Summary
The regulatory Lines/Drumstick/Bowl gene network is implicated in the integration of patterning information at several stages during development. Here, we show that during Drosophila wing development, Lines prevents Bowl accumulation in the wing primordium, confining its expression to the peripodial epithelium. In cells that lack lines or over-expressing Drumstick, Bowl stabilization is responsible for alterations such as dramatic overgrowths and cell identity changes in the proximodistal patterning owing to aberrant responses to signaling pathways. The complex phenotypes are explained by Bowl repressing the Wingless pathway, the earliest effect seen. In addition, Bowl sequesters the general co-repressor Groucho from repressor complexes functioning in the Notch pathway and in Hedgehog expression, leading to ectopic activity of their targets. Supporting this model, elimination of the Groucho interaction domain in Bowl prevents the activation of the Notch and Hedgehog pathways, although not the repression of the Wingless pathway. Similarly, the effects of ectopic Bowl are partially rescued by co-expression of either Hairless or Master of thickveins, co-repressors that act with Groucho in the Notch and Hedgehog pathways, respectively. We conclude that by preventing Bowl accumulation in the wing, primordial Lines permits the correct balance of nuclear co-repressors that control the activity of the Wingless, Notch and Hedgehog pathways.
Keywords: Lines, Drumstick, Bowl, Wingless, Hedgehog, Notch, Groucho, Hairless, Drosophila wing development
INTRODUCTION
Development of a multicellular organism requires coordination in space and time of cellular specification and cellular proliferation. This coordination relies in part on the activity of several signaling pathways that contribute to gene regulation by the activation of specific transcription factors. Some of these conserved signaling pathways are Notch (N), Wnt, Hedgehog (Hh), TGFβ/BMP, EGFR/Ras and JAK/STAT pathways. Knowing the mechanism that integrates these pathways is fundamental to understanding the development of multicellular tissues.
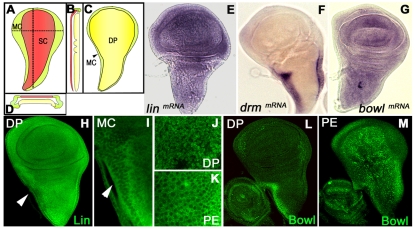
The Drosophila wing is a discrete organ that has been used to study the coordination of signaling pathways during development. The developing wing disc is a sac-like structure composed of the columnar epithelium or disc proper cells (DP), the cuboidal marginal cells (MC) and the overlying squamous cells (SC); MC and SC constitute the peripodial epithelium (PE) (Fig. 1A-D). During larval development, imaginal cells proliferate extensively and are patterned. After metamorphosis, the DP cells differentiate into the cuticle that forms the adult wing and notum, whereas PE cells contribute little to these structures (Milner et al., 1984).
Fig. 1.
Lin/Drm/Bowl regulatory interaction in the wing imaginal disc. (A-D) Schematic representation of the epithelial layers of the wing imaginal disc: peripodial epithelium (containing the squamous cells and the marginal cells) (A), longitudinal section (B), disc proper (C) and cross-section (D), showing SC in red, MC in green and DP in yellow. (E-G) In situ hybridization in a wild-type disc showing lin (E), drm (F) and bowl (G) transcripts. (H-K) Lin protein is in the nucleus and cytoplasm in DP cells (H) but is cytoplasmic in MC (arrowheads in H,I) and SC cells (K). (L) Bowl protein is stabilized in MC cells but is not present in DP cells. (M) Bowl expression in the PE.
Here, we summarize key signaling events that take place in the DP during development. At very early stages, localized Wingless (Wg) signaling restricts the activity of the EGFR pathway to the proximal region to subdivide the wing imaginal disc into wing and body wall (notum) precursors, where it appears to be required continuously to allocate notal cell fates from neighboring wing fates (Wang et al., 2000; Zecca and Struhl, 2002b). This subdivision is the primary manifestation of the proximodistal (PD) patterning.
The growth and patterning of the wing disc depends on the establishment of two organizing/signaling centers. One is established at the boundary between anterior (A) and posterior (P) cells through the activity of Hh, produced in the P compartment. Hh induces expression of the secreted signaling molecule Decapentaplegic (Dpp; a member of the TGF-beta family) in a thin stripe of A cells that acts as a long-range morphogen to coordinate patterning and growth along the AP boundary (reviewed by Blair, 2007). A second organizer is established during the second larval instar to subdivide the wing disc into dorsal (D) and ventral (V) compartments (Diaz-Benjumea and Cohen, 1995), resulting in a stripe of cells with elevated N activation at the interface of DV cells (de Celis et al., 1996; Diaz-Benjumea and Cohen, 1995; Doherty et al., 1996). N in turn activates the expression of Wg in cells along the DV boundary (Diaz-Benjumea and Cohen, 1995; Rulifson et al., 1996), and further refinement involves a series of positive- and negative-feedback loops between both pathways. In addition to these primary signaling events, several other pathways are also important for coordination of cell proliferation in developing tissues, including the JAK/STAT pathway (Mukherjee et al., 2005).
One key question is how can different pathways be integrated and coordinated during the wing development? In the nucleus, several of these pathways use to share common regulators that act simultaneously on their transcriptional control. One of these components is the co-repressor Groucho/TLE (Gro). Gro is recruited to target promoters by association with DNA-binding proteins through conserved eh1 or WRPW domains (Buscarlet and Stifani, 2007). Transcriptional regulators of the N, Wnt, Dpp, EGFR and Hh pathways all interact with Gro (Hasson et al., 2005). A second possible integrator is the regulatory cassette formed by Lines (Lin) and the Odd-skipped gene family of zinc finger proteins [bowl, drumstick (drm), odd-skipped (odd) and sister of odd and bowl (sob)] (Bras-Pereira et al., 2006; de Celis Ibeas and Bray, 2003; Hao et al., 2003; Hatini et al., 2005; Iwaki et al., 2001). The cassette Drm/Lin/Bowl controls the morphogenesis at several stages: in the embryo, they coordinate epidermal cell differentiation through regulating Hh and Wg signaling inputs (Bokor and DiNardo, 1996; Hatini et al., 2000; Hatini et al., 2005); and, in the gut, they regulate morphogenesis by controlling the JAK-STAT proliferative pathway (Green et al., 2002; Iwaki et al., 2001). During imaginal disc development, they are regulated by the N signaling pathway in the leg disc (de Celis Ibeas and Bray, 2003; Hao et al., 2003); in the eye disc, the Odd-skipped family regulates the activation of Hh during retinogenesis (Bras-Pereira et al., 2006).
In this work, we investigated whether Lin/Drm/Bowl contributes to signal integration during wing development. As in the embryo, we find that Lin prevents Bowl accumulation in imaginal cells (DP), except where Drm is expressed (PE). Mutations in lin or overexpression of Drm cause tissue hyper-proliferation. This phenotype appears to be a consequence of a deregulation of Wg, Hh and N pathways, which has long-term effects on JAK-STAT, EGFR and Dpp pathways. Most of the effects can be partially reverted by either Bowl mutations that affect its interaction with the co-repressor Gro or by the co-expression of the Hh and N pathway repressors Master of thickveins (Mtv) and Hairless (H). This suggests that Lin/Drm/Bowl are mediators of signal integration. We conclude that wing development requires Lin in the DP cells to restrict Bowl expression to the PE and permit the function of the Wg, N and Hh pathways at the appropriate levels.
MATERIALS AND METHODS
Drosophila stocks
We used the following fly stocks: linG1 (Bokor and DiNardo, 1996), bowl2 (Wang and Coulter, 1996), gro1 (Preiss et al., 1988), STAT92E-lacZ (http://flystocks.bio.indiana.edu/), Hh-lacZ (Lee et al., 1992), brk-lacZ (Minami et al., 1999), kekkon-lacZ (Musacchio and Perrimon, 1996) and puckered-lacZ (pucE69) (Martin-Blanco et al., 1998). The following Gal4 drivers were used: ptc-Gal4 (Hinz et al., 1994), ubx-Gal4 (Pallavi and Shashidhara, 2003), pnr-Gal4, ap-Gal4 (Calleja et al., 1996) and sd-Gal4 (Mullor et al., 1997).
Transgenic fly lines previously described are: UAS-bowl (de Celis Ibeas and Bray, 2003), UAS-drm (gift from Judith Lengyel), UAS-armS10 (Pai et al., 1997), UAS-dTcf (van de Wetering et al., 1997), UAS-H (Klein et al., 2000), UAS-gro (Apidianakis et al., 2001), UAS-mtv (Funakoshi et al., 2001), UAS-dicer (Dietzl et al., 2007) and UAS-linRNAi, UAS-bowlRNAi (VDRC, http://stockcenter.vdrc.at). To generate the Bowleh1- construct, a fragment of the bowl cDNA lacking the last 13 codons, which encodes the eh-1 domain (RTGFFSIEDI), was amplified. To generate the Bowleh1-VP16 construct, this bowl cDNA was ligated in frame with herpes virus protein VP16 (pHK3NVP16).
Overexpression experiments and generation of clones
Mitotic recombination clones Random clones were generated by FLP-mediated recombination. Flies of the genotype FRT42D linG1/CyO were crossed to flies FLP; FRT42D arm-lacZ/CyO or FLP; FRT42D Ubi-GFP/CyO, and mosaic clones were induced by incubating larvae at 37°C for 30 minutes at 24-48, 48-72 and 72-96 hours after egg laying (AEL).
Flip-out clones
The transgene abx/ubx<FRT, stop, f+, FRT< Gal4-UAS-lacZ (de Celis et al., 1998) and the transgene Act>CD2>Gal4 (Pignoni and Zipursky, 1997) were used to generate ectopic expression clones by incubating larvae at 37°C for 15 minutes at 48-72 hours AEL.
MARCM clones
To generate linG1 clones that ectopically express ArmS10 or Gro males UAS-armS10; FRT42DlinG1/CyO or FRT42DlinG1/CyO; UAS-Gro were crossed to females: y,w,FLP,Tub Gal4,UAS-GFP; FRT42D Gal80/CyO. To generate bowl2 clones marked by UAS-GFP, males bowl2 FRT40A were crossed to females y,w,FLP,Tub Gal4,UAS-GFP; Gal80 FRT40A/CyO. In all cases, larvae were incubated at 37°C for 30 minutes at 48-72 hours AEL.
Transient expression of transgenes
Transient expression of UAS transgenes was induced using different Gal4 drivers and maintaining crosses at 18°C and inactivating the Gal80ts for 7 to 36 hours at the restrictive temperature (29°C).
In situ hybridization
Dioxigenin (Roche) probes were used to detect lin, bowl and drm mRNA in imaginal discs. To prepare the antisense riboprobes, fragments from lin (clone LD 43682), drm (clone LD 26791) and bowl (clone LD 15350) cDNAs were cloned into pGEMT, pOT2 or pBS SK vectors.
Generation of the anti-Lin antibody
For generating the anti-Lin antibody, a region of 1.6 kb of the lin cDNA was amplified and subcloned in the BamHI/KpnI site of the expression vector pT7-7. The induced Lin protein was purified by electrophoresis in acrylamide-SDS gels and extracted and injected in guinea pigs.
Antibodies and immunohistochemistry
Immunostaining was performed according to standard protocols. Antibodies were used at the following dilutions: rabbit anti-β-gal 1/1000 (Jackson Laboratories); rabbit anti-β-gal 1/100 (Promega); anti-Dl 1/5, anti-Wg 1/50 and anti-Ubx antibody 1/10 from the Hybridoma Bank; rabbit anti-Bowl 1/500 (de Celis Ibeas and Bray, 2003); rabbit anti-Hth 1/200 (Aldaz et al., 2005); rat anti-Iro 1/200 (Diez del Corral et al., 1999); mouse anti-MAPK-P 1/1000 (Sigma); mouse anti-Nub 1/50 (Yeo et al., 1995); guinea-pig anti-Sens 1/1000 (Nolo et al., 2000); rat anti-STAT92E 1/20 (gift from Aurel Betz); rabbit anti-STAT-p 1/1000 (Cell Signaling Technology); rabbit anti-Tsh 1/1000 (Gallet et al., 1998); rabbit anti-Zfh2 1/250 (Whitworth and Russell, 2003); rabbit anti-Caspase3 1/50 (Hybridoma bank); mouse anti-Ptc 1/50 (Capdevila and Guerrero, 1994); rabbit anti Hh antibody 1/800 (Takei et al., 2004); and rabbit anti-phospho-Histone-3 1/400 (Cell Signaling Technology).
RESULTS
Lin function involves deployment of the Lin/Drm/Bowl regulatory cassette
During embryonic development, Lin expression is ubiquitous and is largely cytoplasmic, except in those epidermic cells where lin function is required (Green et al., 2002; Hatini et al., 2000; Hatini et al., 2005). To investigate whether similar mechanisms apply in the wing imaginal disc, we monitored lin mRNA and protein distribution. Both, the lin transcript and Lin protein, were ubiquitously expressed (Fig. 1E,H); however, the subcellular localization of the protein was clearly modulated. Whereas Lin was detected in both nucleus and cytoplasm in most of DP cells (Fig. 1H,J), it was restricted to the cytoplasm in the cubiodal MC, a narrow stripe of cells along the anterior border of the notum and pleura (Fig. 1I), and in SC (Fig. 1K). If the Lin/Drm/Bowl cassette functions as in the embryo, the cytoplasmic localization should indicate where Lin is inactive. Conversely, the presence of nuclear Lin in most of the wing imaginal cells would be suggestive of a functional role.
To analyze the role of lin during wing development, we induced mitotic recombinant clones of the null allele linG1 (lin-) (Bokor and DiNardo, 1996). Clones induced mid-way through larval development (48-72 hours AEL) gave rise to dramatic overgrowths that segregated from wild-type tissue, forming smooth borders. The increased in division rate in lin- clones is monitored by higher expression of phospho-Histone 3 (PH3), a marker of cell division. Only few of such clones persisted into adult structures, most probably as a consequence of cell death observed using the apoptotic markers puckered-lacZ, and activated caspase 3. Notably, clones induced earlier (24-48 hours AEL) fail to survive even to larval stages, as only wild-type twin clones were detected under these conditions. Strikingly, some late induced clones (72 hours AEL) were able to regenerate a complete wing or notum when they were located near the wing hinge region (see Fig. S1 in the supplementary material).
In many developmental contexts, Lin is regulated by Drm, which prevents the interaction between Lin and Bowl, allowing nuclear accumulation of Bowl (Bras-Pereira et al., 2006; de Celis Ibeas and Bray, 2003; Hao et al., 2003; Hatini et al., 2005; Iwaki et al., 2001). In the wing imaginal disc, drm transcript was detected in both the MC along the anterior notum border and in the SC, where Lin is cytoplasmic (Fig. 1F). However, although bowl transcript was present uniformly throughout the disc (Fig. 1G), Bowl protein was found only in the nucleus of drm-expressing cells (Fig. 1L,M). As predicted, in lin- clones Bowl protein accumulated dramatically, independently of the position of the clone in the DP cells (Fig. 2A). Therefore, we conclude that in the wing disc Lin regulates Bowl protein stability, probably through similar mechanisms as during embryonic development (Hatini et al., 2005).
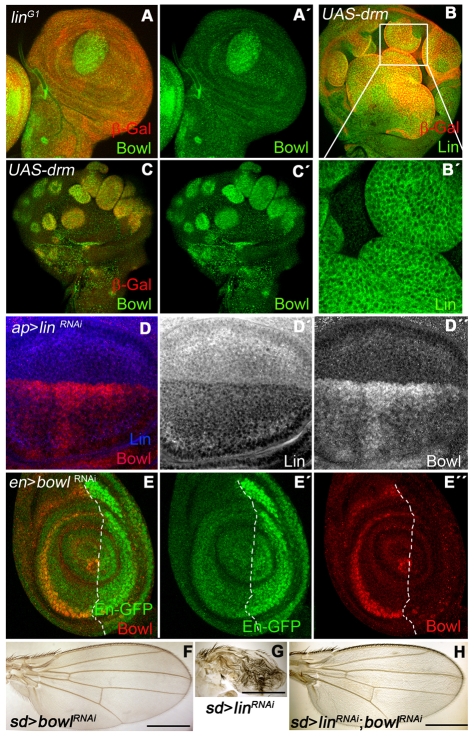
Fig. 2.
Functional interaction between Lin, Drm and Bowl in wing development. (A,A′) Bowl protein expression (green) in a wing disc containing a linG1 clone. (B,B′) Subcellular localization of Lin protein in Drm GOF clones (red in B). In a higher magnification of B, Lin protein (green in B,B′) is relocalized to the cytoplasm. (C,C′) Bowl protein stabilization (green in C,C′) in Drm GOF clones (red in C). (D-D″) ap-Gal4>UAS-linRNAi wing disc shows the absence of Lin protein (blue in D, grey in D′) and the stabilization of Bowl (red in D, grey in D″). (E-E″) en-Gal4>UAS-bowlRNAi leg disc (labeled with GFP, green in E,E′) shows absence of Bowl protein in the P compartment (red in E,E″). (F) A sd-Gal4>UAS-bowlRNAi wing with Bowl expression knocked down in the wing pouch. (G) The sd-Gal4>UAS-linRNAi wing shows a severe phenotype in terms of reduction of wing size. (H) This lin- phenotype is suppressed by co-expression of UAS-linRNAi and UAS-bowlRNAi using sd-Gal4. Scale bars: 500 μm.
Furthermore, ectopic expression of Drm in the DP cells results in a relocalization of Lin to the cytoplasm (Fig. 2B) and a corresponding stabilization of Bowl (Fig. 2C) supporting our model. Therefore, Drm overexpression in DP cells should cause the same phenotypes as lin- clones. Indeed, ectopic Drm expression gave overgrowths and cell identity changes similar to lin- clones (see below) (see Fig. S2 in the supplementary material). Therefore, in subsequent experiments, we use both Drm gain-of-function (GOF) and lin- clones for analyzing the lin requirement in the wing imaginal disc.
To determine whether the phenotypes observed in lin- (or Drm GOF) clones were a consequence of Bowl stabilization, we knocked out both lin and bowl functions by co-expressing RNAi against both genes. We first tested the functionality of the corresponding RNAi. Expression of Lin-RNAi in D cells of the wing disc severely compromised Lin protein expression and led to Bowl protein stabilization (Fig. 2D). Expression of Bowl-RNAi in P cells in the leg disc, where Bowl has a characteristic rings pattern corresponding to the joint primordia (de Celis Ibeas and Bray, 2003; Hao et al., 2003; Hatini et al., 2005), is able to ablate Bowl expression in these cells, demonstrating efficient knock down (Fig. 2E).
To confirm that the effects of removing Lin depend on Bowl, we examined the expression of either Lin-RNAi or Bowl-RNAi, or both, in DP cells of the wing pouch. Expression of Bowl-RNAi did not cause any wing alteration (Fig. 2F). However, Lin-RNAi expression resulted in a drastic reduction of wing size, indicating that the development was severely compromised (Fig. 2G). This phenotype was completely suppressed when Lin-RNAi and Bowl-RNAi were co-expressed (Fig. 2H). Hence, we can conclude that Bowl is the primary effector of the changes observed in lin- (and Drm GOF) clones in the wing disc.
Role of Lin/Drm/Bowl on the regulation of Wg responses.
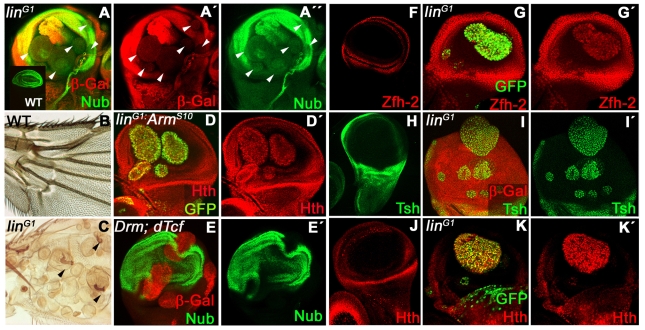
As mentioned above, early induced lin- or Drm GOF clones (48-60 hours AEL) over-proliferate in the wing pouch. When lin- clones survived to the adulthood, they look segregated from the wild-type tissue and produced ectopic structures, such as macrochaetae, suggestive of cell identity transformation into proximal identity (notum, pleura or hinge transformation) (Fig. 3C). In agreement, these clones do not express specific genes for wing pouch. For example, the nubbin (nub) gene, a distal marker (Cifuentes and Garcia-Bellido, 1997; Ng et al., 1995; Whitworth and Russell, 2003) was absent in lin- clones in the wing pouch (Fig. 3A). Conversely, genes normally expressed in the hinge and notal regions of the disc, such as the zinc finger homeodomain-2 (zfh-2), teashirt (tsh) and homothorax (hth) (Fig. 3F,H,J) (Azpiazu and Morata, 2000; Casares and Mann, 2000; Terriente et al., 2008; Whitworth and Russell, 2003; Wu and Cohen, 2002; Zirin and Mann, 2007), were now expressed at high levels in lin- cells, indicating a change in their PD identity (Fig. 3G-I).
Fig. 3.
Repression of Wg responses; P/D transformation of linG1 clones in the wing disc. (A-A″) Nub repression (green in A,A″) in linG1clones induced in a wing disc (lack of red, arrowheads in A-A″). Inset in A shows Nub expression in the wild-type wing disc. (B) Proximal part of an adult wild-type wing. (C) Proximal part of a wing containing linG1 clones labeled by the cuticle marker forked. Arrowheads indicate ectopic macrocheatae. (D,D′) Hth ectopic expression (red) in MARCM linG1 clones overexpressing ArmS10 (labeled with GFP, green). (E,E′) Nub repression (green) in random clones co-expressing Drm and dTcf (red in E). (F,H,J) Wild-type expression of Zfh-2 (F), Tsh (H) and Hth (J) in the wing disc. (G,G′,I,I′,K,K′) Ectopic activation of Zfh-2 (red in G,G′), Tsh (green in I,I′) and Hth (red in K,K′) in linG1 clones (labeled with GFP in G and K, and lack of red in I). All clones were induced between 48 and 72 hours AEL.
As the PD specification involves an antagonistic interaction between Wg and EGFR (Zecca and Struhl, 2002a; Zecca and Struhl, 2002b), the phenotypes might be caused by a requirement of lin for the activation of Wg target genes. To test whether this was the case, we generated lin- clones that simultaneously expressed a constitutively active form of Arm (ArmS10) or dTcf (Pangolin), transcriptional effectors of the Wg pathway (reviewed by Tolwinski and Wieschaus, 2004). Neither rescue of over-proliferation nor aberrant gene expression was detected in either combination (Fig. 3D,E), suggesting that Lin acts in parallel to or downstream of Arm and dTcf/Pangolin to regulate Wg pathway, as it was observed in the embryo (Hatini et al., 2000; Hatini et al., 2005).
Lin/Bowl regulate Wg signaling at the D/V compartment border of the wing disc
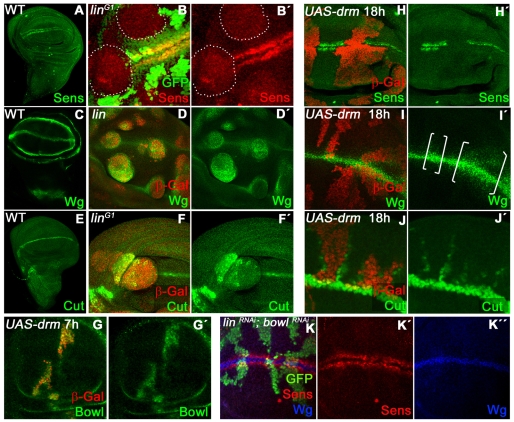
Wg is required at multiple stages during wing development. To investigate whether Lin could regulate the later Wg function at the DV compartment boundary, we analyzed Senseless (Sens) expression, a specific Wg target, and found that its expression was absent in both lin- and Drm GOF clones (Fig. 4B). These results are consistent with the role of Lin regulating Wg signaling, as at earlier stages. Moreover, the initial DV border specification involves an antagonistic interaction between N and Wg pathway; thus, an alternate possibility could be that N signaling is ectopically activated in the lin- cells. In support of this hypothesis, we observed that lin- clones induced close to the DV boundary ectopically expressed two N targets, as Wg and Cut (Ct) (Fig. 4D,F). This result suggests that the effect on Sens expression, a Wg target gene, could be an indirect consequence of N pathway activation.
Fig. 4.
Deregulation of Wg and Notch responses at the DV border of the wing disc. (A,C,E) Expression of Sens (A), Wg (C) and Ct (E) in a wild-type wing disc. (B,B′) Sens repression (red) in a wing disc containing linG1 clones (lack of GFP and outlined). (D,D′) Wg is ectopically activated (green) in linG1 clones (red) induced in the wing pouch. (F,F′) Ct activation (green) in some linG1 clones in the wing pouch (red). Very large clones do not activate Ct probably because they were induced before the onset of the DV border. (G-J′) Wing discs containing transient ectopic UAS-drm clones (red) produced by the tubGal80ts technique. After 7 hours at the restrictive temperature, Bowl protein (green) (G,G′) is stabilized. After 18 hours, Sens (H,H′) is autonomously repressed (green) and Wg (see brackets in I,I′) and Ct (J,J′) are activated. (K-K″) Ectopic clones co-expressing Lin RNAi and Bowl RNAi (green). The effects of knocking down lin by ectopic Lin RNAi are suppressed by co-expression of Bowl RNAi.
To distinguish whether the effects on the Wg activity is caused directly or through N activation, we used the Gal4/Gal80ts technique to induce Drm expression (representing lin- function) at different developmental times. Using this approach, we examined the effect of Drm on targets of both Wg and N pathways after the DV boundary is established. The earliest effect caused by ectopic Drm expression was Bowl stabilization, 7 hours after induction (Fig. 4G). Next, we detected total repression of Sens before 18 hours (Fig. 4H); at that time, the ectopic expression of N targets Wg and Ct was also observed in and adjacent to the Drm-expressing clones (Fig. 4I,J). Thus, upregulation of N and Wg targets appear at similar times, suggesting that effects on these pathways are independent. We note that the strongest induction of Ct occurs at the boundary of the Drm-expressing clone, suggesting that it might be augmented due to the induction of N ligand expression within these clones (see Fig. S2B in the supplementary material), a characteristic of ectopic Notch pathway activity in the late stages of wing margin development (de Celis and Bray, 1997).
Vestigial (Vg), is another gene regulated independently by both N (at the DV boundary) and Wg (elsewhere in the wing pouch) (Zecca and Struhl, 2007). We therefore analyzed Vg expression in Drm-expressing clones induced for 25 hours. We observed Vg expression in clones at the DV border, but in the clones located at a distance from the DV border Vg is repressed. These spatially distinct phenotypes indicate that Drm-expressing cells can both upregulate N pathway activity (to maintain Vg at DV) and downregulate Wg pathway activity (to repress Vg at distant positions; see Fig. S2C in the supplementary material).
As Bowl appears to be the primary effector in lin- or Drm GOF clones in the wing disc, we examined N and Wg targets in clones where Bowl and Lin were simultaneously eliminated by co-expression of UASLin-RNAi and UASBowl-RNAi. Under these conditions, neither the repression of Sens nor the activation of the N targets was detected (Fig. 4K). These data suggest that the inhibition of Bowl by Lin is essential for normal Wg and N functions.
Lin/Drm/Bowl regulates Hh expression
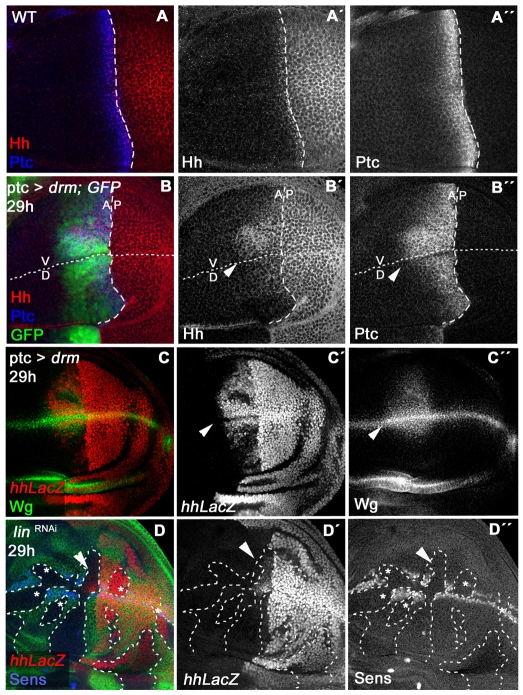
In the dorsal embryonic epidermis lin plays an essential role regulating the antagonistic interaction between the Wg and Hh pathways (Hatini et al., 2005). We therefore analyzed whether the Hh pathway was also affected in lin- or Drm GOF cells in the wing disc. We transiently overexpressed Drm in a stripe in the A compartment and monitored the expression of Hh and its target, Patched (Ptc). Both were ectopically expressed in the A compartment cells (Fig. 5B, compare with Fig. 5A). Using the hh-lacZ reporter, we confirmed that this regulation occurs at transcriptional level. The Hh derepression was more pronounced in V cells, as we also observed for N pathway targets (Fig. 5C, see also Fig. 4I,J), although the reason for this is unclear. Next, inducing UAS-linRNAi clones randomly we observed that hh was only activated in the A compartment clones close to the AP border (Fig. 5D,D′). However, as discussed earlier, repression of the Wg target Sens occurred in all ectopic UAS-linRNAi clones that touch the DV border (Fig. 5D,D″). The spatially restricted hh induction (in A clones close to the AP compartment border) is similar to that seen in gro (Apidianakis et al., 2001) and mtv (Apidianakis et al., 2001; Bejarano et al., 2007) mutant clones. Mtv is a target of Hh at the AP compartment border, which, together with Gro, helps to maintain hh repression in the responding cells. Taken together, these results suggest that Lin/Bowl plays a similar role in the wing pouch to that observed in the dorsal embryonic epidermis, regulating the antagonistic interaction between the Wg and Hh pathways in both contexts (Hatini et al., 2000; Hatini et al., 2005).
Fig. 5.
Drm/Bowl induces the ectopic activation of Hh in the wing disc. (A-A″) Hh (red in A and grey in A′) and Ptc (blue in A and grey in A″) expression in a wild-type wing disc. (B-B″) Ptc and Hh expressions in a ptc-Gal4> tubGal80ts; UAS-drm/UAS-GFP wing disc (after 29 hours of Drm induction). Note the ectopic expression of Hh (red in B, grey in B′) and Ptc (blue in B, grey in B″) in the A compartment (arrowheads). (C-C″) Expression of hh-lacZ (red in C and grey in C′) and Wg (green in C and grey in C″) in a ptcGal>tubGal80ts; UAS-drm/hh-lacZ wing disc (after 29 hours of Drm induction). Hh and Wg are activated within the Ptc domain (arrowheads). (D-D″) UAS-linRNAi clones (lack of green and marked with broken lines) induced using Act>CD2>Gal4; tubGal80ts system (after 29 hours of linRNAi induction). hh-lacZ (red in D and grey in D′) is activated only in the clones touching the AP border (arrowheads), and the repression of Sens (blue in D and grey in D″) is present in all clones touching the DV border (asterisks).
Gro is involved in the deregulation of N and Hh by Lin/Bowl
The co-repressor Gro is a component shared by the repressor complexes regulating Hh, Wg and N pathways (Apidianakis et al., 2001; Barolo et al., 2002; Bejarano et al., 2007; Cavallo et al., 1998; de Celis and Ruiz-Gomez, 1995; Lawrence et al., 2000; Morel et al., 2001; Nagel et al., 2005). As Bowl contains an eh-1 motif that recruits Gro, one way that Lin could exert its effects is by regulating Bowl/Gro interactions (Goldstein et al., 2005). Thus, nuclear Bowl in lin- or Drm GOF cells could interact with Gro and sequester it from the N and Hh repressor complex.
To investigate whether the effects of Lin/Bowl could be mediated through sequestration of Gro, we tested whether the phenotypes caused by ectopic Bowl could be suppressed by co-expressing Gro. On its own, ectopic Bowl induces expression of Ct (or Wg) and Hh (Fig. 6A,E; and data not shown) in a similar manner to lin- or Drm GOF clones (albeit to a much weaker extent because Lin is still competent to destabilize the ectopically expressed Bowl protein). Co-expression of Gro and Bowl was sufficient to prevent the activation of these targets (Fig. 6B). However, when the eh-1 motif was eliminated in Bowleh1- (Fig. 6C,F) or substituted by the VP16 activation domain in Bowleh1-VP16 (Fig. 6D), Bowl is unable to activate ectopic expression of Ct, Wg or Hh. These results indicate that Bowl needs to interact with Gro to activate N targets and Hh expression. Therefore, we propose that Lin prevents the Bowl/Gro interaction. As Gro is required for repression in the N pathway and Hh expression, in lin- or Drm GOF cells, Bowl sequesters Gro from the repressor complexes, triggering ectopic expression of the target genes.
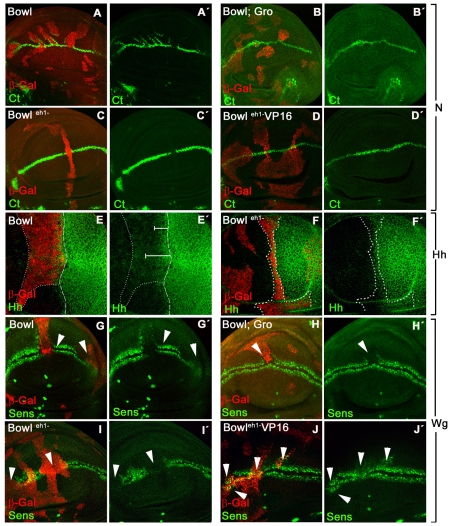
Fig. 6.
Bowl sequesters Gro from the N and Hh repressors complexes. Effects of expressing Bowl or Bowl variants on Notch (A-D′), Hh (E-F′) and Wg (G-J′) targets genes. (A-D′) Ct expression (green) in wing discs containing clones (red) expressing ectopic Bowl (A,A′), Gro and Bowl (B,B′), Bowleh1- (C,C′) or Bowleh1-VP16 (D,D′). Ectopic Ct expression is associated with ectopic Bowl (A,A′) but not with ectopic Gro and Bowl (B,B′), Bowleh1- (C,C′) or Bowleh1-VP16 (D,D′). (E-F′) Hh expression (green) in ectopic Bowl clones and Bowleh1- clones (F,F′), all labeled in red and outlined. (G-J′) Sens expression (green) in clones expressing ectopic Bowl (G,G′), Gro and Bowl (H,H′), Bowleh1- (I,I′) or Bowleh1-VP16 clones (J,J′). All clones are labeled in red (arrowheads). Bowl represses Sens (G) in the presence (G-H′) or absence (I) of the Gro-binding domain eh-1. Replacement of the eh-1 domain by VP16 converts Bowl to an activator of Sens close to the Wg source. The absence of Ct expression in a Bowleh1- clone (C,C′) is probably due to the block of both N and Wg pathways and Wg responses in most of the Bowleh1- clones (I′).
Conversely, the effect on Sens expression suggests that Bowl might act through a different mechanism to regulate the Wg pathway. First, co-expression of Bowl and Gro yields the same effects on Wg targets (Fig. 6H) as when either Gro (see Fig. S4A in the supplementary material) or Bowl (Fig. 6G) are expressed alone, arguing against the sequestration model. Second, expression of Bowleh1-, still repressed Sens (Fig. 6I), indicating that Bowl may acts as a transcriptional repressor independently of its interaction with Gro. Third, expression of Bowleh1-VP16 can activate Sens expression (Fig. 6J), although activation was variable and primarily detected in clones close to the endogenous source of Wg. Nevertheless these results suggest that the repression of Sens by Bowl can be reversed by the presence of an activation domain (VP16) and it is independent of its interaction with Gro. Therefore, Bowl represses Wg targets via a Gro-independent mechanism.
Our model implies a functional relationship for lin/bowl and gro, which might be detected by genetic interactions between alleles of lin and gro genes. gro1 individuals display tufts of bristles in the dorsal head and in the scutelum. Removing one dose of lin in this background (linG1/+; gro1/+) results in a high incidence of lethality and the few escapers showed enhanced phenotypes, such as ectopic eyes, leg truncations and duplications, loss of proboscis, duplication of antenna segments and nicks in the wing margin (see Fig. S3A-H in the supplementary material). The interaction between lin and gro was also evident from the rescue of ectopic Wg expression when Gro was overexpressed in lin- clones (see Fig. S3I,I′ in the supplementary material).
Bowl recruits Gro from the N and Hh repressor complexes
Our results suggest that the effects of lin on the N and Hh pathways are a consequence of the ability of Bowl to bind Gro, a crucial component for repression of both pathways. Thus, the sequestration of Gro by Bowl can explain both the ectopic activation of N targets and the Hh expression.
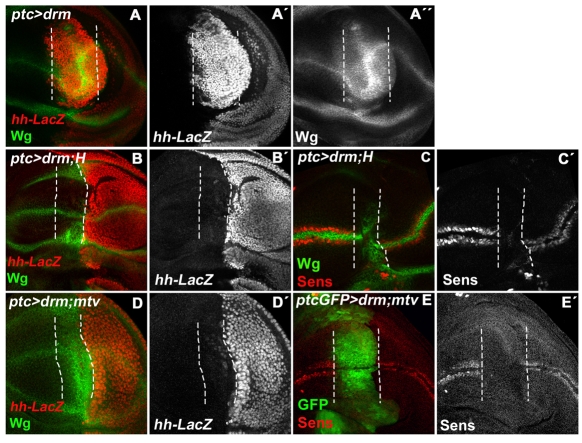
Repression of N target genes by Gro is mediated by Suppressor of Hairless [Su(H)], and Hairless (H), the adaptor that binds directly to Gro (Barolo et al., 2002; Furriols and Bray, 2000; Morel et al., 2001). If the activation of N targets in lin- or Drm GOF clones is caused by the sequestration of Gro, it might be possible to overcome this by increasing the availability of H. We therefore tested whether co-expression of H and Drm was sufficient to suppress the lin- phenotypes. In agreement, the overproliferation and the deregulation of the N pathway, caused by Drm overexpression (Fig. 7A), were largely normalized by H (Fig. 7B-C′). However, Sens expression was not recovered (Fig. 7C). Moreover, the normal Hh activity in the A cells was also restored by the ectopic H (Fig. 7B). Similarly, we tested whether overexpression of Mtv was able to recover the ectopic Hh expression induced in Drm GOF, as predicted if this is also caused by Bowl sequestering Gro from the Mtv/Gro repressor complex. Co-expression of Mtv with Drm prevents the ectopic activation of Hh (Fig. 7D). However, like H, Mtv was unable to normalize the expression of Wg (Fig. 7D) or Sens (Fig. 7E). These results indicate that Bowl can unbalance the Mtv/Gro and H/Su (H)/Gro repressor complexes by sequestering Gro.
Fig. 7.
H or Mtv partially suppress the effects of ectopic Drm in the wing. (A-A″) ptc-Gal4> UAS-drm/hh-lacZ wing disc showing a huge overgrowth and the induction of hh (red in A, grey in A′) and Wg (green in A, grey in A″) in the AP compartment region where Drm is induced. (B,B′) ptc-Gal4>UAS-H;UAS-drm/hh-lacZ wing disc. The co-expression of H and Drm largely normalizes the overgrowth and the expression of hh-lacZ (red in B and grey in B′) and partially rescues at the DV border by the ectopic Wg (green in B) caused by ectopic Drm. (C,C′) ptc-Gal4>UAS-H;UAS-drm. Note that Sens is still repressed (red in C, grey in C′). (D,D′) ptc-Gal4/UAS-mtv;UAS-drm/hh-lacZ wing disc. Overgrowth and activation of hh-lacZ are largely normalized (red in D and grey in D′) but the ectopic Wg expression (green in D) is not. (E,E′) ptc-Gal4>UAS-mtv;UAS-drm/UAS-GFP wing disc (GFP labels the Ptc expression domain in E). Sens repression is not normalized (red in E, grey in E′). The Ptc expression domain is marked with a broken line in all panels.
Proximal/distal transformation of lin- mutant clones
One remaining issue is whether the PD cell identity changes (notum, pleura or hinge) observed in a few lin- clones that survive to the adult (Fig. 3C) can be explained by the Bowl/Gro interaction. The EGFR pathway in proximal cells is one of the earliest signals influencing PD axis specification (Wang et al., 2000). In distal lin- clones (48-60 hours AEL), we observed expression of several EGFR targets (Kekkon, Iroquois and phosphorylated MAPK), suggesting aberrant EGFR signaling and changes in PD identity. Expression of other markers, such as STAT92E-lacZ, phospho-STAT92E and the Dpp targets genes brinker (brk) and spalt (sal), was also altered (see Fig. S5 in the supplementary material). The activation of proximal markers can be explained as a long-term consequence of ectopic repression of the early Wg by ectopic stabilized Bowl in lin- cells, because a pulse (24 hours) of Drm expression at the end of the second instar was sufficient to induce changes in the PD identity, even though Drm was not present continuously (see Fig. S2D in the supplementary material).
As several of the upregulated genes in lin- clones, such as STAT92E, brk, hth and tsh, are also normally expressed in the PE cells, it is possible that lin- cells are transformed to PE identity (rather than proximal DP identity). However, as lin- (48-60 AEL) clones also upregulated genes that are not present in the SC of the PE cells, such as dachsous (ds) (data not shown), iro, zfh2 or kekkon, this cannot be the whole explanation. Second DP lin- cells could be transformed to MC fate. Supporting this possibility, all the genes upregulated in lin- cells are normally expressed in the MC of the disc, including Bowl. Moreover, lin- clones differentiate tricomes and macrochaetes, compatible with a transformation to proximal structures such as pleura/hinge/notum (Fig. 3C). However, as the early-induced lin- clones died and only twin clones were recovered, we cannot exclude the possibility that Lin might have an early function in preventing the transformation to both MC and SC of the PE, as has been recently proposed (Nusinow et al., 2008).
Bowl function in the peripodial epithelium
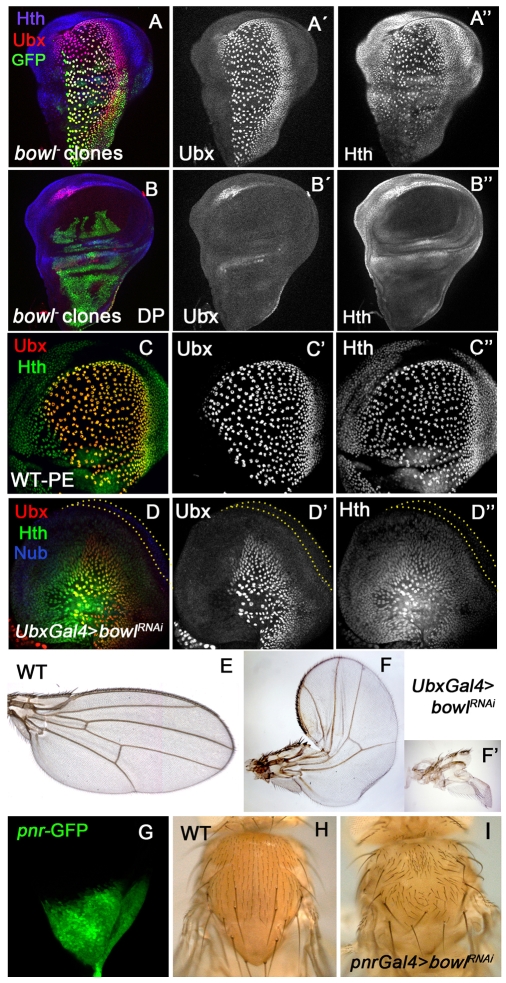
The regulatory interaction between Lin, Drm and Bowl restricts Bowl protein to the SC and MC within the PE. To determine the function of Bowl in these domains, we induced early bowl- clones, marked by the expression of GFP (MARCM clones). Although the frequency of recovered clones in PE (Fig. 8A) is usually lower than in the DP (Fig. 8B), we could visualize large clones in the PE and observed that they still expressed the peripodial markers Ubx (Fig. 8A,A′) and Hth (Fig. 8A,A″). We also expressed Bowl-RNAi in the PE and in the MC using ubx-Gal4 (Pallavi and Shashidhara, 2003). We found that some Ubx-Gal>UAS-bowlRNAi wing discs were smaller than wild-type discs (Fig. 8C) and showed altered expression of Ubx (Fig. 8D,D′) and Hth (Fig. 8D,D″). ubx-Gal4>UAS-bowlRNAi adult wings display (30%) reduction of the proximal wing and occasionally the whole wing was missing (Fig. 8F,F″). Likewise, expressing UAS-bowlRNAi with pnr-Gal4 (expressed in the notum primordium, including the MC expressing Bowl) results in a cleft in the thorax and absence of dorso/central bristles (Fig. 8I). These results suggest that Bowl is required for normal wing and notum development, possibly differentiating the signaling response between the SC/MC and DP cells.
Fig. 8.
Bowl requirement in the peripodial cells of the wing disc. (A-B″) Wing discs containing MARCM bowl2 clones (green) in the PE (A) and in the DP (B). Note that Ubx (red in A,B and grey in A′,B′) and Hth (blue in A,B and grey in A″,B″) expression is not modified in bowl2 clones in either the PE (A) or in the DP (B). (C-C″) PE cells of a wild-type disc showing the expression of Ubx (red in C and grey in C′) and Hth (green in C and grey in C″). (D-D″) ubx-Gal4>UAS-bowlRNAi wing discs showing the expression of Ubx (red in D and grey in D′), Hth (green in D and grey in D″) and Nub (blue in D). Both the size of the PE and the Ubx and Hth expression domains are reduced. The outlined area corresponds to the DP cells expressing Nub (blue in D). (E) Wild-type adult wing. (F,F′) ubx-Gal4>UAS-bowlRNAi wings. (G) Wild-type pnr expression domain (GFP in green) in the notum region. (H) Wild-type adult notum. (I) pnr-Gal4>UAS-bowl RNAi notum. Note the cleft in the notum and the disorganization of micro and macrocheatae.
DISCUSSION
The Lin/Drm/Bowl cassette is emerging as an important molecular mechanism with which to coordinate various pathways in different developmental contexts (de Celis Ibeas and Bray, 2003; Hao et al., 2003; Hatini et al., 2005; Nusinow et al., 2008). In all cases, the steady-state accumulation of Bowl is regulated by the relative levels of Drm and Lin proteins. High levels of Drm impede binding of Lin to Bowl and, thus, this transcriptional repressor becomes stabilized in the nucleus. Here, we have found that regulatory interaction Lin/Drm/Bowl also functions during wing development. In lin- or Drm GOF cause ectopic expression of Bowl and dramatic overgrowths within the wing disc. These overgrowths frequently showed altered cell identity, resembling more proximal disc margin cells. Some of the effects can be explained by the ability of Bowl to interact with Gro co-repressor through the eh-1 motif, forming a complex that sequesters Gro from other repressors complexes such as Su(H)/H/Gro and Mtv/Gro.
Lin/Drm/Bowl regulative interaction
Although Bowl is ubiquitously transcribed in the wing disc, Bowl protein is present only in the SC and MC, being normally absent from the DP cells. The spatial distribution of nuclear Bowl is dependent on Drm, which causes Lin to relocalize to the cytoplasm. Drm is absent from most of the DP cells and, therefore, Lin turns down the steady-state accumulation of Bowl protein in these cells. In the absence of Lin, Bowl accumulates in the DP cell nuclei and elicits the dramatic alterations observed in lin- mutant cells. Therefore, the main function of Lin is to prevent Bowl accumulation in the DP cells, restricting Bowl protein to MC and SC of the PE.
The main alterations in lin-, Drm GOF or Bowl GOF clones can be classified according to the signaling pathways temporally affected. The earliest defect observed is the repression of Wg pathway responses and the evidence suggests that Bowl functions as a repressor of the Wg pathway. However, activated forms of nuclear Wg pathway components, such as ArmS10 or dTcf, cannot restore the expression of the proximal-distal markers owing to repression of the Wg targets in lin-, indicating that Bowl must act in parallel to or downstream of Arm and dTcf, as was previously suggested (Green et al., 2002; Hatini et al., 2005).
Gro acts in the crosstalk of different signaling pathways
Bowl is a zinc-finger protein that can interact with the co-repressor Gro directly through the eh-1 motif (Goldstein et al., 2005). Our results indicate that this mechanism is also important under conditions where Bowl accumulates in the wing disc. Most of the alterations observed in lin- or Drm GOF clones can be explained by Bowl sequestering Gro from other repression complexes (causing activation of N targets and Hh). Several results support this model. First, the strong genetic interaction between lin and gro alleles, where trans-heterozygous combinations between lin and gro alleles result in dramatic phenotypes, argue that Gro is a limiting factor. Second, removal of eh-1 motif that recruits Gro, eliminates the effects of Bowl on the Hh and N pathways. Third, ectopic expression of Gro, H or Mtv partially suppress the phenotypes of ectopic Drm or Bowl. These observations imply a `tug of war' between Bowl, H and Mtv for Gro. Increased H or Mtv would shift the balance back in favor of N target repression and Hh repression.
By contrast, the repression of Wg pathway observed in lin- cells appears to involve a different mechanism. Although the effect is Bowl dependent, repression of Wg targets also occurs with Bowleh1-, indicating that Gro sequestration is not required. Similarly, co-expression of Bowl with H or Mtv cannot re-establish the repression of the Wg targets. These results show that Bowl is able to repress Wg targets independently of Gro and the observation that Bowleh1- VP16 can cause some ectopic expression of Sens suggests that this may involve a direct effect of Bowl on Wg targets.
Wnt/Wg, N and Hh signaling represent major conserved signaling channels to control cell identity and behavior during development. An antagonistic interaction between the Wg and Hh has also been described in the embryo (Hatini et al., 2005) and at the intersection of the D/V and A/P compartment borders of the wing disc (Glise et al., 2002). Similarly, Wnt/Wg and N activities are closely entangled in many different systems. Mutual dependent interactions between N and Wnt signaling have been observed in vertebrate skin precursors (Estrach et al., 2006), in rhombomere patterning (Cheng et al., 2004) and in somitogenesis (Aulehla et al., 2003; Dale et al., 2003; Hofmann et al., 2004). It has also been reported that orthologues of the Odd-skipped family, Osr1 and Osr2, function as transcriptional repressors during kidney formation (Tena et al., 2007). It is possible therefore that Lin/Bowl/Gro interaction is evolutionary conserved and it will be interesting to discover whether lin is an important regulatory factor in other systems.
Bowl function in wing development
By analyzing lin- clones in the wing primordium, we have uncovered the consequences of stabilizing Bowl in the DP cells. There are, however, two regions where Bowl accumulates normally, in the MC and SC within the PE. Removal of Bowl in the PE might lead to ectopic Wg protein and thus to ectopic activity of the Wg signaling to transform PE from squamous to columnar cells (Baena-Lopez et al., 2003). In this context, recently, it has shown that Bowl inhibition by ectopic expression of Lin results in the replacement of the PE by a mirror image duplication of the DP cells (Nusinow et al., 2008). However, we did not observe much alteration in cell morphology nor in the expression of markers such as Ubx or Hth when Bowl was depleted in PE cells (bowl- clones and UAS-BowlRNAi). It could be that the recovered bowl- clones were not induced early enough or that the levels of Bowl-RNAi were not sufficient to completely eliminate the Bowl function in these cells. Nevertheless, our manipulations revealed that bowl- phenotypes in the proximal wing and notum were consistent with a functional role in MC. Therefore, we conclude that Lin has an important role in restricting Bowl to the MC (and PE), delimiting a Bowl-free territory that forms the DP cells and enables their responsiveness to key signaling pathways such as Wg.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/7/1211/DC1
Supplementary Material
We thank Aphrodite Bilioni and Fernando Díaz-Benjumea for critical reading of the manuscript, and members of the Bray and Guerrero laboratories for discussions and advice, especially Maki Daniels and Ainhoa Callejo. We are very grateful to Carmen Ibáñez, Emma Harrison and Marie Quick for excellent technical assistance; to A. Betz, N. Azpiazu, H. Bellen, C. Delidakis, F. J. Diaz-Benjumea, S. Kerridge, J. Modolell, T. Tabata and the Developmental Studies Hybridoma Bank for providing antibodies; and to V. Hatini, M. Calleja, S. DiNardo, C. Delidakis, J. Lengyel, C. Rauskolb, S. Shashidara, T. Tabata, A. Martinez-Arias, Vienna Drosophila RNAi Center and the Bloomington Stock Center for Drosophila stocks. This work has been supported by grants BFU2005-04183 and CSD2007-00008 from the Spanish MEC by an institutional grant from Fundación Areces given to the Centro de Biología Molecular `Severo Ochoa' to I.G. and MEC fellowship (BMC2002-03839) to E.B. Work in the Bray laboratory was supported by a Programme Grant from the Medical Research Council (MRC) to S.J.B., with additional funding from EMBO Short Term Fellowship (ASTF 143-2006) to E.B. and Royal Society Travelling Fellowship to I.G. Deposited in PMC for release after 6 months.
References
- Aldaz, S., Morata, G. and Azpiazu, N. (2005). Patterning function of homothorax/extradenticle in the thorax of Drosophila. Development 132, 439-446. [DOI] [PubMed] [Google Scholar]
- Apidianakis, Y., Grbavec, D., Stifani, S. and Delidakis, C. (2001). Groucho mediates a Ci-independent mechanism of hedgehog repression in the anterior wing pouch. Development 128, 4361-4370. [DOI] [PubMed] [Google Scholar]
- Aulehla, A., Wehrle, C., Brand-Saberi, B., Kemler, R., Gossler, A., Kanzler, B. and Herrmann, B. G. (2003). Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell 4, 395-406. [DOI] [PubMed] [Google Scholar]
- Azpiazu, N. and Morata, G. (2000). Function and regulation of homothorax in the wing imaginal disc of Drosophila. Development 127, 2685-2693. [DOI] [PubMed] [Google Scholar]
- Baena-Lopez, L. A., Pastor-Pareja, J. C. and Resino, J. (2003). Wg and Egfr signalling antagonise the development of the peripodial epithelium in Drosophila wing discs. Development 130, 6497-6506. [DOI] [PubMed] [Google Scholar]
- Barolo, S., Stone, T., Bang, A. G. and Posakony, J. W. (2002). Default repression and Notch signaling: hairless acts as an adaptor to recruit the corepressors Groucho and dCtBP to Suppressor of Hairless. Genes Dev. 16, 1964-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano, F., Perez, L., Apidianakis, Y., Delidakis, C. and Milan, M. (2007). Hedgehog restricts its expression domain in the Drosophila wing. EMBO Rep. 8, 778-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair, S. S. (2007). Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu. Rev. Cell Dev. Biol. 23, 293-319. [DOI] [PubMed] [Google Scholar]
- Bokor, P. and DiNardo, S. (1996). The roles of hedgehog, wingless and lines in patterning the dorsal epidermis in Drosophila. Development 122, 1083-1092. [DOI] [PubMed] [Google Scholar]
- Bras-Pereira, C., Bessa, J. and Casares, F. (2006). Odd-skipped genes specify the signaling center that triggers retinogenesis in Drosophila. Development 133, 4145-4149. [DOI] [PubMed] [Google Scholar]
- Buscarlet, M. and Stifani, S. (2007). The `Marx' of Groucho on development and disease. Trends Cell Biol. 17, 353-361. [DOI] [PubMed] [Google Scholar]
- Calleja, M., Moreno, E., Pelaz, S. and Morata, G. (1996) Visualization of gene expression in living adult Drosophila. Science 274, 252-255. [DOI] [PubMed] [Google Scholar]
- Capdevila, J. and Guerrero, I. (1994). Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 13, 4459-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares, F. and Mann, R. S. (2000). A dual role for homothorax in inhibiting wing blade development and specifying proximal wing identities in Drosophila. Development 127, 1499-1508. [DOI] [PubMed] [Google Scholar]
- Cavallo, R. A., Cox, R. T., Moline, M. M., Roose, J., Polevoy, G. A., Clevers, H., Peifer, M. and Bejsovec, A. (1998). Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 395, 604-608. [DOI] [PubMed] [Google Scholar]
- Cifuentes, F. J. and Garcia-Bellido, A. (1997). Proximo-distal specification in the wing disc of Drosophila by the nubbin gene. Proc. Natl. Acad. Sci. USA 94, 11405-11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y. C., Amoyel, M., Qiu, X., Jiang, Y. J., Xu, Q. and Wilkinson, D. G. (2004). Notch activation regulates the segregation and differentiation of rhombomere boundary cells in the zebrafish hindbrain. Dev. Cell 6, 539-550. [DOI] [PubMed] [Google Scholar]
- Dale, J. K., Maroto, M., Dequeant, M. L., Malapert, P., McGrew, M. and Pourquie, O. (2003). Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature 421, 275-278. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F. and Ruiz-Gomez, M. (1995). groucho and hedgehog regulate engrailed expression in the anterior compartment of the Drosophila wing. Development 121, 3467-3476. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F. and Bray, S. (1997). Feed-back mechanisms affecting Notch activation at the dorsoventral boundary in the Drosophila wing. Development 124, 3241-3251. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., Garcia-Bellido, A. and Bray, S. J. (1996). Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development 122, 359-369. [DOI] [PubMed] [Google Scholar]
- de Celis, J. F., Tyler, D. M., de Celis, J. and Bray, S. J. (1998). Notch signalling mediates segmentation of the Drosophila leg. Development 125, 4617-4626. [DOI] [PubMed] [Google Scholar]
- de Celis Ibeas, J. M. and Bray, S. J. (2003). Bowl is required downstream of Notch for elaboration of distal limb patterning. Development 130, 5943-5952. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea, F. J. and Cohen, S. M. (1995). Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121, 4215-4225. [DOI] [PubMed] [Google Scholar]
- Dietzl, G., Chen, D., Schnorrer, F., Su, K. C., Barinova, Y., Fellner, M., Gasser, B., Kinsey, K., Oppel, S., Scheiblauer, S. et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156. [DOI] [PubMed] [Google Scholar]
- Diez del Corral, R., Aroca, P., Gómez-Skarmeta, J. L., Cavodeassi, F. and Modolell, J. (1999). The Iroquois homeodomain proteins are required to specify body wall identity in Drosophila. Genes Dev. 13, 1754-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, D., Feger, G., Younger-Shepherd, S., Jan, L. Y. and Jan, Y. N. (1996). Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes Dev. 10, 421-434. [DOI] [PubMed] [Google Scholar]
- Estrach, S., Ambler, C. A., Lo Celso, C., Hozumi, K. and Watt, F. M. (2006). Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 133, 4427-4438. [DOI] [PubMed] [Google Scholar]
- Funakoshi, Y., Minami, M. and Tabata, T. (2001). mtv shapes the activity gradient of the Dpp morphogen through regulation of thickveins. Development 128, 67-74. [DOI] [PubMed] [Google Scholar]
- Furriols, M. and Bray, S. (2000). Dissecting the mechanisms of suppressor of hairless function. Dev. Biol. 227, 520-532. [DOI] [PubMed] [Google Scholar]
- Gallet, A., Erkner, A., Charroux, B., Fasano, L. and Kerridge, S. (1998). Trunk-specific modulation of wingless signalling in Drosophila by teashirt binding to armadillo. Curr. Biol. 8, 893-902. [DOI] [PubMed] [Google Scholar]
- Glise, B., Jones, D. L. and Ingham, P. W. (2002). Notch and Wingless modulate the response of cells to Hedgehog signalling in the Drosophila wing. Dev. Biol. 248, 93-106. [DOI] [PubMed] [Google Scholar]
- Goldstein, R. E., Cook, O., Dinur, T., Pisante, A., Karandikar, U. C., Bidwai, A. and Paroush, Z. (2005). An eh1-like motif in odd-skipped mediates recruitment of Groucho and repression in vivo. Mol. Cell. Biol. 25, 10711-10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, R. B., Hatini, V., Johansen, K. A., Liu, X. J. and Lengyel, J. A. (2002). Drumstick is a zinc finger protein that antagonizes Lines to control patterning and morphogenesis of the Drosophila hindgut. Development 129, 3645-3656. [DOI] [PubMed] [Google Scholar]
- Hao, I., Green, R. B., Dunaevsky, O., Lengyel, J. A. and Rauskolb, C. (2003). The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation. Dev. Biol. 263, 282-295. [DOI] [PubMed] [Google Scholar]
- Hasson, P., Egoz, N., Winkler, C., Volohonsky, G., Jia, S., Dinur, T., Volk, T., Courey, A. J. and Paroush, Z. (2005). EGFR signaling attenuates Groucho-dependent repression to antagonize Notch transcriptional output. Nat. Genet. 37, 101-105. [DOI] [PubMed] [Google Scholar]
- Hatini, V., Bokor, P., Goto-Mandeville, R. and DiNardo, S. (2000). Tissue- and stage-specific modulation of Wingless signaling by the segment polarity gene lines. Genes Dev. 14, 1364-1376. [PMC free article] [PubMed] [Google Scholar]
- Hatini, V., Green, R. B., Lengyel, J. A., Bray, S. J. and Dinardo, S. (2005). The Drumstick/Lines/Bowl regulatory pathway links antagonistic Hedgehog and Wingless signaling inputs to epidermal cell differentiation. Genes Dev. 19, 709-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, U., Giebel, B. and Campos-Ortega, J. A. (1994). The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell 76, 77-87. [DOI] [PubMed] [Google Scholar]
- Hofmann, M., Schuster-Gossler, K., Watabe-Rudolph, M., Aulehla, A., Herrmann, B. G. and Gossler, A. (2004). WNT signaling, in synergy with T/TBX6, controls Notch signaling by regulating Dll1 expression in the presomitic mesoderm of mouse embryos. Genes Dev. 18, 2712-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki, D. D., Johansen, K. A., Singer, J. B. and Lengyel, J. A. (2001). drumstick, bowl, and lines are required for patterning and cell rearrangement in the Drosophila embryonic hindgut. Dev. Biol. 240, 611-626. [DOI] [PubMed] [Google Scholar]
- Klein, T., Seugnet, L., Haenlin, M. and Martinez Arias, A. (2000). Two different activities of Suppressor of Hairless during wing development in Drosophila. Development 127, 3553-3566. [DOI] [PubMed] [Google Scholar]
- Lawrence, N., Dearden, P., Hartley, D., Roose, J., Clevers, H. and Arias, A. M. (2000). dTcf antagonises Wingless signalling during the development and patterning of the wing in Drosophila. Int. J. Dev. Biol. 44, 749-756. [PubMed] [Google Scholar]
- Lee, J. J., von Kessler, D. P., Parks, S. and Beachy, P. A. (1992). Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell 71, 33-50. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco, E., Gampel, A., Ring, J., Virdee, K., Kirov, N., Tolkovsky, A. M. and Martinez-Arias, A. (1998). puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12, 557-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner, M., Bleasby, A. and Kelly, S. (1984). The role of the peripodial membrane of leg and wing imaginal discs of Drosophila melanogaster during evagination and differentiation in vitro. Wilhelm Roux's Arch. Dev. Biol. 193, 180-186. [DOI] [PubMed] [Google Scholar]
- Minami, M., Kinoshita, N., Kamoshida, Y., Tanimoto, H. and Tabata, T. (1999). brinker is a target of Dpp in Drosophila that negatively regulates Dpp-dependent genes. Nature 398, 242-246. [DOI] [PubMed] [Google Scholar]
- Morel, V., Lecourtois, M., Massiani, O., Maier, D., Preiss, A. and Schweisguth, F. (2001). Transcriptional repression by suppressor of hairless involves the binding of a hairless-dCtBP complex in Drosophila. Curr. Biol. 11, 789-792. [DOI] [PubMed] [Google Scholar]
- Mukherjee, T., Hombria, J. C. and Zeidler, M. P. (2005). Opposing roles for Drosophila JAK/STAT signalling during cellular proliferation. Oncogene 24, 2503-2511. [DOI] [PubMed] [Google Scholar]
- Mullor, J. L., Calleja, M., Capdevila, J. and Guerrero, I. (1997). Hedgehog activity, independent of decapentaplegic, participates in wing disc patterning. Development 124, 1227-1237. [DOI] [PubMed] [Google Scholar]
- Musacchio, M. and Perrimon, N. (1996). The Drosophila kekkon genes: novel members of both the leucine-rich repeat and immunoglobulin superfamilies expressed in the CNS. Dev. Biol. 178, 63-76. [DOI] [PubMed] [Google Scholar]
- Nagel, A. C., Krejci, A., Tenin, G., Bravo-Patino, A., Bray, S., Maier, D. and Preiss, A. (2005). Hairless-mediated repression of notch target genes requires the combined activity of Groucho and CtBP corepressors. Mol. Cell. Biol. 25, 10433-10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, M., Diaz-Benjumea, F. J. and Cohen, S. M. (1995). Nubbin encodes a POU-domain protein required for proximal-distal patterning in the Drosophila wing. Development 121, 589-599. [DOI] [PubMed] [Google Scholar]
- Nolo, R., Abbott, L. A. and Bellen, H. J. (2000). Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102, 349-362. [DOI] [PubMed] [Google Scholar]
- Nusinow, D., Greenberg, L. and Hatini, V. (2008). Reciprocal roles for bowl and lines in specifying the peripodial epithelium and the disc proper of the Drosophila wing primordium. Development 135, 3031-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai, L. M., Orsulic, S., Bejsovec, A. and Peifer, M. (1997). Negative regulation of Armadillo, a Wingless effector in Drosophila. Development 124, 2255-2266. [DOI] [PubMed] [Google Scholar]
- Pallavi, S. K. and Shashidhara, L. S. (2003). Egfr/Ras pathway mediates interactions between peripodial and disc proper cells in Drosophila wing discs. Development 130, 4931-4941. [DOI] [PubMed] [Google Scholar]
- Pignoni, F. and Zipursky, S. L. (1997). Induction of Drosophila eye development by decapentaplegic. Development 124, 271-278. [DOI] [PubMed] [Google Scholar]
- Preiss, A., Hartley, D. A. and Artavanis-Tsakonas, S. (1988). The molecular genetics of Enhancer of split, a gene required for embryonic neural development in Drosophila. EMBO J. 7, 3917-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson, E. J., Micchelli, C. A., Axelrod, J. D., Perrimon, N. and Blair, S. S. (1996). wingless refines its own expression domain on the Drosophila wing margin. Nature 384, 72-74. [DOI] [PubMed] [Google Scholar]
- Takei, Y., Ozawa, Y., Sato, M., Watanabe, A. and Tabata, T. (2004). Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 131, 73-82. [DOI] [PubMed] [Google Scholar]
- Tena, J. J., Neto, A., de la Calle-Mustienes, E., Bras-Pereira, C., Casares, F. and Gomez-Skarmeta, J. L. (2007). Odd-skipped genes encode repressors that control kidney development. Dev. Biol. 301, 518-531. [DOI] [PubMed] [Google Scholar]
- Terriente, J., Perea, D., Suzanne, M. and Diaz-Benjumea, F. J. (2008). The Drosophila gene zfh2 is required to establish proximal-distal domains in the wing disc. Dev. Biol. 320, 102-112. [DOI] [PubMed] [Google Scholar]
- Tolwinski, N. S. and Wieschaus, E. (2004). A nuclear escort for beta-catenin. Nat. Cell Biol. 6, 579-580. [DOI] [PubMed] [Google Scholar]
- van de Wetering, M., Cavallo, R., Dooijes, D., van Beest, M., van Es, J., Loureiro, J., Ypma, A., Hursh, D., Jones, T., Bejsovec, A. et al. (1997). Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789-799. [DOI] [PubMed] [Google Scholar]
- Wang, L. and Coulter, D. E. (1996). bowel, an odd-skipped homolog, functions in the terminal pathway during Drosophila embryogenesis. EMBO J. 15, 3182-3196. [PMC free article] [PubMed] [Google Scholar]
- Wang, S. H., Simcox, A. and Campbell, G. (2000). Dual role for Drosophila epidermal growth factor receptor signaling in early wing disc development. Genes Dev. 14, 2271-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth, A. J. and Russell, S. (2003). Temporally dynamic response to Wingless directs the sequential elaboration of the proximodistal axis of the Drosophila wing. Dev. Biol. 254, 277-288. [DOI] [PubMed] [Google Scholar]
- Wu, J. and Cohen, S. M. (2002). Repression of Teashirt marks the initiation of wing development. Development 129, 2411-2418. [DOI] [PubMed] [Google Scholar]
- Yeo, S. L., Lloyd, A., Kozak, K., Dinh, A., Dick, T., Yang, X., Sakonju, S. and Chia, W. (1995). On the functional overlap between two Drosophila POU homeo domain genes and the cell fate specification of a CNS neural precursor. Genes Dev. 9, 1223-1236. [DOI] [PubMed] [Google Scholar]
- Zecca, M. and Struhl, G. (2002a). Control of growth and patterning of the Drosophila wing imaginal disc by EGFR-mediated signaling. Development 129, 1369-1361. [DOI] [PubMed] [Google Scholar]
- Zecca, M. and Struhl, G. (2002b). Subdivision of the Drosophila wing imaginal disc by EGFR-mediated signaling. Development 129, 1357-1368. [DOI] [PubMed] [Google Scholar]
- Zecca, M. and Struhl, G. (2007). Recruitment of cells into the Drosophila wing primordium by a feed-forward circuit of vestigial autoregulation. Development 134, 3001-3010. [DOI] [PubMed] [Google Scholar]
- Zirin, J. D. and Mann, R. S. (2007). Nubbin and Teashirt mark barriers to clonal growth along the proximal-distal axis of the Drosophila wing. Dev. Biol. 304, 745-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.