Abstract
Actin stress fiber (SF), focal adhesion (FA) and adherens junction (AJ) are known structures whose formation and development are mechanical force-dependent. At these structures, actin is actively polymerized, which in turn contributes the development of these structures. Recently, we reported that actin polymerization at FAs is facilitated by mechanical forces, which was critically dependent on the force-induced recruitment of the LIM protein zyxin to FAs. Zyxin enhances actin polymerization with the aid of Ena/VASP proteins. Both zyxin and Ena/VASP proteins are localized not only to FAs but also to AJs and SFs, facilitating actin polymerization at these structures. We discuss here the possibility that zyxin is a common mechanotransducer element regulating actin polymerization at FAs, AJs and SFs.
Key words: zyxin, Ena/VASP, mechanotransduction, actin polymerization, focal adhesion, stress fiber, adherens junction
Within tissues, cells are exposed to various mechanical perturbations originating from muscle contraction, blood flow, tissue wounding, etc. In response to changes in mechanical environments, cells alter their morphology mostly associated with changes in the architecture of cytoskeletons and cell adhesions. A growing body of research has shed light on the molecular mechanisms underlying the cellular responses to these mechanical stimuli. In this review, we focus on the recent progress in studies on the role of the LIM protein zyxin in the mechano-dependent regulation of the actin cytoskeleton and cell adhesions.
Zyxin in Mechanotransduction at Focal Adhesions
One of the most studied examples of cellular responses against mechanical environments is accommodation of cell-to-extracellular matrix (ECM) adhesion. Adherent cells such as fibroblasts and endothelial cells form specialized sites for adhesion to ECM, called focal adhesions (FAs). At an FA, ECM receptors integrins are clustered and bind to ECM, while the actin cytoskeleton is anchored to the integrin cluster through a plaque of cytoplasmic proteins.
Cells exert contractile forces mainly from the actin cytoskeleton to ECM at FAs, while mechanical forces in ECM are transduced through FAs into intracellular structures including the actin cytoskeleton,1 which highlights the FA as a mechanical interface between the actin cytoskeleton and ECM (Fig. 1A). These mechanical forces are known to influence on the structure of FAs. Development of FAs is driven by actomyosin-based cellular contractility, which is mediated by the small GTPase Rho.2 When larger forces are exerted on FAs, the size of FAs becomes larger.3 Application of external forces can substitute for the cellular contractile forces in the development of FAs,4 indicating that mechanical forces are the “true” stimuli that induce the FA development. In addition to the FA size control, mechanical loads on integrin clusters strengthen the mechanical linkage between integrin and the actin cytoskeleton.5,6 Mechanical stability of this linkage is essential for the development of FAs, because dissection of this linkage leads to the disassembly of FAs.7 Force-dependent accumulation of actin filaments at integrin clusters is responsible for the hardening of the integrin-actin cytoskeleton linkage, and filamin and stretch-activated ion channels are involved in this process.8 The local accumulation of actin filaments could be based, primarily, on the redistribution of pre-existing actin filaments and/or de novo actin polymerization on site. However, the effect of mechanical forces on the redistribution of actin filaments and on actin polymerization at FAs has not been clarified. Recently, we revealed that mechanical forces on FAs induce actin polymerization at FAs.9
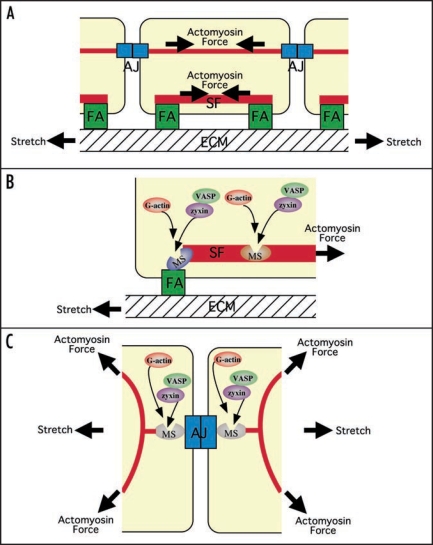
Figure 1.
Hypothetical diagrams showing zyxin as a common regulator of force-dependent actin polymerization at focal adhesions (FA), stress fibers (SF) and adherens junctions (AJ). (A) Side-view of cells adhering to ECM via FAs and to each other via AJs. FAs are connected each other by SFs, and AJs are linked to actomyosin cables. Actomyosin interaction and/or stretching the substratum generate tension in FAs, SFs and AJs. (B and C) Side-view of an FA and a SF (B) and top-view of an AJ (C). In response to mechanical forces, the conformations of hypothetical mechanosensor molecules (MS) at FAs, SFs and AJs are changed, rendering binding-sites for zyxin. The force-dependent recruitment of zyxin to FAs, SFs and AJs leads to the accumulation of Ena/VASP proteins, facilitating the actin polymerization at these structures.
Involvement of actin-regulatory proteins, Arp2/3 complex, mammalian Diaphanous (mDia)-related formins and Ena/VASP proteins, has been frequently implicated in the process of actin polymerization at FAs.10–12 Among them, the contributions of mDia1 and mDia2 have been demonstrated.13–15 However, it is uncertain whether mDia and Arp2/3 play a crucial role in the force-dependent regulation of actin polymerization at FAs, because these proteins do not exhibit apparent localization at FAs, whereas force-induced accumulation of actin filaments is restricted to the adhesion site where force is applied. In contrast to mDia and Arp2/3, Ena/VASP proteins are obviously localized at FAs, which implies a role of Ena/VASP in the local actin polymerization at FAs.
Recruitment of Ena/VASP to FAs depends on the accumulation of the LIM protein zyxin at FAs.16–19 Zyxin dissociated from FAs when the mechanical loads on the FAs were reduced by inhibiting the actomyosin interaction, by ablating individual stress fibers with a focused laser, or by softening the substratum,9,20,21 suggesting that mechanical loads on FAs are required for the recruitment of zyxin to FAs. Stretching the substratum restored zyxin accumulation at FAs in cells devoid of the actomyosin interaction,9 demonstrating that mechanical forces are responsible for the localization of zyxin to FAs. This force-dependent accumulation of zyxin is crucial for the force-induced actin polymerization at FAs.9 These results demonstrate the role of zyxin as a crucial element of the mechanotransducing system at FAs; mechanical signals are transduced into the actin polymerizing response via zyxin accumulation accompanied by Ena/VASP recruitment (Fig. 1B). Other recent findings also support the importance of zyxin in mechanotransduction at FAs; genetic ablation of zyxin or delocalization of zyxin from FAs leads to unusual and integrin-independent migration of cells.16,19 Integrin-dependent mechanotransduction at cell-ECM adhesions is necessary for proper cell migration.22
Given the role of zyxin in the force-dependent facilitation of actin polymerization at FAs, the fundamental question raised here asks which molecule(s) recruits zyxin to FAs in a force-dependent manner. Direct interaction between zyxin and α-actinin was found in vitro more than fifteen years ago,23 and an α-actinin-binding site was identified in the N-terminal region of zyxin.16,24 Enforced localization of recombinant zyxin at the mitochondrial surface could induce a recruitment of α-actinin there, and the N-terminal region of zyxin was necessary for this recruitment,24 suggesting the existence of interaction between zyxin and α-actinin in the intracellular environment. The contribution of this interaction to the recruitment of zyxin to FAs has been suggested based on the observations that introduction of the peptide encoding the α-actinin-binding site of zyxin into cells caused delocalization of zyxin from FAs,16 and that deletion of the N-terminal region in zyxin resulted in a decrease in the zyxin accumulation at FAs.24 However, recent studies have revealed that the interaction of zyxin with α-actinin via the zyxin N-terminal region is not necessary for force-dependent localization of zyxin to FAs. The C-terminal region of zyxin, which consists of three tandem LIM domains, is sufficient for locating zyxin to FAs,9,18 and the recruitment of this C-terminal region of zyxin to FAs is force-dependent.9 Furthermore, the α-actinin-binding site of zyxin alone does not locate to FAs (Hirata et al., unpublished result). Therefore, the molecular interaction(s) via the C-terminal LIM domains of zyxin is crucial for the force-dependent zyxin accumulation at FAs.
Several proteins are known to interact with the zyxin C-terminal region. These include cysteine-rich protein (CRP), H-warts/LATS1 and p130Cas.25 H-warts/LATS1 recruits zyxin to the mitotic apparatus but not to FAs.26 p130Cas is a potential mechanosensor molecule at FAs,27 but zyxin accumulates at FAs in p130Cas-deficient cells,28 indicating that the interaction between zyxin and p130Cas is not necessary for the recruitment of zyxin to FAs. Although CRP is located to FAs, its role in zyxin localization remains unrevealed. Interestingly, siRNA-mediated knock-down of the FA protein Lasp-1 resulted in a decrease in the accumulation of zyxin at FAs.29,30 However, Lasp-1 interacts via its SH3 domain with the N-terminal proline-rich region but not LIM domains of zyxin.31 Thus, the molecule that binds to the C-terminal region of zyxin and is responsible for the force-dependent recruitment of zyxin to FAs is not known yet.
Role of Zyxin in Mechano-Responses of Stress Fibers and Adherens Junctions
FAs are connected each other through the actomyosin bundles, stress fibers (SFs) (Fig. 1A). These SFs generate contractile forces, and the resulting mechanical tension is required for the maintenance and development of SFs. When mechanical forces are externally applied to a cell by stretching the substratum, by fluid flow or by pulling a cell with a microneedle, both the orientation and the thickness of SFs are altered.32–34 We recently reported that the actomyosin meshwork itself has a nature to transform into actomyosin bundles orienting parallel to the direction of tension in cytosol-free semi-intact cells.35 However, the molecular mechanism underlying the regulation of SF thickness in response to mechanical stimuli is not known. Actin-polymerizing sites are distributed in a periodic manner along SFs. Zyxin and Ena/VASP are localized to these actin-polymerizing sites, and Ena/VASP plays a crucial role in actin polymerization there.20,36 These findings imply that zyxin, in concert with Ena/VASP, facilitates actin polymerization along SFs (Fig. 1B), resulting in thickening of SFs. Indeed, recent studies have clearly demonstrated that zyxin recruits Ena/VASP along SFs, which is necessary for thickening of SFs in response to stretching the substratum or fluid flow.19,34,36 Thus, zyxin is a crucial player in the mechano-induced thickening of SFs.
Cadherin-mediated adherens junctions (AJs) contribute to the intercellular recognition and adhesion. At AJs, cadherin clusters are linked to actomyosin cables (Fig. 1A).37 The development of AJs is also dependent on actomyosin-based forces.38–40 The AJ is an active site for actin polymerization, and the actin polymerization drives the development of AJs.41 Recent studies demonstrated the contribution of mDia1, probably through promoting actin polymerization, to the actomyosin-dependent development of AJs.42,43 On the other hand, zyxin and Ena/VASP localize at AJs, and Ena/VASP is involved in the actin polymerization at AJs and is required for the development of AJs.41,44 The localization of Ena/VASP at cadherin clusters was abolished by the inhibition of Rho kinase,44 suggesting that the Rho kinase-mediated actomyosin force is necessary for the recruitment of Ena/VASP to AJs. Although the effect of mechanical forces on zyxin recruitment to AJs remains unrevealed, it is plausible that zyxin-mediated recruitment of Ena/VASP to AJs enhances actin polymerization (Fig. 1C) and contributes to the force-induced development of AJs.
Zxyin as a Common Component of Mechanotransducing Systems?
All above considerations lead to a hypothesis that zyxin is a common regulator of force-dependent actin polymerization in the actin cytoskeleton-cell adhesion system, e.g., FAs, SFs and AJs (Fig. 1). Given such important role of zyxin, it seems controversial that mice with disruption of zyxin gene exhibit no obvious histological abnormalities, and FAs and SFs are observed in zyxin-null cells.19,45 This may be, in part, due to a compensation for zyxin-deficiency by other zyxin family proteins. The zyxin family proteins, including zyxin, thyroid hormone interacting protein 6 (TRIP6), lipoma preferred partner (LPP) and Ajuba, share a proline-rich region and a C-terminal region with three tandem LIM domains.25 These proteins have high sequence similarity, particularly within the LIM domains. On the other hand, zyxin-null cells exhibit abnormalities in dynamic remodeling of the actin cytoskeleton and FAs in response to cyclic stretching, fluid shear or mechanical wounding,19,45 suggesting that zyxin is particularly important in the dynamic phase of mechano-responses of the actin cytoskeleton-cell adhesion system. Interestingly, cyclic stretching the substratum results in the translocation of zyxin from FAs to nuclei in smooth muscle cells.46 This translocation of zyxin may be involved in the mechano-induced gene transcription, because zyxin family proteins have been suggested to mediate transcription process through their interaction with transcription factors.25
In this review, we discuss the role of zyxin as a key element of mechanotransducing systems at cell adhesive structures: force-dependent recruitment of zyxin to FAs, SFs and AJs would facilitate actin polymerization at these structures. The remaining fundamental question is which molecule(s) senses mechanical forces and recruits zyxin to FAs, SFs, AJs and nuclei. In addition, redundancy and specificity of the role of zyxin family members in cellular responses to mechanical stimuli have not been examined. Studies in these directions will reveal the overall picture of the molecular mechanism underlying cellular mechanotransduction mediated by zyxin proteins.
Acknowledgements
This work was supported in part by Grants-in-aid for General Scientific Research (#13480216 to Masahiro Sokabe and #20570151 to Hitoshi Tatsumi), Scientific Research on Priority Areas (#15086270 to Masahiro Sokabe) and Creative Research (#16GS0308 to Masahiro Sokabe) from the Ministry of Education Science Sports and Culture, and a grant from Japan Space Forum (to Masahiro Sokabe and Hitoshi Tatsumi).
Footnotes
Previously published online as a Communicative & Integrative Biology E-publication: http://www.landesbioscience.com/journals/cib/article/7001
References
- 1.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 2.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 4.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1185. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 6.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 7.Pavalko FM, Burridge K. Disruption of the cytoskeleton after microinjection of proteolytic fragments of α-actinin. J Cell Biol. 1991;114:481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glogauer M, Arora P, Chou D, Janmey PA, Downey GP, McCulloch CAG. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J Biol Chem. 1998;273:1689–1698. doi: 10.1074/jbc.273.3.1689. [DOI] [PubMed] [Google Scholar]
- 9.Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci. 2008;121:2795–2804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- 10.Beckerle MC. Spatial control of actin filament assembly: lessons from Listeria. Cell. 1998;95:741–748. doi: 10.1016/s0092-8674(00)81697-9. [DOI] [PubMed] [Google Scholar]
- 11.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 12.Blystone SD. Integrating an integrin: a direct route to actin. Biochim Biophys Acta. 2004;1692:47–54. doi: 10.1016/j.bbamcr.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Butler B, Gao C, Mersich AT, Blystone SD. Purified integrin adhesion complexes exhibit actin-polymerization activity. Curr Biol. 2006;16:242–251. doi: 10.1016/j.cub.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupton SL, Eisenmann K, Alberts AS, Waterman-Storer CM. mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J Cell Sci. 2007;120:3475–3487. doi: 10.1242/jcs.006049. [DOI] [PubMed] [Google Scholar]
- 16.Drees BE, Andrews KM, Beckerle MC. Molecular dissection of zyxin function reveals its involvement in cell motility. J Cell Biol. 1999;147:1549–1559. doi: 10.1083/jcb.147.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drees B, Friederich E, Fradelizi J, Louvard D, Beckerle MC, Golsteyn RM. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J Biol Chem. 2000;275:22503–22511. doi: 10.1074/jbc.M001698200. [DOI] [PubMed] [Google Scholar]
- 18.Nix DA, Fradelizi J, Bockholt S, Menichi B, Louvard D, Friederich E, Beckerle MC. Targeting of zyxin to sites of actin membrane interaction and to the nucleus. J Biol Chem. 2001;276:34759–34767. doi: 10.1074/jbc.M102820200. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman LM, Jensen CC, Kloeker S, Wang CLA, Yoshigi M, Beckerle MC. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. J Cell Biol. 2006;172:771–782. doi: 10.1083/jcb.200512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rottner K, Krause M, Gimona M, Small JV, Wehland J. Zyxin is not colocalized with vasodilator-stimulated phosphoprotein (VASP) at lamellipodial tips and exhibits different dynamics to vinculin, paxillin, and VASP in focal adhesions. Mol Biol Cell. 2001;12:3103–3113. doi: 10.1091/mbc.12.10.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- 22.Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Crawford AW, Michelsen JW, Beckerle MC. An interaction between zyxin and α-actinin. J Cell Biol. 1992;116:1381–1393. doi: 10.1083/jcb.116.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reinhard M, Zumbrunn J, Jaquemar D, Kuhn M, Walter U, Trueb B. An α-actinin binding site of zyxin is essential for subcellular zyxin localization and α-actinin recruitment. J Biol Chem. 1999;274:13410–13418. doi: 10.1074/jbc.274.19.13410. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Gilmore TD. Zyxin and paxillin proteins: focal adhesion plaque LIM domain proteins go nuclear. Biochim Biophys Acta. 2003;1593:115–120. doi: 10.1016/s0167-4889(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 26.Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T, Masuko N, Inagaki M, Hatakeyama K, Saya H. Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumor suppressor. J Cell Biol. 2000;149:1073–1086. doi: 10.1083/jcb.149.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi J, Kloeker S, Jensen CC, Bockholt S, Honda H, Hirai H, Beckerle MC. Members of the zyxin family of LIM proteins interact with members of the p130Cas family of signal transducers. J Biol Chem. 2002;277:9580–9589. doi: 10.1074/jbc.M106922200. [DOI] [PubMed] [Google Scholar]
- 29.Grunewald TGP, Kammerer U, Schulze E, Schindler D, Honig A, Zimmer M, Butt E. Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res. 2006;312:974–982. doi: 10.1016/j.yexcr.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Grunewald TGP, Kammerer U, Winkler C, Schindler D, Sickmann A, Honig A, Butt E. Overexpression of LASP-1 mediates migration and proliferation of human ovarian cancer cells and influences zyxin localization. Br J Cancer. 2007;96:296–305. doi: 10.1038/sj.bjc.6603545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Zhuang L, Trueb B. Zyxin interacts with the SH3 domains of the cytoskeletal proteins LIM-nebulette and Lasp-1. J Biol Chem. 2004;279:20401–20410. doi: 10.1074/jbc.M310304200. [DOI] [PubMed] [Google Scholar]
- 32.Kolega J. Effects of mechanical tension on protrusive activity and microfilament and intermediate filament organization in an epidermal epithelium moving in culture. J Cell Biol. 1986;102:1400–1411. doi: 10.1083/jcb.102.4.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wechezak AR, Viggers RF, Sauvage LR. Fibronectin and F-actin redistribution in cultured endothelial cells exposed to shear stress. Lab Invest. 1985;53:639–647. [PubMed] [Google Scholar]
- 34.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirata H, Tatsumi H, Sokabe M. Dynamics of actin filaments during tension-dependent formation of actin bundles. Biochim Biophys Acta. 2007;1770:1115–1127. doi: 10.1016/j.bbagen.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Furman C, Sieminski AL, Kwiatkowski AV, Rubinson DA, Vasile E, Bronson RT, Fässler R, Gertler FB. Ena/VASP is required for endothelial barrier function in vivo. J Cell Biol. 2007;179:761–775. doi: 10.1083/jcb.200705002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mége R, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, Yonemura S. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp Cell Res. 2006;312:1637–1650. doi: 10.1016/j.yexcr.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 40.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 42.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci. 2007;120:3870–3882. doi: 10.1242/jcs.014365. [DOI] [PubMed] [Google Scholar]
- 44.Scott JA, Shewan AM, den Elzen NR, Loureiro JJ, Gertler FB, Yap AS. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell. 2006;17:1085–1095. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffman LM, Nix DA, Benson B, Boot-Hanford R, Gustafsson E, Jamora C, Menzies AS, Goh KL, Jensen CC, Gertler FB, Fuchs E, Fässler R, Beckerle MC. Targeted disruption of the murine zyxin gene. Mol Cell Biol. 2003;23:70–79. doi: 10.1128/MCB.23.1.70-79.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cattaruzza M, Lattrich C, Hecker M. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension. 2004;43:726–730. doi: 10.1161/01.HYP.0000119189.82659.52. [DOI] [PubMed] [Google Scholar]