Abstract
Serum strongly induces the yeast-to-hypha growth transition in the human fungal pathogen Candida albicans, playing an important role in infection. However, identity of the serum inducer(s) and its sensor remain poorly defined. We used NMR to analyze the chromatographic serum fractionations enriched for the hypha-inducing activity and found structures resembling subunits of bacterial peptidoglycan (PGN). We then confirmed that several purified and synthetic muramyl dipeptides (MDPs), subunits of PGN, can indeed strongly promote C. albicans hyphal growth. Taking cue from the recognition of MDPs by the mammalian bacterial sensor Nod2 using its leucine-rich-repeat (LRR) domain, we discovered that MDPs activate the adenylyl cyclase Cyr1 by binding to its LRR domain. The cAMP/PKA signaling pathway is well known to control hyphal morphogenesis and other infection-related traits. Given the abundance of PGN at the large intestinal epithelial surface, a natural habitat and invasion site for C. albcians, our findings have important implications in the mechanisms of infection by this pathogen.
Key words: muramyl dipeptide, adenylyl cyclase, signal sensing, fungal infection
The AIDS pandemic has seen the rapid emergence of C. albicans from a largely benign commensal to one of the most deadly pathogens in humans.1,2 A key virulence trait of this fungus is its ability to switch between yeast and hyphal growth in the host.3,4 A long held question is: what host molecules trigger the hyphal growth? It has been known for more than 50 years that serum contains potent hypha-inducing activities.5 Since Feng and colleagues6 first showed that the active molecules are smaller than 1 kDa, there have been several candidates including proline, methionine and N-acetylglucosamine (GlcNAc).7–10 However, the concentrations of the two amino acids required for hyphal induction are much higher than what is available in serum, and there has been no report of existence of free GlcNAc molecules in serum.
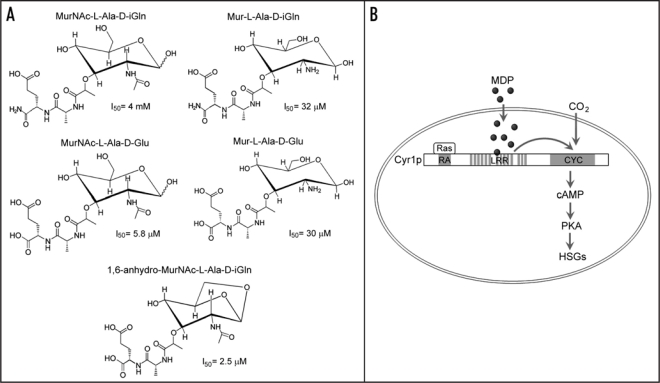
We performed chromatographic fractionations of human and bovine serum and analyzed by NMR the fractions enriched for the hypha-inducing activity.11 We found molecules resembling muramic acid (Mur), alanine (Ala) and isoglutamine (iGln). Since Mur and D-iGln are uniquely present in bacterial PGN in nature,12 we proposed a structure Mur-L-Ala-D-iGln based on the conserved PGN subunit MurNAc-L-Ala-D-iGln. We then synthesized Mur-L-Ala-D-iGln together with several related compounds (Fig. 1A). Test of hyphal induction showed that all the compounds are active. The most active ones are 1,6-anhydro-MurNAc-L-Ala-D-Glu and MurNAc-L-Ala-D-Glu with I50s of 2.5 and 5.8 µM respectively, while MurNAc-L-Ala-D-iGln is the least active with an I50 of 4 mM. The initially proposed structure Mur-L-Ala-D-iGln has an I50 of 32 µM. For comparison, the I50 of GlcNAc is ∼1.8 mM. Thus, the data not only confirm that our proposed molecule is indeed an inducer of C. albicans hyphal growth but also revealed molecules that are much more potent. We also determined the structural requirement for the activity. While Mur and Ala exhibited no activity either singly or as a mixture, Mur-L-Ala was found to be active with an I50 of ∼100 µM, which is the smallest molecule we have found so far with significant activity. In addition, the L configuration of Ala is essential, because Mur-D-Ala and Mur-D-Ala-D-iGln had no activity at all.
Figure 1.
(A) Structure and hypha-inducing activity of several muramyl dipeptides (MDPs). For hyphal induction, 5 × 105 yeast cells/ml were incubated at 37°C for 2 hr in Hank's buffered salt solution supplemented with different concentrations of each MDP. I50 is the concentration of a MDP that caused hyphal growth in 50% of the yeast cells. (B) Schematic description of the mechanism by which MDPs activate Cyr1 and promote hyphal growth. RA, Ras-association domain; CYC, catalytic domain; HSG, hypha-specifc gene.
The most likely source of PGN in blood is the microflora in the human body.13,14 Due to the heterogeneity of PGN in different bacterial species and many possible ways for PGN degradation, PGN-derived molecules in serum likely exist in diverse forms. What are the levels of these molecules in serum? Can they account for serum's hypha-inducing activity? Using HPLC and mass spectrometry (MS), we detected in multiple serum samples 0.1 to 0.5 µM of Mur, an indication of the minimal amount of Mur-containing compounds. These levels are apparently several times lower than the I50 of the most active compound we found. A plausible explanation is that serum contains other unidentified PGN-derived molecules with higher activity than those we have tested. Consistent with this hypothesis, hydrolysis of MurNAc-L-Ala-D-iGln resulted in lysates with an I50 of ∼40 nM. However, we cannot conclude that the PGN-derived molecules are predominantly responsible for the hyphal-inducing activity in serum because of the lack of means to specifically remove these molecules. The use of germ-free mice was thought to be a solution, but we found that serum samples from such mice are equally active and contain similar levels of Mur as serum from normal mice. It is likely that bacteria killed by sterilization can still enter the body of the germ-free animals through food and water intake.
What is the sensor of PGN in C. albicans? Pathogen-associated molecules are recognized by pattern-recognition receptors (PRRs) in diverse organisms.15,16 PRRs commonly contain a long LRR domain for ligand binding. Using the LRR domain of the mammalian PRR Nod2,17,18 to search the C. albicans genome database revealed 14 LRRs in the adenylyl cyclase Cyr1, a component of the cAMP/protein kinase A (PKA) pathway essential for hyphal development.19,20 We obtained several lines of compelling evidence strongly supporting the idea that the LRR domain of Cyr1 acts as a sensor of MDPs. First, deleting the entire or part of the LRR domain abolished the cAMP spike that normally occurs following hyphal induction without affecting the cAMP level in yeast cells. Second, these mutants are defective in hyphal growth. Third, MDP conjugated-beads could specifically pull down the Cyr1 LRR domain expressed in E. coli and Cyr1 from C. albicans cell lysates. Cyr1 is an intracellular protein, indicating that MDPs must first cross the plasma membrane. Using H3-labelling and MS, we detected intact MDPs in the cell, although the mechanism of transport remains unknown.
In summary, we discovered that PGN-derived molecules in serum are strong inducers of C. albicans hyphal growth. We propose that these molecules enter the cell, bind to the LRR domain of the adenylyl cyclase Cyr1 and enhance cAMP synthesis (Fig. 1B). Our data suggest that Cyr1 is a sensor/effector molecule. But do other hyphal-inducing molecules activate Cyr1 by a similar mechanism? CO2 has been shown to directly act on the catalytic domain of Cyr1,21 while the GTPase Ras1 has been shown to activate Cyr1 by binding to the Ras-association domain.22,23 Thus, it remains to be elucidated how Cyr1 receives and responds to different hyphainducing signals leading to proper hyphal morphogenesis.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6870
References
- 1.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 2.Berman J, Sudbery PE. Candida Albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet. 2002;3:918–930. doi: 10.1038/nrg948. [DOI] [PubMed] [Google Scholar]
- 3.Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 4.Zheng X, Wang Y, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds R, Braude AI. The filament inducing property of blood for Candida albicans; its nature and significance. Clin Res Proc. 1956;4:40. [Google Scholar]
- 6.Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol. 1999;181:6339–6346. doi: 10.1128/jb.181.20.6339-6346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst JF. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology. 2000;146:1763–1774. doi: 10.1099/00221287-146-8-1763. [DOI] [PubMed] [Google Scholar]
- 8.Maidan MM, Thevelein JM, Van Dijck P. Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-protein-coupled receptor Gpr1. Biochem Soc Trans. 2005;33:291–293. doi: 10.1042/BST0330291. [DOI] [PubMed] [Google Scholar]
- 9.Simonetti N, Strippoli V, Cassone A. Yeast-mycelial conversion induced by N-acetyl-D-glucosamine in Candida albicans. Nature. 1974;250:344–346. doi: 10.1038/250344a0. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez FJ, Konopka JB. Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans. Mol Biol Cell. 2007;18:965–975. doi: 10.1091/mbc.E06-10-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu XL, Lee RTH, Fang HM, Wang YM, Li R, Zou H, Zhu Y, Wang Y. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host & Microbe. 2008;4:28–29. doi: 10.1016/j.chom.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Cloud-Hansen KA, Peterson SB, Stabb EV, Goldman WE, McFall-Ngai MJ, Handelsman J. Breaching the great wall: peptidoglycan and microbial interactions. Nat Rev Microbiol. 2006;4:710–716. doi: 10.1038/nrmicro1486. [DOI] [PubMed] [Google Scholar]
- 13.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoetendal EG, Vaughan EE, de Vos WM. A microbial world within us. Mol Microbiol. 2006;59:1639–1650. doi: 10.1111/j.1365-2958.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 15.Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol. 2004;41:1099–1108. doi: 10.1016/j.molimm.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Delbridge LM, O'Riordan MX. Innate recognition of intracellular bacteria. Curr Opin Immunol. 2007;19:10–16. doi: 10.1016/j.coi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 18.Royet J, Dziarski R. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defenses. Nat Rev Microbiol. 2007;5:264–277. doi: 10.1038/nrmicro1620. [DOI] [PubMed] [Google Scholar]
- 19.Leberer E, Harcus D, Dignard D, Johnson L, Ushinsky S, Thomas DY, Schroppel K. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol Microbiol. 2001;42:673–687. doi: 10.1046/j.1365-2958.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- 20.Rocha CR, Schroppel K, Harcus D, Marcil A, Dignard D, Taylor BN, Thomas DY, Whiteway M, Leberer E. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell. 2001;12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schröppel K, Naglik JR, Eckert SE, Mogensen EG, Haynes K, Tuite MF, Levin LR, Buck J, Mühlschlegel FA. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang HM, Wang Y. RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol Microbiol. 2006;61:484–496. doi: 10.1111/j.1365-2958.2006.05248.x. [DOI] [PubMed] [Google Scholar]
- 23.Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985;40:27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]