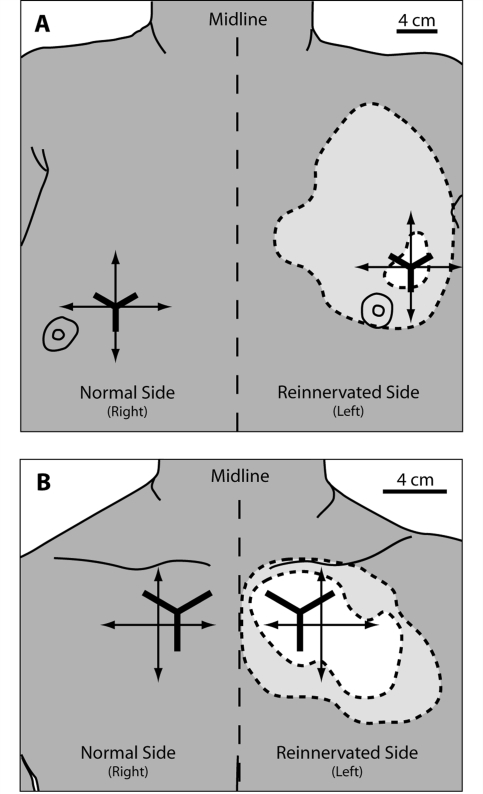
Targeted reinnervation amputees were tested over an area of the reinnervated skin site where they felt only hand sensation, and on normal chest skin contralateral to the reinnervated skin (Kuiken et al., 2007a). Reference points were marked on the chest of each amputee through a grid of holes drilled in thermoplastic test sockets that fit intimately to the left shoulder of each individual. These sockets were created following each amputee's targeted reinnervation surgery and used to create reference marks for sensory reinnervation experiments. Once the reference points were placed, a cross was drawn on the chest of each targeted reinnervation subject to guide placement of the dome gratings (Fig. 1). On TR2, all testing tools were applied to reinnervated skin within the bounds of the region deemed to be insensate following transection of the supraclavicular cutaneous nerve (Kuiken et al., 2007b). Each side of both amputees’ chests was tested three separate times. The control site on the right chest of TR1 was shifted to a position that was medial and inferior in relation to the testing site on the reinnervated side to avoid chest skin that was repaired by skin grafts. Controls were tested once on their right upper chest, in a position homologous to the reinnervation site of the targeted reinnervation amputees, midway between the collarbone and the nipple.
Figure 1.
Diagrams of testing regions on the chests of the targeted reinnervation amputees. (A) TR1, Male bilateral shoulder disarticulation amputee. (B) TR2, female unilateral short trans-humeral amputee (functional shoulder disarticulation). The dashed line surrounding the light grey area on the left chest of the amputees denotes the extent of sensation that is referred to the missing limb. Within the light grey area the amputees feel a mix of referred hand sensation and native chest sensation. The white area denotes where the targeted reinnervation amputees feel only referred hand sensation with no native chest sensation. The crossed arrows show the vertical and horizontal axes of the grating placements for the grating orientation task. The Y shaped lines denote the placement of the grids used for the point localization task.
Point localization task
Targeted reinnervation amputee subjects were blindfolded and seated comfortably in a quiet room. A reference point was drawn, using the thermoplastic socket described previously, on the amputees’ reinnervated skin at the center of a region of hand-only sensation (Kuiken et al., 2007a). From this reference point, evenly spaced testing points at 2.5 mm intervals (out to 2.5 cm) were marked in three directions forming a Y-shape (Weinstein, 1968). In each amputee subject, the testing grid was placed entirely within the hand-only region of reinnervated chest. A 5.07—Semmes-Weinstein monofilament (North Coast Medical, Morgan Hill, CA) delivering 10 g of force was used as the stimulus. This force was above the threshold of detection for control subjects (Weinstein, 1968), and was above the threshold at which previously tested reinnervated subjects detected pressure in their missing limb (Kuiken et al., 2007a). Testing points were selected in random order. For each trial, the stimulus was first applied to the reference point and then to the test location. Subjects reported whether they perceived the stimuli as being applied to the same or to different locations. All stimuli were applied following the timing scheme of 1.5 s stimulus application, 1.5 s contact and 1.5 s removal (Bell-Krotoski and Buford, 1997; Ylioja et al., 2004). Reference and comparison stimuli were separated by 2 s (Ylioja et al., 2004). Each point was tested three times, including the reference point for ‘blank’ trials, totaling 93 trials. For each direction, the percentage of time the subject reported ‘different’ was plotted versus the distance from the reference point (Ylioja et al., 2006). A psychometric curve was fit to the data and the 75% difference point was taken as the discrimination threshold. The sum of the squared error (SSE) was calculated for each curve fit and is indicative of the goodness of fit for the psychometric curve.
Results
Grating orientation thresholds
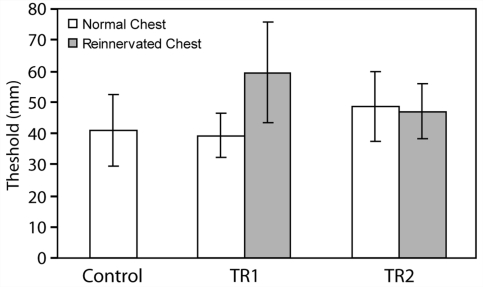
The mean grating orientation threshold for the reinnervated left chest of subject TR1 was 59.6 mm (SD = 16.2 mm) and the mean threshold for his non-reinnervated right chest was 39.2 mm (SD = 7.1 mm) (Fig. 2). The mean grating orientation threshold for the reinnervated skin of the left chest of TR2 was 47.0 mm (SD = 8.8 mm) and the mean threshold for her non-reinnervated right chest was 48.6 mm (SD = 11.2 mm) (Fig. 2). There were no statistical differences between thresholds for contralateral skin, reinnervated skin or controls. Since TR2 was a unilateral amputee and had a fully intact and normal right side, we also tested the grating orientation threshold for her right index fingertip and the back of her hand. The grating orientation threshold values for these locations were 1.1 mm (SD = 0.29 mm) and 9.7 mm (SD = 2.66 mm). The intact fingertip testing site was chosen to mirror the location of the fingertip sensation that she feels at the center of the grating orientation threshold testing site on her reinnervated chest. The mean grating orientation threshold for the right chest of the 14 control subjects was 41.1 mm (SD = 11.5 mm) (Fig. 2).
Figure 2.
Grating orientation thresholds in millimeters for the reinnervated (grey) and normal chest (white) for each targeted reinnervation amputee and the control population (white). There were 15 individuals in the control population. Error bars indicate 1 SD. This test employed a two-interval-forced-choice staircase routine to converge at a threshold value corresponding to 70.7% correct responses.
Point localization thresholds
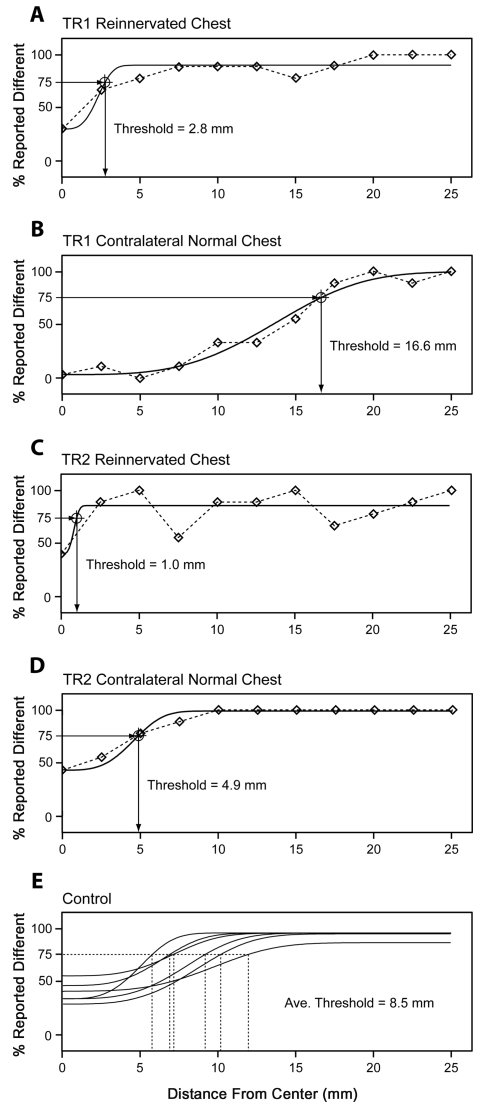
The mean point localization threshold for the reinnervated left chest of subject TR1 was 2.8 mm (SSE = 0.060) and the mean threshold for his non-reinnervated right chest was 16.6 mm (SSE = 0.050) (Fig. 3). The mean point localization threshold for the reinnervated left chest of subject TR2 was 1.0 mm (SSE = 0.20) and the mean threshold for her non-reinnervated right chest was 4.9 mm (SSE = 0.015) (Fig. 3). The mean point localization threshold for the right chest of the six control subjects was 8.5 mm (Avg. SSE = 0.16, SD = 2.30) (Fig. 3).
Figure 3.
Point localization thresholds in millimeters for the reinnervated and normal chest for TR1 (A and B), TR2 (C and D) and controls (E). There were six individuals in the control population. Touch stimuli were applied with a 10 g Semmes-Weinstein monofilament first to the central reference point then to a point on an arm of the grid. The percentage of times the subject reported a difference was plotted as a function of the distance from the centre of the grid. A psychometric curve was fit to the data with the 75% difference point taken as threshold.
Discussion
The targeted reinnervation nerve redirection procedure provided a condition where afferents from the hand (a region of the body with high mechanoreceptor innervation density, high functional importance and significant cortical representation) were displaced to a cutaneous surface within an entirely different dermatome (Johansson and Vallbo, 1979b; Sur et al., 1980; Kelly et al., 2005). The denervated target skin of the chest has a much lower mechanoreceptive innervation density and less functional significance than the hand (Sur et al., 1980; Lacour et al., 1991). Interestingly, following the targeted reinnervation surgery, these amputees were able to orient gratings at thresholds similar to their contralateral normal side, while they were able to localize point stimuli more effectively on their reinnervated side than their contralateral normal side. These results suggest that the sensation of the reinnervated target skin of these individuals appears to be functionally intact and that the influence of central processing mechanisms may play a role in the level of recovery afforded by the regenerating afferents.
The skin of TR2's chest was completely denervated by cutting the supraclavicular cutaneous nerve; her chest skin went numb after the surgery. The sensation that is evident in this region was likely entirely a product of regeneration (Fig. 1). The dome gratings and point localization grid used to test the subjects had footprints that fell entirely within the previously denervated skin region. Therefore, it was unlikely that the native innervation of the chest played a significant role in determining the orientation of the grating and the point localization for this subject. In contrast to TR2, subject TR1 had overlapping sensation from the native chest afferents and the reinnervated limb afferents (Kuiken et al., 2007a) (Fig. 1). It can be argued that the native afferents that were left in his chest following removal of the subcutaneous tissue may have been responsible for mediating the ability to orient the gratings. However, it is also possible that the reinnervated limb afferents contributed to the ability to orient the gratings. In either case, it is likely that since the native chest and reinnervating afferents shared space in the skin, there were a reduced number of receptor terminals devoted to either population of afferents. Because the number of functional terminals in the skin was likely divided between the native and reinnervated afferents, the grating orientation thresholds for TR1 might be expected to be higher regardless of which population contributed to the ability to orient the gratings. This might be an explanation for the observation that the average grating orientation threshold for TR1's reinnervated chest was higher than his normal chest.
Although the targeted reinnervation amputee patients have not undergone training to improve tactile spatial acuity, they appeared to have the ability to orient grating stimuli at threshold levels similar to their normal chest skin (Fig. 2). This is interesting because even though the sensory reinnervation occurring from the nerve transfers appeared to be extensive, there was likely a disrupted somatotopy at the skin surface from the random regeneration of the afferents (Horch, 1979; Horch and Burgess, 1980). The disruption of the topographic organization of the receptors at the skin surface following nerve regeneration likely created a new and different central representation of the reafferented region (Hansson and Brismar, 2003). A disrupted central representation would be expected to cause difficulties in processing and interpreting tactile information from the reinnervated regions (Hsiao et al., 2002). Given this, it would be expected that the ability to discern grating orientation would be lost. However, instead of showing a substantial functional deficit, both amputees were able to distinguish grating orientation at gap distances near the normal range. There is the possibility that having the regenerated afferents residing together in the new skin surface and being stimulated together may have allowed the central processing mechanisms to adapt to the new input. For instance, it has been demonstrated that receptive fields in cortex rapidly remap when even disparate tactile input is temporally correlated (Clark et al., 1988; Wang et al., 1995).
Grating orientation threshold values correspond well to sensory receptor innervation density (Johnson and Phillips, 1981; Phillips and Johnson, 1981; Bensmaia et al., 2006). Electrophysiological evidence suggests that threshold values are related to the physical spacing of Merkel cell (SA 1) receptors in the skin (Phillips and Johnson, 1981). The grating orientation thresholds observed for the reinnervated chest in the targeted reinnervation amputees were reflective of both their contralateral normal skin and the chest thresholds observed within the control population. These data suggest that the innervation density of the reinnervated skin in targeted reinnervation amputees appeared to be at near-normal levels. It appears that supplying the denervated skin with an excess of regenerating afferents (hyper-reinnervation) likely provided a mechanism to reinnervate a large number of target terminal receptors in the denervated skin. The hyper-reinnervation of muscle improves functional recovery considerably, resulting in approximately three times more motor unit formation than self-reinnervation alone (Kuiken et al., 1995). These data suggest that hyper-reinnervation also plays a role in the high level of functional recovery of cutaneous sensation.
While grating orientation thresholds were similar on both reinnervated and normal sides for the targeted reinnervation amputees, the ability of targeted reinnervation amputees to localize sequentially applied touch stimulation was better on their reinnervated side with respect to their normal side (Fig. 3). For TR1, the difference between localization thresholds for the reinnervated skin and the normal chest skin was marked. Point localization threshold values for the reinnervated chest skin were similar to those described for the hand by others (Weinstein, 1968; Ylioja et al., 2006). However, because of differences in stimulus intensity and methodology for estimation of localization error, similarities in threshold values between studies are likely not directly comparable. As described above, the grating orientation thresholds are indicative of spacing between receptors, and these data suggest that the innervation density of the reinnervated skin does not appear to be greater than normal for the chest. The point localization task involves cortical interpretation (Bell-Krotoski et al., 1993). The increase in touch localization ability observed in these subjects may be reflective of a connection from the reinnervated skin to the large, functionally relevant, central processing regions of the hand and limb.
The relative acuity of peripheral receptors appears to be related to the amount of central processing territory devoted to the inputs. For instance, in the visual system of rhesus monkeys, the cortical representation of the fovea is larger than would be predicted by the density of the ganglion cells and likely explains observed effects of increased acuity in the central visual field (Azzopardi and Cowey, 1993). Conversely, regions of the human hand that have less cortical territory devoted to them appear to have a lower tactile spatial acuity than would be predicted based solely on the distance between receptors in the skin surface (Craig and Lyle, 2002). This discrepancy may stem from the differences in central processing territory devoted to the palm of the hand (which is much less involved with haptic exploration) versus the fingertips (Craig and Lyle, 2002). The targeted reinnervation nerve redirection surgery likely connects the skin of the chest with the central processing regions once connected to the skin of the hand. Regions of the body with the highest tactile spatial acuity have larger representations in primary somatosensory cortex than parts of the body with lower tactile spatial acuity (Adrian, 1943; Catania and Kaas, 1997; Sur et al., 1980). Following targeted reinnervation, the reinnervated terminals in the skin of the chest may gain greater spatial representation within a highly functionally important central processing region. This condition may be manifested in a high degree of recovery of tactile spatial acuity for the denervated and subsequently reinnervated skin of the chest than would otherwise be expected. This may be a possibility because it has been shown that disorganized spatial relationships resulting from nerve section can be overcome to restore sensory function, particularly in regions of high functional importance such as the hand and fingers (Dykes et al., 1979; Johansson and Vallbo, 1979a; Duncan and Boynton, 2007). It has been demonstrated that increasing the size of cortical representational maps likely provides recruitment of processing resources to increase tactile spatial acuity (Godde et al., 1996; Dinse et al., 1997). Furthermore, there is evidence suggesting that increasing the size of the cortical territory devoted to repaired hand nerves through selective cutaneous forearm anesthesia provides improved sensory function (Rosen et al., 2006).
From the results of this study, it appears that it is feasible to use the reinnervated sensation as a pathway to return sensory feedback from a prosthetic device to the amputee. These subjects appeared to develop a high degree of sensory function, even without a training regime. Although the tactile spatial acuity of the reinnervated skin appears to be coarse with respect to the original tactile spatial acuity of the fingertips, the level of sensory function seen in these amputees suggests that a high level of function is possible. It appears that there are mechanisms in place to make the most of re-established touch input in these amputees. We suggest that providing sensory feedback for a prosthetic limb would help to increase the function of an amputee by better integrating a prosthetic device from a control prospective, but also by helping to assimilate the device with the user's self image (Van Dorsten, 2004; Dhillon and Horch, 2005; Murray, 2008; Rybarczyk and Behel, 2008).
Funding
National Institutes of Health (R01-HD-4-3137, R01-HD-4-4798, N01-HD-5-3402 to T.K); the Searle Funds at The Chicago Community Trust; Defense Advanced Research Projects Agency (number 908090 under Prime Contract number N66001-06-C-80060 to T.K.). Funding to pay the Open Access publication charges for this article was provided by a National Institutes of Health grant, number N01-HD-5-3402.
References
- Adrian ED. Afferent areas in the brain of ungulates. Brain. 1943;66:89–103. [Google Scholar]
- Azzopardi P, Cowey A. Preferential representation of the fovea in the primary visual cortex. Nature. 1993;361:719–21. doi: 10.1038/361719a0. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Shelton P, Hallett M. Spatial discrimination is abnormal in focal hand dystonia. Neurology. 2000;55:1869–73. doi: 10.1212/wnl.55.12.1869. [DOI] [PubMed] [Google Scholar]
- Bell-Krotoski J, Weinstein S, Weinstein C. Testing sensibility, including touch-pressure, two-point discrimination, point localization, and vibration. J Hand Ther. 1993;6:114–23. doi: 10.1016/s0894-1130(12)80292-4. [DOI] [PubMed] [Google Scholar]
- Bell-Krotoski JA, Buford WL The force/time relationship of clinically used sensory testing instruments. J Hand Ther. 1997;10:297–309. doi: 10.1016/s0894-1130(97)80045-2. [DOI] [PubMed] [Google Scholar]
- Bensmaia SJ, Craig JC, Johnson KO. Temporal factors in tactile spatial acuity: evidence for RA interference in fine spatial processing. J Neurophysiol. 2006;95:1783–91. doi: 10.1152/jn.00878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyenheuft Y, Thonnard JL. Tactile spatial resolution measured manually: a validation study. Somatosens Mot Res. 2007;24:111–4. doi: 10.1080/08990220701496639. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Fishman S, Edwards A, Jennings CL, Stojanovic M, et al. Acute plasticity in the human somatosensory cortex following amputation. Neuroreport. 1998;9:1013–7. doi: 10.1097/00001756-199804200-00011. [DOI] [PubMed] [Google Scholar]
- Catania KC, Kaas JH. Somatosensory fovea in the star-nosed mole: behavioral use of the star in relation to innervation patterns and cortical representation. J Comp Neurol. 1997;387:215–33. [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature. 1988;332:444–5. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- Craig JC, Lyle KB. A correction and a comment on Craig and Lyle (2001) Percept Psychophys. 2002;64:504–6. doi: 10.3758/bf03194721. [DOI] [PubMed] [Google Scholar]
- Dhillon GS, Horch KW. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans Neural Syst Rehabil Eng. 2005;13:468–72. doi: 10.1109/TNSRE.2005.856072. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Godde B, Hilger T, Haupt SS, Spengler F, Zepka R. Short-term functional plasticity of cortical and thalamic sensory representations and its implication for information processing. Adv Neurol. 1997;73:159–78. [PubMed] [Google Scholar]
- Duncan RO, Boynton GM. Tactile hyperacuity thresholds correlate with finger maps in primary somatosensory cortex (S1) Cereb Cortex. 2007;17:2878–91. doi: 10.1093/cercor/bhm015. [DOI] [PubMed] [Google Scholar]
- Dykes RW, Terzis JK, Strauch B. Sensations from surgically transferred glabrous skin; central versus peripheral factors. Can J Neurol Sci. 1979;6:437–9. doi: 10.1017/s0317167100023842. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Muhlnickel W, Pantev C, Wienbruch C, Taub E. Cortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputees. Exp Brain Res. 1998;119:205–12. doi: 10.1007/s002210050334. [DOI] [PubMed] [Google Scholar]
- Flor H, Muhlnickel W, Karl A, Denke C, Grusser S, Kurth R, et al. A neural substrate for nonpainful phantom limb phenomena. Neuroreport. 2000;11:1407–11. doi: 10.1097/00001756-200005150-00011. [DOI] [PubMed] [Google Scholar]
- Gibson GO, Craig JC. The effect of force and conformance on tactile intensive and spatial sensitivity. Exp Brain Res. 2006;170:172–81. doi: 10.1007/s00221-005-0200-1. [DOI] [PubMed] [Google Scholar]
- Godde B, Spengler F, Dinse HR. Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. Neuroreport. 1996;8:281–5. doi: 10.1097/00001756-199612200-00056. [DOI] [PubMed] [Google Scholar]
- Grant AC, Fernandez R, Shilian P, Yanni E, Hill MA. Tactile spatial acuity differs between fingers: a study comparing two testing paradigms. Percept Psychophys. 2006;68:1359–62. doi: 10.3758/bf03193734. [DOI] [PubMed] [Google Scholar]
- Gutmann E. The reinnervation of muscle by sensory nerve fibres. J Anat. 1945;79:1–84. [PMC free article] [PubMed] [Google Scholar]
- Hansson T, Brismar T. Loss of sensory discrimination after median nerve injury and activation in the primary somatosensory cortex on functional magnetic resonance imaging. J Neurosurg. 2003;99:100–5. doi: 10.3171/jns.2003.99.1.0100. [DOI] [PubMed] [Google Scholar]
- Horch K. Guidance of regrowing sensory axons after cutaneous nerve lesions in the cat. J Neurophysiol. 1979;42:1437–49. doi: 10.1152/jn.1979.42.5.1437. [DOI] [PubMed] [Google Scholar]
- Horch KW, Burgess PR. Functional specificity and somatotopic organization. In: Jewett DL, McCarroll HR, editors. Nerve repair and regeneration: its clinical and experimental basis. St Louis: Mosby; 1980. pp. 105–14. [Google Scholar]
- Hsiao SS, Lane J, Fitzgerald P. Representation of orientation in the somatosensory system. Behav Brain Res. 2002;135:93–103. doi: 10.1016/s0166-4328(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Detection of tactile stimuli. Thresholds of afferent units related to psychophysical thresholds in the human hand. J Physiol. 1979a;297:405–22. doi: 10.1113/jphysiol.1979.sp013048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand - relative and absolute densities of 4 types of mechanoreceptive units in glabrous skin. J Physiol London. 1979b;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KO, Phillips JR. Tactile spatial resolution. I. Two-point discrimination, gap detection, grating resolution, and letter recognition. J Neurophysiol. 1981;46:1177–92. doi: 10.1152/jn.1981.46.6.1177. [DOI] [PubMed] [Google Scholar]
- Kelly EJ, Terenghi G, Hazari A, Wiberg M. Nerve fibre and sensory end organ density in the epidermis and papillary dermis of the human hand. Br J Plast Surg. 2005;58:774–9. doi: 10.1016/j.bjps.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Kuiken TA, Childress DS, Rymer WZ. The hyper-reinnervation of rat skeletal muscle. Brain Res. 1995;676:113–23. doi: 10.1016/0006-8993(95)00102-v. [DOI] [PubMed] [Google Scholar]
- Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Ortho Int. 2004;28:245–53. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- Kuiken TA, Marasco PD, Lock BA, Harden RN, Dewald JP. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc Natl Acad Sci USA. 2007a;104:20061–6. doi: 10.1073/pnas.0706525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, et al. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007b;369:371–80. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- Lacour JP, Dubois D, Pisani A, Ortonne JP. Anatomical mapping of Merkel cells in normal human adult epidermis. Br J Dermatol. 1991;125:535–42. doi: 10.1111/j.1365-2133.1991.tb14790.x. [DOI] [PubMed] [Google Scholar]
- Manger PR, Woods TM, Jones EG. Plasticity of the somatosensory cortical map in macaque monkeys after chronic partial amputation of a digit. Proc Biol Sci. 1996;263:933–9. doi: 10.1098/rspb.1996.0138. [DOI] [PubMed] [Google Scholar]
- Murray C. Embodiment and prosthetics. In: Gallagher P, Desmond D, MacLachlan M, editors. Psychoprosthetics. London: Springer-Verlag; 2008. pp. 119–29. [Google Scholar]
- O'Shaughnessy KD, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield K, Kuiken TA. Targeted reinnervation to improve prosthesis control in transhumeral amputees. A report of three cases. J Bone Joint Surg Am. 2008;90:393–400. doi: 10.2106/JBJS.G.00268. [DOI] [PubMed] [Google Scholar]
- Phillips JR, Johnson KO. Tactile spatial resolution. II. Neural representation of Bars, edges, and gratings in monkey primary afferents. J Neurophysiol. 1981;46:1192–203. doi: 10.1152/jn.1981.46.6.1192. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science. 1992a;258:1159–60. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Stewart M, Rogers-Ramachandran DC. Perceptual correlates of massive cortical reorganization. Neuroreport. 1992b;3:583–6. doi: 10.1097/00001756-199207000-00009. [DOI] [PubMed] [Google Scholar]
- Rosen B, Bjorkman A, Lundborg G. Improved sensory relearning after nerve repair induced by selective temporary anaesthesia—a new concept in hand rehabilitation. J Hand Surg [Br] 2006;31:126–32. doi: 10.1016/j.jhsb.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Rybarczyk B, Behel J. Embodiment and prosthetics. In: Gallager P, Desmond D, MacLachlan M, editors. Psychoprosthetics. London: Springer-Verlag; 2008. pp. 23–31. [Google Scholar]
- Sathian K, Zangaladze A. Feeling with the mind's eye: contribution of visual cortex to tactile perception. Behav Brain Res. 2002;135:127–32. doi: 10.1016/s0166-4328(02)00141-9. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A, Hoffman JM, Grafton ST. Feeling with the mind's eye. Neuroreport. 1997;8:3877–81. doi: 10.1097/00001756-199712220-00008. [DOI] [PubMed] [Google Scholar]
- Schlereth T, Magerl W, Treede R. Spatial discrimination thresholds for pain and touch in human hairy skin. Pain. 2001;92:187–94. doi: 10.1016/s0304-3959(00)00484-x. [DOI] [PubMed] [Google Scholar]
- Schultz AE, Marasco PD, Kuiken TA. Brain Res. Vol. 1251. 2009. Vibrotactile detection thresholds for chest skin of amputees following targeted reinnervation surgery. pp. 121–9. [DOI] [PubMed] [Google Scholar]
- Shaw WW, Orringer JS, Ko CY, Ratto LL, Mersmann CA. The spontaneous return of sensibility in breasts reconstructed with autologous tissues. Plast Reconstr Surg. 1997;99:394–9. doi: 10.1097/00006534-199702000-00013. [DOI] [PubMed] [Google Scholar]
- Stark G, LeBlanc M. Overview of body-powered upper extremity prostheses. In: Meier R, Atkins D, editors. Functional restoration of adults and children with upper extremity amputation. New York: Demos Medical Publishing, Inc.; 2004. pp. 175–86. [Google Scholar]
- Sur M, Merzenich MM, Kaas JH. Magnification, receptive-field area, and “hypercolumn” size in areas 3b and 1 of somatosensory cortex in owl monkeys. J Neurophysiol. 1980;44:295–311. doi: 10.1152/jn.1980.44.2.295. [DOI] [PubMed] [Google Scholar]
- Travieso D, Lederman SJ. Assessing subclinical tactual deficits in the hand function of diabetic blind persons at risk for peripheral neuropathy. Arch Phys Med Rehabil. 2007;88:1662–72. doi: 10.1016/j.apmr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Valentini M, Kischka U, Halligan PW. Residual haptic sensation following stroke using ipsilateral stimulation. J Neurol Neurosurg Psychiatry. 2008;79:266–70. doi: 10.1136/jnnp.2007.120279. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Johnson KO. A psychophysical study of the mechanisms of sensory recovery following nerve injury in humans. Brain. 1994;117(Pt 1):149–67. doi: 10.1093/brain/117.1.149. [DOI] [PubMed] [Google Scholar]
- Van Dorsten B. Integrating psychological and medical care: practice recommendations for amputation. In: Meier R, Atkins D, editors. Functional restoration of adults and children with upper extremity amputation. New York: Demos Medical Publishing, Inc.; 2004. pp. 73–88. [Google Scholar]
- Vega-Bermudez F, Johnson KO. Differences in spatial acuity between digits. Neurology. 2001;56:1389–91. doi: 10.1212/wnl.56.10.1389. [DOI] [PubMed] [Google Scholar]
- Vega-Bermudez F, Johnson KO. Spatial acuity after digit amputation. Brain. 2002;125:1256–64. doi: 10.1093/brain/awf129. [DOI] [PubMed] [Google Scholar]
- Walsh R, O’Dwyer JP, Sheikh IH, O’Riordan S, Lynch T, Hutchinson M. Sporadic adult onset dystonia: sensory abnormalities as an endophenotype in unaffected relatives. J Neurol Neurosurg Psychiatry. 2007;78:980–3. doi: 10.1136/jnnp.2006.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378:71–5. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- Weinstein S. Intensive and extensive aspects of tactile sensitivity as a function of body part, sex and laterality. In: Kenshalo DR, editor. The skin senses. 1968. pp. 195–222. Springfield; Charles C. Thomas. [Google Scholar]
- Weiss P, Edds MVJR. Sensory-motor nerve crosses in the rat. J Neurophysiol. 1945;8:173–93. [Google Scholar]
- Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002;5:936–8. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]
- Wetherill GB, Levitt H. Sequential estimation of points on a psychometric function. Br J Math Stat Psychol. 1965;18:1–10. doi: 10.1111/j.2044-8317.1965.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Ylioja S, Carlson S, Raij TT, Pertovaara A. Localization of touch versus heat pain in the human hand: a dissociative effect of temporal parameters on discriminative capacity and decision strategy. Pain. 2006;121:6–13. doi: 10.1016/j.pain.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Ylioja S, Pertovaara A, Koivisto J, Korvenoja A, Artchakov D, Carlson S. The effect of interstimulus interval on somatosensory point localization. Somatosens Motor Res. 2004;21:3–7. doi: 10.1080/0899022042000201245. [DOI] [PubMed] [Google Scholar]
- Zeuner KE, Hallett M. Sensory training as treatment for focal hand dystonia: a 1-year follow-up. Mov Disord. 2003;18:1044–7. doi: 10.1002/mds.10490. [DOI] [PubMed] [Google Scholar]
- Zhou P, Lowery MM, Englehart KB, Huang H, Li G, Hargrove L, et al. Decoding a new neural machine interface for control of artificial limbs. J Neurophysiol. 2007;98:2974–82. doi: 10.1152/jn.00178.2007. [DOI] [PubMed] [Google Scholar]