Abstract
Tyrosine kinase receptors (RTKs) represent a large family of transmembrane proteins,1,2 present in all metazoans, whose function is to transduce signals from the extracellular milieu to the inside of the cells. The common features of this protein family are: the extracellular domain, devoted to the binding to the specific receptor ligand, a single transmembrane region, and an intracellular chain featuring the conserved protein tyrosine kinase domain. RTKs control many aspects of cellular physiology both during development and in adult life, such as cell proliferation, migration, survival and differentiation.1
Key words: receptor tyrosine kinase, PDGF receptor signaling, endocytosis
The common activation mechanism of RTKs consists, upon ligand binding, in the receptor dimerization (with the notable exception of insulin receptor) that leads to autophosphorylation on specific tyrosine residues that act as docking proteins for intracellular signaling proteins.3 The recruitment of these proteins leads to the activation of many signaling pathways, including ERK1/2, phosphatidylinositol 3-phosphate kinase (PI-3K), phospholipase C-γ (PLC-γ), the Src family of tyrosine kinases, the SHP-2 tyrosine phosphatase and the signal transducers and activators of transcription (STATS) whose function is to transduce the activation signals to the nucleus eliciting the corresponding transcriptional response.
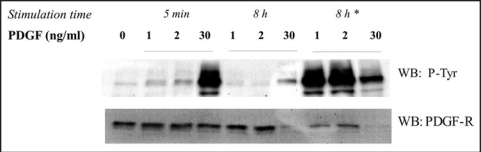
The fact that: (i) all the members of RTKs share very similar signaling modules to elicit their physiologic functions and that (ii) every single RTK in a given cellular type is able to induce different, if no opposite (i.e., for a single cell at a given time, proliferation and movement are two mutually exclusive phenotypes), cellular outcomes, raises the important question of RTK signaling specificity.4 It is conceivable that ontogenesis requires receptors belonging to the same family devoted, for example, to cell proliferation induction, being expressed in different cell types in order to guide the selective and regulated growth of different cell population. In this view it is not surprising the use of the same signaling modules by different RTKs to achieve the same phenotypic outcome. More difficult, in our opinion, is to explain how a single RTK in a given cell type can induce different phenotypic response. PDGF-R, a member of RTKs family, is largely reported to be able to induce many cellular response in mesenchymal-derived cell lines.5,6 In particular PDGF stimulation leads to both cell migration and proliferation, but for a single cell at one time these two cellular response are mutually exclusive. We have shown that cellular behavior induced by PDGF stimulation is different, depending on the ligand concentration sensed by the cell. At low PDGF concentration (1–2 ng/ml) NIH-3T3 cells are induced to migrate, whereas at high (>10 ng/ml) ligand concentration the phenotypic response is cell proliferation.7 The analysis of intracellular signaling pathways activation revealed that in these two different conditions distinct signaling modules were activated. In particular, at low PDGF concentration there is a strong activation of pathways related to cytoskeleton rearrangements correlated to cell migration such as Rac, Rho and FAK whereas Ras/Erks and PI-3K pathways, more strictly devoted to cell proliferation, are not active.7 On the contrary, high PDGF concentration induces the activation of the set of signaling modules that are involved in the induction of cell cycle progression whereas the signaling pathways related to cytoskeleton remodeling are poorly or not active at all. Hence, the ligand concentration drives the cellular outcome by inducing a differential PDGF-R signaling output which, in turn, is mediated by different intracellular localization of PDGF-R itself. PDGF-induced cell migration represents a reversible phenotype. In fact, a single cell migrate in the direction of the increasing ligand gradient until it reaches a zone in which it starts dividing as a results of the gain of the appropriate PDGF threshold that irreversibly commits the cell to mitosis. Figure 1 shows the time-dependent PDGF-R activation in response to different ligand concentration. After 5 min from stimulation, 1 and 2 ng/ml PDGF doses induce a very weak PDGF-R phosphorylation, leading to cell migration, whereas 30 ng/ml PDGF leads to a relevant PDGF-R phosphorylation correlated to mitogenesis induction.7 After 8 hour from stimulation (Fig. 1) it is notable that cells stimulated with low PDGF still show high level of receptor, whereas high dose of PDGF leads to a strong decrease in PDGF-R expression. Consequently, a second 30 ng/ml PDGF stimulation after 8 hours lead to a high phosphorylation response only in cells previously treated with low doses (Fig. 1). These results demonstrate that stimulation with low PDGF doses leads to the retention of the majority of PDGF-R at the plasma membrane likely acting like a sensor to direct cell migration along the ligand gradient. When PDGF concentration reaches the mitotic threshold the number of phosphorylated PDGF-R molecules increase massively leading to the activation of the mitogenic signaling pathway modules that, irreversibly, induce the shift from a migrating phenotype to a proliferating one. Consequently, PDGF-R undergoes endocytosis and degradation having already started the non-reversible mitotic phenotype (Fig. 1). Indeed, after 8 h of 30 ng/ml PDGF treatment the PDGF-R expression level is much lower respect to cell stimulated with low ligand doses. Another direct evidence that the mitosis induced by high PDGF stimulation dose is an irreversible phenotype, derives from the cell cycle analysis of cells that have been treated for variable times with 30 ng/ml PDGF and subsequently washed with PBS an put again in starvation medium (Table 1). The results show that the percentage of cells that enter S phase after 20 hour is the same irrespective of the ligand incubation time. The presence of the ligand in the external medium is needed only for ten minutes, or even less, and it is sufficient to irreversibly commit the cell to proliferate as well as the constant presence of the ligand for 20 hours.
Figure 1.
NIH-3T3 cells were serum starved for 24 hours and then stimulated with the indicated PDGF-BB concentration for the indicated times and then lysed with SDS-sample buffer. Lysates were used for western blot analysis. The membranes were treated with anti phosphotyrosine specific antibodies or with anti-PDGF receptor specific antibodies. *2nd stimulation time with 30 ng/ml PDGF for 5 min after 8 hours from the first stimulation.
Table 1.
Cytofluorimetric analysis
| Time | 0 | 10′ | 20′ | 30′ | 1 h | 2 h | 20 h |
| G1 | 93.0 | 60.35 | 58.26 | 58.11 | 56.13 | 55.91 | 53.17 |
| S | 5.09 | 29.76 | 31.17 | 29.65 | 33.17 | 29.67 | 34.18 |
| G2/M | 1.98 | 9.88 | 10.57 | 12.24 | 10.69 | 14.42 | 12.65 |
5 × 105 NIH-3T3 cells were seeded onto 10 mm dishes and after 24 hours of starvation cells were stimulated with 30 ng/ml PDGF-BB. After the indicated times cells were washed extensively with PBS and in put in starvation medium. After 20 h cells were lysed in 1 ml propidium iodide solution and analyzed with Becton Dickinson FACScanto cytofluorimeter using the FacsDiva and Mod Fit cell analysis software.
How can we explain the biphasic behavior of PDGF-R signaling as a function of ligand concentration? Analogously to EGF-R,8 PDGF-R undergoes different endocytotic routes in response to different ligand concentrations. Low PDGF doses induce exclusively clathrin-mediated endocytosis (CME) in which most or the receptor is not degraded, but it is recycled back to the plasma membrane and its function is to induce and guide directional cell movements.7 High PDGF doses induce PDGF-R internalization also through Rafts/Caveolin-mediated endocytosis (RME) which is not only sufficient (9) but also necessary for PDGF-induced cell proliferation.7 RME endocytotic route leads also to PDGF-R degradation since the presence of the receptor is no further requested having started an irreversible program. The key points of PDGF signaling specificity reside in: (i) ligand concentration that determines the number of activated PDGF-R molecules; (ii) when a low number of receptors are activated, PDGF-R acts as a sensor inducing directional movement through the activation of Rho/Rac/FAK signaling pathways while the receptor is mainly retained at the plasma membrane; (iii) when the number of activated PDGF-R molecules increase (in response to an increased extracellular ligand concentration) over a “mitotic” threshold there is the activation of an additional endocytotic route (RME) that relocates PDGF-Rs prevalently to the endosomal compartment. The relocation of the receptor changes the kind of signaling proteins recruited by the receptor itself and, therefore, this event leads the to the activation of pro-mitogenic signaling modules (MAPK, PI-3K, etc.,); (iv) in the meanwhile the recycle rate of PDGF-R to the cell surface is reduced and the receptor is addressed to late endosome/lysosomal compartment for degradation, consistently with the fact that now the cell is committed irreversibly to mitosis and PDGF-R has ended its function.
In conclusion, PDGF-R, and likely many other RTKs, can induce specific cellular behavior by activating different subsets of signaling modules as a result of a quantitative modulation of its own activation that is controlled by extracellular ligand concentration.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7098
References
- 1.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 2.Grassot J, Gouy M, Perriíre G, Mouchiroud G. Origin and molecular evolution of receptor tyrosine kinases with immunoglobulin-like domains. Mol Biol Evol. 2006;23:1232–1241. doi: 10.1093/molbev/msk007. [DOI] [PubMed] [Google Scholar]
- 3.Pawson T, Gish GD, Nash P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 2001;11:504–511. doi: 10.1016/s0962-8924(01)02154-7. [DOI] [PubMed] [Google Scholar]
- 4.Madhani HD. Accounting for specificity in receptor tyrosine kinase signaling. Cell. 2001;106:9–11. doi: 10.1016/s0092-8674(01)00422-6. [DOI] [PubMed] [Google Scholar]
- 5.Rönnstrand L, Heldin CH. Mechanisms of platelet-derived growth factor-induced chemotaxis. Int J Cancer. 2001;91:757–762. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1136>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Donatis A, Comito G, Buricchi F, Vinci MC, Parenti A, Caselli A, Camici G, Manao G, Ramponi G, Cirri P. Proliferation versus migration in platelet-derived growth factor signaling: the key role of endocytosis. J Biol Chem. 2008;283:19948–19956. doi: 10.1074/jbc.M709428200. [DOI] [PubMed] [Google Scholar]
- 8.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Pennock SD, Chen X, Kazlauskas A, Wang Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J Biol Chem. 2004;279:8038–8046. doi: 10.1074/jbc.M311494200. [DOI] [PubMed] [Google Scholar]