Summary
Integrin receptors for the extracellular matrix and receptor tyrosine kinase growth factor receptors represent two of the major families of receptors that transduce into cells information about the surrounding environment. Wnt proteins are a major family of signaling molecules that regulate morphogenetic events. There is presently little understanding of how the expression of Wnt genes themselves is regulated. In this study, we demonstrate that α3β1 integrin, a major laminin receptor involved in the development of the kidney, and c-Met, the receptor for hepatocyte growth factor, signal coordinately to regulate the expression of Wnt7b in the mouse. Wnt signals in turn appear to regulate epithelial cell survival in the papilla of the developing kidney, allowing for the elongation of epithelial tubules to form a mature papilla. Together, these results demonstrate how signals from integrins and growth factor receptors can be integrated to regulate the expression of an important family of signaling molecules so as to regulate morphogenetic events.
Keywords: Integrins, Signal transduction, Wnt genes, Receptor tyrosine kinase
INTRODUCTION
Integrins are a major family of heterodimeric transmembrane receptors through which cells receive information about their surrounding extracellular matrix (ECM) (Hynes, 1992). From the very first stages of embryogenesis, cells establish interactions with the ECM that are essential for proper differentiation and tissue organization (Hogan, 1999; Hynes, 1999). Upon interaction with the ECM, integrin-mediated signal transduction elicits behavioral responses that include cell proliferation, survival and differentiation, and migration (Boudreau and Bissell, 1998; Giancotti and Ruoslahti, 1999; Howe et al., 1998; Huang and Ingber, 1999; Miranti and Brugge, 2002; Ruoslahti, 1999; Schwartz and Baron, 1999). Integrins signal through multiple biochemical pathways; however, there is limited information relating distinct pathways to specific biological processes in tissues and organs. Nevertheless, genetic studies have demonstrated the importance of integrin-mediated signaling during the development of invertebrates and mammals (for a review, see Danen and Sonnenberg, 2003). For example, in nematodes and flies, integrin-mediated cell adhesion and signaling can regulate development of the muscular system, central nervous system and gut (for a review, see Hynes and Zhao, 2000). Similarly, integrin-mediated signaling can regulate organ development in mammals. In mice, inactivation of the β1 integrin gene, which eliminates expression of the entire class of β1 integrins, results in very early embryonic lethality (Fassler et al., 1996; Fassler and Meyer, 1995; Stephens et al., 1995). Furthermore, mutation of distinct α subunits that heterodimerize with β1 has provided information about the function of different integrin heterodimers in development. For example, loss of α5β1 integrin (Goh et al., 1997; Taverna et al., 1998; Yang et al., 1993), or of α4β1 integrin (Yang et al., 1995), leads to mesodermal or to placental and cardiac defects, respectively. The α6-containing integrins α6β1 and α6β4 have been found to play important roles in development of the skin and nervous system (Georges-Labouesse et al., 1998; Georges-Labouesse et al., 1996; Gorski and Olsen, 1998), whereas α1β1- and α2β1-deficient mice show no apparent developmental defects. Mutation of the α3 integrin gene leads to defects in development of the kidney, skin, brain and salivary glands (Anton et al., 1999; DiPersio et al., 1997; Kreidberg et al., 1996; Menko et al., 2001).
Wnts are secreted signaling molecules that have been implicated in a wide array of biological processes including morphogenesis, cell proliferation, survival and tumorigenesis (Wodarz and Nusse, 1998). In the developing kidney, Wnt9b is expressed in the ureteric bud and is essential for inducing the metanephric mesenchyme to form nephrons (Carroll et al., 2005). Wnt4, which is expressed in the pretubular aggregates, is required for the mesenchymal-to-epithelial transformation of these aggregates into the simple tubules that develop into nephrons (Kispert et al., 1998; Stark et al., 1994). Wnt11 is expressed at the tip of the ureteric buds, where it appears to have a role in modulating branching morphogenesis of the derivatives of the ureteric bud (Kispert et al., 1996; Majumdar et al., 2003). Wnt7b is expressed in ureteric bud stalks and collecting ducts (Kispert et al., 1996). Mice deficient in Wnt7b have lung hemorrhages caused by vascular smooth muscle defects, and lung hypoplasia resulting from impaired branching morphogenesis, mesenchyme proliferation and epithelial differentiation (Shu et al., 2002), but the Wnt7b kidney phenotype is unreported.
α3β1 integrin has been best characterized as a receptor for certain isoforms of laminin, including laminin 5, 10 and 11. Our recent work (Chattopadhyay et al., 2003) also ascribes a role for α3β1 integrin in cell-cell interaction as a component of the E-cadherin adhesion complex. In α3β1-deficient mice, several abnormalities of epithelial development are evident, including malformation of the papilla in the developing kidney (Kreidberg et al., 1996). The papilla is a cone-shaped collection of densely packed epithelial tubules that includes collecting ducts and loops of Henle. Although its formation is not completely understood, it clearly involves several rounds of branching morphogenesis, followed by tubular extension, differentiation and tissue remodeling (Cebrian et al., 2004; Osathanondh and Potter, 1963). In α3β1 integrin-deficient kidneys, although differentiated collecting ducts are present, the papilla is much smaller and does not extend out as a projection from the main body of the kidney. These observations suggest that α3β1 integrin might be involved in regulating the patterning that results in the formation of the mature papilla.
In our previous study we observed increased levels of β-catenin in the presence of α3β1 integrin (Chattopadhyay et al., 2003). Although higher levels of β-catenin might be a consequence of α3β1 integrin stimulation of cadherin-mediated cell-cell adhesion, it is also possible that higher levels of β-catenin might reflect increased Wnt signaling through the canonical Wnt pathway. Here we report that α3β1 integrin, acting in coordination with the hepatocyte growth factor (Hgf) receptor c-Met (also known as Met), regulates expression of two of the three Wnt7b transcripts expressed in the developing papilla. Furthermore, the expression of Wnt7b appears to regulate cell survival. Thus, these results demonstrate how integrin-receptor tyrosine kinase complexes may regulate the expression of signaling molecules involved in pattern formation during development.
MATERIALS AND METHODS
Antibodies, reagents and constructs
The following were used: fetal calf serum (FCS) (SH30071.03, Hyclone), Hgf (H1404, Sigma), X-Gal (B9146, Sigma), the mouse Wnt customized MultiGene-12 RT-PCR Profiling Kit (SuperArray), Hgf-neutralizing antibody (AF-294-NA, R&D Systems), IGF-neutralizing antibody (AF-291-NA, R&D Systems), paraformaldehyde (PFA) (76240, Fluka), anti-digoxigenin antibody (1109327490, Roche, Indianapolis, USA), BM Purple alkaline phosphatase substrate (11442074001, Roche), anti-c-Met antibody (3127, Cell Signaling), anti-α3 integrin antibody (Invitrogen, Carlsbad, USA), anti-PI3K antibody (4292, Cell Signaling), anti-phospho PI3K p85(Thr458)/p55(Thr199) antibody (Cell Signaling), anti-phospho-AKT (Thr308) antibody (9275, Cell Signaling), ImmunoPure immobilized Protein G (20398, Pierce), ImmunoPure immobilized Protein A (20333, Pierce), protease-free BSA (A3059, Sigma), anti-phosphotyrosine antibody 4G10 (05-321, Upstate Biotech, Lake Placid, USA), ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (S7111, Chemicon, Temecula, USA), Lipofectamine reagent (11668-019, Invitrogen) and DMEM/F12 media (10-092-CV, Cellgro).
Axin, Fz8CRD and Dkk1 plasmid expression constructs and a control construct expressing only the Fc region of the Fz8CRD construct were obtained from Xi He (Children's Hospital, Boston, MA, USA).
Conditional allele of the α3 integrin gene
A vector to target a conditional mutation to the α3 integrin gene was constructed using the pDELBOY vector. LoxP sites flank exon 3, and a Frt-NeoR-Frt cassette was placed downstream of exon 2 (see Fig. S1 in the supplementary material). Deletion of exon 3 creates translational termination codons in exon 4. After homologous recombination in embryonic stem cells and derivation of mice containing this allele, the Frt-NeoR-Frt cassette was excised by mating these mice with mice expressing FlpE in the germ line (obtained from Dr Susan Dymecki, Harvard Medical School, Boston, MA, USA). This left a single Frt site between exon 2 and the loxP site upstream of exon 3. Further details of the construction and genotyping are available upon request. For conditional mutation of the α3 integrin gene, mice were mated with HoxB7-Cre/GFP mice (Zhao et al., 2004).
Laminin mutant mice
Lama5-null mice have been described (Miner and Li, 2000).
Cell culture
Generation and maintenance of wild-type (WT), α3 integrin knockout (KO), α3 and α6 integrin stalk and laminin binding mutant cells have been described previously (Chattopadhyay et al., 2003; Wang et al., 1999). For the present studies, WT and KO cells were generated a second time from E18 papillae of mice carrying a temperature-sensitive T-antigen and the results obtained with the previously and newly developed cell lines were identical. Cells were routinely cultured on Matrigel-coated plates (Becton Dickinson). Hgf stimulation experiments involved a 16-hour incubation in serum-free medium followed by the addition of medium containing 50 ng/ml Hgf. To study the effect of the Hgf-neutralizing antibody with cell lines, WT cells were treated either with 10 μg/ml Hgf-neutralizing antibody or 10 μg/ml IGF-neutralizing antibody (as control) for 12 hours. To study the effect of Wnt3a, HEK293T cells were transfected with plasmids expressing the Wnt inhibitors Fz8CRD (or control IgG) or Dkk1 using Lipofectamine (Invitrogen) and incubated for 24 hours. After 16 hours, the medium was collected and replaced with fresh DMEM/F12. After an additional 36 hours incubation, the conditioned medium was collected, centrifuged, and used to incubate kidney papillae for 24 hours (or to treat cells in the experiments shown in the supplementary figures).
Organ culture
To analyze apoptosis in organ cultures, kidney papillae were isolated from E18.5 mouse embryos using fine-needle microdissection to remove the papilla from the cortex and outer medulla. The kidney papillae were then kept under standard organ culture conditions on Nuclepore membranes for 24 hours in DMEM/10% FCS in the presence of inhibitors or activators as described. The preparation of Wnt- or inhibitor-conditioned medium was as described above. To study the effect of Hgf, kidney papillae were treated for 24 hours in the presence of 20 μg/ml Hgf-neutralizing antibody or IGF-neutralizing antibody or 50 ng/ml Hgf prior to fixation. After 24 hours in culture, the kidneys were snap frozen in OCT (Sakura, Torrance, USA) to provide frozen sections for TUNEL and DAPI staining.
Wnt reporter staining
Mice carrying a Tcf/β-catenin-responsive luciferase reporter were obtained from Dr Benjamin Allman (University of Toronto, Ontario, Canada). Frozen sections were stained for β-galactosidase expression as described (Sanes et al., 1986).
RT-PCR
Specific isoforms of Wnt7b were measured using semi-quantitative RT-PCR, as the PCR reactions required to define specific transcripts result in PCR products that are too long for use in real-time PCR assays. Cycle number for each reaction was minimized to assure that amplification was in the linear range for each assay. Total RNA was isolated from cells as described (Chomczynski and Sacchi, 1987). Seven micrograms of total RNA was used for the reverse transcription reaction using the Superscript III First-Strand cDNA Synthesis Kit (Invitrogen). The resulting cDNA was subjected to PCR using the following primers (shown 5′ to 3′). For Wnt7b, AAGCACCCACGTAGGTAACG (primer i), AAACCAAGTGACCACCAAGC (ii), AGGTGTCTCTTTGGAGCCG (iii), TCTATTGCCCGCAGATCTTT (iv), GCGACAGGAGGAGCATACTT (v), CTTCACGTAGAGGACGCCAA (vi), CTCTCGACTCCCTACTCGGA (vii); and for Wnt4, GGCGTAGCCTTCTCACAGTC and AGCACGTCTTTACCTCGCAG. PCR products were cloned using the PCR-II-TOPO Cloning Kit (Invitrogen) and sequenced before preparing in situ hybridization probes.
Quantitative PCR
Quantitative PCR to detect all Wnt7b transcripts was performed using a Cephiad Smart Cycler II. Primers (shown 5′ to 3′) were: forward, TTTGGCGTCCTCTACGTGAAG and reverse, CCCGACTCCCCACTTTGAG. Cycles: 94°C for 30 seconds; 40 cycles of 94°C for 15 seconds, 58°C for 30 seconds, 72°C for 30 seconds; a final 72°C for 120 seconds. All reactions were validated by examining a melt curve for a single peak between 58°C and 95°C. Results were normalized to 18S rRNA.
In situ hybridization
Mouse kidneys were fixed in 4% PFA overnight and cryopreserved in 30% sucrose. Kidneys were then fixed in OCT and sectioned. Frozen kidney sections (10 μm) were refixed in 4% PFA and treated with 15 μg/ml proteinase K. After refixing and acetylation, sections were hybridized with 500 ng/ml digoxigenin-labeled probes (sense and antisense) in 1.3× SSC buffer. Sections were then washed extensively, blocked in sheep serum and reacted with alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche). The signals were detected using BM Purple alkaline phosphatase substrate.
Immunoprecipitation and western blot
Cells were grown in 10-cm dishes, washed with PBS and lysed in lysis buffer (20 mM Tris-HCl pH 7.6, 1% Triton X-100, 2 mM CaCl2, 1 mM benzamidine, 0.1 mM ammonium molybdate, 1 mM PMSF, 20 μg/ml aprotinin, 10 μg/ml leupeptin). Insoluble materials were cleared from the lysate by centrifugation at 14,000 rpm (10,000 g) for 20 minutes. For western blotting, 20 μg protein was subjected to SDS-PAGE, followed by western blot with the specific antibody. For immunoprecipitation, 100 μg protein was subjected to immunoprecipitation with the specific antibody followed by western blot.
For the c-Met phosphorylation assay, cells were incubated in 1 mM sodium orthovanadate for 30 minutes, washed with ice-cold PBS and lysed. All the buffers used for cell lysis contained 2 mM sodium orthovanadate. Cellular protein (100 mg) was subjected to immunoprecipitation with anti-c-Met antibody and Protein G-agarose. The immunoprecipitate was separated on a 7.5% SDS-PAGE gel and transferred to nitrocellulose membranes. The membranes were blocked with 5% protease-free BSA, and then probed with an anti-phosphotyrosine antibody (4G10).
To reprobe the blot, the membrane was stripped using 62.5 mM Tris-HCl pH 6.7, 2% SDS, 0.7% β-mercaptoethanol, then reblocked and probed with specific antibody.
TUNEL assay
The fluorescent TUNEL assay was used to determine apoptosis in cells, following the manufacturer's protocol (Chemicon).
RESULTS
α3β1 integrin-dependent Wnt signaling in mouse kidney epithelial cells
The papilla of the mammalian kidney forms through a complex process that includes branching morphogenesis, tubular elongation and additional tissue remodeling. The number of branching events known to occur prior to E15.5 can account for the number of collecting ducts observed in the mature papilla (Cebrian et al., 2004), without the need for any additional branching within the papilla during subsequent development of the kidney. Rather, the enlargement of the papilla is due to tubular elongation (Cebrian et al., 2004), maturation of the epithelia in collecting ducts that involves significant apical-basal lengthening, and the penetration of loops of Henle from nephrons located in the cortex down into the medulla and papilla.
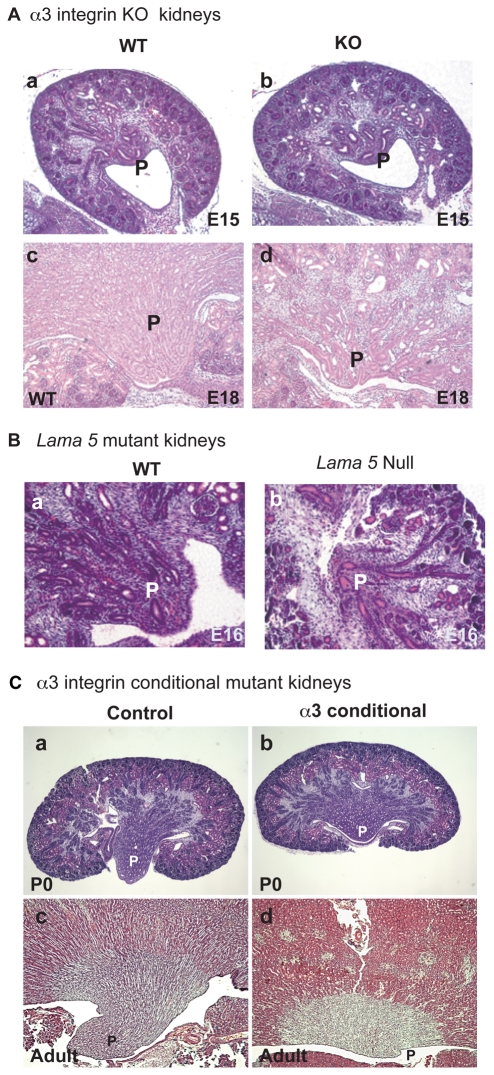
At E15.5, wild-type (WT) and α3 integrin mutant (KO) embryonic kidneys were indistinguishable with regard to branching morphogenesis (Fig. 1A,B). However, by E18.5, there was a marked difference between the WT and KO kidneys, primarily reflected in the failure of papillary outgrowth (Fig. 1C,D). The failure of papillary outgrowth was completely penetrant and observed in all α3 integrin KO kidneys examined (n>6).
Fig. 1.
Histology of α3β1 integrin-deficient or Lama5-deficient kidney papillae. (A) Histology and proliferation of (a,c) wild-type (WT) and (b,d) α3 integrin KO kidneys. (a,b) E15; (c,d) detail of papilla (P) from E18 kidneys. (B) WT (a) and Lama5-null (b) papillae at E16. (C) Control (a,c) and α3 integrin conditional KO (b,d) kidneys, obtained using HoxB7-Cre deleter mice, at (a,b) P0 and (c,d) at 7 months. In d, the P label is adjacent to the minimal papilla present. Genotypes: (a) α3 integrinflox/-; (b,d) α3 integrinflox/-, HoxB7-Cre+; (c) WT.
The malformation of the papilla in α3β1 integrin-deficient embryonic kidneys may relate to the role of this integrin as a laminin receptor (Elices et al., 1991), or to its recently described role in the E-cadherin cell-cell adhesion complex (Chattopadhyay et al., 2003). Genetic tools are not presently available to test the role of α3β1 integrin in cell-cell adhesion in vivo. However, it is possible to examine papillary development in embryonic kidneys from mice carrying a mutation in the α5 laminin gene (Lama5), α5 being the subunit of laminin 10 that contains the α3β1 integrin binding site (Kikkawa et al., 1998). Similar to kidneys of α3 integrin KO embryos, kidneys of α5 laminin-null mutant embryos (Miner and Li, 2000) also had malformed papillae at E16.5 (Fig. 1B); these embryos did not survive to E18.5. Although these results do not exclude a role for α3β1 integrin stimulation of E-cadherin-mediated cell-cell adhesion in formation of the papilla, they do support the possibility that the integrin-laminin interaction is required for normal papillary development.
Malformed papillae are not the only kidney defect observed in α3β1 integrin-deficient kidneys (Kreidberg et al., 1996). Glomeruli are also highly abnormal, raising the question of whether the papillary phenotype could be secondary to glomerular dysfunction, which could expose the tubules of the developing papillae to a protein-rich filtrate that affects its development. To exclude this possibility, we used the HoxB7-Cre/GFP mouse (Zhao et al., 2004) to conditionally delete the α3 integrin gene in the derivatives of the ureteric bud, which include the collecting duct epithelia of the papilla (see Fig. S1 in the supplementary material). Although variable in phenotype, some neonatal and adult conditional mutant mice were observed in which the kidney papillae were largely absent, and the majority of conditionally mutant kidneys had abnormal papillae (Fig. 1C, Table 1). For those conditional mutant kidneys in which some degree of papillary formation occurred, this might be due to non-cell-autonomous rescue of α3β1 integrin-deficient cells by Wnt7b expression (see below) from cells that retained expression of α3β1 integrin.
Table 1.
Abnormal papilla development in α3 integrin conditional mutant kidneys at different ages
|
Abnormal papilla
|
|||
|---|---|---|---|
| Stage | n | n | % |
| PO | 6 | 4 | 67 |
| P5 | 5 | 3 | 60 |
| 8.5 months | 10 | 9 | 90 |
Abnormal papillae were defined as those that were less than 50% of the length of a normal papilla as judged from a section through the mid-line of the kidney.
n, number of animals.
In our previous report, we observed higher levels of β-catenin in cells expressing α3β1 integrin (Chattopadhyay et al., 2003). To examine whether this might reflect an increase in signaling through the canonical Wnt pathway, the TOPFLASH vector containing a luciferase reporter gene under control of a Tcf/β-catenin-responsive promoter was transfected into immortalized WT and α3β1 integrin-deficient (KO) collecting duct epithelial cell lines (Chattopadhyay et al., 2003; Wang et al., 1999). Luciferase activity was several fold higher in WT than in KO cells or in cells expressing a mutant form of the α3 subunit that is unable to bind laminin (Zhang et al., 2003). The elevated luciferase activity in WT cells was sensitive to Wnt signaling blockade with Dkk1, Fz8CRD (a truncated form of frizzled 8 that contains only the Wnt-association domain) or Axin (see Fig. S2A,B in the supplementary material).
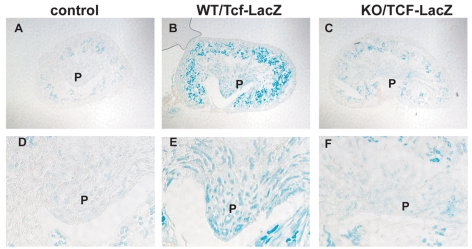
In our previous study we demonstrated that an interaction of the α3 integrin subunit `stalk' domain with the tetraspanin Cd151 was required to stimulate E-cadherin-mediated cell-cell interaction (Chattopadhyay et al., 2003). However, TOPFLASH luciferase activity was not affected by the interaction of α3β1 integrin with Cd151 (see Fig. S2A in the supplementary material). We then examined whether Wnt/β-catenin activity was affected in the absence of α3β1 integrin in vivo, utilizing embryos containing a Tcf-responsive lacZ transgene (Cheon et al., 2002) that were homozygous for the α3 integrin-null allele. Abundant β-galactosidase expression was observed in collecting ducts of papillae from WT E17.5 embryos (WT/Tcf-lacZ in Fig. 2), whereas greatly diminished β-galactosidase staining was apparent in the collecting ducts of KO papillae (KO/Tcf-lacZ in Fig. 2). Thus, canonical Wnt signaling, as reflected by β-catenin levels, indeed appeared to be decreased in the absence of α3β1 integrin and dependent on the interaction with laminin. That higher levels of Wnt signaling could be observed in both cell lines and in vivo in the presence of α3β1 integrin suggested that it might be a cell-autonomous feature of α3β1-expressing cells, rather than being due to a heterotypic interaction between the epithelial tubules and the adjacent stroma, which is known to express additional Wnt genes (Itaranta et al., 2006). This hypothesis was supported by additional in vitro experiments in which TOPFLASH activity was rescued in KO cells that were exposed to conditioned media from WT cells; this rescue could be blocked by addition of the Wnt blockers Dkk1 or Fz8CRD (see Fig. S3A,B in the supplementary material).
Fig. 2.
Tcf/β-catenin reporter transgene expression in wild-type and α3β1 integrin KO kidney papillae. All kidneys are from E17.5 mice. (A,D) Control WT mice without the Tcf-lacZ transgene, showing background staining in the cortex, but minimal staining in the papilla (P). (B,E)WT/Tcf-lacZ mice showing heavy lacZ staining in cortex and papilla. (C,F)KO/Tcf-lacZ mice showing background lacZ staining in the region from where the papilla would emerge, and decreased staining within the cortex. The results shown are representative of those obtained from three sets of kidneys.
Differential regulation of Wnt7b expression by α3β1 integrin
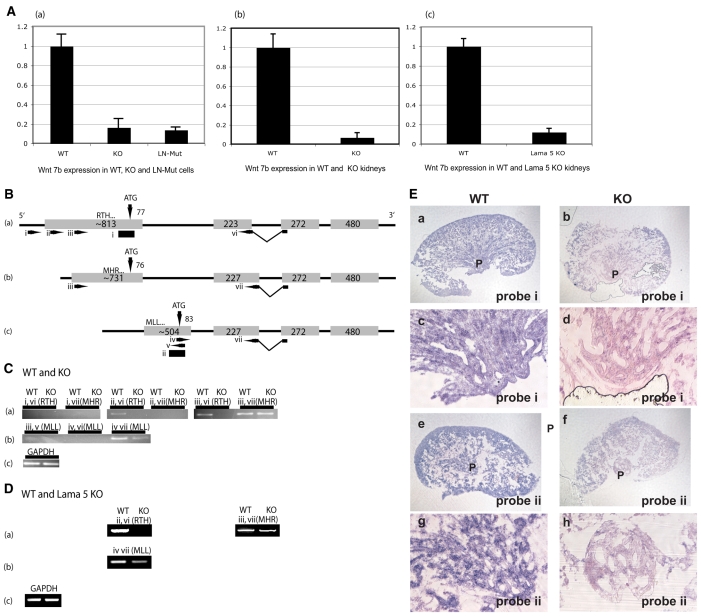
Our studies then focused on Wnt7b, which is known to be expressed in the derivatives of the ureteric bud (Kispert et al., 1996) that form the epithelia of the papilla. Wnt7b expression was decreased several fold in α3 integrin KO cells or in a cell line expressing the laminin binding mutant of the α3 integrin subunit (Zhang et al., 2003) in place of the wild-type subunit, and also in papillae of both α3 integrin KO kidneys and laminin α5 mutant kidneys (Fig. 3A). Three mammalian Wnt7b transcripts are described in the Ensembl database and in recent publications (Rajagopal et al., 2008): RTH, MHR and MLL (here designated by the first three amino acids of the putative peptides). RTH and MHR [recently published as Wnt7b-1 (Rajagopal et al., 2008)] share a common first exon, although the transcription start site of the RTH mRNA has been reported to be upstream of the MHR mRNA. Additionally, the predicted peptide from this first exon differs between the RTH and MHR isoforms owing to the use of different splice donor sites at the end of exon 2 that require the use of different translational start sites in exon 1 to maintain an open reading frame through exon 4. The third isoform, MLL [recently published as Wnt7b-2 (Rajagopal et al., 2008)], is encoded by an entirely different first exon from the other two isoforms, but shares common exons 2, 3 and 4 with RTH and MHR, and the same reading frame as MHR beginning in exon 2 (Fig. 3B).
Fig. 3.
Differential expression of Wnt7b transcripts in α3β1 integrin KO papillae. (A) Real-time PCR for total Wnt7b in (a) cell line G165A B12 that expresses an α3 integrin subunit with a mutation in the laminin-binding domain, (b) papillae of WT and α3 integrin KO E18 mouse kidneys, and (c) WT and Lama5-null E16 whole kidneys. (B) Schematic of the exon/intron structure of the three known mouse Wnt7b transcripts, designated RTH, MHR and MLL according to the first three amino acids of the predicted peptides. The intron lengths are not in proportion to those of the exons (gray boxes). The length of each exon is indicated. The number of predicted translated nucleotides is designated above the exons, adjacent to the arrows that mark the predicted translational start sites (ATG). The locations of PCR primers are shown (arrows, i-vii). The locations of in situ probes are shown as black rectangles below the RTH and MLL exons. See text for further description of the PCR strategy. (C) Detection of Wnt7b expression from RNA prepared from E17 papillae of WT and α3 integrin KO kidneys. The primers used and the transcript identified are designated above each panel. (a) Detection of RTH and MHR transcripts. The RTH transcript is only detected in WT, whereas MHR is detected in both WT and KO. (b) Detection of MLL transcript. Less MLL is detected in the KO than in the WT. (c) Gapdh RT-PCR on WT and KO. (D) Detection of Wnt7b expression from RNA prepared from E16 papillae of WT and Lama5 KO kidneys. The designations are as in C. (E) In situ hybridization for Wnt7b in WT and α3 integrin KO E17 papillae. Probe `i' recognizes both the RTH and MHR transcripts, whereas probe `ii' recognizes only the MLL transcript (see B). (a,b,e,f) Low-magnification views of the entire kidney. (c,d,g,h) High-magnification views of the papilla or area from which the papilla emerges in KO. (a-d) Expression of RTH and MHR. (e-h) Expression of MLL transcript. Each experiment was repeated a minimum of three times.
To confirm the published data regarding these isoforms, and to delimit the 5′ boundaries of the RTH and MHR transcripts in the embryonic kidney, we designed a nested set of 5′ PCR primers spaced 100 bp apart, and 3′ primers were designed to bridge the second and third exons so as to distinguish the RTH and MHR transcripts based on their distinct splice acceptor and donor sites (Fig. 3B). Detection of the MLL transcript utilized a distinct 5′ primer and the same 3′ primer used to detect the MHR transcripts. As shown in Fig. 3C, all three transcripts were detected in WT E17.5 renal papillae, and the RTH transcript appeared to initiate ∼100 bp upstream of the MHR transcript. α3β1 integrin-dependent expression was observed for the RTH and MLL isoforms, whereas the level of MHR transcript did not appear to differ between the WT and α3 integrin KO kidney papillae. There was a similar pattern of decreased Wnt7b isoform expression in kidneys of α5 laminin-mutant embryos (Fig. 3D).
In situ hybridization confirmed the α3β1 integrin-dependent expression of the Wnt7b transcripts in the developing papillae. The MLL transcript of Wnt7b was less abundant in α3β1-deficient kidney papillae, and a probe recognizing both RTH and MHR mRNAs also demonstrated decreased expression in the absence of α3β1 integrin (Fig. 3E). These results indicate that α3β1 integrin differentially regulates the expression of Wnt7b isoforms in the developing kidney.
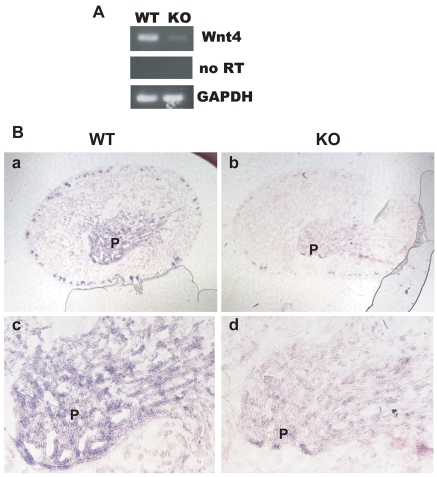
The expression of a second Wnt gene expressed in the papilla, Wnt4 (Itaranta et al., 2006), was also examined. When assessed by RT-PCR or in situ hybridization (Fig. 4), Wnt4 expression also appeared to be decreased in the absence of α3β1 integrin.
Fig. 4.
Expression of Wnt4 by wild-type and α3β1 integrin-deficient kidney papillae. (A) Wnt4 RT-PCR from WT and α3β1 integrin KO mouse papillae. The middle panel is a control reaction that omitted reverse transcriptase (RT). (B) In situ hybridization for Wnt4 in (a,c) WT or (b,d) KO papillae (P). (a,b) Low-magnification views of entire kidneys. (c,d) High-magnification views of the papilla or area from which the papilla would have emerged in the KO.
Coordinate signaling between α3β1 integrin and a receptor tyrosine kinase
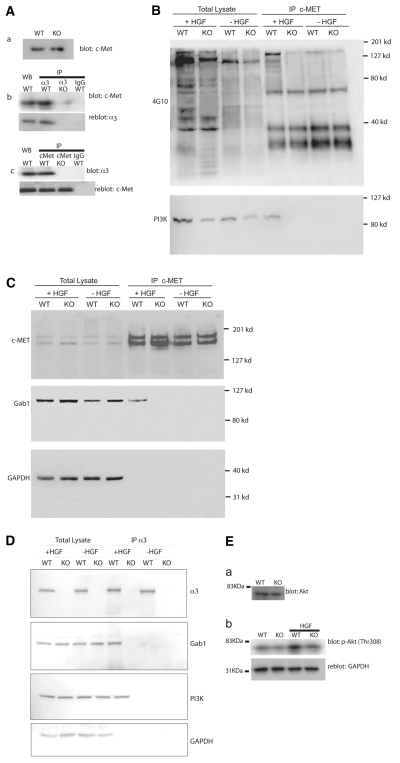
Integrins and growth factor receptors signal coordinately to integrate signaling by soluble growth factors and the ECM. In some instances, a physical association between integrins and growth factor receptors has been identified (Comoglio et al., 2003; Eliceiri, 2001). Therefore, we investigated whether α3β1 integrin interacts with a growth factor receptor to stimulate Wnt gene expression and thus regulate kidney morphogenesis. Among the growth factor receptors known to be expressed in the developing kidney and to potentially regulate branching morphogenesis, the Hgf receptor c-Met was detected in immortalized collecting duct epithelial cell lines by western blotting irrespective of the presence or absence of α3β1 integrin (Fig. 5Aa). Two other related growth factor receptors, c-Ret and c-Ron (Mst1r - Mouse Genome Informatics), were not present in these cells (not shown). Although Hgf has been reported to induce branching morphogenesis in vitro with MDCK cells (Jeffers et al., 1996; Montesano et al., 1991; Stoker et al., 1987; Zhang and Vande Woude, 2003), no kidney defects were reported in c-Met or Hgf mutant kidneys (Birchmeier and Gherardi, 1998; Schmidt et al., 1995; Uehara et al., 1995). However, c-Met- and Hgf-deficient embryos die of hepatic failure prior to the time when defects are observed in α3β1 integrin-deficient kidneys, potentially obscuring later defects in kidney development owing to the absence of c-Met or Hgf. It was possible to co-immunoprecipitate α3β1 integrin and c-Met, indicating that there may indeed be coordinate signaling by these receptors (Fig. 5Ab,c).
Fig. 5.
Coordinate signaling between α3β1 integrin and c-Met. WT and α3β1 integrin KO immortalized epithelial cell lines were used as indicated. (A) Co-immunoprecipitation of α3β1 integrin and c-Met. (a) Western blot of c-Met. (b) Immunoprecipitation with anti-α3 integrin or control rabbit IgG followed by western blot with anti-c-Met. Lower panel is a reblot for the α3 integrin subunit. (c) Immunoprecipitation with anti-c-Met or control mouse IgG followed by western blot with anti-α3 integrin subunit. Lower panel is a reblot for c-Met. The first four lanes in b and c are direct western blots of the lysates prior to immunoprecipitation. (B) Tyrosine phosphorylation of c-Met. Hgf treatment is designated above the panel. Total lysate indicates a direct western blot for phosphotyrosine. On the right are cell lysates immunoprecipitated with anti-c-Met antibody, followed by western blot with anti-phosphotyrosine antibody (4G10). Lower panel is a reblot with anti-PI3K, indicating the PI3K only co-immunoprecipitated in WT cells after stimulation with Hgf. (C) Association of Gab1 with c-Met. The same extracts and immunoprecipitates were used in B and C. Total lysate indicates a direct western blot for c-Met, Gab1 and Gapdh as a loading control. On the right are cell lysates immunoprecipitated with anti-c-Met antibody. The upper panel is a positive control for the immunoprecipitations in B and C. The lower panel is a reblot with anti-Gab1, showing co-immunoprecipitation with c-Met only in WT cells after stimulation with Hgf. (D) Co-immunoprecipitation of Gab1 and PI3K with α3β1 integrin. The first four lanes are a direct western blot of WT or α3 integrin KO cells treated with Hgf, or untreated. The right-hand four lanes are an immunoprecipitation with anti-α3 integrin, followed by western blot using antibodies noted at the right of each panel. Gab1 and PI3K only co-immunoprecipitate with α3β1 integrin in WT cells stimulated with Hgf. The overall levels of Gab1 and PI3K in whole-cell extracts are unaffected by stimulation with Hgf. (E) Activation of AKT. (a) Western blot for AKT. (b) Western blot with anti-phosphothreoine 308 AKT antibody.
A previous study has demonstrated a requirement for α3β1 integrin and its associated tetraspanin CD151 for activation of c-MET in cells derived from human salivary gland carcinomas (Klosek et al., 2005). As a first determination of whether α3β1 integrin has a modulatory effect on signaling by c-Met that could relate to the α3 integrin mutant kidney phenotype, tyrosine phosphorylation of c-Met in response to Hgf was examined in WT and α3β1-deficient (KO) cells. Hgf treatment induced higher levels of tyrosine phosphorylation of several proteins in WT cells as compared with KO cells (Fig. 5B). Moreover, a c-Met immunoprecipitate, blotted with anti-phosphotyrosine, also detected increased tyrosine phosphorylation and additional bands not present in KO cells (Fig. 5B), suggesting that multi-molecular complexes that are assembled upon activation of c-Met are dependent on the presence of α3β1 integrin.
In many cell types, c-Met has been found to signal through a complex that includes Gab1, which recruits phosphatidyl inositol-3-kinase (PI3K; Pik3) and signals to activate AKT (Akt1) (reviewed by Vivanco and Sawyers, 2002). To determine whether α3β1 integrin augmented signaling through PI3K and AKT, we studied the association of Gab1 and PI3K with the c-Met-α3β1 complex. Gab1 (Fig. 5C) and PI3K (Fig. 5B) could be co-immunoprecipitated with c-Met only in the presence of α3β1 integrin. Gab1 could also be directly immunoprecipitated with α3β1 integrin (Fig. 5D), demonstrating that the association of Gab1 with c-Met does not occur in a complex that is distinct from that containing α3β1 and c-Met.
Activation of PI3K leads to phosphorylation of AKT, which results in the activation or deactivation of diverse biological responses (Vivanco and Sawyers, 2002). A similar amount of AKT was present in WT and KO cells (Fig. 5Ea), but AKT was more highly phosphorylated at the threonine 308 residue in response to Hgf in WT cells (Fig. 5Eb). Together, these results suggest that signaling through the c-Met/PI3K/AKT pathway is dependent on an interaction with α3β1 integrin.
Regulation of Wnt7b expression by Hgf
To examine whether coordinate signaling by α3β1 and c-Met regulates the expression of Wnt genes in the papilla, WT cells were treated with a Hgf-neutralizing antibody. This completely blocked the expression of the RTH, MHR and MLL Wnt7b isoforms, as detected by RT-PCR (Fig. 6A). By contrast, a similarly prepared neutralizing antibody that blocks Igf1 function had no effect on the expression of these Wnt7b mRNAs. The discrepancy between the effect of the Hgf-neutralizing antibody, which blocks expression of all Wnt7b isoforms, and the absence of α3β1 integrin, which only blocks two out of the three isoforms, might indicate an absolute requirement for signals downstream of c-Met that are augmented by association with α3β1 integrin. In contrast to Wnt7b, Wnt4 expression by immortalized cell lines was not sensitive to c-Met blockade with the Hgf-neutralizing antibody (Fig. 6B).
Fig. 6.
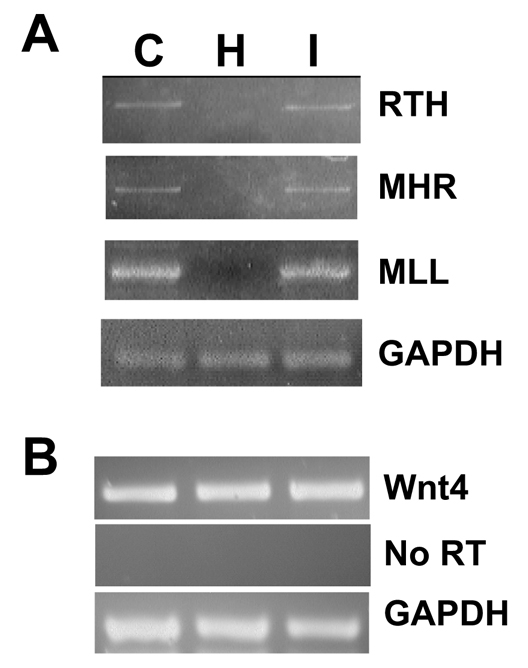
Regulation of Wnt7b but not Wnt4 expression by Hgf. WT mouse cells were treated with a Hgf-neutralizing antibody (H) or IGF-neutralizing antibody (I) before RNA extraction. C, control untreated cells. (A) RT-PCR was used to amplify the three isoforms (RTH, MHR and MLL) of Wnt7b as shown in Fig. 3. (B) RT-PCR for Wnt4 from cells treated as in A.
Hgf and Wnt stimulate cell survival in the developing papilla
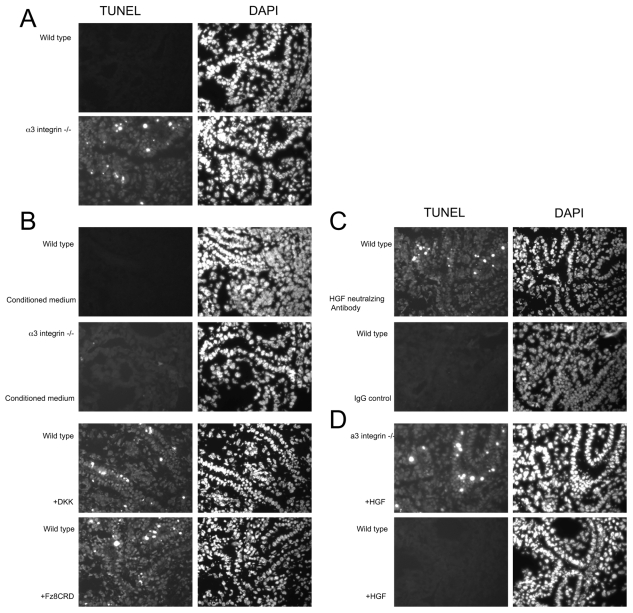
Many of the proposed mechanisms of integrin-mediated regulation of morphogenesis have focused on integrin function in cell adhesion, cytoskeletal organization and cell migration. Although fewer tubules were present in α3β1 integrin-deficient kidneys, those tubules present displayed normal morphology (Fig. 1A). Cell survival is known to require integrin engagement, particularly in epithelial cells. Therefore, we examined whether increased apoptosis could have a role in the failure of papillary outgrowth in α3β1 integrin-deficient kidneys. Indeed, TUNEL staining of papillae showed significantly more apoptosis in α3 integrin KO than in WT kidneys (Fig. 7A).
Fig. 7.
Effect of Wnt and Hgf on cell survival in kidney papilla. Isolated mouse papillae were sectioned and TUNEL/DAPI stained to reveal apoptotic cells prior to (A) or after (B,C,D) culture under various conditions. TUNEL staining is on the left with the corresponding DAPI staining on the right. (A) Papillae directly sectioned without organ culture. Significantly more apoptosis was observed in α3 integrin KO than in WT kidney papillae. The difference in background TUNEL staining between WT and KO was reproducible and considered significant. This difference was still observed in WT treated with Wnt or Hgf blockade. (B) Effect of conditioned medium from WT immortalized cells and of Wnt blockade. WT-cell-conditioned medium prevented apoptosis in KO papillae. Wnt blockers Dkk1 and Fz8CRD stimulated apoptosis in WT papillae. Identical results were obtained using a Wnt3a-conditioned medium prepared with HEK293 cells (see Fig. S4 in the supplementary material). Control conditioned media made using a vector expressing only the Fc region used in the Fz8CRD construct had no effect on WT kidneys (not shown). (C) The effect of Hgf-neutralizing antibody or control rabbit IgG on cell survival. An Hgf-neutralizing antibody stimulated apoptosis in WT papillae. A control anti-Igf1 antibody had no effect. (D) Hgf did not prevent apoptosis in KO papillae. Addition of Hgf to cultures of KO kidneys did not prevent apoptosis. Hgf had no effect on WT kidneys. Each experiment was repeated a minimum of three times.
Wnt signaling has been found to prevent apoptosis in various cell types (Chen et al., 2001; Hwang et al., 2004). To examine whether Wnts are required for regulating apoptosis in vivo, α3 integrin KO papillae were placed in culture for 24 hours with conditioned medium from Wnt3a-expressing HEK293 cells. This treatment prevented apoptosis in these kidney papillae, suggesting a potential role of Wnt proteins in maintaining cell survival. In addition, treatment of WT kidney papillae with Wnt inhibitors (Fz8CRD and Dkk1) induced apoptosis in WT kidneys (Fig. 7B), further supporting the role of Wnts in preventing apoptosis. As shown in Fig. 7, the number of obviously apoptotic cells was low, and may not completely account for the failure of papillary outgrowth, although TUNEL staining is a relatively late marker of apoptosis and these results might underestimate the total amount of apoptosis that will occur between E18.5 and P0. (For lower-magnification images of these organ cultures, see Fig. S4 in the supplementary material.)
To determine whether Hgf-mediated Wnt expression was involved in regulating apoptosis, WT kidney papillae were treated with the Hgf-neutralizing antibody. This treatment also induced apoptosis in kidney papillae (Fig. 7C). By contrast, treatment with Hgf could not prevent apoptosis in α3β1 integrin-deficient kidney papillae (Fig. 7D), consistent with the previous results indicating that c-Met is largely unresponsive to Hgf in the absence of α3β1 integrin. Together, these results suggest that Hgf and laminin signaling through c-Met and α3β1 integrin can regulate cell survival via stimulation of Wnt gene expression, and that this is dependent on the presence of the α3β1 integrin. Thus, these results suggest a novel role of α3β1 integrin: acting coordinately with c-Met to regulate Wnt gene expression, which in turn can regulate cell survival and contribute to the proper patterning of the renal papilla.
Cell proliferation was also examined in WT and α3 integrin KO kidneys. There is little proliferation at the tip of the papilla in WT kidneys. Instead, most proliferation is found at the mid-papilla and extending into the cortex. The frequency of proliferating cells within the collecting ducts appeared similar in WT and KO kidneys (see Fig. S5 in the supplementary material).
DISCUSSION
We present two novel findings in this report. First, that α3β1 integrin and the receptor tyrosine kinase c-Met signal coordinately to regulate a morphogenetic event. Secondly, that coordinate signals by integrins and receptor tyrosine kinases can regulate the expression of Wnt genes. That Wnt signals can act to maintain cell survival has been demonstrated previously. Here, we place this function within a developmental context downstream of signals from diffusible growth factors and the ECM. These findings also underscore that it is important to consider the ECM, and not only diffusible growth factors, as biological information that groups of cells integrate to produce morphogenetic events.
Mice deficient in α3β1 integrin die during the neonatal period with multiple developmental defects, including abnormal development of the renal glomerulus and the papillary region of the kidney (Kreidberg et al., 1996). Although it is more likely that the observed neonatal death is due to glomerular dysfunction, the papillary defects are nonetheless informative with regard to integrin and receptor tyrosine kinase function in the regulation of Wnt gene expression and epithelial morphogenesis. The present study establishes an important role for integrins in maintaining epithelial cell survival in vivo.
Wnt signaling is transduced through several biochemical pathways, which are categorized as canonical and non-canonical (Pandur et al., 2002). In non-mammalian species, it has been possible to relate specific biochemical pathways to morphogenetic processes. These relationships are less well defined in mammals. For example, tubular extension during development of the papilla of the embryonic kidney involves cell proliferation, cell survival and vectorial tubular extension. Proliferation and cell survival are likely to be consequences of canonical Wnt signaling. This is supported by our observations using β-catenin/Tcf reporter transgenes. Wnt7b is the most likely candidate to mediate cell proliferation and survival. A role in proliferation was recently demonstrated in the developing lung (Rajagopal et al., 2008). By contrast, vectorial tubular extension may be related to planar cell polarity (PCP) pathways (Fischer et al., 2006), which are generally thought to be a consequence of non-canonical Wnt signaling. In this model, tubular extension results in elongation of tubules that maintain a constant diameter. If PCP pathways were non-functional, one prediction is that cysts would form instead of tubules as a consequence of random rather than ordered cell divisions (Fischer et al., 2006). Wnt4 has been suggested to be involved in non-canonical Wnt pathways (Cohen et al., 2002; Lim et al., 2005; Osafune et al., 2006), but this does not exclude a role in canonical Wnt signaling. Indeed, it remains unclear whether it is possible to strictly classify specific Wnts into exclusively canonical or non-canonical signaling ligands. Whether Wnt4 is responsible for PCP signaling in the developing kidney is unknown. However, the observation that those tubules that are present, albeit fewer in number, appear normal and without cysts, suggests that PCP pathways might be intact. Possibly, there is sufficient Wnt4 to maintain PCP, even though levels appear to be diminished. It is also possible that Wnt7b regulates the pattern of proliferation in the developing papilla and this might be the focus of future studies.
Note added in proof
Since the acceptance of this manuscript, two reports of relevance to our studies have been published that describe the phenotypes of the conditional mutation of Wnt7b (Yu et al., 2009) and of c-Met (Ishibe et al., 2009) in the kidney.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/5/843/DC1
Supplementary Material
We thank Drs Susan Dymecki for FlpE deleter mice, Xi He for advice and expression vectors, Andrew McMahon and Jing Yu for valuable discussions and Wnt probes. J.A.K. acknowledges support from R01 DK57604 and R01 DK50118-01, J.H.M. from R01 DK064687 and R01 GM060432, H.A.C. from HL44712 and C.M.B. from R01 DK070030-01. T.V. was supported by a NIH T32 Training Grant in Pediatric Nephrology. C.S. was supported by a Fellowship Grant from the National Kidney Foundation. S.Q. was supported by a Fellowship Grant from the PKD Foundation. Deposited in PMC for release after 12 months.
References
- Anton, E. S., Kreidberg, J. A. and Rakic, P. (1999). Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron 22, 277-289. [DOI] [PubMed] [Google Scholar]
- Birchmeier, C. and Gherardi, E. (1998). Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 8, 404-410. [DOI] [PubMed] [Google Scholar]
- Boudreau, N. and Bissell, M. J. (1998). Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr. Opin. Cell Biol. 10, 640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll, T. J., Park, J. S., Hayashi, S., Majumdar, A. and McMahon, A. P. (2005). Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283-292. [DOI] [PubMed] [Google Scholar]
- Cebrian, C., Borodo, K., Charles, N. and Herzlinger, D. A. (2004). Morphometric index of the developing murine kidney. Dev. Dyn. 231, 601-608. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay, N., Wang, Z., Ashman, L. K., Brady-Kalnay, S. M. and Kreidberg, J. A. (2003). alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion. J. Cell Biol. 163, 1351-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S., Guttridge, D. C., You, Z., Zhang, Z., Fribley, A., Mayo, M. W., Kitajewski, J. and Wang, C. Y. (2001). Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J. Cell Biol. 152, 87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon, S. S., Cheah, A. Y., Turley, S., Nadesan, P., Poon, R., Clevers, H. and Alman, B. A. (2002). beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc. Natl. Acad. Sci. USA 99, 6973-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski, P. and Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156-159. [DOI] [PubMed] [Google Scholar]
- Cohen, E. D., Mariol, M. C., Wallace, R. M., Weyers, J., Kamberov, Y. G., Pradel, J. and Wilder, E. L. (2002). DWnt4 regulates cell movement and focal adhesion kinase during Drosophila ovarian morphogenesis. Dev. Cell 2, 437-448. [DOI] [PubMed] [Google Scholar]
- Comoglio, P. M., Boccaccio, C. and Trusolino, L. (2003). Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 15, 565-571. [DOI] [PubMed] [Google Scholar]
- Danen, E. H. and Sonnenberg, A. (2003). Integrins in regulation of tissue development and function. J. Pathol. 201, 632-641. [DOI] [PubMed] [Google Scholar]
- DiPersio, C. M., Hodivala-Dilke, K. M., Jaenisch, R., Kreidberg, J. A. and Hynes, R. O. (1997). alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 137, 729-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri, B. P. (2001). Integrin and growth factor receptor crosstalk. Circ. Res. 89, 1104-1110. [DOI] [PubMed] [Google Scholar]
- Elices, M. J., Urry, L. A. and Hemler, M. E. (1991). Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J. Cell Biol. 112, 169-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler, R. and Meyer, M. (1995). Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 9, 1896-1908. [DOI] [PubMed] [Google Scholar]
- Fassler, R., Georges-Labouesse, E. and Hirsch, E. (1996). Genetic analyses of integrin function in mice. Curr. Opin. Cell Biol. 8, 641-646. [DOI] [PubMed] [Google Scholar]
- Fischer, E., Legue, E., Doyen, A., Nato, F., Nicolas, J. F., Torres, V., Yaniv, M. and Pontoglio, M. (2006). Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 38, 21-23. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse, E., Messaddeq, N., Yehia, G., Cadalbert, L., Dierich, A. and Le Meur, M. (1996). Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 13, 370-373. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse, E., Mark, M., Messaddeq, N. and Gansmuller, A. (1998). Essential role of alpha 6 integrins in cortical and retinal lamination. Curr. Biol. 8, 983-986. [DOI] [PubMed] [Google Scholar]
- Giancotti, F. G. and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028-1032. [DOI] [PubMed] [Google Scholar]
- Goh, K. L., Yang, J. T. and Hynes, R. O. (1997). Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development 124, 4309-4319. [DOI] [PubMed] [Google Scholar]
- Gorski, J. P. and Olsen, B. R. (1998). Mutations in extracellular matrix molecules. Curr. Opin. Cell Biol. 10, 586-593. [DOI] [PubMed] [Google Scholar]
- Hogan, B. L. (1999). Morphogenesis. Cell 96, 225-233. [DOI] [PubMed] [Google Scholar]
- Howe, A., Aplin, A. E., Alahari, S. K. and Juliano, R. L. (1998). Integrin signaling and cell growth control. Curr. Opin. Cell Biol. 10, 220-231. [DOI] [PubMed] [Google Scholar]
- Huang, S. and Ingber, D. E. (1999). The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1, E131-E138. [DOI] [PubMed] [Google Scholar]
- Hwang, S. G., Ryu, J. H., Kim, I. C., Jho, E. H., Jung, H. C., Kim, K., Kim, S. J. and Chun, J. S. (2004). Wnt-7a causes loss of differentiated phenotype and inhibits apoptosis of articular chondrocytes via different mechanisms. J. Biol. Chem. 279, 26597-26604. [DOI] [PubMed] [Google Scholar]
- Hynes, R. O. (1992). Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11-25. [DOI] [PubMed] [Google Scholar]
- Hynes, R. O. (1999). Cell adhesion: old and new questions. Trends Cell Biol. 9, M33-M37. [PubMed] [Google Scholar]
- Hynes, R. O. and Zhao, Q. (2000). The evolution of cell adhesion. J. Cell Biol. 150, F89-F96. [DOI] [PubMed] [Google Scholar]
- Ishibe, S., Karihaloo, A., Ma, H., Zhang, J., Marlier, A., Mitobe, M., Togawa, A., Schmitt, R., Czyczk, J., Kashgarian, M., Geller, D. S., Thorgeirsson, S. S. and Cantley, L. G. (2009). Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development 136, 337-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaranta, P., Chi, L., Seppanen, T., Niku, M., Tuukkanen, J., Peltoketo, H. and Vainio, S. (2006). Wnt-4 signaling is involved in the control of smooth muscle cell fate via Bmp-4 in the medullary stroma of the developing kidney. Dev. Biol. 293, 473-483. [DOI] [PubMed] [Google Scholar]
- Jeffers, M., Rong, S. and Vande Woude, G. F. (1996). Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-met signalling in human cells concomitant with induction of the urokinase proteolysis network. Mol. Cell. Biol. 16, 1115-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa, Y., Sanzen, N. and Sekiguchi, K. (1998). Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J. Biol. Chem. 273, 15854-15859. [DOI] [PubMed] [Google Scholar]
- Kispert, A., Vainio, S., Shen, L., Rowitch, D. H. and McMahon, A. P. (1996). Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development 122, 3627-3637. [DOI] [PubMed] [Google Scholar]
- Kispert, A., Vainio, S. and McMahon, A. P. (1998). Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125, 4225-4234. [DOI] [PubMed] [Google Scholar]
- Klosek, S. K., Nakashiro, K., Hara, S., Shintani, S., Hasegawa, H. and Hamakawa, H. (2005). CD151 forms a functional complex with c-Met in human salivary gland cancer cells. Biochem. Biophys. Res. Commun. 336, 408-416. [DOI] [PubMed] [Google Scholar]
- Kreidberg, J. A., Donovan, M. J., Goldstein, S. L., Rennke, H., Shepherd, K., Jones, R. C. and Jaenisch, R. (1996). Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122, 3537-3547. [DOI] [PubMed] [Google Scholar]
- Lim, J., Norga, K. K., Chen, Z. and Choi, K. W. (2005). Control of planar cell polarity by interaction of DWnt4 and four-jointed. Genesis 42, 150-161. [DOI] [PubMed] [Google Scholar]
- Majumdar, A., Vainio, S., Kispert, A., McMahon, J. and McMahon, A. P. (2003). Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130, 3175-3185. [DOI] [PubMed] [Google Scholar]
- Menko, A. S., Kreidberg, J. A., Ryan, T. T., Van Bockstaele, E. and Kukuruzinska, M. A. (2001). Loss of alpha3beta1 integrin function results in an altered differentiation program in the mouse submandibular gland. Dev. Dyn. 220, 337-349. [DOI] [PubMed] [Google Scholar]
- Miner, J. H. and Li, C. (2000). Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev. Biol. 217, 278-289. [DOI] [PubMed] [Google Scholar]
- Miranti, C. K. and Brugge, J. S. (2002). Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4, E83-E90. [DOI] [PubMed] [Google Scholar]
- Montesano, R., Matsumoto, K., Nakamura, T. and Orci, L. (1991). Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 67, 901-908. [DOI] [PubMed] [Google Scholar]
- Osafune, K., Takasato, M., Kispert, A., Asashima, M. and Nishinakamura, R. (2006). Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development 133, 151-161. [DOI] [PubMed] [Google Scholar]
- Osathanondh, V. and Potter, E. L. (1963). Development of the human kidney as shown by microdissection II. Renal pelvis, calyces, and papillae. Arch. Pathol. 76, 53-65. [PubMed] [Google Scholar]
- Pandur, P., Maurus, D. and Kuhl, M. (2002). Increasingly complex: new players enter the Wnt signaling network. BioEssays 24, 881-884. [DOI] [PubMed] [Google Scholar]
- Rajagopal, J., Carroll, T. J., Guseh, J. S., Bores, S. A., Blank, L. J., Anderson, W. J., Yu, J., Zhou, Q., McMahon, A. P. and Melton, D. A. (2008). Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development 135, 1625-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti, E. (1999). Fibronectin and its integrin receptors in cancer. Adv. Cancer Res. 76, 1-20. [DOI] [PubMed] [Google Scholar]
- Sanes, J. R., Rubenstein, J. L. and Nicolas, J. F. (1986). Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 5, 3133-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, C., Bladt, F., Goedecke, S., Brinkmann, V., Zschiesche, W., Sharpe, M., Gherardi, E. and Birchmeier, C. (1995). Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373, 699-702. [DOI] [PubMed] [Google Scholar]
- Schwartz, M. A. and Baron, V. (1999). Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol. 11, 197-202. [DOI] [PubMed] [Google Scholar]
- Shu, W., Jiang, Y. Q., Lu, M. M. and Morrisey, E. E. (2002). Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 129, 4831-4842. [DOI] [PubMed] [Google Scholar]
- Stark, K., Vainio, S., Vassileva, G. and McMahon, A. P. (1994). Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679-683. [DOI] [PubMed] [Google Scholar]
- Stephens, L. E., Sutherland, A. E., Klimanskaya, I. V., Andrieux, A., Meneses, J., Pedersen, R. A. and Damsky, C. H. (1995). Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9, 1883-1895. [DOI] [PubMed] [Google Scholar]
- Stoker, M., Gherardi, E., Perryman, M. and Gray, J. (1987). Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 327, 239-242. [DOI] [PubMed] [Google Scholar]
- Taverna, D., Disatnik, M. H., Rayburn, H., Bronson, R. T., Yang, J., Rando, T. A. and Hynes, R. O. (1998). Dystrophic muscle in mice chimeric for expression of alpha5 integrin. J. Cell Biol. 143, 849-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara, Y., Minowa, O., Mori, C., Shiota, K., Kuno, J., Noda, T. and Kitamura, N. (1995). Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 373, 702-705. [DOI] [PubMed] [Google Scholar]
- Vivanco, I. and Sawyers, C. L. (2002). The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2, 489-501. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Symons, J. M., Goldstein, S. L., McDonald, A., Miner, J. H. and Kreidberg, J. A. (1999). (Alpha)3(beta)1 integrin regulates epithelial cytoskeletal organization. J. Cell Sci. 112, 2925-2935. [DOI] [PubMed] [Google Scholar]
- Wodarz, A. and Nusse, R. (1998). Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59-88. [DOI] [PubMed] [Google Scholar]
- Yang, J. T., Rayburn, H. and Hynes, R. O. (1993). Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development 119, 1093-1105. [DOI] [PubMed] [Google Scholar]
- Yang, J. T., Rayburn, H. and Hynes, R. O. (1995). Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development 121, 549-560. [DOI] [PubMed] [Google Scholar]
- Yu, J., Carroll, T. J., Rajagopal, J., Kobayashi, A., Ren, Q. and McMahon, A. P. (2009). A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136, 161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F., Tom, C. C., Kugler, M. C., Ching, T. T., Kreidberg, J. A., Wei, Y. and Chapman, H. A. (2003). Distinct ligand binding sites in integrin alpha3beta1 regulate matrix adhesion and cell-cell contact. J. Cell Biol. 163, 177-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. W. and Vande Woude, G. F. (2003). HGF/SF-met signaling in the control of branching morphogenesis and invasion. J. Cell. Biochem. 88, 408-417. [DOI] [PubMed] [Google Scholar]
- Zhao, H., Kegg, H., Grady, S., Truong, H. T., Robinson, M. L., Baum, M. and Bates, C. M. (2004). Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev. Biol. 276, 403-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.