Abstract
It is a fundamental question in neuroscience how long-term memory formation is regulated at the molecular level. Although widely considered a highly complex process requiring numerous molecular players, it also has been speculated that a single protein could play a pivotal role. This “astonishing hypothesis” has made a significant impact on memory research and has led to a reevaluation of concepts regarding memory formation.1,2
Key words: PKMzeta, AMPA-receptor trafficking, long-term memory, long-term potentiation, actin cytoskeleton, metaplasticity
A recent publication by Yao et al.3 in The Journal of Neuroscience has provided compelling evidence that protein kinase M zeta (PKMζ), an atypical protein kinase C (aPKC) isoform, could mediate such a central function in memory formation by maintaining a stable enhancement of synaptic transmission at neuronal synapses, thus regulating the late phase of LTP (LTP3).4 PKMζ is a constitutively active, second-messenger independent, kinase produced from a brain specific PKMζ-mRNA which is transported from the nucleus to the dendritic tree. Once initiated, PKMζ facilitates the delivery of AMPA-receptors (AMPAR) into the synapse.5 Since PKMζ seems necessary and sufficient for preserving late-LTP and long-term memory in vivo,6 it can be considered a key player responsible for plastic properties of central neurons, thus providing the prospect of further exciting research on memory functions. However, the precise mechanism how PKMζ exerts its actions on AMPAR trafficking remained unclear.
Yao and colleagues tackled this issue by probing how PKMζ modulates major AMPAR trafficking pathways. In particular, they focused on the mobilization of AMPAR from extrasynaptic sites and AMPAR delivery via the exocytotic pathway. First, the possible involvement of N-ethylmaleimide-Sensitive Factor (NSF)/GluR2 signaling in PKMζ-mediated upregulation of AMPAR-dependent neurotransmission was studied (their Fig. 1, http://www.jneurosci.org/cgi/content/full/28/31/7820/F1). Through a whole-cell recording pipette, PKMζ and inhibitors of the NSF/GluR2 interaction were perfused into CA1 pyramidal cells and subsequent changes of AMPAR responses at Schaffer collateral/commissural-CA1 pyramidal cell synapses were recorded. Both pep2m, a protein that mimics the NSF-binding site on GluR2, and pep-NSF3, a peptide that blocks the ATPase activity of NSF, inhibited PKMζ-mediated potentiation of AMPAR responses. These results indicate that PKMζ enhances lateral diffusion of AMPAR through NSF/GluR2 interactions. To exclude the possibility that PKMζ could act via enhancing AMPAR-exocytosis, the authors also applied botulinum toxin B light chain (Botox B). This toxin blocks the exocytotic AMPAR trafficking pathway by cleaving vesicle-associated membrane protein (VAMP)/synaptobrevin. Since this approach did not affect the PKMζ-mediated potentiation of AMPAR responses (their Fig. 1D, http://www.jneurosci.org/cgi/content/full/28/31/7820/F1), the authors concluded that PKMζ did not act via this mechanism. Although these interpretations are plausible, we would like to add a note of caution: Botox B application produced a significant baseline rundown making it difficult to safely conclude that the exocytotic pathway is not engaged in PKMζ-mediated potentiation of AMPAR responses, even if baseline subtraction shows no significant difference in the recordings. Thus, the interpretation of the authors, although compelling, could be strengthened by additional corroborating data.
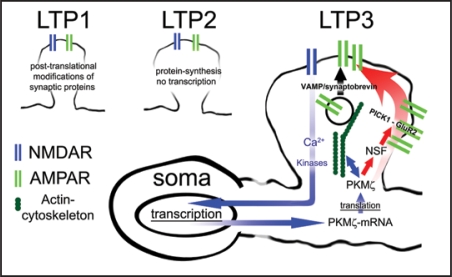
Figure 1.
Based on duration and biochemical mechanisms, LTP has been classified into three distinct phases4 that have been termed LTP1, LTP2, and LTP3: LTP1 is a short-lasting (1 hour) early form of LTP that requires post-translational modification of synaptic proteins but is independent of protein synthesis. LTP2 is slowly decaying (1–3 hours) and dependent on protein translation but does not require gene transcription. Finally, LTP3 represents the long-lasting phase of LTP and is believed to be both translation- and transcription-dependent. PKMζ maintains late-LTP (LTP3) through upregulation of an NSF/GluR2-dependent AMPAR trafficking pathway (red pathway). PKMζ acts through NSF by releasing GluR2-containing receptors from a reserve pool held at extrasynaptic sites by PICK1 and thereby promoting their trafficking to the postsynaptic density. The VAMP/synaptobrevin dependent exocytotic pathway does not seem to be influenced by PKMζ. Interactions between PKMζ and the actin cytoskeleton remain to be clarified as well as upstream regulatory pathways (e.g., role of calcium and kinases), initiating transcription and translation of PKMζ (blue pathway).
Next, Yao et al.3 carefully studied the molecular events downstream of PKMζ. They focused on NSF ATPase which disrupts the interaction between GluR2 and PICK1 (a protein interacting with C-kinase 1) required for AMPAR trafficking.7 To unravel the mechanism of the NSF-dependent PKMζ action, the authors selectively disrupted GluR2/PICK1 interaction by intracellular pep2-EVKI infusion. This manipulation mimicked and occluded the PKMζ-mediated AMPAR potentiation (their Fig. 2, http://www.jneurosci.org/cgi/content/full/28/31/7820/F2), indicating that PKMζ potentiates AMPAR responses via NSF mediated release of GluR2 from PICK1. To show directly that PKMζ mediates NSF/GluR2-dependent AMPAR trafficking during LTP, the authors studied AMPAR-trafficking using biochemical fractionating techniques. In agreement with their hypothesis, both pep2m and the PKMζ inhibitor ZIP blocked the LTP-related synaptosomal GluR2 and GluR3 subunit increase after LTP-inducing tetanization (their Fig. 3, http://www.jneurosci.org/cgi/content/full/28/31/7820/F3). These experiments were performed in a time window of 1 h after LTP induction. To directly test whether PKMζ-dependent NSF/GluR2 signaling is crucial for establishing late-LTP, pep2m and ZIP were applied 3 h after tetanization, successfully reversing persistent synaptic potentiation without affecting a second, independent non-tetanized pathway recorded within each of the slices (their Fig. 4, http://www.jneurosci.org/cgi/content/full/28/31/7820/F4 and Suppl. Fig. 7). These results support NSF/GluR2 dependent AMPAR trafficking as a downstream mechanism of PKMζ. Nevertheless, some additional experiments are conceivable which could make the data even more robust and compelling. Since PKMζ is believed to be acting upstream of NSF, washing out the blocker of NSF/GluR2 interaction, myr-pep2m, should result in a repotentiation. Also, since the authors show that it is possible to bring the established LTP back to baseline levels in a fairly fast time window, one could test whether, in the presence of myr-pep2m, an additional tetanus would induce a short lasting potentiation or cause a full blown LTP. According to the metaplasticity theory,8,9 the depotentiation of a pre-established LTP allows the field to reproduce LTP once a tetanic stimulus is reapplied. In this respect, if one could show that normal LTP can be evoked following myr-pep2m treatment, an interesting and counterintuitive hypothesis could be formulated (their Fig. 4): Blocking PKMζ signaling might paradoxically increase the possibility to form new memories, allowing the re-establishment of LTP once a new tetanus is applied. It appears to be prudent not to exclude this possibility at the current stage of knowledge.
The authors report a pivotal role of PKMζ in sustaining lateral movement of AMPAR to synaptic sites. They emphasize, however, that their interpretation allows room for additional downstream mechanisms targeting AMPAR trafficking. Since AMPAR are linked to the spine cytoskeleton, PKMζ could also direct AMPAR to the synapse by regulating actin dynamics. This hypothesis is attractive since actin-based structural remodelling is important during the late phase of LTP and might be required for the replacement of transient synaptic GluR1 homomers with more stable GluR2-containing AMPAR.10 However, previous experiments of the authors have shown that preventing actin polymerization during LTP with latrunculin B has no dramatic effects on the potentiation of AMPA receptors by PKMζ.11 Nevertheless, in these experiments latrunculin B blocked de novo synthesis of PKMζ indicating the importance of actin dynamics for PKMζ regulation. Thus, further examination of the involvement of PKMζ in regulating actin-dependent AMPAR movement and turnover appears to be called for and may provide new insight into the interplay between AMPAR trafficking, actin-dependent spine morphology and late LTP.
In summary, this work shows that PKMζ maintains late-LTP through upregulation of an NSF/GluR2-dependent AMPAR trafficking pathway. PKMζ acts through NSF by releasing GluR2-containing receptors from a reserve pool held at extrasynaptic sites by PICK1 and promoting their trafficking to the postsynaptic density (Fig. 1). These interesting data raise several important questions regarding possible upstream and downstream PKMζ signaling pathways and provide new insights on the role of PKMζ in long-term memory formation.
Acknowledgements
We thank Dr. Thomas Deller for critical reading of the manuscript and helpful discussions.
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/6889
References
- 1.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 2.Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKMzeta. Science. 2007;317:951–953. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- 3.Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC. PKMzeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the “long” in long-term potentiation. Trends Neurosci. 2007;30:167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Ling DS, Benardo LS, Sacktor TC. Protein kinase Mzeta enhances excitatory synaptic transmission by increasing the number of active postsynaptic AMPA receptors. Hippocampus. 2006;16:443–452. doi: 10.1002/hipo.20171. [DOI] [PubMed] [Google Scholar]
- 6.Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- 7.Hanley JG, Khatri L, Hanson PI, Ziff EB. NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron. 2002;34:53–67. doi: 10.1016/s0896-6273(02)00638-4. [DOI] [PubMed] [Google Scholar]
- 8.Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 9.Jedlicka P. Synaptic plasticity, metaplasticity and BCM theory. Bratisl Lek Listy. 2002;103:137–143. [PubMed] [Google Scholar]
- 10.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 11.Kelly MT, Yao Y, Sondhi R, Sacktor TC. Actin polymerization regulates the synthesis of PKMzeta in LTP. Neuropharmacology. 2007;52:41–45. doi: 10.1016/j.neuropharm.2006.07.002. [DOI] [PubMed] [Google Scholar]