Abstract
Siboglinid worms are a group of gutless marine annelids which are nutritionally dependent upon endosymbiotic bacteria.1,2 Four major groups of siboglinids are known including vestimentiferans, Osedax spp., frenulates and moniliferans.3–5 Very little is known about the diversity of bacterial endosymbionts associated with frenulate or monoliferan siboglinids. This lack of knowledge is surprising considering the global distribution of siboglinids; this system is likely among the most common symbioses in the deep sea. At least three distinct clades of endosymbiotic γ-proteobacteria associate with siboglinid annelids.6 Frenulates harbor a clade of γ-proteobacteria that are divergent from both the thiotrophic bacteria of vestimentiferans and monoliferans as well as the heterotrophic bacteria of Osedax spp.6,7 We also discuss priorities for future siboglinid research and the need to move beyond descriptive studies. A promising new method, laser-capture microdissection (LCM), allows for the precise excision of tissue regions of interest.8 This method, when used in concert with molecular and genomic techniques, such as Expressed Sequence Tag (EST) surveys using pyrosequencing technology, will likely enable investigations into physiological processes and mechanisms in these symbioses. Furthermore, adopting a comparative approach using different siboglinid groups, such as worms harboring thiotrophic versus methanotrophic endosymbionts, may yield considerable insight into the ecology and evolution of the Siboglinidae.
Key words: endosymbiont, Frenulata, Osedax, Pogonophora, Siboglinidae, symbiosis, Vestimentifera, γ-proteobacteria symbiont
Although symbiotic relationships in marine environments occur widely from the ocean depths to intertidal systems, research on marine symbioses have largely been descriptive, rather than experimental, in nature. A limited number of systems (e.g., cnidarian/Symbiodinium, squid/Vibrio) have received considerable attention, making them excellent candidates for future experimental work. Here we briefly describe one of these well known but under-studied systems, siboglinid tubeworms and their bacterial endosymbionts. We also discuss their promise for future experimental research as a model system for marine symbioses. Substantial research in chemosynthetic environments has focused on hydrothermal vents, hydrocarbon seeps and whale falls, where symbiotic relationships drive diverse ecosystems with much higher faunal densities than their surroundings.9–13 Interestingly, many of the taxa living in chemosynthetic environments are derived from non-symbiotic lineages (e.g., vesicomyid clams, bathymodiolid mussels and siboglinid annelids), which raises questions on how these symbiotic relationships originate and evolve, as well as why some organisms (e.g., tubeworms and bivalves) dominate these ecosystems?5,14,15 Siboglinid annelids are arguably one of the best systems to address such questions since they: (1) occur in a diversity of habitats where they are numerically and ecologically dominant, (2) associate with a variety of physiologically and metabolically different bacteria, and (3) encompass a number of species, allowing for comparative studies.
Siboglinid annelid tubeworms are comprised of four major lineages: vestimentiferans, frenulates, and the genera Sclerolinum (= Monolifera) and Osedax.3–5 As adults, all lack a functional gut, mouth, and anus and instead derive their energy from bacterial endosymbionts housed within a unique storage organ, the trophosome.16 This symbiosis has also allowed them to invade and adapt to extreme environments.9,10,17 Whereas vestimentiferans and Osedax spp. have received considerable attention,10 symbioses in the more diverse frenulates has only been cursorily examined.18–20 This is particularly surprising considering that frenulates occupy a wider range of marine habitats than either vestimentiferans or Osedax spp.,17 suggesting that they and their symbiotic partners may more readily adapt to varying environments.
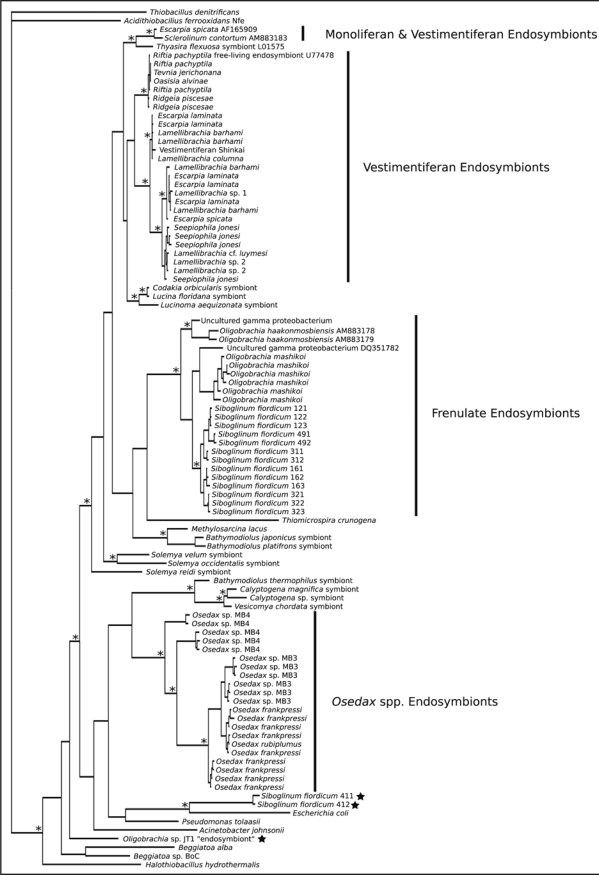
Recent studies have significantly advanced our knowledge of the bacterial symbionts of frenulate and monoliferan siboglinids.6,7 Specifically, Figure 1 presents the phylogeny of known γ-proteobacteria siboglinid symbionts, including newly characterized endosymbionts of the frenulates Siboglinum fiordicum and Oligobrachia haakonmosbiensis, as well as the monoliferan Sclerolinum contortum. Thornhill et al. demonstrated that S. fiordicum associates with a distinct group of γ-proteobacteria that are divergent from those harbored by vestimentiferan and Osedax spp. hosts.6 Notably, symbionts of S. fiordicum from Norway are most closely related to symbionts of the frenulate O. mashikoi from Japan,18,19 suggesting that symbionts of frenulates share a common evolutionary history and (potentially) similar metabolic features.6 Likewise, the symbionts of O. haakonmosbiensis belong to a clade that includes the congeneric O. mashikoi, S. fiordicum, and several strains of uncultured γ-proteobacteria.7 Lösekmann et al. also characterized the first endosymbionts from a monoliferan, S. contortum.7 This monoliferan endosymbiont was phylogenetically affiliated with a symbiont found in hydrothermal vent Escarpia spicata (Vestimentifera) and part of a larger clade that includes symbionts from other Vestimentifera as well as lucinid and thyasirid clams.7 Taken together, these preliminary results are interesting because they suggest specific associations between hosts and symbionts at higher taxonomic levels.6 Such a pattern is unexpected because siboglinids acquire symbiotic bacteria horizontally from the environment each generation, rather than vertically from the maternal parent.
Figure 1.
(Previous page) Bayesian inference phylogeny of known γ-proteobacteria siboglinid endosymbionts based on 1458 bp of 16S rRNA gene sequences. Nodal support (posterior probabilities) values ≥0.95 are designated with an asterisk. Unusual bacteria isolated from siboglinids but not definitively localized to host trophosome tissue are noted with a star symbol. GenBank accession numbers provided for taxa that were not included in Thornhill et al.6 Topologies were produced using MrBayes25 implementing the GTR+I+G model of substitution, suggested by MrModeltest 2.2.26 Two sets of four chains (three hot, one cold) were run for 1 × 106 generations and sampled every 100 generations. The first 2 × 105 generations were discarded as burn-in and a 50% majority-rule consensus tree was calculated from the resulting 16,000 trees (8,000 trees from each set).
Despite these advances, development of a more holistic understanding of these symbioses requires new data specifically aimed at elucidating the complex host-symbiont associations and interactions in frenulate and monoliferan siboglinids.
Future Directions in Siboglinid Endosymbiosis Research
Frenulata, the most species-rich and ecologically diverse siboglinid lineage, represents an ideal model for studying marine endosymbioses, since they are highly conducive to experimental, as well as descriptive, research questions. For instance, S. fiordicum can be collected from relatively shallow waters (∼30 m deep)6 and can be easily maintained in the laboratory. Furthermore, asymbiotic (or nearly so) embryos of S. fiordicum can be readily obtained from laboratory stock (Halanych, unpublished observations). In contrast, vestimentiferans are much more difficult to collect as well as expensive to maintain (e.g., vestimentiferans typically require high-pressure systems to maintain live worms).21 Likewise, Osedax spp. are highly specialized in terms of habitat requirements and utilize heterotrophic, not chemoautotrophic, endosymbionts for their nutrition.3,4,22 Lastly, Sclerolinum includes only seven recognized species with limited abundances and distributions.23 In light of this, frentulates are the most amenable system for experimental manipulation to investigate how physiological mechanisms that underlie the symbiosis react to environmental or physiological change.
Based on our observations, we suggest that future studies seek to characterize symbiont diversity and the mechanisms that underlie the establishment and maintenance of these symbioses. Specifically, among marine symbiotic systems, we have little understanding of the molecular machinery promoting regulation, signaling and ultimate maintenance of mutualistic relationships between organisms. In particular for siboglinid research, we see the following as areas of highest priority:
Characterize endosymbiont diversity among siboglinid lineages
Identify physiological mechanisms that help establish symbioses in siboglinids
Determine mechanisms that promote, maintain and/or regulate functional associations between the host and endosymbiont(s)
Elucidate evolutionary trends in siboglinid symbioses via a comparative approach
Recently, Thornhill et al.6 utilized Laser-Capture Microdissection (LCM)8 to isolate targeted endosymbiont cell populations from histological sections uncontaminated by surrounding cells. This work demonstrated that endosymbiont nucleic acids can be successfully attained from excised tissue sections for molecular analysis at extremely fine (<10 µm) scales.6 This provides the opportunity for LCM to be utilized in concert with genomic-scale techniques, such as Expressed Sequence Tag (EST) surveys using pyrosequencing technology, to investigate the genetic and physiological mechanisms underling symbiosis in this group. If these comparisons are conducted between various life history stages of a host (e.g., aposymbiotic larvae vs. symbiotic juveniles and adults) and/or among hosts harboring symbionts with different metabolic modes (e.g., thiotrophic S. fiordicum2 and methanotrophic S. poseidoni24), potentially key physiological pathways and de novo mechanisms could be revealed. Following identification of candidate mechanisms, environment or physiology could then be manipulated as a means of further elucidating the interactions facilitating and maintaining the symbiotic state. We envision that comparison across siboglinid species will provide important insights into the evolution of chemoautotrophic symbiosis mechanisms that operate in widespread marine habitats. Since chemoautorophic symbioses are likely more commonplace than currently realized, information collected from siboglinids in this manner will provide critical working hypotheses that can be explored in both siboglinids as well as other symbiotic systems.
Acknowledgements
This work was funded by National Science Foundation grants (EAR-0120646, OCE-0425060) to Kenneth M. Halanych. This is Auburn Marine Biology Program contribution #45.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7108
References
- 1.Felbeck H. Chemoautotrophic potential of the hydrothermal vent tube worm, Riftia pachyptila Jones (Vestimentifera) Science. 1981;213:336–338. doi: 10.1126/science.213.4505.336. [DOI] [PubMed] [Google Scholar]
- 2.Southward AJ, Southward EC, Dando PR, Barrett RL, Ling R. Chemoautotrophic function of bacterial symbionts in small Pogonophora. J Mar Biol Assoc UK. 1986;66:415–437. [Google Scholar]
- 3.Rouse GW, Giffredi SK, Vrijenhoek RC. Osedax: bone-eating marine worms with dwarf males. Science. 2004;305:668–671. doi: 10.1126/science.1098650. [DOI] [PubMed] [Google Scholar]
- 4.Glover AG, Kallstrom B, Smith CR, Dahlgren TG. World-wide whale worms? A new species of Osedax from the shallow north Atlantic. Proc R Soc London B. 2005;272:2587–2592. doi: 10.1098/rspb.2005.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halanych KM. Molecular phylogeny of siboglinid annelids (a.k.a. pogonophorans): a review. Hydrobiologia. 2005;535:297–307. [Google Scholar]
- 6.Thornhill DJ, Wiley AA, Campbell AL, Bartol FF, Teske A, Halanych KM. Endosymbionts of Siboglinum fiordicum and the phylogeny of bacterial endosymbionts in Siboglinidae (Annelida) Biol Bull. 2008;214:135–144. doi: 10.2307/25066670. [DOI] [PubMed] [Google Scholar]
- 7.Lösekann T, Robador A, Niemann H, Knittel K, Boetius A, Dubilier N. Endosymbioses between bacteria and deep-sea siboglinid tubeworms from and Arctic cold seep (Haakon Mosby Mud Volcano, Barents Sea) Environ Microbiol. 2008 doi: 10.1111/j.1462-2920.2008.01712.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Espina V, Wulfkuhle JD, Calvert VS, VanMeter A, Zhou W, Coukos G, Geho DH, Petricoin EF, III, Liotta LA. Laser-capture microdissection. Nat Protoc. 2006;1:586–603. doi: 10.1038/nprot.2006.85. [DOI] [PubMed] [Google Scholar]
- 9.Fisher CR. Chemoauthotrophic and methanotrophic symbiosis in marine invertebrates. Aquat Sci. 1990;2:399–436. [Google Scholar]
- 10.McMullin ER, Hourdez S, Schaeffer SW, Fisher CR. Phylogeny and biogeography of deep sea vestimentiferan tubeworms and their bacterial symbionts. Symbiosis. 2003;34:1–41. [Google Scholar]
- 11.Van Dover CL. The Ecology of Deep-Sea Hydrothermal Vents. Princeton University Press; 2000. [Google Scholar]
- 12.Dahlgren TG, Glover AG, Baco A, Smith CRD. Fauna of whale falls: Systematics and ecology of a new polychaete (Annelida: Chrysopetalidae) from the deep Pacific Ocean. Deep Sea Res Part I Oceanogr Res Pap. 2004;51:1873–1887. [Google Scholar]
- 13.Levin LA, Michener RH. Isotopic evidence for chemosynthesis-based nutrition of macrobenthos: the lightness of being at pacific methane seeps. Limnol Oceanogr. 2002;47:1336–1345. [Google Scholar]
- 14.Distel DL, Lane DJ, Olsen GJ, Giovannoni SJ, Pace P, Pace NR, Stahl DA, Felbeck H. Sulfur-oxidizing bacterial endosymbionts: Analysis of phylogeny and specificity by 16S rRNA sequences. J Bacteriol. 1988;170:2506–2510. doi: 10.1128/jb.170.6.2506-2510.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peek AS, Feldman RA, Lutz RA, Vrijenhoek RC. Cospeciation of chemoautotrophic bacteria and deep sea clams. Proc Nat Acad Sci USA. 1998;95:9962–9966. doi: 10.1073/pnas.95.17.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Southward EC, Schulze A, Gardiner SL. Pogonophora (Annelida): form and function. Hydrobiologia. 2005;535:227–251. [Google Scholar]
- 17.Schulze A, Halanych KM. Siboglinid evolution shaped by habitat preference and sulfide tolerance. Hydrobiologia. 2003;496:199–205. [Google Scholar]
- 18.Kimura H, Sato M, Sasayama Y, Naganuma T. Molecular characterization and in situ localization of endosymbiotic 16S rRNA and RuBisCO genes in the pogonophoran tissue. Mar Biotechnol. 2003;5:261–269. doi: 10.1007/s10126-002-0073-2. [DOI] [PubMed] [Google Scholar]
- 19.Kubota N, Kanemori M, Sasayama Y, Aida M, Fukumori Y. Indentification of endosymbionts in Oligobrachia mashikoi (Siboglinidae, Annelida) Microbes Environ. 2007;22:136–144. [Google Scholar]
- 20.Naganuma T, Elsaied HE, Hoshii D, Kimura H. Bacterial endosymbioses of gutless tube-dwelling worms in nonhydrothermal vent habitats. Mar Biotechnol. 2005;7:416–428. doi: 10.1007/s10126-004-5089-3. [DOI] [PubMed] [Google Scholar]
- 21.Nyholm SV, Robidart J, Girguis PR. Coupling metabolite flux to transcriptomics: Insights into the molecular mechanisms underlying primary productivity by the hydrothermal vent tubeworm Ridgeia piscesae. Biol Bull. 2008;214:255–265. doi: 10.2307/25470667. [DOI] [PubMed] [Google Scholar]
- 22.Goffredi SK, Johnson SB, Vrijenhoek RC. Genetic diversity and potential function of microbial symbionts associated with newly discovered species of Osedax polychaete worms. App Environ Microbiol. 2007;73:2314–2323. doi: 10.1128/AEM.01986-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halanych KM, Feldman RA, Vrijenhoek RC. Molecular evidence that Sclerolinum brattstromi is closely related to vestimentiferans, not to frenulate pogonophorans (Siboglinidae, Annelida) Biol Bull. 2001;201:65–75. doi: 10.2307/1543527. [DOI] [PubMed] [Google Scholar]
- 24.Schmaljohann R, Flügel HJ. Methane-oxidizing bacteria in Pogonophora. Sarsia. 72:91–98. [Google Scholar]
- 25.Huelsenbeck JP, Ronquist F. MrBAYES: Baysian inference of phylogenetic trees. Bioinformatices. 17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 26.Nylander JAA. MrModeltest v2 [online] Evolutionary Biology Centre, Uppsala University; Available: http://www.abc.se/~nylander/ Program distributed by the author. [Google Scholar]