Abstract
Chlamydiae are obligate intracellular pathogens that cause a wide range of human diseases. Chlamydia resides in a membrane bound vacuole (“inclusion”) that expands to accommodate replicating bacteria. We recently reported that Chlamydia remodels and recruit two major cytoskeletal components of the host cell- F-actin and Intermediate filaments-to form a dynamic scaffold that provides structural stability to the inclusion. As the inclusion expands, a secreted chlamydial protease progressively modifies the intermediate filaments scaffold, presumably to increase the inclusion's flexibility and accommodate the increased bacterial load. This represents a unique mechanism employed by an intracellular pathogen to support its intracellular niche and may be linked to immune evasion by this pathogen. Here, we discuss the potential consequences of Chlamydia-mediated alteration of host cytoskeletal dynamics on the pathogenesis of chlamydial infections.
Key words: Chlamydia, intermediate filaments, Rho GTPases, cell motility, inflammation
Introduction
Chlamydiae are Gram-negative obligate intracellular bacterial pathogens that infect pulmonary, ocular and genital epithelial surfaces to cause ailments ranging from pneumonia to sexually transmitted diseases. Chronic inflammation from recurrent chlamydial infections can result in blinding trachoma, pelvic inflammatory disease and infertility.1 Chlamydiae have a biphasic lifecycle with an infectious, metabolically-inactive Elementary body (EB) that transitions into a replicative Reticulate body (RB) form soon after invasion.2 During the intracellular phase of infection, the bacteria are sequestered within a membrane-bound vacuole termed the ‘inclusion’ that dramatically expands in size to accommodate exponential bacterial replication.3 The inclusion membrane serves both as an interface through which the bacteria import nutrients as well as a barrier that prevents exposure of bacterial products to cytosolic innate immune surveillance. In addition, it has been proposed that the morphology of the inclusion may regulate the biphasic transition of RBs to EBs.4 How Chlamydiae maintain the shape and stability of the inclusion was unknown.
F-actin and Intermediate Filaments Form a Scaffold that Stabilizes the Chlamydia Inclusion
Because of the central role of the mammalian cytoskeleton in providing mechanical support, we hypothesized that it played a role in maintaining inclusion morphology. This was consistent with previous reports suggesting alterations of host cytoskeleton in Chlamydiae infected cells.5,6 We determined that the inclusion is encased in a dynamic meshwork consisting of host F-actin and Intermediate filaments (IFs)7 (Fig. 1). Disruption of F-actin or IFs with inhibitors or using IF-deficient cells reduced the stability of the inclusion and resulted in distortion of inclusion shape as well as spillage of bacterial components into the host cytosol. Although actin microfilaments are well known targets of several intracellular pathogens during invasion,8 their role during the intracellular phase of infection is poorly understood. For example, Salmonella recruits F-actin filaments to the surface of the Salmonella containing vacuole (SCV) at 4–8 hrs post-invasion9 and prolonged treatment with F-actin inhibitors resulted in reduced vacuole integrity.9 We speculate that actin recruitment may be a strategy to promote stability of intracellular parasitic vacuole membranes in at least a subset of intracellular pathogens. The mechanisms underlying actin assembly at the vacuole surface are likely to be varied. For example actin assembly at the Chlamydia inclusion requires small GTPase RhoA but not canonical RhoA effectors,7 while actin recruitment at the SCV was independent of host Rho GTPases.10
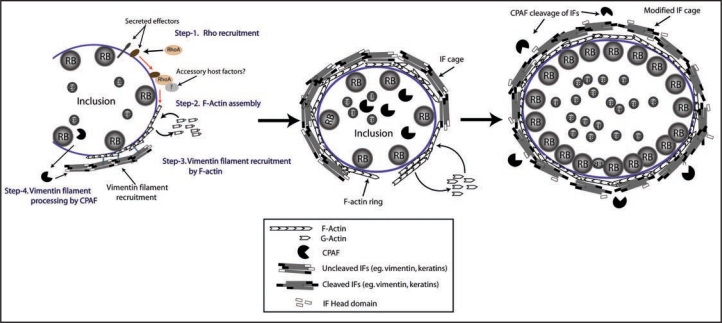
Figure 1.
A host-derived cytoskeletal scaffold stabilizes the chlamydial inclusion. The chlamydial inclusion is encased in a network of F-actin and Intermediate filaments (IFs). In this model, F-actin assembly at the inclusion surface is orchestrated by RhoA, which is presumably recruited to the inclusion by putative bacterial effectors. RhoA possibly in cooperation of other host accessory proteins orchestrates F-actin assembly (Left). Inclusion-associated F-actin further recruits IFs completing the cytoskeletal cage (middle). Inclusion expansion in response to bacterial replication is facilitated by modification of IFs by the chlamydial protease CPAF (Right).
In addition to F-actin, the chlamydial inclusion stability was also dependent on IFs. IFs are one of the most abundant and diverse cytoskeletal systems in mammalian cells with functions ranging from mechanical support to vesicular trafficking and organelle positioning.11 A role for IF alterations has been proposed in viral12 as well as protozoan13 infections, but are less common in bacterial pathogens.14,15 Our studies suggest that Chlamydia not only recruits IFs to the pathogenic vacuoles but also modifies the cytoskeletal properties of these filaments7 during the course of infection.
Identification of chlamydial and host factors that are recruited by Chlamydia to regulate actin and IF assembly is likely to provide novel insights into how this pathogen manipulates inclusion integrity (see below) and to explore its role in pathogenesis.
The Stability of the Inclusion Is Linked to Immune-Evasion by Chlamydia
The innate immune surveillance machinery in mammalian cells is the first line of defense against infection by intracellular pathogens and consists of Pathogen recognition receptors (PRRs) that sense pathogen associated molecular patterns (PAMPs). These receptors activate signaling cascades resulting in production and release of pro-inflammatory cytokines.16 For pathogens such as Listeria17 and Francisella,18 studies with mutants incapable of escaping the vacuoles indicated that sequestration in membrane-bound compartments limits the activation of cytoplasmic surveillance pathways. By analogy, we hypothesized that stability of the inclusion may limit the detection of Chlamydia by PRRs. Indeed, destabilization of the inclusion via reversible host-actin disruption led to spillage of bacterial products into the host cytosol and resulted in increased pro-inflammatory cytokine production.7 We propose that chlamydial modulation of host cytoskeleton may constitute a mechanism of immune-modulation. Because tissue damage in the form of scarring of genital, ocular or bronchial mucosal epithelia is due mostly to inflammation,19,20 a detailed understanding of factors governing pathogenic vacuole stability will offer new insights into the interaction of Chlamydiae with host innate immune pathways.
The Impact of Chlamydia Infections on Cytoskeleton-Dependent Host Cell Functions
The mammalian cytoskeleton plays important roles in a variety of cellular functions including cell migration, cell division, vesicular trafficking, and organelle architecture.21–23 It is possible that disruption of F-actin and IF architecture at infection sites may impact cytoskeleton-dependent functions of the mucosal epithelia. We tested if cell migration, an essential process required for wound healing and repair following tissue damage, may be impaired in Chlamydia infected cells. As shown in Figure 2, infection by Chlamydia LGV-L2 serovar inhibited host cell motility in a wound healing assay. During viral infections, modulation of epithelial cell motility has been linked to both the spread of infection as well as reduced tissue repair following inflammation. For instance inhibition of bronchial epithelial cell migration by bovine herpesvirus-1 infection compromises respiratory epithelial repair after injury contributing to lung disease.24 In contrast vaccinia virus infection stimulates host cell motility via activation of RhoA by its F11L gene product to facilitate dissemination.25 Whether Chlamydia inhibits cell motility directly by cytoskeletal disruption or indirectly via sequestration of cell migration regulators such as RhoA, remains to be determined. It is also possible that impaired migration is the result of a failure to properly polarize the Golgi apparatus, either due to centrosomal miss-positioning26 or cytoskeletal alterations in infected cells. The full impact of inhibition of cell migration on pathogenesis will require a detailed analysis of the cell biology of infected cells in animal models of chlamydial disease.27
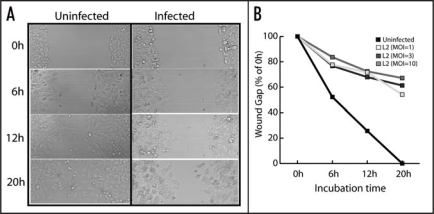
Figure 2.
Chlamydia inhibits host cell motility. The ability of HeLa cells to migrate in response to injury was tested using an in vitro scratch wound assay.28 HeLa cell monolayers were either untreated or infected with C. trachomatis LGV-L2 serovar for 12 h with various indicated multiplicity of infection (MOI) and a scratch wound was made and imaged over a period of 20 h (A). The wound gap was measured every 6 h and is expressed as percent of 0 h (B).
Conclusion
Chlamydiae hijacks host cytoskeletal components to stabilize its intracellular vacuolar niche. The stability of the chlamydial inclusion and possibly other pathogenic vacuoles may be linked to evasion of innate immune pathways. In addition, alteration of host cytoskeleton by Chlamydiae, may impact previously unappreciated cytoskeleton-dependent functions such as cell motility and wound repair contributing to disease progression.
Acknowledgements
This work was supported by funds from the Whitehead Foundation, the NIH (AI068032), and the Burroughs Wellcome Trust Fund.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7146
References
- 1.Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis and Immunity. Washington, DC: ASM; 1999. p. 31. [Google Scholar]
- 2.Belland R, Ojcius DM, Byrne GI. Chlamydia. Nat Rev Microbiol. 2004;2:530–531. doi: 10.1038/nrmicro931. [DOI] [PubMed] [Google Scholar]
- 3.Fields KA, Hackstadt T. The chlamydial inclusion: escape from the endocytic pathway. Annu Rev Cell Dev Biol. 2002;18:221–245. doi: 10.1146/annurev.cellbio.18.012502.105845. [DOI] [PubMed] [Google Scholar]
- 4.Peters J, Wilson DP, Myers G, Timms P, Bavoil PM. Type III secretion a la Chlamydia. Trends Microbiol. 2007;15:241–251. doi: 10.1016/j.tim.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Campbell S, Richmond SJ, Yates PS. The effect of Chlamydia trachomatis infection on the host cell cytoskeleton and membrane compartments. J Gen Microbiol. 1989;135:2379–2386. doi: 10.1099/00221287-135-9-2379. [DOI] [PubMed] [Google Scholar]
- 6.Stuart ES, Brown MV. chlamydial inclusions: Accumulation of fibers bearing an intermediate filament epitope. Curr Microbiol. 1992;24:329–335. [Google Scholar]
- 7.Kumar Y, Valdivia RH. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe. 2008;4:159–169. doi: 10.1016/j.chom.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rottner K, Stradal TE, Wehland J. Bacteria-host-cell interactions at the plasma membrane: stories on actin cytoskeleton subversion. Dev Cell. 2005;9:3–17. doi: 10.1016/j.devcel.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Meresse S, et al. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell Microbiol. 2001;3:567–577. doi: 10.1046/j.1462-5822.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 10.Unsworth KE, Way M, McNiven M, Machesky L, Holden DW. Analysis of the mechanisms of Salmonella-induced actin assembly during invasion of host cells and intracellular replication. Cell Microbiol. 2004;6:1041–1055. doi: 10.1111/j.1462-5822.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581–1597. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- 12.Stefanovic S, Windsor M, Nagata KI, Inagaki M, Wileman T. Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II. J Virol. 2005;79:11766–11775. doi: 10.1128/JVI.79.18.11766-11775.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halonen SK, Weidner E. Overcoating of Toxoplasma parasitophorous vacuoles with host cell vimentin type intermediate filaments. J Eukaryot Microbiol. 1994;41:65–71. doi: 10.1111/j.1550-7408.1994.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 14.Batchelor M, et al. Involvement of the intermediate filament protein cytokeratin-18 in actin pedestal formation during EPEC infection. EMBO Rep. 2004;5:104–110. doi: 10.1038/sj.embor.7400038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson SA, Omary MB, Jones BD. Identification of cytokeratins as accessory mediators of Salmonella entry into eukaryotic cells. Life Sci. 2002;70:1415–1426. doi: 10.1016/s0024-3205(01)01512-0. [DOI] [PubMed] [Google Scholar]
- 16.Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that cooperate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 17.O'Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci USA. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry T, Monack DM. Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell Microbiol. 2007;9:2543–2551. doi: 10.1111/j.1462-5822.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 19.Cohen CR, Brunham RC. Pathogenesis of Chlamydia induced pelvic inflammatory disease. Sex Transm Infect. 1999;75:21–24. doi: 10.1136/sti.75.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.el-Asrar AM, Van de Oord JJ, Geboes K, Missotten L, Emarah MH, Desmet V. Immunopathology of trachomatous conjuctivitis. Br J Ophthamol. 1989;73:276–282. doi: 10.1136/bjo.73.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musch A. Microtubule organization and function in epithelial cells. Traffic. 2004;5:1–9. doi: 10.1111/j.1600-0854.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 22.Toivola DM, Tao GZ, Habtezion A, Liao J, Omary MB. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 2005;15:608–617. doi: 10.1016/j.tcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 24.Spurzem JR, et al. Bovine herpesvirus-1 infection reduces bronchial epithelial cell migration to extracellular matrix proteins. Am J Physiol. 1995;268:214–220. doi: 10.1152/ajplung.1995.268.2.L214. [DOI] [PubMed] [Google Scholar]
- 25.Morales I, et al. The vaccinia virus F11L gene product facilitates cell detachment and promotes migration. Traffic. 2008;9:1283–1298. doi: 10.1111/j.1600-0854.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 26.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Vanrompay D, Lyons JM, Morre SA. Animal models for the study of Chlamydia trachomatis infections in the female genital infection. Drugs Today (Barc) 2006;42:55–63. [PubMed] [Google Scholar]
- 28.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]