Abstract
Convergent evolution of trophic life style and morphological characters are very common in the fungal kingdom. Recently, we have shown that the same molecular machinery containing a tetraspanin and a NADPH oxidase has been recruited in two different fungal species for the same purpose (exiting from a melanized re-enforced cell at a focal weakened point), but at different stages of their development (ascospore germination and appressorium mediated penetration). Although this molecular machinery is required at these key developmental steps, it is also likely involved in specialized cellular functions at other stages of fungal development, as shown here for nutrient acquisition by Podospora anserina.
Key words: convergent evolution, tetraspanin, NADPH oxidase, fungi, Podospora anserina, Magnaporthe grisea
How single hyphae can emerge from a melanized cell remains largely unknown, although this phenomenon is a key developmental process in many fungi. The filamentous fungus Podospora anserina differentiate heavily melanized ascospores that germinate under inducing conditions through a specific germ pore that corresponds to a specific region of the ascospore with a non-melanized weakened cell wall. On the contrary, the rice blast fungus Magnaporthe grisea produces non-melanized ascospores that germinate spontaneously from multiple points. However, M. grisea develops a heavily melanized cell specifically devoted to the penetration of the fungus into its host plant, the appressorium. Specialized hyphae, known as penetration pegs, are formed at the base of these appressoria in a region called the pore with also a non-melanized weakened cell wall. The penetration peg breaches plant cuticle and cell wall, allowing the fungus to penetrate into host tissues. Up to now, the mechanisms involved in these two processes were thought to be completely different, since these two melanized structures are so different either morphologically or in their biological role. Moreover, P. anserina, a non-pathogen saprobe, and M. grisea, a devastating phytopathogen, are not closely related phylogenetically, since they belong to two different clades of the Sordariomycetes.
Genetic studies undertaken to isolate M. grisea non pathogenic mutants showed that appressorium mediated penetration and the formation of penetration pegs require a functional MgPLS1 gene, whereas the formation of appressoria themselves does not.1 MgPLS1 encodes a membrane protein from the tetraspanin family identified in animals and fungi.2,3 Surprisingly, Pls1 orthologs were also identified in fungi devoid of appressorium, such as P. anserina.4 What could be the role of such a protein in an appressorium-less non-pathogenic fungus? To address this question, we performed a deletion of PaPls1, the P. anserina MgPLS1 ortholog.4 P. anserina mutant strains lacking PaPls1 can complete their whole life cycle, except that their ascospores do not germinate. Although morphologically identical to those issued from a wild-type cross, ascospores from a cross between PaPls1 null mutants are unable to form a germ tube normally extruded from the pore. We also observed that hyaline (melanine mutant) ascospores of PaPls1 mutants germinate as wild-type, demonstrating that the inactivation of PaPls1 has no effect on the germination of non-melanized ascospores. Finally, we showed that MgPLS1 can functionally replace PaPls1 (positive complementation of a PaPls1 null mutant with MgPLS1) indicating that these two genes are true functional orthologs.
Interestingly, the specific and autonomous defect in ascospore germination of the PaPls1 mutants is identical to the mutant phenotype previously observed for the PaNox2 null mutants.5 PaNox2 encodes a NADPH oxidase, which is a membrane enzyme involved in the production of superoxide for either signaling or defense toward competitors in a broad range of eukaryotes.6 Deletion of the corresponding MgNOX2 gene led to non-pathogenic mutants impaired in appressorium mediated penetration.7 Altogether, these results show that the germination of P. anserina melanized ascospores and the formation of the penetration peg in M. grisea at the base of melanized appressoria share similar molecular determinants, namely a tetraspanin and a NADPH oxidase. Therefore, we postulated that these two different developmental processes are driven by the recruitment of highly specialized machinery (PLS1/NOX2) through convergent evolution. The co-occurrence of Pls1 and Nox2 in fungal genomes strengthens this hypothesis.4
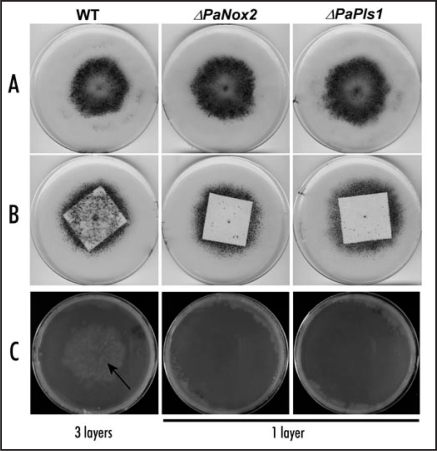
P. anserina ESTs data4,8 showed that both PaPls1 and PaNox2 are expressed throughout the life cycle of this fungal species. Recent micro-arrays experiments (Bidard F, unpublished data) showed that both genes are upregulated during P. anserina sexual development. Indeed the peak of PaPls1 and PaNox2 expression occurs during meiosis, a few hours prior ascospore formation. This expression pattern strengthens our finding that both act together on P. anserina melanized ascospore germination. In addition to the detection of their upregulation during sexual development, these micro-arrays data confirmed that both genes were expressed in vegetative hyphae (Bidard F, unpublished data). However, we were not able to observe any vegetative phenotypes in PaPls1 and PaNox2 null mutants. But, while testing the influence of different sources of carbohydrates on growth and reproduction of various P. anserina mutants, we recently discovered a new role for PaPls1 and PaNox2. Indeed, P. anserina is usually cultivated on minimal medium containing dextrin as sole carbon source. On such a medium, PaPls1 and PaNox2 mutants are indistinguishable from wild-type (Fig. 1A). However, when grown on a medium with 3 cm × 3 cm piece of filter paper as sole carbon source, we observed clear differences between wild-type and these two mutants (Fig. 1B). In wild-type crosses, a large number of fructifications were formed on the filter paper, while hardly any fruiting bodies were observed in both mutant crosses. In both wild-type and mutant crosses, fully mature fructifications were formed at the margin of the filter paper in the surrounding agar medium. However, in mutant crosses, a reduced number of scattered fructifications were observed with a delayed development. These results suggest that PaPls1/PaNox2 molecular machinery has a specific role during the vegetative growth needed before fructification formation, related to its expression in vegetative hyphae. Indeed, the mycelium must efficiently scavenge nutrients and needs to translocate them to the developing fructifications. It appears that the PaPls1 and PaNox2 mutants are affected in this process when grown on filter paper as sole carbon source. As both mutants are delayed in their ability to penetrate a cellophane membrane (Fig. 1C), their defect observed on filter paper is likely related to a reduced ability to metabolize this complex carbon source that needs to be degraded to be assimilated. This novel role for PaPls1/PaNox2 fits well with a possible role of reactive oxygen species produced by NADPH oxidases in the degradation of polymers and complex carbohydrate sources. This hypothesis could be transposed to the interpretation of the defects observed in P. anserina ascospore germination and M. grisea appressorium mediated penetration.4 Indeed, according to this novel hypothesis, PaPLS1 and PaNOX2, as well as MgPls1 and MgNox2 could be involved in the site-specific degradation of the plugs that close the pores of melanized ascospores and appressoria, respectively. A similar role for MgPLS1 and MgNOX2 can account for the impaired growth of the corresponding M. grisea mutants in planta, which is presently unexplained.
Figure 1.
(A) After seven days of growth on a medium with dextrin as sole carbon source, wild-type, PaPls1 and PaNox2 mutants differentiate fructifications (small black dots) mostly in a region surrounding inoculum's explants located at the center of the plates. (B) After seven days of growth on 3 cm × 3 cm filter papers as sole carbon source, wild-type differentiates about fifty times more fructifications than PaPls1 and PaNox2 mutants on the filter paper. (C) After seven days of growth on a medium overlaid with cellophane, wild-type has reached the underneath medium (arrow) through three layers of cellophane, whereas both PaPls1 and PaNox2 mutants have not breached a single layer of cellophane.
Now, that the first two players of the PLS1/NOX2 molecular machinery are identified, it will be of great interest to unravel the members of this “pathway” to understand in depth, how a similar cellular machinery was recruited by convergent evolution, to allow a focalized exit from melanized cells in unrelated organisms and to mediate degradation of natural carbon polymers.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7198
References
- 1.Clergeot PH, Gourgues M, Cots J, Laurans F, Latorse MP, Pepin R, Tharreau D, Notteghem JL, Lebrun MH. PLS1, a gene encoding a tetraspanin-like protein, is required for penetration of rice leaf by the fungal pathogen Magnaporthe grisea. Proc Natl Acad Sci USA. 2001;98:6963–6968. doi: 10.1073/pnas.111132998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang S, Yuan S, Dong M, Su J, Yu C, Shen Y, Xie X, Yu Y, Yu X, Chen S, Zhang S, Pontarotti P, Xu A. The phylogenetic analysis of tetraspanins projects the evolution of cell-cell interactions from unicellular to multicellular organisms. Genomics. 2005;86:674–684. doi: 10.1016/j.ygeno.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Lambou K, Tharreau D, Kohler A, Sirven C, Marguerettaz M, Barbisan C, Sexton AC, Kellner EM, Martin F, Howlett BJ, Orbach MJ, Lebrun MH. Fungi have three tetraspanin families with distinct functions. BMC Genomics. 2008;9:63. doi: 10.1186/1471-2164-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambou K, Malagnac F, Barbisan C, Tharreau D, Lebrun MH, Silar P. The crucial role during ascospore germination of the Pls1 tetraspanin in Podospora anserina provides an example of the convergent evolution of morphogenetic processes in fungal plant pathogens and saprobes. Eukaryot Cell. 2008;7:1809–1818. doi: 10.1128/EC.00149-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malagnac F, Lalucque H, Lepere G, Silar P. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet Biol. 2004;41:982–997. doi: 10.1016/j.fgb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Silar P. Peroxide accumulation and cell death in filamentous fungi induced by contact with a contestant. Mycol Res. 2005;109:137–149. doi: 10.1017/s0953756204002230. [DOI] [PubMed] [Google Scholar]
- 7.Egan MJ, Wang ZY, Jones MA, Smirnoff N, Talbot NJ. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc Natl Acad Sci USA. 2007;104:11772–11777. doi: 10.1073/pnas.0700574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espagne E, Lespinet O, Malagnac F, Da Silva C, Jaillon O, Porcel BM, Couloux A, Aury JM, Segurens B, Poulain J, Anthouard V, Grossetete S, Khalili H, Coppin E, Dequard-Chablat M, Picard M, Contamine V, Arnaise S, Bourdais A, Berteaux-Lecellier V, Gautheret D, de Vries RP, Battaglia E, Coutinho PM, Danchin EG, Henrissat B, Khoury RE, Sainsard-Chanet A, Boivin A, Pinan-Lucarre B, Sellem CH, Debuchy R, Wincker P, Weissenbach J, Silar P. The genome sequence of the model ascomycete fungus Podospora anserina. Genome Biol. 2008;9:77. doi: 10.1186/gb-2008-9-5-r77. [DOI] [PMC free article] [PubMed] [Google Scholar]