Abstract
Sporadic inclusion-body myositis (sIBM) is the most common disabling, adult-onset, inflammatory myopathy histologically characterized by intense inflammation and vacuolar degeneration. In spite of T cell-mediated cytotoxicity and persistent, clonally expanded and antigen-driven endomysial T cells, the disease is resistant to immunotherapies. Alemtuzumab is a humanized monoclonal antibody that causes an immediate depletion or severe reduction of peripheral blood lymphocytes, lasting at least 6 months. We designed a proof-of-principle study to examine if one series of Alemtuzumab infusions in sIBM patients depletes not only peripheral blood lymphocytes but also endomysial T cells and alters the natural course of the disease. Thirteen sIBM patients with established 12-month natural history data received 0.3 mg/kg/day Alemtuzumab for 4 days. The study was powered to capture ≥10% increase strength 6 months after treatment. The primary end-point was disease stabilization compared to natural history, assessed by bi-monthly Quantitative Muscle Strength Testing and Medical Research Council strength measurements. Lymphocytes and T cell subsets were monitored concurrently in the blood and the repeated muscle biopsies. Alterations in the mRNA expression of inflammatory, stressor and degeneration-associated molecules were examined in the repeated biopsies. During a 12-month observation period, the patients’ total strength had declined by a mean of 14.9% based on Quantitative Muscle Strength Testing. Six months after therapy, the overall decline was only 1.9% (P < 0.002), corresponding to a 13% differential gain. Among those patients, four improved by a mean of 10% and six reported improved performance of daily activities. The benefit was more evident by the Medical Research Council scales, which demonstrated a decline in the total scores by 13.8% during the observation period but an improvement by 11.4% (P < 0.001) after 6 months, reaching the level of strength recorded 12 months earlier. Depletion of peripheral blood lymphocytes, including the naive and memory CD8+ cells, was noted 2 weeks after treatment and persisted up to 6 months. The effector CD45RA+CD62L cells, however, started to increase 2 months after therapy and peaked by the 4th month. Repeated muscle biopsies showed reduction of CD3 lymphocytes by a mean of 50% (P < 0.008), most prominent in the improved patients, and reduced mRNA expression of stressor molecules Fas, Mip-1a and αB-crystallin; the mRNA of desmin, a regeneration-associated molecule, increased. This proof-of-principle study provides insights into the pathogenesis of inclusion-body myositis and concludes that in sIBM one series of Alemtuzumab infusions can slow down disease progression up to 6 months, improve the strength of some patients, and reduce endomysial inflammation and stressor molecules. These encouraging results, the first in sIBM, warrant a future study with repeated infusions (Clinical Trials. Gov NCT00079768).
Keywords: Alemtuxumab, IBM, muscle inflammation, muscle degeneration, lymphocyte depletion, endomysial inflammation, stressor molecules
Introduction
Sporadic inclusion-body myositis (sIBM), the most common muscle disease in patients above the age of 50, is a relentlessly progressive disorder that ultimately results in severely restricted mobility, dysphagia or even death (Dalakas, 1991, 2004; Mikol and Engel, 2004; Askanas and Engel, 2006; Needham and Mastaglia, 2007). A combination of autoimmune and degenerative features plays a role in the disease pathogenesis. The immunopathological hallmarks of sIBM include prominent endomysial inflammation characterized by a T cell-mediated and MHC-I-restricted cytotoxicity; clonal expansion of the autoinvasive CD8+ T cells and B cells and upregulation of cytokines, chemokines, and of co-stimulatory molecules. The degenerative features consist of vacuolization in fibres not invaded by T cells and intracellular deposits of amyloid and related proteins. In spite of the antigen-driven T cell response and the strong immunopathology, immunotherapies have been either unsuccessful or of minimal benefit.
We have hypothesized that strategies targeting T cells may be of clinical benefit in sIBM if they also have the potential to deplete or reduce some of the endomysial T cells responsible for the T cell-mediated muscle fibre injury. Alemtuzumab (Campath®, Campath-1H, or MabCAMPATH) is a recombinant DNA-derived humanized monoclonal antibody directed against CD52, a 21–28 KD cell surface glycoprotein, predominantly expressed on the surface of mature T lymphocytes and monocytes (Hale and Waldmann, 2000). With conventional doses, neutrophils are not affected, hence the reasonably good safety record of the drug (Hale et al., 1988; Hale and Waldmann, 2000). Alemtuzumab, approved for the treatment of T cell leukemias, induces a profound and persisting—over 6 months—dose-dependent lymphocyte depletion (Hale et al., 1988). Monocytes and macrophages are also depleted, but they return after 1–2 weeks due to redistribution (Armstrong et al., 1998; Kirk et al., 2003). Among non-oncologic applications, Alemtuzumab is being explored in rheumatoid arthritis, vasculitis, multiple sclerosis and organ transplantation, with very promising results (Weinblatt et al., 1995; Flynn and Byrd, 2000; Kirk et al., 2003; Reiff, 2005; Coles et al., 2006). Recently, a controlled study demonstrated efficacy in relapsing–remitting multiple sclerosis (The CAMMS233 Trial Investigation, 2008).
The profound T cell depletion achieved with Alemtuzumab in the periphery and lymphoid tissues, prompted us to examine whether it also reduces endomysial T cells. Because B cells are also affected, it may exert an additional benefit considering the emerging role of these cells in sIBM. If in sIBM the endomysial T cells are myotoxic, as proposed (Dalakas, 2006; Mikol and Engel, 2007), a reduction of these cells after 4–6 months is expected to result in some clinical benefit. Accordingly, in this proof-of-principle study we examined the effect of Alemtuzumab in endomysial T cells and disease progression.
Study Design and Methods
Patient selection
Patients with sIBM were selected from a pool of patients participating in a natural history longitudinal study. These patients had been followed in the outpatient clinic of the NIH Clinical Centre with serial quantitative strength measurements every 6 months for up to 12–18 months, prior to enrolment in the CAMPATH study. All enrolled patients were ambulatory and had not been taking any immunomodulating or immunosuppressive therapies for at least a year. Patients were excluded if they had malignancy, coagulopathy, low-platelet count, anaemia, cardiac insufficiency or thyroiditis.
The patients’ mean age was 60 (range 55–80), and the mean disease duration from the time of diagnosis was 10 years (range 5–15). Thirteen patients were chosen (four female, nine male) according to the order of enrolment in the natural history protocol. The diagnosis was made on the basis of the typical clinical and histopathological features, determined by a muscle biopsy performed at NIH at the time of enrolment in the longitudinal study (baseline biopsy). Prior to starting CAMPATH, all patients had a second biopsy (pre-CAMPATH biopsy) to assess changes in the endomysial T cells that might have occurred during the natural history period, and examine the direct effect of CAMPATH on the endomysial T cells based on a third (post-CAMPATH) biopsy, as described below.
Study drug
All patients were admitted to the inpatient unit of the Clinical Centre, NIH, under an IRB-approved protocol and after signing informed consent. This was an investigator-initiated study, funded by the NINDS and the Clinical Center under a ‘bench-to-bedside’ competitive award to the principal investigator (M.C.D.). The study began after an IND granted by the FDA to M.C.D. Infusions were conducted under telemetry monitoring. Alemtuzumab, provided by the NIH pharmacy, was administered by intravenous drip at a continuous rate of 0.1 mg/kg/h or over a 4-h period. The infusions were repeated every other day for a total of four doses at 0.3 mg/kg/day, not exceeding 30 mg/day. Patients were pre-medicated with IV methylprednisolone 250 mg 2 h prior to dose 1, 125 mg 2 h prior to dose 2 and 60 mg 2 h prior to doses three and four and with oral diphenhydramine 50 mg and acetaminophen 650 mg prior to each infusion. In addition, they received trimethoprim/sulfamethoxazole DS (double strength) daily three times per week, clotrimazole troche 10 mg BID, and valganciclovir 450 mg once daily upon initiation of treatment and up to 6 months thereafter or until the absolute lymphocyte count (ALC) was >500 cells/µl, whichever occurred later. Total blood counts, liver enzymes and lymphocyte subsets were monitored daily for the first 10 days and weekly thereafter until CD4+ counts were equal or >200 cells/µl or the ALC had exceeded 500. Afterwards, lymphocyte repopulation was assessed monthly.
Assessment of efficacy
Quantitative Muscle Strength Testing (QMT), which provides a total summed score of strength in kilograms was performed to measure maximum voluntary isometric muscle contractions (MVIC) for grip, wrist and elbow flexion and extension, shoulder abduction, knee extension, hip flexion, and ankle dorsiflexion. The average of two MVIC trials for each muscle group was analysed. Interrater and intrarater reliability was assessed in 10 healthy volunteers with excellent reliability based on intraclass correlation coefficients of 0.95 and 0.93, respectively. In addition, manual muscle testing (MMT) was administered by the same neurologist using the modified Medical Research Council (MRC) scale, a validated 0–10 scale with excellent interrrater reliability, to record the strength of over 30 muscle groups bilaterally in arms and legs. All patients were tested every 6 months for at least 12 months to obtain natural history data prior to entering the trial. For the CAMPATH study, muscle strength was assessed at baseline (pre-treatment) and at 2, 4, 6, 8, 10 and 12 months by the same assessors who had also obtained the QMT and MRC scores during the natural history period. Changes in the ability to perform fundamental activities of daily living were assessed with a brief questionnaire, which allowed for the subjective reporting of changes after 6 months of therapy. The following questions were asked: (i) did any of your symptoms improve? If so, when did you notice the improvement and how long did it last?; (ii) what specific tasks were you able to do after the infusions that you could not do before?; (iii) did you consider your clinical benefit sufficient that you would like to be re-treated?; (iv) on the basis of a subjective 0–10 scale, how did you rate your overall ability to perform daily tasks prior to therapy, 6 months after therapy and 12 months later?; and (v) did you notice any side-effects?
Muscle biopsies
The patients underwent two open muscle biopsies, one prior to Alemtuzumab (pre-CAMPATH) and another 6 months later (post-CAMPATH) from the same extremity. All patients also had a prior biopsy by us (baseline biopsy) performed at the time of enrolment in the longitudinal study, 12–18 months prior to entering the trial. All three biopsies were obtained under separate consents and were processed for muscle enzyme histochemistry, immunocytochemistry and molecular studies. The lymphocytic infiltrates in all specimens were quantified as in our previous trial (Dalakas et al., 2001). In brief, the total number of endomysial T cells immunostained with monoclonal antibodies against CD3+ and CD8+ were counted. The total number of CD3+ and CD8+ cells and the total number of fibres were counted blindly in three randomly selected fields at 20×. The ratio of CD3+ and CD8+ cells per fibre was calculated and compared between pre- and post-treatment specimens by applying the two-sided sample t-test. Lymphocyte count was also performed on the baseline biopsy and compared with the pre- and post-CAMPATH biopsies. Double immunohistochemical staining was also performed for CD8 and CXCL-9 as previously described (Schmidt et al., 2008).
Immunologic studies in peripheral blood lymphocytes and muscle
Lymphocyte subsets (CD3, 4, 8, 16, 20, CD45RO, CD45RA, CD52, CD80, CD86, CD154, CD19), utilizing specific monoclonal antibodies, were determined in the peripheral blood lymphocytes with the flow cytometry before the infusion, and every 2 months thereafter, as previously described (Pearl, 2005). Briefly, polychromatic flow cytometry was used to ask whether post-depletional T cells (CD3+) were predominantly CD4+ or CD8+, naïve (CD45RA+CD62L+); one of three phenotypes associated with functional memory cells: CD45RA-CD62L (effector), CD45RA-CD62L+ (central) or CD45RA+CD62L-(RA+) memory; or memory cells with potential regulatory function (CD45RA-CD4+CD25+). To determine these phenotypes, clinical whole blood samples were stained with the following Mab conjugates: CD3 Cascade Blue, CD4 Cy5.5 Phycoerythrin (PE), CD8 Texas Red PE, CD11a Cy7 allophycocyanin (APC), CD14 Cy7PE, CD25 PE, CD45RA Cy5PE, CD45RO Cy5.5APC, CD62L fluorescein isothiocyanate (FITC), CD56 APC, and CCR7 PE. Purified antibodies to the cell-surface markers above were obtained from BD/Pharmingen (Franklin Lakes, NJ, USA) and conjugated to the indicated fluorochromes using standard protocols (http://drmr.com/abcon). Cascade blue, FITC, and Texas Red PE were obtained from Molecular Probes (Eugene, OR, USA). Phycoerythrin and APC were obtained from ProZyme (San Leandro, CA, USA). Cy5, Cy5.5, and Cy7 were obtained from Amersham Life Sciences (Pittsburgh, PA, USA). Compensation was set using the fluorescence minus one method for all fluorochromes. Data were collected on a modified FACSDiVa (Becton–Dickinson, Franklin Lakes, NJ, USA) or a modified LSRII (Beckton–Dickinson) and analysed with FlowJo software (Tree Star, San Carlos, CA, USA).
Quantitative immunocytochemistry and PCR methodology were applied in the muscle biopsy specimens before and after therapy to determine changes in the mRNA of the following cells and inflammatory mediators: CD3, CD8,CD68, CD28, ICOS, ICOS-L, CD52,CD80,IL1β, TGF-β,IFN-γ, TNF-α, IL6, CXCL9, CCL4,CCL3 and MHC-I. In brief, quantitative (real-time) PCR was performed on an Opticon 2 DNA engine (MJ research/Applied Biosystems), using specific primers with 6-carboxy-fluorescein (FAM)-labelled probes (Applied Biosystems, Foster City, CA, USA) and following the standard cycle protocol and instructions given by the supplier. Target mRNA-expression was quantified using the Δc(t) method in relation to expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as housekeeping gene. These inflammatory markers were correlated with the mRNA of amyloid and degeneration/regeneration-associated molecules including APP, αB crystalin, NCAM, desmin and Ubiquitin, as previously described (Schmidt et al., 2008).
Sample size, outcome measures and power analysis
Clinical changes were evaluated on the basis of the total muscle strength scores obtained at baseline (pre-treatment) to scores obtained after treatment, as determined at 2-month intervals. Assessment of clinical improvement was based on the difference in the total muscle strength scores from three sequential time periods; 12 months natural history (pre-treatment, baseline, period), 6 months after CAMPATH (treatment period) and 6 months without treatment after completion of CAMPATH (post-treatment period). The percentage of change in the summed muscle strength scores obtained from each of the three periods was compared using a repeated measures analysis of variance (ANOVA). The ANOVA results were used to determine how the treatment periods differed from the baseline measurements. All data were checked for sphericity assumptions and Tukey's Least Significant Difference procedure was used for the post hoc analyses. A change in the muscle strength at 6 months by ≥10% was considered an improvement. Based on our previous assessments of muscle strength in inclusion-body myositis patients (Dalakas et al., 1997, 2001) and a 0.12 (or 12%) standard deviation of difference, a power analysis of a two-sided paired t-test, 13 patients were needed to provide 80% power to detect a change by 10% at 6 months (test significance level, α 0.050 with a two-sided t-test). Power analysis was not performed for detecting changes in disease progression, as the percentage of strength decline was not known at the time the protocol began.
Results
Change in muscle strength
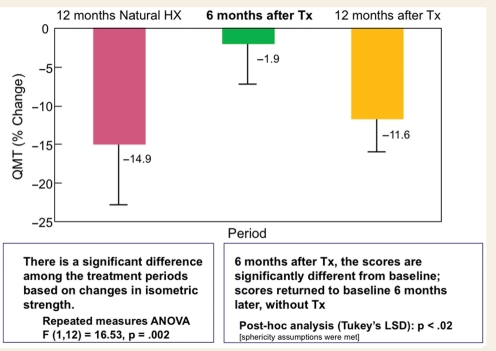
The summed total strength measured by QMT in all patients declined by a mean of 14.9% during a 12-month observational period that immediately preceded the inclusion in the CAMPATH trial (Fig. 1). In contrast, after 6 months of CAMPATH, the total strength score had declined only by 1.9% from baseline corresponding to a mean of 13% differential gain. Among those patients, four (31%) had a mean strength gain by 10% (4–19%), which translates to a mean of 10 kg added force. Among all patients, bilateral wrist flexion and extension accounted for the four largest percent increases of >25% each. The right quadriceps improved by 14.4% for all patients while the left and right hip flexion improved by 3.4 and 5.6%, respectively. Follow-up evaluations at month 12 (6 additional months after the primary end-point, and without further treatment), showed a decline in strength by a mean of 11.6% (P < 0.01) indicating that the reversal of disease progression, observed during the first 6 months of therapy, could not be maintained without re-treatment (Fig. 1).
Figure 1.
Changes in muscle strength using QMT. During a 12-month natural history period there is a decline in muscle strength based on QMT measurements by a mean of −14.9%. After 6 months of CAMPATH treatment the mean total muscle strength scores changed by a −1.9% from baseline (P = 0.002). At month 12 from CAMPATH initiation (6 months of follow-up without therapy), the patients’ strength had declined by a mean of −9.7% (P < 0.01) reaching almost the baseline.
The QMT measurements were reinforced with the MRC data. A mean of 13.8% decline in the strength of all patients was noted during a 24 month natural history period (P < 0.001) and a 9.1% decline during the 12-month period before therapy (P < 0.09). After 6 months of CAMPATH, there was a reversal in the disease decline, as the patients had gained a mean of 11.4% of their strength (P = 0.001), reaching almost the level of strength they had exhibited 12-18 months earlier (Fig. 2).
Figure 2.
Total muscle strength scores obtained with MRC measurements before and after CAMPATH. A mean of 13.8% decline was noted during a 24-month natural history period and 9.1% decline during a 12-month period. Six months after CAMPATH, the total strength improved be a mean of 11.4% (P = 0.0001) reaching almost the level of strength the patients had 2.5 years earlier.
Functional significance of increased strength in the improved patients
The noted strength changes were of functional importance to the daily activities of some of the patients when asked to judge the clinical significance of their benefit. Five patients reported absolutely no benefit, three experienced a moderate but clear effect; and five had a definite improvement, requesting another infusion. The improved patients experienced the benefit 2 months after treatment, for up to 5–8 months and reported an increased ability to perform the following: walk further distances with better balance and less falls; get up from a chair; step up into the car or go up steps; button shirts or use utensils; and lift heavier objects (one patient who was exercising regularly, became able to lift more weights and increased his repetitions).
Lymphocyte depletion in peripheral blood lymphocytes and muscle
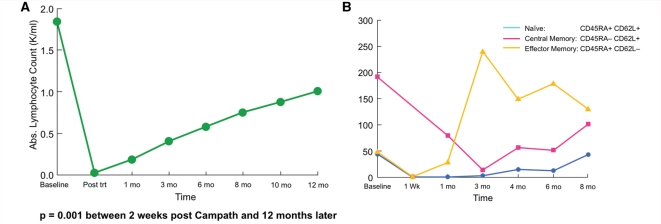
Peripheral blood lymphocytes of all phenotypes were markedly reduced immediately after treatment. Repopulation began slowly thereafter and by the 6th month (the study's primary efficacy endpoint and the time a repeated muscle biopsy was performed) the ALC was 30% of the baseline level; by month 12, the ALC had reached the 50% level (Table 1 and Fig. 3A). Depletion occurred, and repopulation proceeded, in an inhomogeneous fashion with respect to the surface phenotype for both CD4 and CD8+ T cells. As reported (Pearl et al., 2005), naïve cells were more effectively depleted compared to antigen-experienced cells, particularly those of the effector memory subset (Table 2 and Fig. 3B for the kinetics of a representative patient). The peripheral population became predominantly memory phenotype and remained largely comprised of effector and central memory cells throughout the study period, although some regeneration of naïve cells was evident within 3 months and later thereafter (Table 2 and Fig. 3B).
Table 1.
Absolute lymphocyte count in the peripheral blood before and 12 months after CAMPATH
| Patient # | Baseline | Post-Campath | 1 month | 3 months | 6 months | 8 months | 10 months | 12 months |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.897 | 0.012 | 0.08 | 0.248 | 0.271 | 0.433 | 0.693 | 0.705 |
| 2 | 1.285 | 0.058 | 0.213 | 0.349 | 0.661 | 0.595 | 0.798 | 0.867 |
| 3 | 1.206 | 0.071 | 0.564 | 0.688 | 0.938 | 0.729 | 1.067 | 1.183 |
| 4 | 1.528 | 0.026 | 0.101 | 0.39 | 0.458 | 0.456 | 0.369 | 0.653 |
| 5 | 1.341 | 0.004 | 0.145 | 0.178 | 0.339 | 0.442 | 0.358 | 0.617 |
| 6 | 2.933 | 0.062 | 0.268 | 0.412 | 0.621 | 1.658 | 2.118 | 2.284 |
| 7 | 1.132 | 0.033 | 0.381 | 0.543 | 0.59 | 0.685 | 0.519 | 0.599 |
| 8 | 2.323 | 0.03 | 0.187 | 0.591 | 0.706 | 0.76 | 0.8 | 1.005 |
| 9 | 2.532 | 0 | 0.097 | 0.29 | 1.126 | 1.115 | 1.072 | 1.308 |
| 10 | 1.622 | 0 | 0.053 | 0.383 | 0.379 | 0.534 | 0.666 | 0.724 |
| 11 | 2.076 | 0 | 0.231 | 0.381 | 0.46 | 0.621 | 0.799 | 0.882 |
| 12 | 1.612 | 0 | 0.059 | 0.563 | 0.607 | 0.725 | 0.936 | 1.043 |
| 13 | 2.576 | 0 | 0.032 | 0.351 | 0.407 | 1.049 | 1.284 | 1.352 |
| Mean | 1.851 | 0.02 | 0.185 | 0.412 | 0.581 | 0.754 | 0.883 | 1.017 |
Figure 3.
Changes in peripheral blood lymphocytes after CAMPATH. (A) A reduction of total peripheral blood lymphocytes immediately after CAMPATH was noted with a steady repopulation (occurring slowly up to 12 months) in all patients. (B) The kinetics of CD8 + T cells subsets after CAMPATH, including changes in the memory T cells, is shown for a representative patient. A depletion of naïve CD4+ and CD8+ T cells, compared to effector memory cells, was noted in nine patients (shown in Table 2).
Table 2.
Longitudinal changes (±SD) up to 6 months from baseline, in the percentage of naïve and memory T cells in nine patients treated with CAMPATH
| Base | 1 Month | 3 Months | 6 Months | |
|---|---|---|---|---|
| CD4 | ||||
| Naïve (CD45RA+ CD62L+) | 23.7 ± 14.7 | 0.9 ± 1.8 | 8.2 ± 8.7 | 7.6 ± 9.4 |
| Central memory (CD45RA− Cd62+) | 44.4 ± 12.9 | 46.0 ± 26.1 | 45.4 ± 14.4 | 47.6 ± 13.7 |
| Effector memory (CD45RA− CD62−) | 28.1 ± 17.6 | 37.1 ± 23.4 | 45.3 ± 17 | 43.4 ± 16.3 |
| RA+ effector memory (CD45RA+ CD62−) | 4.5 ± 4.8 | 6.2 ± 13.3 | 7.4 ± 17.1 | 3.3 ± 5.4 |
| CD8 | ||||
| Naïve (CD45RA+ CD62L+) | 26.8 ± 17.4 | 0.9 ± 1.6 | 8.2 ± 12.3 | 7.6 ± 27.4 |
| Central memory (CD45RA− Cd62+) | 14.7 ± 14.2 | 17.6 ± 28 | 10.3 ± 8.8 | 17 ± 4.3 |
| Effector memory (CD45RA− CD62−) | 29.6 ± 14.8 | 36.8 ± 20 | 46.4 ± 21 | 37.9 ± 23 |
| RA+ Effector memory (CD45RA+ CD62−) | 28.9 ± 23.9 | 31.6 ± 24.2 | 34.6 ± 25.1 | 24.3 ± 15.4 |
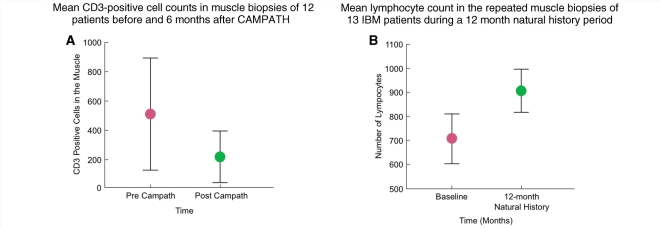
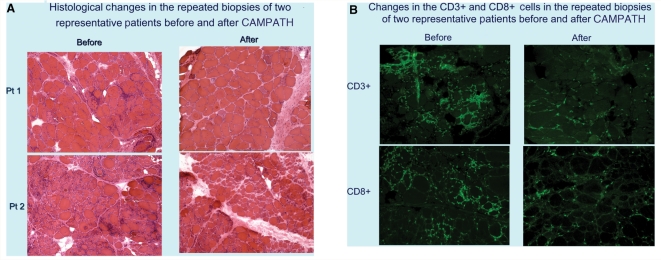
In the repeated biopsies, there was an overall reduction in the lymphocytes and an improvement in the muscle cytoarchitecture. Quantification of the lymphocytes, in all biopsies before and after CAMPATH, demonstrated a significant decline in the total number of CD3+ cells (P = 0.008; Fig. 4A). This was in contrast to the mean number of endomysial lymphocytes during the 12-month natural history period that had increased, from 708 ± 350 to 907 ± 313 (Fig. 4B). In four of the five patients who improved, the repeated muscle biopsies showed a >50% depletion of the CD3+ T cells. The reduction of endomysial T cells and improved muscle cytoarchitecture are depicted in the samples of two representative patients before and after therapy (Fig. 5A–B).
Figure 4.
Quantification of T cells in all biopsies showed a significant (P < 0.008) decline in the total number of CD8+ T cells (A). In contrast, during the natural history period prior to CAMPATH, the total lymphocyte count had remained unchanged or increased (B).
Figure 5.
(A) Representative muscle biopsy samples performed before and 6 months after treatment (stained with H&E) demonstrate reduction of lymphocytic infiltrates. With immunocytochemistry (B), a reduction of CD3- and CD8-positive lymphocytes was noted.
Effect of CAMPATH on mRNA expression of inflammatory mediators and degeneration-associated molecules in the repeated muscle biopsies
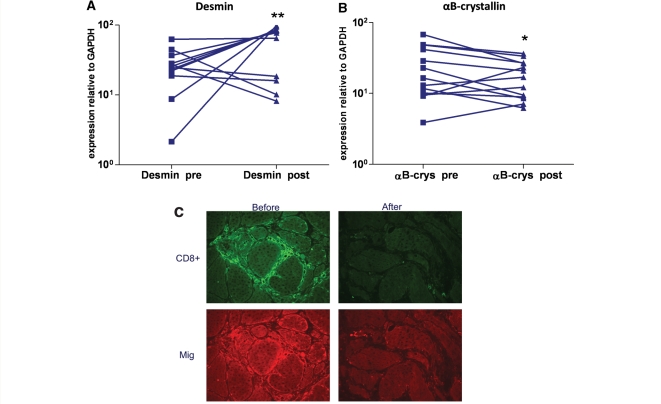
The mRNA of Fas, Mip-1α, and αB crystallin associated with degeneration or cell stress of myofibres, was significantly reduced after treatment both at the mRNA and protein level (Fig. 6B). In contrast, the mRNA of desmin, associated with regeneration, was statistically increased after treatment (Fig. 6A). No significant changes were noted in the mRNA expression for ILβ, IL-6, CD28, CD40, CD52, CD86, CD15, Granzyme B, MCP-1, Mip-3β, μig, MMP-2 and MMP-9, APP and ubiquitin. Protein expression of the chemokine CXCL-9 was clearly reduced in some patients as indicated by the double immunohistochemical staining for CXCL9 and CD8 (Fig. 6C).
Figure 6.
Quantitative mRNA expression of desmin (A) and aB crystalline (B) in the patients’ repeated muscle biopsies before and 6 months after CAMPATH. There is a significant increase of desmin and reduction of αB-crystallin. A significant decline of mRNA expression of Mip-1α (CCL-3) was also noted (not shown); at the protein level, CXCL9 was reduced in some patients, as observed in double immunohistochemical staining for CXCL-9 and CD8 (C). *P < 0.05; **P < 0.01.
Safety and tolerance
The drug was well tolerated. No secondary infections, thyroiditis or idiopathic thrombocytopenic purpura (ITP) were observed up to 2 years after treatment. Infusion-related events were observed in three patients manifested as transient hypotension. The only complication—not directly related to the drug—was thrombosis of the subclavian vein in one patient caused by the PIC line.
Discussion
This proof-of-principle study showed that a long-lasting reduction of peripheral T cells, as caused by CAMPATH, results in reduction of endomysial T cells and has the potential to arrest disease progression or improve strength of some patients with inclusion-body myositis (IBM). The study, designed to capture changes in the natural history of IBM, supports the view that aggressive immunotherapy may slowdown the course of the disease, at least for some patients, and opens the opportunity to explore long-term benefits with follow-up infusions.
In spite of the association of IBM with other autoimmune disorders and viruses and the prominent clonal expansion of endomysial cytotoxic T cells (Koffman et al., 1998; Muntzing et al., 2003; Badrising et al., 2004; Dalakas, 2006; Salajegheh et al., 2007; Chanin and Engel, 2008), conventional immunotherapies, including IVIg (Dalakas et al., 1997, 2001), have failed to provide significant benefits. The resistance of IBM to most immunotherapies has generated dilemmas about its pathogenesis, prompting the suggestion that it might be a primary neurodegenerative disorder (Askanas and Engel, 2006). The present study, powered to detect a >10% change in strength, demonstrated improvement in some patient's strength and a slowdown in the overall disease progression based on the natural history data, for up to 6 months. Although the study was unblinded, the results are unbiased because: (i) the evaluators performing the QMT examinations were not participating in the day-to-day activity of the patients; (ii) the data were obtained simultaneously with the other patients participating in the natural history study that involved a large cohort of IBM patients; (iii) the QMT data were collected by the same evaluators who had performed the natural history study in the same patients; (iv) the MRC measurements, which usually display less variability than the QMT scales, independently provided convincing support for a more prominent stability or improvement; (v) IBM has not improved with any intervention in a 6-month period and, as shown from the natural history, it has a steady decline; and (vi) the gains in strength lasted ∼6 months, coinciding with the effect of CAMPATH on T cell depletion, reverting thereafter almost to baseline level. Most importantly, the noted strength changes in the improved patients were meaningful to their daily activities, although some placebo effect cannot be excluded.
The study was designed to examine the relationship between T lymphocyte depletion and clinical response up to 6 months after treatment. It appears that the duration of the beneficial effect correlated with the re-emergence of peripheral CD8+ Memory T cells. Although the naïve T cells remained suppressed after 6 months, the memory T cells were less affected and may be responsible for the persistent inflammatory response in some patients. Follow-up infusions therefore, after 4–6 months, may be needed to sustain or enhance the clinical benefit, as commonly applied to other autoimmune diseases. The incomplete elimination of antigen-experienced cells may have accounted for the lack of clinical response in some of the patients but it might have contributed to the overall good safety profile of the applied intervention. CAMPATH was also effective in reducing the endomysial T cells, as determined from the repeated biopsies. Although there may be some variability in the degree of inflammation from muscle-to-muscle, our data are consistent with a drug-related reduction because: (i) the same muscle was biopsied twice; (ii) the assessment was based on counting the mean number of endomysial lymphocytes in a large number of biopsies; and (iii) the reduction was significant when compared to the natural history biopsies performed on the same patients’ opposite arm 12–18 months before CAMPATH. The observation, that the most prominent T cell reduction was observed in the muscles of those five patients who had the most notable clinical benefit, suggests that in IBM the T cells are related to the immunopathogenesis of the disease and play a central role in tissue destruction. The concomitant improvement in the histopathology along with the downregulation of the cell-stressor molecule αB crystallin, previously noted to be enhanced in sIBM muscles (Banwell and Engel, 2005; Schmidt et al., 2008), supports the view that in IBM the T cell-mediated cytotoxicity is involved in inducing cell-stress to the myofibres (Nagaraju et al., 2005; Dalakas, 2006).
Inclusion-body myositis remains a complex disorder and immune mechanisms may not be the only culprit in the disease mechanisms. Whether the vacuolar degeneration is a consequence of the chronic inflammation related to the long-lasting cytokine upregulation and the MHC-I-induced cell stress, as proposed (Nagaraju et al., 2005; Dalakas, 2006), or it is an independent factor, remains unclear. Although a direct interrelationship between inflammation and degeneration has been demonstrated in sIBM muscles (Nagaraju et al., 2005; Schmidt et al., 2008), we could not find any significant changes in the correlation between the degeneration-associated molecules, such as β-amyloid or ubiquitin, with inflammatory mediators in the repeated biopsies, probably because the period of therapy was too short to capture any appreciable change at the mRNA level. IBM is a chronic disease and any effect on degeneration markers may require long-term therapy.
The drug, as given, was well tolerated without any immediate or long-term side-effects, such as thyroiditis or ITP, as reported in other trials (the CAMMS233 Trial, 2008). Although the sample was small, it was powered to capture changes in muscle strength even in a 6-month period owing to the availability of natural history data in the same patients. Even if the improvement was arguably modest, no other agent has shown any similar effect in the decline of the disease course. Some benefits noted with IVIg or with anti-thymocyte globulin were marginal and short-lived (Dalakas et al., 1997; Lindberg et al., 2003). Because IBM is predictably disabling and notoriously resistant to therapies, the encouraging results from this pilot study and the good tolerance of the drug, provide the impetus to consider a large placebo-controlled trial with repeated infusions to assess the long-term benefits keeping in mind the safety concerns raised in the recent multiple sclerosis trial (the CAMMS233 Trial, 2008).
Funding
Intramural funding from National Institutes of Health.
Acknowledgements
The study has been also awarded the Bench-to-Bedside Award by the NIH Clinical Center to Prof. Marinos Dalakas. We thank Konstanze Barthel for help with data analysis of PCR and IHC.
Glossary
Abbreviations:
- ALC
absolute lymphocyte count
- MRC
Medical Research Council
- QMT
Quantitative Muscle Strength Testing
- sIBM
sporadic inclusion-body myositis
References
- Armstrong N, Buckley P, Oberley T, Fechner J, Jr, Dong Y, Hong X, et al. Analysis of primate renal allografts following T cell depletion with anti-CD3-CRM9. Transplantation. 1998;66:5–13. doi: 10.1097/00007890-199807150-00002. [DOI] [PubMed] [Google Scholar]
- Askanas V, Engel WK. Inclusion-body myositis: a myodegenerative conformational disorder associated with Abeta, protein misfolding, and proteasome inhibition. Neurology. 2006;66(2 Suppl 1):S39–48. doi: 10.1212/01.wnl.0000192128.13875.1e. [DOI] [PubMed] [Google Scholar]
- Badrising UA, Schreuder GM, Giphart MJ, Geleijns K, Verschuuren JJ, Wintzen AR, et al. Associations with autoimmune disorders and HLA class I and II antigens in inclusion body myositis. Neurology. 2004;63:2396–8. doi: 10.1212/01.wnl.0000148588.15052.4c. [DOI] [PubMed] [Google Scholar]
- Banwell BL, Engel AG. AlphaB-crystallin immunolocalization yields new insights into inclusion body myositis. Neurology. 2000;54:1033–41. doi: 10.1212/wnl.54.5.1033. [DOI] [PubMed] [Google Scholar]
- Chahin N, Engel AG. Correlation of muscle biopsy, clinical course, and outcome in PM and sporadic IBM. Neurology. 2008;70:418–24. doi: 10.1212/01.wnl.0000277527.69388.fe. [DOI] [PubMed] [Google Scholar]
- Coles AJ, Lox A, Le Page, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence for monoclonal antibody therapy. J Neurol. 2006;253:98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991;325:1487–98. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Inflammatory disorders of muscle: progress in polymyositis, dermatomyositis and inclusion body myositis. Curr Opin Neurol. 2004;17:561–7. doi: 10.1097/00019052-200410000-00006. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Sporadic inclusion body myositis-diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol. 2006;2:437–47. doi: 10.1038/ncpneuro0261. [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Sonies B, Dambrosia J, Sekul E, Cupler E, Sivakumar K. Treatment of inclusion body myositis with IVIg: a double-blind, placebo-control study. Neurology. 1997;48:712–6. doi: 10.1212/wnl.48.3.712. [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Koffman B, Fujii M, Spector S, Sivakumar K, Cupler E. A controlled study of intravenous immunoglobulin combined with prednisone in the treatment of IBM. Neurology. 2001;56:323–7. doi: 10.1212/wnl.56.3.323. [DOI] [PubMed] [Google Scholar]
- Flynn JM, Byrd JC. Campath-1H monoclonal antibody therapy. Curr Opin Oncol. 2000;12:574–81. doi: 10.1097/00001622-200011000-00010. [DOI] [PubMed] [Google Scholar]
- Hale G, Waldmann H. From laboratory to clinic: the story of CAMPATH-1. Methods Mol Med. 2000;40:243–66. doi: 10.1385/1-59259-076-4:243. [DOI] [PubMed] [Google Scholar]
- Hale G, Dyer MJS, Clark MR, Phillips JM, Marcus R, Riechmann L, et al. Remission induction in non-Hodgkin lymphoma with reshaped human monoclonal antibody CAMPATH-1H. Lancet. 1988;2:1394–9. doi: 10.1016/s0140-6736(88)90588-0. [DOI] [PubMed] [Google Scholar]
- Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody Campath-1H. Transplantation. 2003;76:120–9. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- Koffman BM, Rugiero M, Dalakas MC. Immune-mediated conditions and antibodies associated with sporadic inclusion body myositis. Muscle Nerve. 1998;21:115–7. doi: 10.1002/(sici)1097-4598(199801)21:1<115::aid-mus15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Lindberg C, Trysberg E, Tarkowski A, Oldfors A. Anti-T-lymphocyte globulin treatment in inclusion body myositis: a randomized pilot study. Neurology. 2003;61:260–2. doi: 10.1212/01.wnl.0000071852.27182.c7. [DOI] [PubMed] [Google Scholar]
- Needham M, Mastaglia FL. Inclusion body myositis: current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet Neurol. 2007:620–31. doi: 10.1016/S1474-4422(07)70171-0. [DOI] [PubMed] [Google Scholar]
- Mikol J, Engel AG. Inclusion body myositis. In: Engel AG, Franzini-Armstrong C, editors. Myology. 3rd. New York, NY: McGraw-Hill Book Co.; 2004. pp. 1367–88. [Google Scholar]
- Muntzing K, Lindberg C, Moslemi AR, Oldfors A. Inclusion body myositis: clonal expansions of muscle-infiltrating T cells persist over time. Scand J Immunol. 2003;58:195–200. doi: 10.1046/j.1365-3083.2003.01251.x. [DOI] [PubMed] [Google Scholar]
- Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005;52:1824–35. doi: 10.1002/art.21103. [DOI] [PubMed] [Google Scholar]
- Pearl JP, Paris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy , et al. Immunocometent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–74. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- Reiff A. A review of Campath in autoimmune disease: biologic therapy in the gray zone between immunosuppression and immunoablation. Hematology. 2005;10:79–93. doi: 10.1080/10245330400026139. [DOI] [PubMed] [Google Scholar]
- Salajegheh M, Rakocevic G, Raju R, Shatunov A, Goldfarb LG, Dalakas MC. T cell receptor profiling in muscle and blood lymphocytes in sporadic inclusion body myositis. Neurology. 2007;69:1672–9. doi: 10.1212/01.wnl.0000265398.77681.09. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Barthel K, Wrede A, Salajegheh M, Bähr M, Dalakas MC. Interrelation of inflammation and APP in sIBM: IL-1β induces accumulation of β-amyloid in skeletal muscle. Brain. 2008;131:1228–40. doi: 10.1093/brain/awn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The CAMMS233. Trial investigation. N Engl J Med. 2008;359:1786–801. [Google Scholar]
- Weinblatt ME, Maddison PJ, Bulpitt KJ, Hazleman BL, Urowitz MB, Sturrock RD, et al. CAMPATH-1H, a humanized monoclonal antibody, in refractory rheumatoid arthritis. An intravenous dose-escalation study. Arthritis Rheum. 1995;38:1589–94. doi: 10.1002/art.1780381110. [DOI] [PubMed] [Google Scholar]