Abstract
Synapses are basic units that mediate the communication between neurons and their target cells. The formation of synapse is regulated by secreted factors, receptors, adhesion molecules and intracellular signaling molecules. The interplay between positive and negative factors determines synapse assembling, remodeling and elimination, resulting in the formation of precise synaptic connections. However, compared to the abundant identified positive factors, negative factors are largely unknown. We have recently shown that Wnt3a acts as a negative factor that inhibits postsynaptic differentiation at the neuromuscular junction (NMJ), the synapse formed between motor neurons and skeletal muscle fibers. The clustering of acetylcholine receptor (AChR) guarantees efficient and accurate neurotransmission and is a hallmark for postsynaptic differentiation at the NMJ. We found that treatment with Wnt3a or upregulation of β-catenin inhibited the formation of AChR clusters. Furthermore, we investigated the underlying mechanism and found that Wnt/β-catenin signaling negatively regulated AChR clustering by downregulating the expression of Rapsyn, an AChR-associated protein required for formation and stabilization of AChR clusters.
Key words: AChR, Agrin, β-catenin, Rapsyn, Wnt3a
In the central nervous system (CNS), many secreted molecules have been identified to promote synapse formation. Some of them, including Wnts, Fibroblast growth factors (FGFs), neurotrophins are derived from target cells and act retrogradely to promote presynaptic differentiation,1–4 while others can aggregate postsynaptic apparatus directly.5 At the vertebrate NMJ, major efforts have been put to identify the factors that regulate AChR clustering on skeletal muscle fibers. Agrin, a glycoprotein derived from motor neuron, is known to be a positive factor that is crucial for formation and stabilization of AChR clusters.6,7 In contrast, ACh is shown to be a negative factor that disperses AChR clusters.8,9 The role of Agrin in inducing AChR clustering requires muscle specific receptor tyrosine kinase MuSK and AChR-associated protein Rapsyn.6,7
Recently, we found that ACh disperses AChR clusters by activating Calpain, a calcium-dependent protease.10 Furthermore, we found that Rapsyn binds to and inhibits Calpain to stabilize AChR clusters. Agrin increases the interaction between Rapsyn and Calpain, leading to further inhibition of Calpain and stabilization of AChR clusters.11 Thus, Rapsyn acts not only as a structural protein, but also as an inhibitor of Calpain.
Since ACh can be easily hydrolyzed, there may be other ACh-independent dispersing factors. Early studies indicate that both brain-derived neurotrophic factor (BDNF) and neurotrophin- 4 (NT-4) inhibit Agrin-induced AChR clustering on cultured myotubes,12 however genetic evidence suggests that these factors may not be involved in dispersing AChR clusters in vivo.8 Another report shows that neuregulin inhibits AChR aggregation in myotubes.13 Given that neuregulin may activate Cdk5,14 a cytoplasmic serine/threonine kinase involved in ACh-induced dispersion of AChR clusters,8 it remains possible that neuregulin-1 acts as a dispersing factor. This hypothesis needs further investigation in vivo.
Several components of Wnt signaling pathway, including Dishevelled (Dvl) and adenomatous polyposis coli (APC), regulate AChR clustering at vertebrate neuromuscular junctions,15,16 by interacting with MuSK or AChR respectively. Recently Wnt has been suggested as a local inhibitor for presynaptic assembly of C. elegans and is crucial for establishing the precisely patterned neuromuscular connectivity.17 Nevertheless, the role of Wnt in vertebrate NMJ development remains poorly understood. Recently, we found that Wnt3a could disassemble AChR clusters in cultured muscle cells and in vivo. Upregulation of β-catenin, the condition mimicking the activation of Wnt canonical pathway, inhibited the formation of AChR clusters in vitro and in vivo. Furthermore, treatment with Wnt3a or upregulation of β-catenin suppressed the expression of Rapsyn, and reconstitution of Rapsyn expression that was not responsive to Wnt3a or β-catenin partially prevented the dispersing effect of Wnt3a. This result suggests that the decreased expression of Rapsyn induced by Wnt3a is indeed responsible for the dispersing effect of Wnt3a.11
Almost contemporaneously with our study, Zhang et al., reported that suppression of β-catenin expression inhibited Agrin-induced AChR clustering. Their data indicate that β-catenin binds to Rapsyn and serves as a link between AChRs and a-catenin-associated cytoskeleton.18 Thus β-catenin might have multiple roles in the neuromuscular synaptogenesis. The appropriate level and location of β-catenin are crucial for normal synaptic structures and functions.
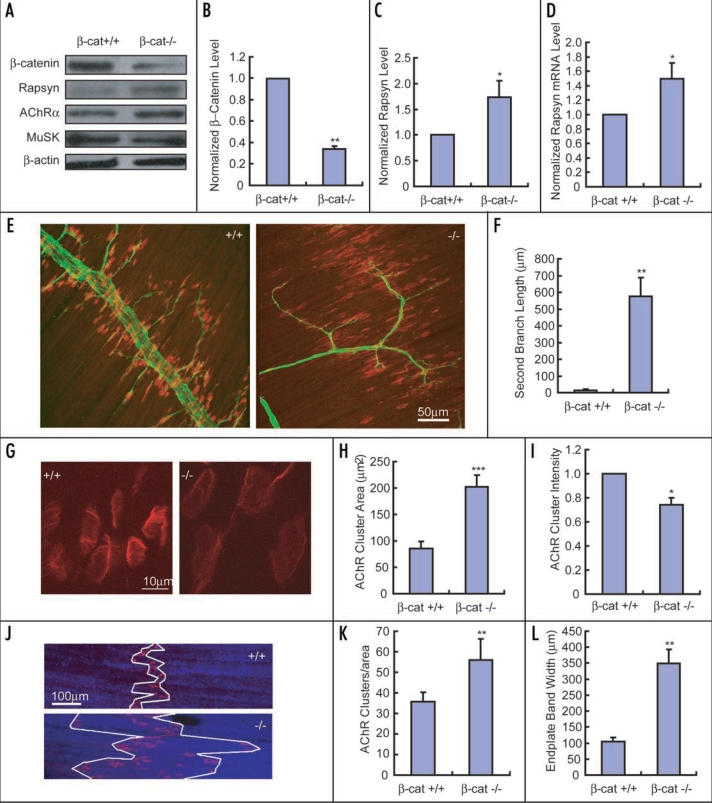
To investigate the role of β-catenin in NMJ development in vivo, we used the cre/loxP approach to specifically ablate the β-catenin gene in skeletal muscle. Mice with loxP sites flanking the second and sixth introns of the β-catenin gene19 were crossed to mice that express the cre gene under the control of human skeletal actin promoter (HSA), which drives muscle-specific expression.20–22 The progeny were lethal immediately after birth. We found that Rapsyn expression was increased in β-catenin mutant muscles, in either protein level (Fig. 1A and C) or mRNA level (Fig. 1D), supporting the notion that β-catenin negatively regulates Rapsyn expression. Interestingly, unlike primary intramuscular nerve trunks in the diaphragms of control mice that run along the middle region of muscle fibers (Fig. 1E, left), the primary trunks of mutant mice traverse along margins of muscles perpendicular to myotubes (Fig. 1E, right). However, mutant diaphragms still developed endplates at the middle region of muscle fibers, whereby a number of AChR clusters were formed (Fig. 1E). AChR clusters were quantitatively analyzed. As shown in Figure 1I, the number of AChR clusters was increased in mutant mice than that of control. In addition, diaphragms of mutant mice exhibited bigger AChR clusters (Fig. 1G and H) and wider endplate bands (Fig. 1F and J). Since β-catenin has multiple functions,23,24 it is possible that defects in mutant mice mentioned above and other defects, if any, might be caused by parallel mechanisms, in addition to the effect on Rapsyn expression as shown here. Of particular interest, Li et al., noted that the presynaptic transmission was affected in the mutant mice with specific ablation of β-catenin in the skeletal muscle.25 Thus the enlarged AChR clusters could be due to the reduced secretion of ACh from motor neurons. It is also possible that there is adaptation or redundant compensatory mechanisms in the β-catenin−/− mice.
Figure 1.
Synaptic phenotypes of mutant mice deficient for β-catenin expression in the skeletal muscle. (A) Homogenates of skeletal muscles isolated from β-catenin knockout mice (β-cat−/−) or littermate wild-type mice (β-cat+/+) at P0 were probed with indicated antibodies. (B and C) Quantification of relative protein levels of β-catenin (B) or Rapsyn (C) as shown in (A). Protein level of wild-type mice was taken as 1.0. n = 3, *p < 0.05, **p < 0.01. (D) mRNA samples isolated from skeletal muscles of mutant or wild-type mice (P0) were analyzed by quantitative-PCR analysis. n = 4, *p < 0.05. (E, G and J) Diaphragm muscles from wild-type or β-cat−/− mice at E18 (E) or P0 (G, J) were stained with antibody against neurofilament (green) to mark nerve branches (E), and R-BTX to label AChRs (E, G and J). Shown in (E) are representative images from the ventral quadrant of the right side hemidiaphragm of wild type (left) or mutant mice (right). Scale bars, 50 µm in (E), 10 µm in (G), and 100 µm in (J). (F) Second branch length. n = 7 for wild-type mice, n = 4 for β-cat−/− mice. ** p < 0.01. (H and I) Average area (Ém2) (H) or intensity (I) of individual AChR clusters from diaphragms of wild-type or mutant mice at P0. n = 22 for wild type mice, n = 16 for β-cat−/− mice. * p < 0.05, *** p < 0.001. (K) Numbers of AChR clusters in an area covered by 500 µm primary branches in the ventral quadrant of the right side hemidiaphragm. n = 5 for wild-type mice at P0, n = 6 for β-cat−/− mice at P0. ** p < 0.01. (L) Average width of endplate bands of mice at P0. n = 5 for wild-type mice, n = 4 for β-cat−/− mice. ** p < 0.01.
In summary these studies reveal complex roles of Wnt or its signaling components in NMJ synaptogenesis. Wnt expression or release is shown recently to be regulated by neuronal activity in cultured hippocampal neurons or slices,26,27 it shall be of interest to determine whether Wnt expression or release in the muscle or motor neuron is regulated by neurotransmitter ACh.
Acknowledgements
This work was supported by National Natural Science Foundation of China (30721004, 30825013), Key State Research Program of China (2006CB806600 and 2006CB943900), Chinese Academy of Sciences Grant (KSCX2-YW-R-102), and Program of Shanghai Subject Chief Scientist (08XD14050).
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7099
References
- 1.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 2.Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- 3.Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- 5.Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 6.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 7.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 8.Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, Lee KF. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc Natl Acad Sci USA. 2005;102:11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Qian L, Yang ZH, Huang Y, Ngo ST, Ruan NJ, Wang J, Schneider C, Noakes PG, Ding YQ, Mei L, Luo ZG. Rapsyn interaction with calpain stabilizes AChR clusters at the neuromuscular junction. Neuron. 2007;55:247–260. doi: 10.1016/j.neuron.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Ruan NJ, Qian L, Lei WL, Chen F, Luo ZG. Wnt/beta-catenin signaling suppresses Rapsyn expression and inhibits acetylcholine receptor clustering at the neuromuscular junction. J Biol Chem. 2008;283:21668–21675. doi: 10.1074/jbc.M709939200. [DOI] [PubMed] [Google Scholar]
- 12.Wells DG, McKechnie BA, Kelkar S, Fallon JR. Neurotrophins regulate agrin-induced postsynaptic differentiation. Proc Natl Acad Sci USA. 1999;96:1112–1117. doi: 10.1073/pnas.96.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trinidad JC, Cohen JB. Neuregulin inhibits acetylcholine receptor aggregation in myotubes. J Biol Chem. 2004;279:31622–31628. doi: 10.1074/jbc.M400044200. [DOI] [PubMed] [Google Scholar]
- 14.Fu AK, Fu WY, Cheung J, Tsim KW, Ip FC, Wang JH, Ip NY. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat Neurosci. 2001;4:374–381. doi: 10.1038/86019. [DOI] [PubMed] [Google Scholar]
- 15.Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, Mei L. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron. 2002;35:489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Jing Z, Zhang L, Zhou G, Braun J, Yao Y, Wang ZZ. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat Neurosci. 2003;6:1017–1018. doi: 10.1038/nn1128. [DOI] [PubMed] [Google Scholar]
- 17.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Luo S, Dong XP, Zhang X, Liu C, Luo Z, Xiong WC, Mei L. Beta-catenin regulates acetylcholine receptor clustering in muscle cells through interaction with rapsyn. J Neurosci. 2007;27:3968–3973. doi: 10.1523/JNEUROSCI.4691-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 20.Brennan KJ, Hardeman EC. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. J Biol Chem. 1993;268:719–725. [PubMed] [Google Scholar]
- 21.Crawford GE, Faulkner JA, Crosbie RH, Campbell KP, Froehner SC, Chamberlain JS. Assembly of the dystrophin-associated protein complex does not require the dystrophin COOH-terminal domain. J Cell Biol. 2000;150:1399–1410. doi: 10.1083/jcb.150.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo ZG, Je HS, Wang Q, Yang F, Dobbins GC, Yang ZH, Xiong WC, Lu B, Mei L. Implication of geranylgeranyltransferase I in synapse formation. Neuron. 2003;40:703–717. doi: 10.1016/s0896-6273(03)00695-0. [DOI] [PubMed] [Google Scholar]
- 23.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 24.Bienz M, Clevers H. Armadillo/β-catenin signals in the nucleus—proof beyond a reasonable doubt? Nat Cell Biol. 2003;5:179–182. doi: 10.1038/ncb0303-179. [DOI] [PubMed] [Google Scholar]
- 25.Li XM, Dong XP, Luo SW, Zhang B, Lee DH, Ting AK, Neiswender H, Kim CH, Carpenter-Hyland E, Gao TM, Xiong WC, Mei L. Retrograde regulation of motoneuron differentiation by muscle beta-catenin. Nat Neurosci. 2008;11:262–268. doi: 10.1038/nn2053. [DOI] [PubMed] [Google Scholar]
- 26.Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]