Abstract
Mitosis is a complex cellular process that is completed by the final abscission step called cytokinesis. The many roles of cytoskeletal proteins in animal cell division have been studied extensively, and are essential for proper daughter cell segregation. Lately, the need for the membrane delivery to the cleavage furrow of dividing cells has been implicated as a necessary and important step in cytokinesis.1–4 Newly published work from several group demonstrate that endosomal membranes are required for cytokinesis and also provide insight into the targeting of these membranes to the cleavage furrow.5–8 Rab11 GTPase and its effector protein FIP3 were shown to play key roles in endosome targeting to the cleavage furrow in a spatially and temporally regulated manner.7–9 Thus, Rab11/FIP3 protein complex have emerged as a regulator of endocytic traffic, essential for the abscission step in cytokinesis.
Key words: centralspindlin complex, cytokinesis, endosomes, FIP3, Rab11
Membrane delivery to the plane of division in mitotic cells is a necessary and important step for the completion of cytokinesis.1–4 Studies from our group, and others, have emphasized the role of membrane delivery from various cellular compartments to the mitotic furrow in several organisms.1–3,8,10,11 Newly published work from our group reinforces the findings that endosomal membranes are required for cytokinesis and provides the insights into the regulated targeting of these membranes to the cleavage furrow.12
Rab proteins belong to a family of small monomeric GTPases that are essential regulators of membrane transport and targeting. Two members of Rab protein family, Rab11 and Rab35, were shown to regulate endocytic recycling and also play a specialized role during mitosis.8,13 Rab11 is enriched at the midbody and has been studied as a major regulator of membrane delivery during cytokinesis.7–9,14 In conjunction with Rab11, FIP3 (Rab11 Family Interacting Protein 3) acts as an “address tag,” delivering endocytic membranes to the cleavage furrow in mammalian cells. Not surprisingly, FIP3 homologues also play important roles in specialized membrane delivery in other organisms. FIP3 was first described in Drosophila malanogaster as Nuf.11 Syncitial Drosophila embryos deficient in Nuf fail to add membrane vesicles to the ingressing cleavage furrow and were unable to complete cellularization.4 Similarly, in mammalian cells depletion of FIP3 causes late stage cytokinesis defects, and a failure to undergo abscission.8 These data indicate that regulated Rab11/FIP3 associated vesicle delivery is indispensable for cell partitioning, including cytokinesis.
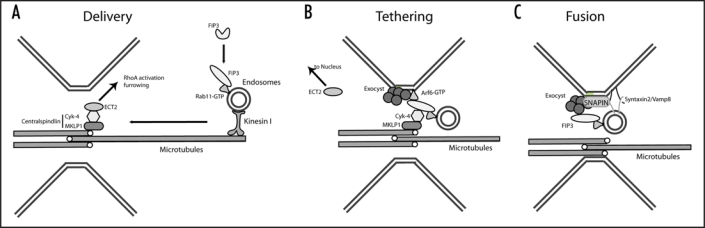
Many mitotic proteins display tightly regulated changes in activity and sub-cellular localization during cell division. Similarly, in early mitosis Rab11/FIP3 vesicles remain diffuse and distributed through the cell. The onset of anaphase sequesters these vesicles to the centrosomes at the opposite poles of the cell. During telophase these vesicles move from the centrosomes, to the furrow, and then to the midbody to aid in abscission.8 The exact mechanism for this directional movement remains unclear; however, unpublished data from both our and Dr. Chavrier's laboratories indicate that Kinesin I may function to move Rab11/FIP3-containing endosomes to the cleavage furrow along midzone microtubules (Fig. 1).
Figure 1.
Mechanisms mediating the targeting and fusion of recycling endosomes with plasma membrane of the cleavage furrow. (A) At anaphase onset, activated Rab11/FIP3 complex is delivered, along midzone microtubules to the cleavage furrow by Kinesin I. (B) The removal of ECT2 allows FIP3 to associate with the Centralspindlin and the Exocyst complexes, tethering the vesicles at the midbody in telophase. (C) Association with the Exocyst, and Snapin allow the SNARE-mediated fusion of Rab11/FIP3-endosomes to the furrow plasma membrane.
Upon the delivery of Rab11/FIP3 endosomes to the midbody, they interact with at least two separate protein complexes that allow the tethering and accumulation of FIP3-endosomes at the midbody during late telophase. Previous studies indicate that FIP3/Rab11 complex interacts with Arf6, the small monomeric GTPase that is enriched at the furrow.7,15,16 Arf6 was shown to bind Sec10 subunit of the Exocyst complex, that is known to tether transport vesicles to the plasma membrane during interphase and mitosis. Interestingly, Rab11 also binds to Sec15, another subunit of the Exocyst complex.7 Thus, the interactions between Rab11/FIP3/Arf6 protein complex with the Exocyst is believed to mediate the tethering of endosomes to the midbody during the abscission step of cytokinesis (Fig. 1).
In addition to Arf6, FIP3 also binds Cyk-4, the subunit of the Centralspindlin complex.12 The Centralspindlin complex, that consists from Cyk-4 and MKLP1, functions to bundle microtubules and mediate RhoA-driven actomyosin ring contraction.17 During early cytokinesis, FIP3 binding to the Centralspindlin is inhibited by ECT2, which competes with FIP3 for binding to of Cyk4. At late cytokinesis, ECT2 is removed from the centralspindlin complex and sequestered back to the nucleus, allowing Rab11/FIP3 endosomes to associate with the centralspindlin complex (Fig. 1). All of these interactions have been demonstrated to be required for abscission, and any perturbation of these processes results in mitotic failure.12
Once delivered and tethered to the midbody the Rab11/FIP3 vesicles are assumed to undergo SNARE-mediated membrane fusion. Consistent with this, it has been shown that SNARE family members syntaxin 1/2 and Vamp 8 are enriched at the midbody during cytokinesis.3,18 Furthermore, the inhibition of SNARE function by dominant-negative mutants leads to failed cytokinesis.18 The link between the SNARE-mediated fusion and vesicle tethering is little understood during cytokinesis, but the centrosomally associated protein centriolin provides some clues as to their relation. Centriolin interacts with SNARE associated protein Snapin and the Exocyst complex.19 The depletion of centriolin disrupted the accumulation of Vamp8 and the Exocyst at the mitotic furrow and prevented completion of cytokinesis.19
The role of membrane vesicle delivery during mitosis is a relatively young field of study. The precise mechanisms that regulate the delivery and tethering of endosomes to the midbody are only beginning to emerge. Furthermore, the functions of these endosomes remain unclear. One hypothesis suggests that the accumulation of membrane vesicles at the furrow could result in a large fusion event that quickly and effectively divides the two cells. Alternatively, Rab11/FIP3-endosomes could be used to deliver proteins that are necessary to disassemble the furrow cytoskeleton or regulate membrane deformation and fusion, the steps that are required for successful abscission. The identification the regulation and functions of furrow endosomes will be an important step in our understanding of the mechanisms regulating mitotic cell division.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6864
References
- 1.Danilchik MV, Funk WC, Brown EE, Larkin K. Requirement for microtubules in new membrane formation during cytokinesis of Xenopus embryos. Dev Biol. 1998;194:47–60. doi: 10.1006/dbio.1997.8815. [DOI] [PubMed] [Google Scholar]
- 2.Bluemink JG, de Laat SW. New membrane formation during cytokinesis in normal and cytochalasin B-treated eggs of Xenopus laevis. I. Electron microscope observations. J Cell Biol. 1973;59:89–108. doi: 10.1083/jcb.59.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jantsch-Plunger V, Glotzer M. Depletion of syntaxins in the early Caenorhabditis elegans embryo reveals a role for membrane fusion events in cytokinesis. Curr Biol. 1999;9:738–745. doi: 10.1016/s0960-9822(99)80333-9. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell WF, Zhang CX, Zelano C, Hsieh TS, Sullivan W. The Drosophila centrosomal protein Nuf is required for recruiting Dah, a membrane associated protein, to furrows in the early embryo. J Cell Sci. 1999;112:2885–2893. doi: 10.1242/jcs.112.17.2885. [DOI] [PubMed] [Google Scholar]
- 5.Chen XW, Inoue M, Hsu SC, Saltiel AR. RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J Biol Chem. 2006;281:38609–38616. doi: 10.1074/jbc.M512847200. [DOI] [PubMed] [Google Scholar]
- 6.Dyer N, et al. Spermatocyte cytokinesis requires rapid membrane addition mediated by ARF6 on central spindle recycling endosomes. Development. 2007;134:4437–4447. doi: 10.1242/dev.010983. [DOI] [PubMed] [Google Scholar]
- 7.Fielding AB, et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson GM, et al. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2005;16:849–860. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickson GR, et al. Arfophilins are dual Arf/Rab11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol Biol Cell. 2003;14:2908–2920. doi: 10.1091/mbc.E03-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riggs B, et al. Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J Cell Biol. 2003;163:143–154. doi: 10.1083/jcb.200305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothwell WF, Fogarty P, Field CM, Sullivan W. Nuclear-fallout, a Drosophila protein that cycles from the cytoplasm to the centrosomes, regulates cortical micr ofilament organization. Development. 1998;125:1295–1303. doi: 10.1242/dev.125.7.1295. [DOI] [PubMed] [Google Scholar]
- 12.Simon GC, et al. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. EMBO J. 2008;27:1791–1803. doi: 10.1038/emboj.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Horgan CP, Walsh M, Zurawski TH, McCaffrey MW. Rab11-FIP3 localises to a Rab11-positive pericentrosomal compartment during interphase and to the cleavage furrow during cytokinesis. Biochem Biophys Res Commun. 2004;319:83–94. doi: 10.1016/j.bbrc.2004.04.157. [DOI] [PubMed] [Google Scholar]
- 15.Schweitzer JK, D'Souza-Schorey C. Localization and activation of the ARF6 GTPase during cleavage furrow ingression and cytokinesis. J Biol Chem. 2002;277:27210–27216. doi: 10.1074/jbc.M201569200. [DOI] [PubMed] [Google Scholar]
- 16.Schonteich E, et al. Molecular characterization of Rab11-FIP3 binding to ARF GTPases. Eur J Cell Biol. 2007;86:417–431. doi: 10.1016/j.ejcb.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Mishima M, Pavicic V, Gruneberg U, Nigg EA, Glotzer M. Cell cycle regulation of central spindle assembly. Nature. 2004;430:908–913. doi: 10.1038/nature02767. [DOI] [PubMed] [Google Scholar]
- 18.Low SH, et al. Syntaxin2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 19.Gromley A, et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]