Abstract
Elevations of the levels of N-acetyl-aspartyl-glutamate (NAAG) and N-acetyl-aspartate (NAA) are associated with myelin loss in the leucodystrophies Canavan's disease and Pelizaeus-Merzbacher-like disease. NAAG and NAA can activate and antagonize neuronal N-methyl-D-aspartate (NMDA) receptors, and also act on group II metabotropic glutamate receptors. Oligodendrocytes and their precursors have recently been shown to express NMDA receptors, and activation of these receptors in ischaemia leads to the death of oligodendrocyte precursors and the loss of myelin. This raises the possibility that the failure to develop myelin, or demyelination, occurring in the leucodystrophies could reflect an action of NAAG or NAA on oligodendrocyte NMDA receptors. However, since the putative subunit composition of NMDA receptors on oligodendrocytes differs from that of neuronal NMDA receptors, the effects of NAAG and NAA on them are unknown. We show that NAAG, but not NAA, evokes an inward membrane current in cerebellar white matter oligodendrocytes, which is reduced by NMDA receptor block (but not by block of metabotropic glutamate receptors). The size of the current evoked by NAAG, relative to that evoked by NMDA, was much smaller in oligodendrocytes than in neurons, and NAAG induced a rise in [Ca2+]i in neurons but not in oligodendrocytes. These differences in the effect of NAAG on oligodendrocytes and neurons may reflect the aforementioned difference in receptor subunit composition. In addition, as a major part of the response in oligodendrocytes was blocked by tetrodotoxin (TTX), much of the NAAG-evoked current in oligodendrocytes is a secondary consequence of activating neuronal NMDA receptors. Six hours exposure to 1 mM NAAG did not lead to the death of cells in the white matter. We conclude that an action of NAAG on oligodendrocyte NMDA receptors is unlikely to be a major contributor to white matter damage in the leucodystrophies.
Keywords: leucodystrophy, Pelizaeus-Merzbacher-like disease, Canavan's disease, glutamate, NMDA
Introduction
The speeding of axon potential propagation which is produced by myelination of axons by oligodendrocytes is essential for normal brain function (Fields, 2008). Failure of the development of myelination, as occurs in genetic leucodystrophies and cerebral palsy, or destruction of myelin, as occurs in multiple sclerosis, produces mental and physical disability. It has become increasingly clear that activation of glutamate receptors on oligodendrocytes, or their precursor cells, can lead to loss of myelin. Periventricular leucomalacia, leading to cerebral palsy, can be caused by a deficient blood flow to the periventricular white matter and/or infection, leading to glutamate release that kills precursor and immature oligodendrocytes (Follett et al., 2000; Káradóttir et al., 2008; Khwaja and Volpe, 2008). In spinal cord injury, secondary ischaemia leads to a release of glutamate by reversed uptake from axons and from oligodendrocytes themselves, which damages oligodendrocytes by activating AMPA/kainate receptors (Li et al., 1999). Oligodendrocytes also express N-methyl-D-aspartate (NMDA) receptors, however, and activation of these receptors by glutamate released in ischaemia causes demyelination and loss of axonal action potential propagation (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006; Bakiri et al., 2008).
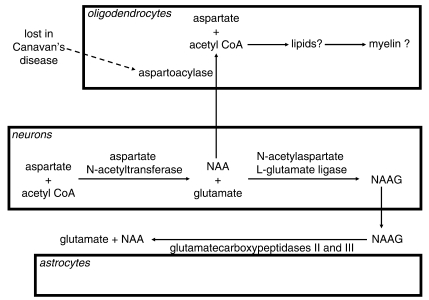
Other endogenous molecules may also act on oligodendrocyte NMDA receptors. In particular, N-acetyl-aspartate (NAA) and N-acetyl-aspartyl-glutamate (NAAG) have been shown to act as both agonists and antagonists at neuronal NMDA receptors (Westbrook et al., 1986; Sekiguchi et al., 1992; Burlina et al., 1994; Koenig et al., 1994; Valivullah et al., 1994; Rubin et al., 1995; Pliss et al., 2000; Bergeron et al., 2005). NAA and NAAG are synthesized in neurons (Baslow, 2007), as shown in Fig. 1. NAA can be exported to oligodendrocytes, where it is metabolized by the enzyme aspartoacylase, to form acetyl-CoA, which has been suggested to be used for myelination (Chakraborty et al., 2001), although this idea has been criticized (Baslow, 2007) on the grounds that NAA turns over much faster than myelin, and that myelin is still formed in a disorder in which NAA is no longer made. NAAG is broken down to NAA and glutamate by glutamatecarboxypeptidases (Berger et al., 1999) located on the surface of astrocytes (Fig. 1).
Figure 1.
Metabolic pathways of NAA and NAAG. NAA is synthesized in neurons from aspartate and acetyl-CoA. It can then be exported to oligodendrocytes, where it is converted back to acetyl-CoA by aspartoacylase (an enzyme lost in Canavan's disease) and may thus be converted into lipids that form part of the myelin. Alternatively, it can be converted to NAAG in neurons and released to the extracellular space, where it is converted back to NAA by carboxypeptidases expressed on the extracellular surface of astrocytes.
In two leucodystrophies there is a defect in the metabolism of NAAG and NAA, associated with a lack of formation of myelin. Canavan's disease is caused by a lack of aspartoacylase activity (Fig. 1) in oligodendrocytes (Kumar et al., 2006), which reduces NAA breakdown and thus causes a large rise in NAA and NAAG levels. Patients have also been reported with a Pelizaeus-Merzbacher-like syndrome, in which there is an absence of myelin similar to that seen when the myelin proteolipid protein is mutated in Pelizaeus-Merzbacher disease (although no mutation was found in these patients), and this is associated with a large rise of NAAG concentration (Wolf et al., 2008). Because NAA and NAAG levels rise in these disorders, and because these agents affect neuronal NMDA receptors, we hypothesized that the demyelination occurring in these disorders might reflect NAA or NAAG acting on oligodendrocyte NMDA receptors. However, the subunit composition of oligodendrocyte NMDA receptors most probably differs from that of neuronal ones (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006), and so may show a different pharmacological profile. We therefore tested whether NAAG or NAA can act on oligodendrocyte NMDA receptors.
Methods
Preparation
Rats (post-natal day 12) were killed by cervical dislocation in accordance with UK government regulations. Cerebellar slices (225 μm thick) were made in solution containing 124 mM NaCl, 26 mM NaHCO3, 1 mM NaH2PO4, 2.5 mM KCl, 2 mM MgCl2, 2.5 mM CaCl2, 10 mM glucose, bubbled with 95% O2/5% CO2, pH 7.4, as well as 1 mM Na-kynurenate to block glutamate receptors. For experiments, slices were superfused at 24 ± 1°C with HEPES-buffered solution containing 144 mM NaCl, 2.5 mM KCl, 10 mM HEPES, 1 mM NaH2PO4, 2.5 mM CaCl2, 10 mM glucose, pH set to 7.4 with NaOH, bubbled with 100% O2. Usually MgCl2 was omitted from the solution, and glycine (100 μM, to ensure activation of the glycine site of NMDA receptors) and strychnine (5 μM, to block glycine-gated chloride channels) were added. Some experiments, shown in Figs 9 and 10, were performed in physiological (1 mM) Mg2+ and with no added glycine. NAA and NAAG were from Tocris, UK. HPLC data supplied by Tocris showed that 1 mM NAAG contained < 0.2 μM glutamate.
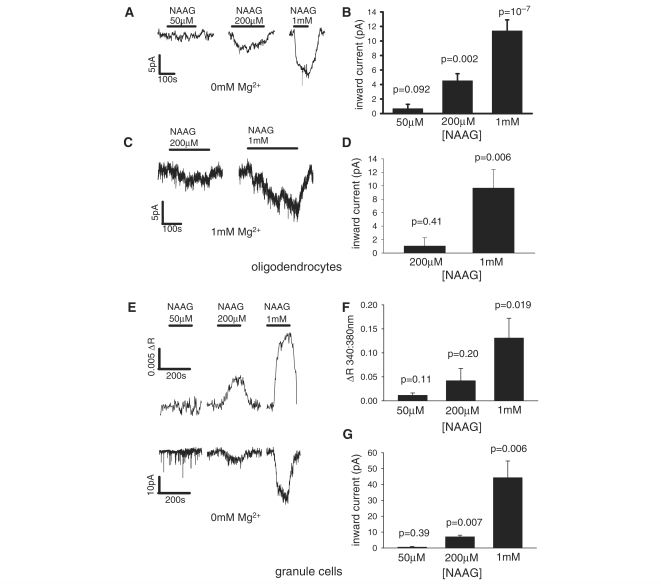
Figure 9.
The current response of white matter oligodendrocytes, and the current response and [Ca2+]i rise of grey matter granule cells, to pathologically relevant concentrations of NAAG. (A) Current responses of a single oligodendrocyte, and (B) mean response of 7, 9 and 25 cells, respectively to 50, 200 and 1000 µM NAAG in 0 mM Mg2+ solution. (C) Responses of a single oligodendrocyte and (D) mean response of 10 and 10 cells, respectively to 200 and 1000 µM NAAG in 1 mM Mg2+ solution with no added glycine. (E) [Ca2+]i rise (upper panel) and current response (lower panel) of a single granule cell, and mean (F) [Ca2+]i rise and (G) current response of three, four and seven cells to 50, 200 and 1000 µM NAAG in 0 mM Mg2+. All P-values are for comparison with zero response.
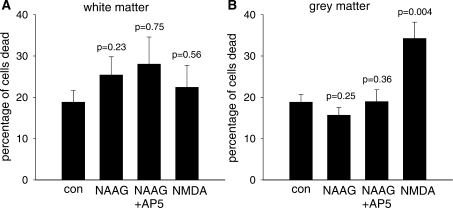
Figure 10.
The death of white matter (A) and grey matter (B) cells evoked by 1 mM NAAG (n = 11 slices), 1 mM NAAG and 100 µM AP5 (n = 6 slices) and 100 µM NMDA (n = 10 slices), compared with the death in control solution (n = 10 slices). Slices were incubated at 36°C for 6 h, and data were averaged from three replicate experiments. The percentage of death was calculated by dividing the number of dead cells (labelled with PI) by the total number of cells (given by the number of nuclei labelled with DAPI).
Electrophysiology
White matter cells (avoiding cerebellar nuclei) were whole-cell clamped with pipettes containing a Cs+-based solution comprising 130 mM Cs-gluconate, 4 mM NaCl, 0.5 mM CaCl2, 10 mM HEPES, 10 mM BAPTA, 4 mM MgATP, 0.5 mM Na2GTP, K-Lucifer yellow 2, pH set to 7.3 with CsOH. Membrane potentials were compensated for the –14 mV junction potential measured with the electrode in the extracellular solution (Fenwick et al., 1982). Series resistance was 10–30 MΩ. Mature oligodendrocytes were identified from their morphology after Lucifer filling, with a small number of cells also being labelled after recording for myelin basic protein (as in Káradóttir et al., 2005; Káradóttir and Attwell, 2006). Granule cells were identified from their position in the granule cell layer, their small soma attached to approximately four dendrites and their high input resistance (> 3 GΩ).
[Ca2+]i imaging with Fluo-4-AM
Slices were loaded with the acetoxymethyl ester of Fluo-4 by incubating slices for 2 h in a 10 μM solution of Fluo-4-AM (a 2 mM Fluo-4-AM stock was made by dissolving 50 μg of the ester in 11.5 μl of 20% pluronic acid in DMSO, and adding 11.5 μl of DMSO; then 10 μl of this stock was added to 2 ml of slicing medium). 95% O2/5% CO2 was blown gently over the solution while loading. Fluorescence was excited at 488 ± 10 nm and emitted light was collected at 535 ± 22 nm. Regions of interest in the white and grey matter were identified from the morphology and anatomy of the slice. If the slices were not loaded with dye the baseline fluorescence was 2.99-fold lower for white matter and 3.37-fold lower for grey matter (comparing 12 loaded and three unloaded slices) and there was a negligible fluorescence (F) change in response to 1 mM NAAG (ΔF/F = –0.00146 ± 0.00146 in three unloaded slices, compared with 0.03611 ± 0.0095 in the white matter of 12 loaded slices).
Single cell [Ca2+]i imaging
White matter oligodendrocytes and grey matter granule cells were patch-clamped with pipettes containing a Cs+-based solution comprising 130 mM Cs-gluconate, 4 mM NaCl, 10 mM HEPES, 0.01 mM BAPTA, 10 mM phosphocreatine, 4 mM MgATP, 0.5 mM Na2GTP, 1 mM Fura-2, pH set to 7.3 with CsOH. Fluorescence was excited sequentially at 340 ± 10 and 380 ± 10 nm, and emitted light was collected at 510 ± 20 nm. The ratio of the emission intensities (340/380 nm) was used as a measure of increased [Ca2+]i, and only ratios that reflected both a decrease of fluorescence excited at 380 nm and an increase in fluorescence excited at 340 nm were used.
Cell death assay
Slices were bathed in a HEPES-buffered solution (containing 144 mM NaCl, 2.5 mM KCl, 10 mM HEPES, 1 mM NaH2PO4, 2.5 mM CaCl2, 1 mM MgCl2, 10 mM glucose, pH set to 7.4 with NaOH, bubbled with 100% O2) containing 1 mM NAAG, or 1 mM NAAG and 100 µM AP5, or 100 µM NMDA, or control solution, at 36°C for 6 h. Propidium iodide (PI, Sigma, 37 μM) was added to reveal a loss of membrane permeability associated with cell death. Slices were then fixed in 4% paraformaldehyde overnight, washed twice for 15 min in phosphate buffered saline (PBS) and preincubated for 4 h in 0.05% Triton and 10% normal goat serum in PBS. To identify the white matter, antibody to myelin basic protein (MBP, mouse, Chemicon, 1:150) was applied overnight, followed by 3 × 30 min PBS washes and 8 h incubation with secondary antibody (goat anti-mouse IgG, AlexaFluor 488, Invitrogen, 1:200). Slices were washed 3 × 30 min with PBS and mounted on coverslips using a mounting solution containing DAPI to label nuclei.
Confocal microscopy
Confocal images of slices were taken for the cell death assay using excitation and emission wavelengths as follows: DAPI (excitation 364 nm, emission > 385 nm), AlexaFluor 488 (excitation 488 nm, emission 505–530 nm) and PI (excitation 543 nm, emission 570–600 nm). A 40× objective was used to take pictures of the white matter (MBP-positive) and grey matter (MBP-negative) in each slice. The percentage of dead cells was calculated as the number of PI-positive cells divided by the total number of cells with DAPI-labelled nuclei.
Statistics
Data are presented as mean ± SEM. P-values are from Student's two-tailed t-tests.
Results
The effect of NAA and NAAG on oligodendrocyte and granule cell currents
We compared the effect of 1 mM NAAG, 1 mM NAA and 60 μM NMDA on mature oligodendrocytes and granule cells in cerebellar slices, both of which have previously been reported to show NMDA-evoked currents (D’Angelo et al., 1993; Káradóttir et al., 2005). High concentrations of NAA and NAAG were used to determine whether there was any possibility of these agonists activating the cells’ NMDA receptors, because earlier work (Westbrook et al., 1986; Rubin et al., 1995) demonstrated a low potency for these compounds’ action on neuronal NMDA receptors. NAA, NAAG and NMDA were usually applied in Mg2+-free solution containing glycine (100 μM) to ensure adequate activation of the receptors’ glycine/d-serine site (although, at least in the grey matter of the cerebellum, this site is saturated by endogenously released glycine or d-serine: Billups and Attwell, 2003), but we show below (Fig. 9) that NAAG also evokes a current in oligodendrocytes in solution lacking added glycine and containing a physiological level of Mg2+ (1 mM).
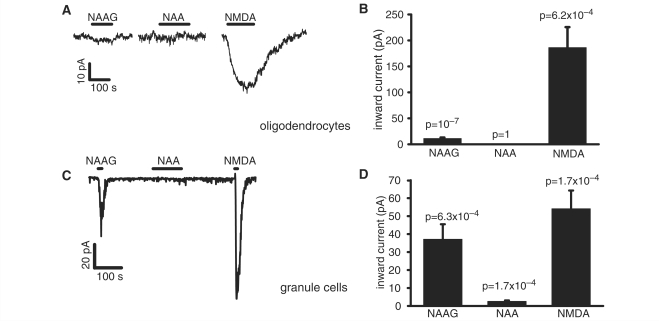
NAAG evoked a significant inward current in oligodendrocytes (Fig. 2A), which was ∼ 7% of the current evoked by NMDA in the same cells (Fig. 2B, which also includes data for NMDA in a small number of cells to which NAA but not NAAG was applied), while NAA evoked no detectable current. In contrast, in granule cells the NAAG-evoked current was 77% of the current evoked by NMDA in the same cells, and NAA also evoked a small but significant current (Fig. 2C and D).
Figure 2.
NAAG, NAA and NMDA-evoked whole cell currents at –74 mV in white matter oligodendrocytes and cerebellar granule cells. (A) Representative trace shows current response to 1 mM NAAG, 1 mM NAA and 60 µM NMDA in a single oligodendrocyte. (B) Average responses to 1 mM NAAG (n = 20 cells), 1 mM NAA (n = 5) and 60 µM NMDA (n = 12) in oligodendrocytes. (C) Representative trace shows response to 1 mM NAAG, 1 mM NAA and 60 µM NMDA in a single granule cell. (D) Average responses to 1 mM NAAG (n = 14), 1 mM NAA (n = 14) and 60 µM NMDA (n = 13) in granule cells.
Pharmacology of the NAAG-evoked current
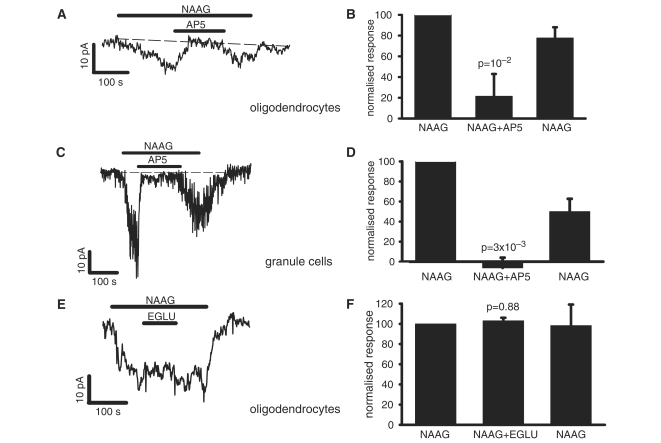
The NMDA receptor blocker D-AP5 (100 μM) reversibly blocked the NAAG-evoked current both in oligodendrocytes (Fig. 3A and B) and in granule cells (Fig. 3C and D). The incomplete block of the NAAG-evoked current in oligodendrocytes in Fig. 3B probably reflects the inaccuracies of measuring the very small current evoked by NAAG in the presence of small baseline current variations. Since NAAG has also been reported to act on metabotropic glutamate (mGluR3) receptors (Wroblewska et al., 1997, 1998), we tested the effect on the NAAG-evoked current of the group II mGluR antagonist EGLU [(2S)-α-ethylglutamic acid]. At a concentration of 200 μM, i.e. three times the Ki value for mGluR3 inhibition (Jane et al., 1996), EGLU had no effect on the NAAG-evoked current in oligodendrocytes (Fig. 3E and F), leading to the conclusion that all of this current is the result of NAAG activating NMDA receptors—however, this does not rule out the possibility that NAAG evokes mGluR3 mediated effects which are not detected as a current.
Figure 3.
Pharmacology of the NAAG-evoked current at –74 mV in white matter oligodendrocytes and cerebellar granule cells. (A) Representative trace shows response to 1 mM NAAG and block of the response by 100 µM AP5 in a white matter oligodendrocyte. (B) Mean effect on the response to 1 mM NAAG of 100 µM AP5 in 10 white matter oligodendrocytes. The second and third bars are normalized to the initial NAAG response (as 100%), and show the response to NAAG in the presence of AP5 and the response to a subsequent application of NAAG alone. P-value compares the response in NAAG to the average of the preceding and subsequent responses without AP5. (C) Representative trace shows response to 1 mM NAAG alone and block of the response by 100 µM AP5 in a granule cell. (D) Mean effect on the response to 1 mM NAAG of 100 µM AP5 in four granule cells. (E) Representative trace shows effect on 1 mM NAAG-evoked response of 200 µM EGLU in white matter oligodendrocyte. (F) Mean effect of 200 µM EGLU on the response to 1 mM NAAG in three white matter oligodendrocytes. Response in EGLU was measured at the end of EGLU application, and response to NAAG after EGLU was measured at the end of NAAG application.
NAAG and NAA are not strong blockers of NMDA receptors
Since the role of NMDA receptors in controlling and maintaining myelination is not yet known, it seemed possible that a demyelinating action of NAAG or NAA might occur if these agents blocked NMDA receptors and thus inhibited normal signalling from axons to oligodendrocytes or their precursors. Block of neuronal NMDA receptors by NAAG has been reported previously (Burlina et al., 1994; Bergeron et al., 2005).
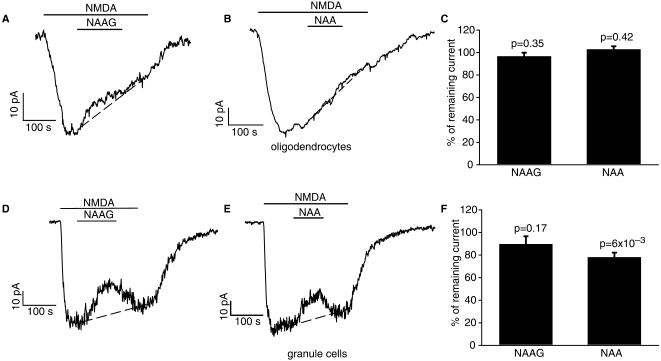
However, when 1mM NAA or NAAG were applied during the response to NMDA in white matter oligodendrocytes, any reduction in the NMDA-evoked current they produced was small compared with the reduction occurring anyway as a result of receptor desensitization (Fig. 4A–C; reduction of the response by NAAG/NAA was estimated by fitting a straight line to the desensitization, and measuring the actual current at the end of the NAAG/NAA application relative to the interpolated value that would occur without NAAG/NAA). In some granule cells both NAA and NAAG produced a small reduction of the NMDA-evoked current (Fig. 4D and E), but in others the reduction was minimal (the mean reduction is shown in Fig. 4F). Thus, even using 1 mM NAA or NAAG produced much less inhibition of the oligodendocyte or granule cell NMDA response than the 80% block reported to be produced by NAAG concentrations above 20 μM in hippocampal pyramidal neurons (Bergeron et al., 2005).
Figure 4.
Test of block of NMDA-evoked current at –74 mV by NAAG and NAA in cerebellar white matter oligodendrocytes (A–C) and granule cells (D–F). (A) Representative trace shows block of 60 µM NMDA-evoked response by 1 mM NAAG in oligodendrocyte. (B) Representative trace shows block of 60 µM NMDA-evoked response by 1 mM NAA in oligodendrocyte. (C) Mean effect of 1 mM NAAG (five cells) and 1 mM NAA (three cells) on the NMDA-evoked current, calculated assuming for simplicity that desensitization of the NMDA-evoked current follows a linear time course (dashed lines in A and B: the NMDA-evoked current at the end of the NAAG or NAA application was divided by the current predicted assuming linear desensitization). (D) Cell showing block of 60 µM NMDA-evoked response by 1 mM NAAG in granule cell (not all cells showed any block). (E) Cell showing block of 60 µM NMDA-evoked response by 1 mM NAA in granule cell (not all cells showed any block). (F) Mean effect of 1 mM NAAG (nine cells) and 1 mM NAA (five cells) on the NMDA-evoked current, calculated assuming for simplicity that desensitization of the NMDA-evoked current follows a linear time course.
Part of the oligodendrocyte NAAG response is produced by hydrolysis to glutamate
Extracellular NAAG, whether generated endogenously or applied exogenously, can be converted to glutamate by carboxypeptidases II and III on the extracellular surface of astrocytes (Fig. 1; Berger et al., 1999; Bzdega et al., 2004). To test whether the response of oligodendrocytes to NAAG was generated in part by secondarily derived glutamate, we blocked these enzymes with 10 μM 2-PMPA [2-(phosphonomethyl)pentanedioic acid], (Jackson et al., 1996): the IC50 values for inhibition of GCP II and III are 6.7 and 0.9 nM, respectively (Bzdega et al., 2004). This concentration of 2-PMPA does not significantly block NMDA receptors (Tortella et al., 2000).
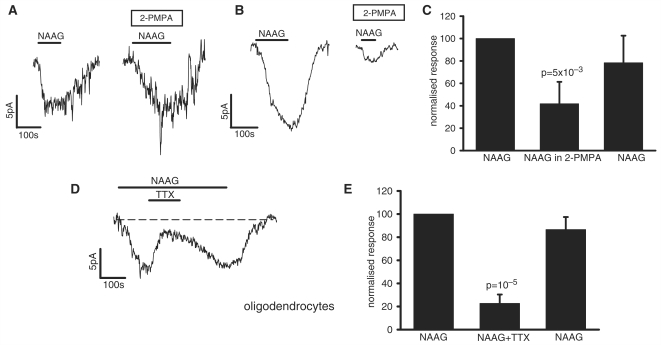
We found an interesting variability in the effect of 2-PMPA. In some oligodendrocytes (Fig. 5A) the NAAG response was unaffected by blocking this enzyme, suggesting that the NAAG-evoked current was produced by NAAG itself (HPLC data provided by Tocris showed that 1 mM NAAG solution contained < 0.2 μM contaminating glutamate), while in other cells the enzyme blocker significantly reduced the response (Fig. 5B) implying that part of the response was produced by conversion of NAAG to glutamate by endogenous carboxypeptidases. On average, about 60% of the response was blocked by 2-PMPA (Fig. 5C).
Figure 5.
The effect of blocking carboxypeptidases and blocking action potentials on the NAAG-evoked current at –74 mV in oligodendrocytes. (A) Specimen trace showing a cell where the carboxypeptidase blocker 2-PMPA (10 μM) had no effect on the NAAG-evoked current. (B) Specimen trace showing a cell where 2-PMPA reduced the NAAG-evoked current. (C) Mean effect of 2-PMPA on the NAAG-evoked current in seven cells. (D) Specimen trace showing the effect of TTX (1 μM) on the NAAG-evoked current. (E) Mean effect of TTX on the NAAG-evoked current in eight cells.
The NAAG response in oligodendrocytes is partly produced by neuronal action potentials
Superfusion of NAAG (or NMDA) could, in principle, evoke a current in oligodendrocytes by acting directly on oligodendrocyte NMDA receptors, or by acting on neuronal NMDA receptors to increase neuronal firing and hence increase the release of factors like K+ from axons, which may generate a current in oligodendrocytes. The large NMDA-evoked current is not significantly affected by tetrodotoxin (TTX) (Káradóttir et al., 2005), suggesting that essentially all of the NMDA-evoked current is generated by a direct action on oligodendrocytes. However, 1 μM TTX reduced the NAAG-evoked current by 77% (Fig. 5D and E), suggesting that much of the NAAG response is generated indirectly by NAAG activating neuronal NMDA receptors and increasing action potential firing (see Discussion section). In contrast, TTX did not affect the NMDA-evoked current in granule cells (reduced by 2.4 ± 8.2%, not significant, P = 0.79).
Effects of NAAG and NAA on [Ca2+]i in the white matter and grey matter
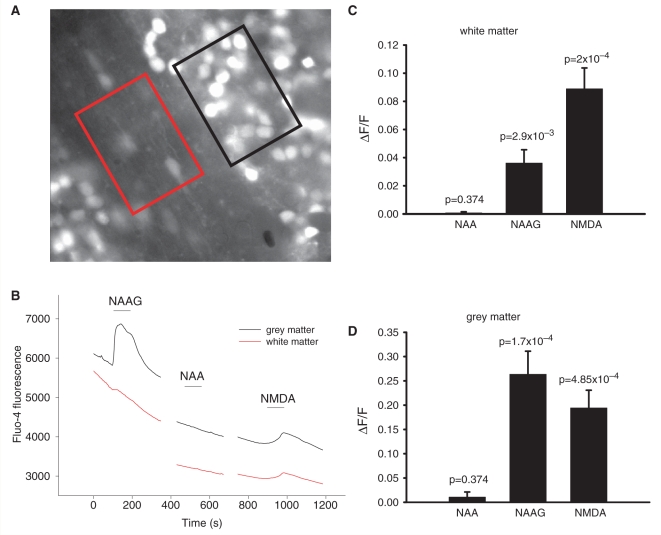
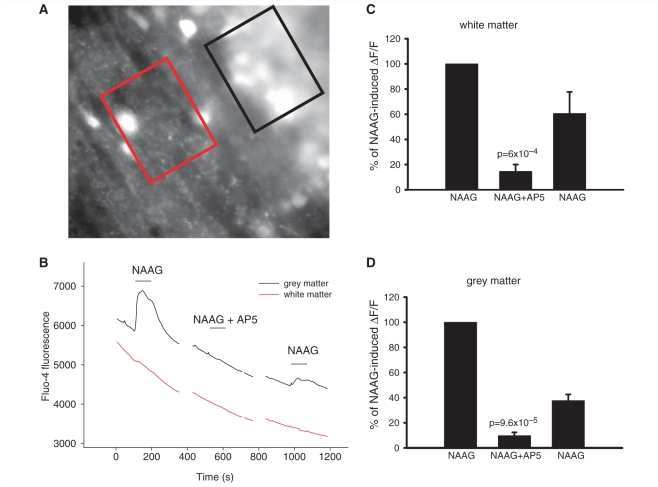
The death of oligodendrocyte lineage cells and lack of myelin that occur in Canavan's disease and in Pelizaeus-Merzbacher-like disease may be generated by a rise of [Ca2+]i in oligodendrocytes. To determine whether NAAG or NAA raise [Ca2+]i in cells of the white matter, we initially employed the Ca2+-sensitive dye Fluo-4, which was loaded into the cells of cerebellar slices as its acetoxymethyl ester. Regions of interest were imaged that included, separately, the white matter and the grey matter (Fig. 6A).
Figure 6.
Change in [Ca2+]i in white and grey matter on application of 1 mM NAA, 1 mM NAAG and 60 µM NMDA. (A) Specimen image of cerebellar white matter (red region) and grey matter (black region) loaded with Fluo-4. (B) Rise of Fluo-4 fluorescence (F) in white matter and grey matter after application of NAAG and NMDA, and lack of a response after application of NAA. (C) Average responses of white matter to NAA (n = 5 slices), NAAG (n = 12) and NMDA (n = 10). (D) Average responses of grey matter to NAA (n = 5), NAAG (n = 12) and NMDA (n = 10).
In both the grey and the white matter, NAAG and NMDA evoked an increase in Fluo-4 fluorescence, while NAA did not (Fig. 6B–D). As for the membrane currents shown for grey matter granule cells and white matter oligodendrocytes in Fig. 2, the response to NAAG, relative to that for NMDA, was larger in the grey matter than in the white matter. In addition, in both areas the ratio of the NAAG response to that for NMDA (measured in the same slice or cell) tended to be larger for the calcium response than for the current response (NAAG/NMDA = 0.37 ± 0.14, n = 9, for white matter [Ca2+]i and 0.066 ± 0.014, n = 10, for oligodendrocyte current, significantly different: P = 0.03; NAAG/NMDA = 1.74 ± 0.57, n = 9, for grey matter [Ca2+]i and 0.77 ± 0.24, n = 7, for granule cell current, not significantly different: P = 0.18). D-AP5 (100 μM) decreased the fluorescence change evoked by NMDA (data not shown) and by NAAG, both in white and grey matter (Fig. 7).
Figure 7.
Block of 1 mM NAAG-induced rise in [Ca2+]i in white and grey matter by 100 µM AP5. (A) Specimen image of cerebellar white matter (red region) and grey matter (black region) loaded with Fluo-4. (B) Block of NAAG-induced rise in Fluo-4 fluorescence in white matter and grey matter by AP5. (C) Average responses of white matter to NAAG, NAAG + AP5 and a subsequent application of NAAG (in four slices), normalized to the amplitude of the first response. (D) Normalized average responses of grey matter to NAAG, NAAG + AP5, and a subsequent application of NAAG (in four slices).
The effect of NAAG on [Ca2+]i in oligodendrocytes and granule cells
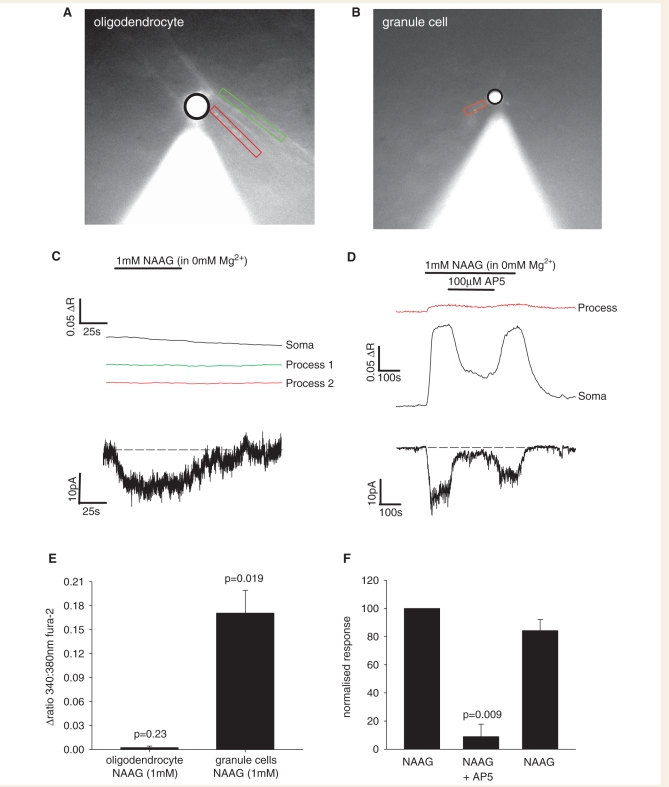
Although AM-loading of the calcium dye Fluo-4 led to granule cells generating sufficient fluorescence for their calcium responses to be reliably distinguished from those generated in surrounding cells, this was not the case for the very fine processes of oligodendrocytes in the white matter. To determine whether the cells in the white matter that respond to NAAG with a [Ca2+]i rise are oligodendrocytes, we patch-clamped cells whose morphology (assessed after Lucifer yellow filling from the patch pipette) corresponded to mature oligodendrocytes, using pipettes containing the ratiometric Ca2+-sensitive dye Fura-2. As a control, we also patch-clamped granule cells in grey matter.
In oligodendrocytes, 1 mM NAAG did not evoke a significant [Ca2+]i rise in either the cell body or the processes, despite NAAG evoking an inward current (Fig. 8A, C and E), and despite spontaneous [Ca2+]i elevations being observed, so we knew that the dye was working. Granule cells, however, responded to 1 mM NAAG with a statistically significant [Ca2+]i rise (both in their processes and cell body) that was blocked by D-AP5 (Fig. 8B, D–F). The implications of these results are considered in the Discussion section.
Figure 8.
NAAG evoked changes of [Ca2+]i in oligodendrocytes and granule cells. (A) Specimen image of a cerebellar white matter oligodendrocyte patch-clamped with a pipette containing Fura-2. Black line encircles soma, green and red rectangles are around processes aligned with axons. (B) Specimen image of a cerebellar granule cell patch-clamped with a pipette containing Fura-2. Black line encircles soma, red rectangle is around a single process (C) Lack of change of Fura-2 fluorescence in both the soma and processes of the oligodendrocyte in A after application of 1 mM NAAG (upper panel), despite NAAG evoking an inward current (lower panel). (D) Change of Fura-2 fluorescence (▵R = change of ratio 340/380 nm) in the granule cell in B after application of 1 mM NAAG, and block of this response by 100 µM AP5 (upper panel), with the simultaneously evoked inward current (lower panel). (E) Average responses of oligodendrocytes (eight cells) and granule cell somata (seven cells) to 1 mM NAAG. (F) Average responses of granule cell somata to NAAG, NAAG + AP5, and a subsequent application of NAAG (three cells), normalized to the initial response.
The effect of pathologically relevant levels of NAAG
In Canavan's disease the extracellular NAA concentration (assessed in the CSF) rises from its baseline value of ∼ 1.5 μM (Jakobs et al., 1991) to ∼ 0.4–0.9 mM (Wevers et al., 1995; Burlina et al., 1999) i.e. less than the 1 mM concentration we have applied which produces no effect on oligodendrocytes, and the NAAG level rises from its normal value of 1–10 to ∼ 20 μM (Burlina et al., 1999). In Pelizaeus-Merzbacher-like disease, the NAAG concentration has been reported to rise to ∼ 50 μM (Sartori et al., 2008) or ∼ 200 μM (Wolf et al., 2004). We therefore, tested the effect of 50 and 200 μM NAAG on the membrane current and [Ca2+]i change of oligodendrocytes and granule cells. In both oligodendrocytes (Fig. 9A and B) and granule cells (Fig. 9E and G) 50 μM NAAG produced a barely detectable current, while 200 μM NAAG evoked a significant current in 0 mM Mg2+. NAAG (200 μM and 1 mM) also evoked a current in oligodendrocytes in the presence of a physiological Mg2+ concentration (1 mM: Fig. 9C and D). As in Fig. 8, a [Ca2+]i rise was seen in granule cells but not in oligodendrocytes, which increased with increasing NAAG concentration (Fig. 9E and F).
The effect of NAAG on cell death in white matter and grey matter
As NAAG evokes an inward current in oligodendrocytes of the cerebellar white matter, and an inward current and [Ca2+]i rise in granule neurons of grey matter, we hypothesized that a direct or indirect effect of this molecule on oligodendrocytes may lead to their death and hence to the myelin loss observed in Canavan's and Pelizaeus-Merzbacher-like diseases. We checked this theory by bathing cerebellar slices in extracellular solution containing a physiological level of Mg2+ (1 mM, with no added glycine) and 1 mM NAAG, 1 mM NAAG with 100 µM AP5, 100 µM NMDA or no NMDA agonists, at 36°C for 6 h. Afterwards, we labelled the slices with antibody to myelin basic protein to define the white matter and with DAPI to define cell nuclei, and quantified the percentage of cells which were dead (i.e. took up propidium iodide into their soma) in the white matter and the grey matter.
Although NMDA evoked significant death of granule cells in the grey matter (P = 0.004), NAAG did not, and neither NAAG nor NMDA evoked significant cell death in the white matter (Fig. 10). The relevance of these data to the loss of myelin occurring in the leukodystrophies is discussed below.
Discussion
The levels of NAA and NAAG rise in several leucodystrophies, and these agents have previously been shown to activate neuronal NMDA receptors. Our data show, for the first time, that the rise of NAAG concentration occurring in the leucodystrophies also evokes a membrane current in oligodendrocytes.
The NAAG evoked current, in both cerebellar granule cells and oligodendrocytes, was blocked by D-AP5 but not by a blocker of mGluR3 receptors (which NAAG can also activate), showing that the current is produced by the activation of NMDA receptors. However, our data show that NAAG and NAA have much less effect on the type of NMDA receptor that is expressed in oligodendrocytes than on those in granule cells, presumably because the oligodendrocyte receptors have a different subunit composition (Káradóttir et al., 2005; Salter and Fern, 2005; Micu et al., 2006). Thus, in granule cells 1 mM NAAG evokes a current which is about 3/4 the size of that evoked by 60 μM NMDA, and 1 mM NAA evokes a detectable current, while in oligodendrocytes the NAAG-evoked current is only about 7% of the NMDA-evoked current, and NAA does not evoke a detectable current (Fig. 2).
The potency of NAAG at oligodendrocyte NMDA receptors is even less than is suggested by inspection of Fig. 2, because a significant fraction of the NAAG response in oligodendrocytes is generated indirectly. A major part of the response is blocked by TTX (Fig. 5E), and so presumably reflects NAAG acting on neuronal NMDA receptors to increase neuronal firing, which then releases a substance, perhaps K+, which generates an inward current in the oligodendrocytes. Blocking carboxypeptidases also reduced the response to NAAG (Fig. 5C), implying that part of the NAAG-evoked current is generated by NAAG being converted to glutamate (Fig. 1), and this will presumably also be the case for endogenously generated NAAG.
NAAG evoked a rise of calcium concentration which was easily detectable in the grey matter but small in the white matter, as assessed with AM-loading of a Ca2+-sensing dye (Figs 6 and 7), and when individual cells were studied by loading Ca2+ dye from the patch pipette, although granule cells in the grey matter showed a robust [Ca2+]i rise mediated by NMDA receptors, oligodendrocytes did not (Fig. 8). These data suggest that the small [Ca2+]i rise detected with AM dye loading in the white matter does not originate from oligodendrocytes. Oligodendrocytes may not generate a [Ca2+]i rise because the presence of NR3 subunits in their NMDA receptors reduces their Ca2+ permeability (Chatterton et al., 2002). In addition, as described above, NAAG does not activate oligodendrocyte NMDA receptors well, and the current it generates in these cells partly reflects an indirect effect (mediated by NAAG activating NMDA receptors on neurons), which may not generate a Ca2+ influx.
Exposure for 6 h to even 1 mM NAAG (in a physiological Mg2+ concentration) did not produce significant cell death in either the grey or the white matter (Fig. 10). We note, however, that 6 h exposure to NAAG may not reflect the effect of the more prolonged pathological exposure occurring in the leucodystrophies and, in addition, NMDA receptor activating agonists can damage the myelinating processes of oligodendrocytes without killing the soma (Salter and Fern, 2005; Micu et al., 2006), which might make propidium iodide labelling an unreliable indicator of myelin loss.
In summary, we have tested the hypothesis that the elevation of NAAG and NAA concentrations that occurs in the leucodystrophies might damage oligodendrocytes by acting on oligodendrocyte NMDA receptors. Our data show that NAAG evokes an inward current both in oligodendrocytes and granule cells, along with a calcium concentration change in the latter. NAAG is a low affinity agonist at NMDA receptors, but does produce a significant current in both cell types at a pathologically relevant concentration of 200 µM (Fig. 9). However, NAAG is a much less effective agonist at oligodendrocyte NMDA receptors than at neuronal NMDA receptors, and the small current generated in oligodendrocytes by NAAG largely reflects a secondary consequence of its activation of neuronal NMDA receptors. Thus, if the elevation of NAAG concentration in the leucodystrophies causes white matter damage, this will most likely be initiated by it activating neuronal NMDA receptors and changing neuronal activity, not by it acting on oligodendrocyte NMDA receptors.
Funding
Funding for this article was provided by the Wellcome Trust, the Royal Society and the EU.
Glossary
Abbreviations:
- mGluR3
metabotropic glutamate receptor 3
- NAA
N-acetyl-aspartate
- NAAG
N-acetyl-aspartyl-glutamate
- NMDA
N-methyl-D-aspartate
- TTX
tetrodotoxin
References
- Bakiri Y, Hamilton NB, Káradóttir R, Attwell D. Testing NMDA receptor block as a therapeutic strategy for reducing ischaemic damage to CNS white matter. Glia. 2008;56:233–40. doi: 10.1002/glia.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslow MH. N-acetylaspartate and N-acetylaspartylglutamate. In: Oja SS, Schousboe A, Saransaari P, editors. Chapter 14 of Handbook of neurochemistry and molecular neurobiology: amino acids and peptides in the nervous system. 3rd. Springer, Berlin; 2007. [Google Scholar]
- Berger UV, Luthi-Carter R, Passani LA, Elkabes S, Black I, Konradi C, et al. Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. J Comp Neurol. 1999;415:52–64. doi: 10.1002/(sici)1096-9861(19991206)415:1<52::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Coyle JT, Tsai G, Greene RW. NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharmacology. 2005;30:7–16. doi: 10.1038/sj.npp.1300559. [DOI] [PubMed] [Google Scholar]
- Billups D, Attwell D. Active release of glycine or D-serine saturates the glycine site of NMDA receptors at the cerebellar mossy fibre to granule cell synapse. Eur J Neurosci. 2003;18:2975–80. doi: 10.1111/j.1460-9568.2003.02996.x. [DOI] [PubMed] [Google Scholar]
- Burlina AP, Ferrari V, Divry P, Gradowska W, Jakobs C, Bennett MJ, et al. N-acetylaspartylglutamate in Canavan disease: an adverse effector? Eur J Pediatr. 1999;158:406–9. doi: 10.1007/s004310051102. [DOI] [PubMed] [Google Scholar]
- Burlina AP, Skaper SD, Mazza MR, Ferrari V, Leon A. Burlina AB. N-acetylaspartylglutamate selectively inhibits neuronal responses to N-methyl-D-aspartic acid in vitro. J Neurochem. 1994;63:1174–7. doi: 10.1046/j.1471-4159.1994.63031174.x. [DOI] [PubMed] [Google Scholar]
- Bzdega T, Crowe SL, Ramadan ER, Sciarretta KH, Olszewski RT, Ojeifo OA, et al. The cloning and characterization of a second brain enzyme with NAAG peptidase activity. J Neurochem. 2004;89:627–35. doi: 10.1111/j.1471-4159.2004.02361.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW. Intraneuronal N-acetylaspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem. 2001;78:736–45. doi: 10.1046/j.1471-4159.2001.00456.x. [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, et al. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–8. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- D’Angelo E, Rossi P, Teglietti V. Different proportions of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptor currents at the mossy fibre-granule cell synapse of developing rat cerebellum. Neuroscience. 1993;53:121–30. doi: 10.1016/0306-4522(93)90290-v. [DOI] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982;331:577–97. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–70. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–41. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PF, Cole DC, Slusher BS, Stetz SL, Ross LE, Donzanti BA, et al. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated alpha-linked acidic dipeptidase, J Med Chem. 1996;39:619–22. doi: 10.1021/jm950801q. [DOI] [PubMed] [Google Scholar]
- Jakobs C, ten Brink HJ, Langelaar SA, Zee T, Stellaard F, Macek M, et al. Stable isotope dilution analysis of N-acetylaspartic acid in CSF, blood, urine and amniotic fluid: accurate postnatal diagnosis and the potential for prenatal diagnosis of Canavan disease. J Inherit Metab Dis. 1991;14:653–60. doi: 10.1007/BF01799929. [DOI] [PubMed] [Google Scholar]
- Jane DE, Thomas NK, Tse HW, Watkins JC. Potent antagonists at the L-AP4- and (1S,3S)-ACPD-sensitive presynaptic metabotropic glutamate receptors in the neonatal rat spinal cord. Neuropharmacology. 1996;35:1029–35. doi: 10.1016/s0028-3908(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Káradóttir R, Attwell D. Combining patch-clamping of cells in brain slices with immunocytochemical labeling to define cell type and developmental stage. Nat Protoc. 2006;1:1977–86. doi: 10.1038/nprot.2006.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–6. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káradóttir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–6. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–61. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig ML, Rothbard PM, DeCoster MA, Meyerhoff JL. N-acetyl-aspartyl-glutamate (NAAG) elicits rapid increase in intraneuronal Ca2+in vitro. Neuroreport. 1994;5:1063–8. doi: 10.1097/00001756-199405000-00012. [DOI] [PubMed] [Google Scholar]
- Kumar S, Mattan NS, de Vellis J. Canavan disease: a white matter disorder. Ment Retard Dev Disabil Res Rev. 2006;12:157–65. doi: 10.1002/mrdd.20108. [DOI] [PubMed] [Google Scholar]
- Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999;19:RC16. doi: 10.1523/JNEUROSCI.19-14-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Pliss L, FitzGibbon T, Balcar VJ, St’astný F. Neurotoxicity of NAAG in vivo is sensitive to NMDA antagonists and mGluR II ligands. Neuroreport. 2000;11:3651–4. doi: 10.1097/00001756-200011090-00050. [DOI] [PubMed] [Google Scholar]
- Rubin Y, LaPlaca MC, Smith DH, Thibault LE, Lenkinski RE. The effect of N-acetylaspartate on the intracellular free calcium concentration in NTera2-neurons. Neurosci Lett. 1995;198:209–12. doi: 10.1016/0304-3940(95)12014-u. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Sartori S, Burlina AB, Salviati L, Trevisson E, Toldo I, Laverda AM, et al. Increased level of N-acetylaspartylglutamate (NAAG) in the CSF of a patient with Pelizaeus-Merzbacher-like disease due to mutation in the GJA12 gene. Eur J Paediatr Neurol. 2008;12:348–50. doi: 10.1016/j.ejpn.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Wada K, Wenthold RJ. N-acetylaspartylglutamate acts as an agonist upon homomeric NMDA receptor (NMDAR1) expressed in Xenopus oocytes. FEBS Lett. 1992;311:285–9. doi: 10.1016/0014-5793(92)81121-2. [DOI] [PubMed] [Google Scholar]
- Tortella FC, Lin Y, Ved H, Slusher BS, Dave JR. Neuroprotection produced by the NAALADase inhibitor 2-PMPA in rat cerebellar neurons. Eur J Pharmacol. 2000;402:31–7. doi: 10.1016/s0014-2999(00)00519-7. [DOI] [PubMed] [Google Scholar]
- Valivullah HM, Lancaster J, Sweetnam PM, Neale JH. Interactions between N-acetylaspartylglutamate and AMPA, kainate, and NMDA binding sites. J Neurochem. 1994;63:1714–9. doi: 10.1046/j.1471-4159.1994.63051714.x. [DOI] [PubMed] [Google Scholar]
- Westbrook GL, Mayer ML, Namboodiri MA, Neale JH. High concentrations of N-acetylaspartylglutamate (NAAG) selectively activate NMDA receptors on mouse spinal cord neurons in cell culture. J Neurosci. 1986;6:3385–92. doi: 10.1523/JNEUROSCI.06-11-03385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers RA, Engelke U, Wendel U, de Jong JG, Gabreëls FJ, Heerschap A. Standardized method for high-resolution 1H-NMR of cerebrospinal fluid. Clin Chem. 1995;41:744–51. [PubMed] [Google Scholar]
- Wolf NI, Willemsen MA, Engelke UF, van der Knaap MS, Pouwels PJ, Harting I, et al. Severe hypomyelination associated with increased levels of N-acetylaspartylglutamate in CSF. Neurology. 2004;62:1503–8. doi: 10.1212/01.wnl.0000123094.13406.20. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Santi MR, Neale JH. N-acetylaspartylglutamate activates cyclic AMP-coupled metabotropic glutamate receptors in cerebellar astrocytes. Glia. 1998;24:172–9. doi: 10.1002/(sici)1098-1136(199810)24:2<172::aid-glia2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–81. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]