Abstract
Anhedonia, the reduced propensity to experience pleasure, is a promising endophenotype and vulnerability factor for several psychiatric disorders, including depression and schizophrenia. In the present study, we used resting electroencephalograms, functional magnetic resonance imaging, and volumetric analyses to probe putative associations between anhedonia and individual differences in key nodes of the brain’s reward system in a non-clinical sample. We found that anhedonia, but not other symptoms of depression or anxiety, was correlated with reduced nucleus accumbens (NAcc) responses to rewards (gains in a monetary incentive delay task), reduced NAcc volume, and increased resting delta current density (i.e., decreased resting activity) in the rostral anterior cingulate cortex (rACC), an area previously implicated in positive subjective experience. In addition, NAcc reward responses were inversely associated with rACC resting delta activity, supporting the hypothesis that delta might be lawfully related to activity within the brain’s reward circuit. Taken together, these results help elucidate the neural basis of anhedonia and strengthen the argument for anhedonia as an endophenotype for depression.
Keywords: depression, anhedonia, striatum, reward, anterior cingulate cortex
Introduction
Early theorists suggested that anhedonia, the reduced propensity to experience pleasure, might constitute a vulnerability factor for psychiatric disorders, including Major Depressive Disorder (MDD) and schizophrenia (e.g., Meehl, 1975; Rado, 1956). Consistent with this view, anhedonia is currently considered a promising endophenotype of MDD, because it is a cardinal symptom of the disorder but is considerably more homogeneous, more easily quantified, and tied to dysfunction in the neural circuitry of reward, which is increasingly well-understood (Hasler et al., 2004; Pizzagalli et al., 2005). Therefore, information on the neural correlates of anhedonia may provide valuable insights into the pathophysiology and etiology of psychiatric disorders and may ultimately allow for early identification of high-risk individuals.
The neural systems underlying reward and pleasure have long been the object of scientific scrutiny (for a recent review, see Berridge and Kringelbach, 2008). Starting from early self-stimulation studies in rodents performed by Olds and Milner (1954), a large body of animal work has emphasized the role of mesocorticolimbic pathways in incentive motivation and the experience of pleasure. Even before the advent of modern neuroimaging techniques, Heath (1972) demonstrated that activation of these areas has powerful, positive motivational effects in humans by documenting fervent self-stimulation in a patient implanted with electrodes into the dopamine-rich mesolimbic septum/nucleus accumbens (NAcc) region. More recently, functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies have described increased activation in the basal ganglia, including the ventral striatum, in response to various appetitive cues (see Phan et al., 2002, for a review). Furthermore, PET studies using dopaminergic tracers have shown that the positive subjective effects of amphetamine are correlated with receptor binding in the ventral striatum (e.g., Drevets et al., 2001; Leyton et al., 2002; Oswald et al., 2005). Thus, the role of the ventral striatum in reward processing has been firmly established using multiple methods.
Neuroimaging studies have also linked the experience of pleasure to neural activity in the medial prefrontal cortex (Berridge and Kringelbach, 2008; Phan et al., 2002). In particular, Rolls and colleagues (de Araujo et al., 2003; Grabenhorst et al., 2008; Rolls et al., 2003, 2008) have described an association between subjective ratings of pleasantness for a wide variety of stimuli from different modalities and responses to these stimuli in ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (rACC) regions (Figure 1). These cortical areas receive dense dopaminergic inputs (Gaspar et al., 1989), project to the striatum (in particular the NAcc) and the ventral tegmental area (Haber et al., 2006; Öngür and Price, 2000; Sesack and Pickel, 1992), show activity increases in response to dopamine-inducing drugs (Udo de Haes et al., 2007; Völlm et al., 2004), and have been implicated in preference judgments (e.g., Paulus and Frank, 2003), consistent with a role in reward-guided decision making (Rushworth et al., 2007).
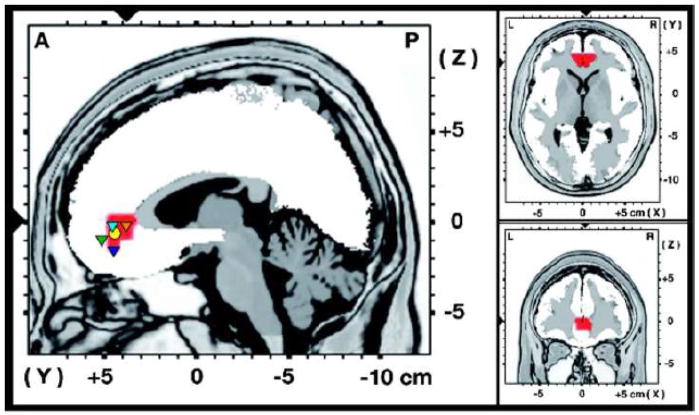
Figure 1.
LORETA whole-brain analyses. Results of voxel-by-voxel correlations between the Anhedonic Depression scale of the Mood and Anxiety Symptom Questionnaire (MASQ AD) and log-transformed delta (1.5–6.0 Hz) current density. The statistical map is thresholded at p < .001 (uncorrected). The red area indicates the only cluster with significant positive correlations. The slices displayed run through the voxel with the maximal correlation (r = .534, N = 41, p = .0003; MNI coordinates: 4, 38, 1). The circle and the triangles in the sagittal slice represent projections of peak activations from prior fMRI studies (x-coordinates for all peaks within ± 7). The yellow circle represents the area where two prior studies independently observed a significant positive correlation between anhedonia and the BOLD response to positive stimuli (Harvey et al., 2007; Keedwell et al., 2005). The triangles represent areas in which correlations between ratings of subjective pleasantness and the BOLD response to odors (green triangle, Rolls et al., 2003), tastes (light blue triangle, Grabenhorst et al., 2008), water in fluid-deprived subjects (dark blue triangle, de Araujo et al., 2003), as well as warm and cold stimuli applied to the hand (orange triangle, Rolls et al., 2008) have been described.
Complementing these findings, emerging evidence from neuroimaging studies in clinical samples indicates that anhedonic symptoms are linked to reward responses in key nodes of the reward system (Epstein et al., 2006; Juckel et al., 2006a, 2006b; Keedwell et al., 2005; Mitterschiffthaler et al., 2003; Tremblay et al., 2005). For example, Epstein et al. (2006) reported that depressed subjects were characterized by reduced ventral striatal responses to positive pictures, and the strength of these responses was negatively correlated with self-reported anhedonia. Similarly, in a sample of twelve patients with MDD, Keedwell et al. (2005) found a negative correlation between anhedonia (but not depression severity) and ventral striatal responses to positive stimuli. Interestingly, these authors also found a positive correlation between anhedonia and responses in the vmPFC (BA10) and rACC (BA24/32). In what seems to be the only neuroimaging study on the brain correlates of anhedonia in healthy subjects, Harvey et al. (2007) did not observe a significant correlation between anhedonia and ventral striatal responses to positive pictures. They did, however, replicate Keedwell et al.’s (2005) observation of a positive correlation between anhedonia and responses to positive stimuli in a region in the vmPFC, again extending into the rACC. In addition, Harvey et al. (2007) found that anhedonia was associated with reduced volume in caudate regions extending into the NAcc.
Taken together, these previous findings suggest that anhedonia may be associated with weaker responses to positive stimuli and reduced volume in the striatum, as well as with increased responses to positive stimuli in vmPFC/rACC. The latter association is surprising, given that activity in vmPFC/rACC is also positively related to ratings of pleasure as detailed above (e.g., de Araujo et al., 2003; Grabenhorst et al., 2008; Rolls et al., 2008; Rolls et al., 2003). Importantly, the vmPFC/rACC figures prominently in the brain’s default network, which is activated during resting, task-free states and becomes deactivated when participants engage in a task (Buckner et al., 2008). Indeed, converging lines of evidence raise the possibility that associations between anhedonia and task-related activation in medial frontal regions may reflect individual differences in resting state activity.
First, depression has been associated with dysfunctional resting activity in vmPFC/rACC, with some studies reporting decreased (e.g., Drevets et al., 1997; Ito et al., 1996; Mayberg et al., 1994) and others increased (e.g., Kennedy et al., 2001; Videbech et al., 2002) activity, and decreased resting rACC activity has been found to predict a poor response to treatment (Mayberg et al., 1997; Mülert et al., 2007; Pizzagalli et al., 2001). Second, using both PET and measurements of electroencephalographic (EEG) activity, Pizzagalli et al. (2004) reported decreased resting activity (i.e., reduced glucose metabolism and increased delta activity) in the subgenual ACC (BA 25) in patients with melancholia – a depressive subtype characterized by psychomotor disturbances and pervasive anhedonia. Finally, various conditions and diseases characterized by reduced resting medial PFC activity are associated with reduced task-induced medial PFC deactivation (Fletcher et al., 1998; Kennedy et al., 2006; Lustig et al., 2003), and recent findings by Grimm et al. (2008) indicate that this may also apply to depression. Specifically, these authors observed smaller task-induced deactivations in depressed individuals versus controls in several areas of the default network, including an area closely matching the ones implicated by Keedwell et al. (2005) and Harvey et al. (2007). Collectively, these observations suggest that the seemingly paradoxical positive association between anhedonia and vmPFC/rACC activation to positive stimuli might be due to an association between reduced baseline activity in this area and anhedonia, resulting in smaller deactivations during stimulus-processing. To our knowledge the hypothesis of an association between lower resting vmPFC/rACC activity and anhedonia has not been tested previously.
If such an association exists, it is likely to be evident in the delta frequency band of the EEG. As Knyazev (2007) recently noted in his review of the functional roles of different EEG oscillations, a number of observations support the idea that the delta rhythm is a signature of reward processing and salience detection. First, animal studies have identified generators of delta activity in key nodes of the brain reward system, such as the NAcc (Leung and Yim, 1993), ventral pallidum (Lavin and Grace, 1996), and dopaminergic neurons of the ventral tegmental area (Grace, 1995). Second, although electrical activity in the striatum cannot be measured noninvasively in humans, EEG source localization studies have implicated anterior medial frontal regions in the generation of delta activity (Michel et al., 1992; 1993). Critically, these sources overlap with regions reciprocally connected to the ventral tegmental area and emerging from fMRI studies as being associated with self-reported pleasure responses (see above). Third, the available animal data suggest that dopamine release in the NAcc is associated with decreased delta activity (Chang et al., 1995; Ferger et al., 1994; Kropf and Kuschinsky, 1993; Leung and Yim, 1993; Luoh et al., 1994). Fourth, opioid and cocaine administration have been associated with changes in delta activity in humans (Greenwald and Roehrs, 2005; Reid et al., 2006; Scott et al., 1991). However, in contrast to the animal data, increases instead of decreases in delta activity were observed (see also Heath, 1972). Whereas these apparent discrepancies between animal and human data currently cannot be resolved, the available evidence nonetheless suggests that EEG delta activity may be linked to reward processing. Therefore the present study aims to further elucidate the proposed link between delta and reward.
In sum, the major goals of the present investigation were: (1) to examine whether anhedonia is negatively and positively associated with reward response in the ventral striatum and the vmPFC/rACC, respectively, as assessed by fMRI in conjunction with a monetary incentive delay task known to recruit the brain’s reward network (Dillon et al., 2008); (2) to replicate Harvey et al.’s (2007) observation of an inverse association between anhedonia and striatal volume; (3) to investigate whether anhedonia is associated with increased resting EEG delta current density (i.e., decreased resting activity) in vmPFC/rACC; and (4) to probe the suggested link between EEG delta activity and the brain’s reward system (Knyazev, 2007) by assessing the correlation between striatal reward responses measured via fMRI and resting EEG delta current density in the vmPFC/rACC.
Materials and methods
Participants
Data from the present report stem from a larger study that integrates behavioral, electrophysiological (resting EEG, event-related potentials), and neuroimaging (fMRI, structural MRI) measures as well as molecular genetics to investigate the neurobiology of reward processing and anhedonia in a non-clinical sample. A previous publication on this sample has focused on event-related potential data collected during a reinforcement task (Santesso et al., 2008), and a report on links between candidate genes and the fMRI data is in preparation (Dillon, Bogdan, Fagerness, Holmes, Perlis, and Pizzagalli, in preparation). Unlike prior reports, the primary goal of the current study was to investigate relations between individual differences in anhedonia and (1) resting EEG data, and (2) functional and volumetric measurements of reward-related basal ganglia regions. Secondary analyses aimed to evaluate interrelations among the three neuroimaging modalities.
In an initial behavioral session, 237 healthy adults between 18 and 40 years old completed a two-alternative forced choice task in which correct identification of one of two stimuli was rewarded more frequently. Prior work in independent clinical and non-clinical samples revealed that this probabilistic reward task is sensitive to variation in reward responsiveness and anhedonia (Bogdan and Pizzagalli, 2006; Pizzagalli et al., 2009; Pizzagalli et al., 2005). Based on their performance in the initial session, 47 of the 170 subjects meeting inclusion criteria for the current study (right-handedness; absence of medical or neurological illnesses, pregnancy, current alcohol/substance abuse, smoking, use of psychotropic medications during the last 2 weeks, or claustrophobia) were invited for the EEG and fMRI sessions (session order counterbalanced). Participants were selected to cover a wide range of individual differences in reward learning as measured by the probabilistic reward task: specifically, we first identified participants in the upper and lower 20% of the distribution of reward learning, and then selected remaining participants with the goal of achieving a continuum in reward learning that would be representative of the general population (for further details on the selection criteria, see Santesso et al., 2008).
Of these 47 participants, 41 (5 African American, 5 Asian, 29 Caucasian, 2 other) agreed to participate in the EEG session, and 33 of these also completed the fMRI session. All 41 participants (mean age: 21.2 years, S.D.: 3.1; mean education: 14.2 years, S.D.: 1.5; 20 male) had usable resting EEG data. Of the 33 participants who completed both sessions, five were excluded from the fMRI analyses due to excessive motion artifacts resulting in a sample of N = 28 for the fMRI analyses (mean age: 21.5 years, S.D.: 3.5; mean education: 14.5 years, S.D.: 1.6; 14 male). Apart from one participant with specific phobia and one with minor depressive disorder, none of the participants had current psychiatric disorders, as determined with the Structured Clinical Interview for DSM-IV. There was evidence of past Axis I pathology in a minority of participants (past MDD: n = 1; past depressive disorder not otherwise specified: n = 1; past binge eating disorder: n = 1; past anorexia nervosa: n = 1; past alcohol abuse: n = 1).
Participants received approximately $12, $45, and $80 for the behavioral, EEG, and fMRI sessions, respectively, in task earnings and reimbursement for their time. All participants provided written informed consent and all procedures were approved by the Committee on the Use of Human Subjects at Harvard University and the Partners-Massachusetts General Hospital Internal Review Board.
Procedure
Behavioral session
At both the behavioral and EEG session, the short version of the Mood and Anxiety Symptom Questionnaire (MASQ, Watson et al., 1995) was administered to measure depression-specific symptoms (Anhedonic Depression, AD), anxiety-specific symptoms (Anxious Arousal, AA) and general distress symptoms common to both depression and anxiety (General Distress: Depressive Symptoms, GDD; General Distress: Anxious Symptoms, GDA). Prior studies indicate that all MASQ scales possess excellent reliability (coefficient alpha: .85–.93 in adult and student samples) and convergent/discriminant validity with regard to other anxiety and depression scales (e.g., Watson et al., 1995). In the current sample, the test-retest reliability between the behavioral and EEG session (average interval = 36.6 days; range 2–106 days) of the AD, GDD, AA, and GDA scales was .69, .62, .49, and .70, respectively, indicating moderate to high stability. In the present analyses we only analyzed the MASQ scores from the behavioral session in order to (1) demonstrate the predictive validity of the self-report measures for the physiological measures, and (2) minimize the influence of state effects on the MASQ-physiology correlations by ensuring that both EEG and fMRI measures were obtained at a different session from the MASQ data. However, very similar results emerged when analyzing the averages of the two MASQ administrations (data available upon request). In addition, the state version of the Positive and Negative Affect Schedule (PANAS, Watson et al., 1988) was administered at both the behavioral and the EEG sessions to assess current mood.
Resting EEG session
Participants were instructed to sit still and relax while the resting EEG was recorded for eight minutes (4 minutes with eyes open, 4 minutes with eyes closed in counterbalanced order). Subsequently, participants repeated the probabilistic reward task used for subject selection during event-related potential recordings (Santesso et al., 2008).
MRI session
After collection of structural MRI data, participants performed a monetary incentive delay (MID) task during functional imaging. The MID has been described earlier in an independent sample (Dillon et al., 2008). Briefly, participants completed 5 blocks of 24 trials. Each trial began with the presentation of one of three equally probable cues (duration: 1.5 s) that signaled potential monetary gains (+$), no incentive (0$), or losses (−$). After a jittered inter-stimulus interval (ISI) of 3–7.5 s, a red square was presented to which participants responded with a button press. Following a second jittered ISI (4.4–8.9 s), feedback was presented indicating a gain (range: $1.96 to $2.34; mean: $2.15), no-change, or penalty (range: −$1.81 to −$2.19; mean −$2.00). Participants were told that their reaction time (RT) to the target affected trial outcomes such that rapid RTs increased the probability of receiving gains and decreased the probability of receiving penalties. In fact, 50% of reward and loss trials resulted in delivery of gains and penalties, respectively (see Dillon et al., 2008, for further detail). Outcome delivery was de-coupled from responses in this fashion in order to permit a fully balanced design, with an equal number of trials featuring each outcome. However, to maintain task believability and engagement, for trials leading to a positive outcome (e.g., gains in reward trials), target exposure time corresponded to the 85th percentile of RTs collected during a 40-trial practice session administered immediately before scanning; for trials scheduled to yield a negative outcome (e.g., no gains in reward trials), target exposure time corresponded to the 15th percentile of practice RTs. The order of outcome delivery was based on a pre-determined sequence that optimized the statistical efficiency of the fMRI design (Dale, 1999).
Data Collection and Analyses
EEG recording
Resting EEG was recorded using a 128-channel Electrical Geodesic system (EGI Inc., Eugene, OR) at 250 Hz with 0.1–100 Hz analog filtering referenced to the vertex. Impedances were kept below 50 kΩ. Data were re-referenced off-line to an average reference. After correcting eye-movement artifacts using an Independent Component Analysis implemented in Brain Vision Analyzer (Brain Products GmbH, Germany), the data were visually scored for remaining artifacts, and corrupted channels were interpolated using a spline interpolation.
Following prior procedures (e.g., Pizzagalli et al., 2001, 2004, 2006), Low Resolution Electromagnetic Tomography (LORETA, Pascual-Marqui et al., 1999) was used to estimate resting intracerebral current density in various frequency bands. To this end, spectral analyses were first performed on artifact-free 2048-ms epochs using a discrete Fourier transform and boxcar windowing. LORETA was then used to estimate the intracerebral current density distribution for the following bands: delta (1.5–6.0 Hz), theta (6.5–8.0 Hz), alpha1 (8.5–10.0 Hz), alpha2 (10.5–12.0 Hz), beta1 (12.5–18.0 Hz), beta2 (18.5–21.0 Hz), beta3 (21.5–30.0 Hz), and gamma (36.5–44.0 Hz). Based on prior findings (e.g., Knyazev, 2007; Pizzagalli et al., 2004; Scheeringa et al., 2008), delta activity was the main frequency of interest; other EEG bands were analyzed to evaluate the specificity of possible findings.
At each voxel (n = 2394; voxel resolution = 7 mm3), current density was computed as the squared magnitude of the intracerebral current density within each of the eight frequency bands (unit: amperes per square meter, A/m2). For each subject and band, LORETA values were normalized to a total power of 1 and then log transformed before statistical analyses. Voxel-by-voxel Pearson correlations between MASQ AD and log-transformed delta current density were then computed and displayed on a standard MRI template (MNI space) after thresholding at p < .001 (uncorrected).
In addition to voxel-by-voxel correlations, we also analyzed current density in several a priori defined regions of interest (ROIs) within the ACC. This approach was selected to (1) increase statistical power, (2) allow comparisons between MASQ AD and the other MASQ scales unbiased by statistical thresholding (i.e., evaluation of symptom-specificity), and (3) allow comparisons among various ACC subdivisions (i.e., evaluation of region-specificity). To this end, for each subject and band, average current density was computed for the following subdivisions of the ACC (for details see Bush et al., 2000; Pizzagalli et al., 2006): the more rostral, “affective” subregions, including BA25 (17 voxels, 5.83 cm3), BA24 (12 voxels, 4.12 cm3), and BA32 (17 voxels, 5.83 cm3), and the more dorsal, “cognitive” subregions, including BA32′ (20 voxels, 6.86 cm3) and BA24′ (48 voxels, 16.46 cm3). Location and extent of these subdivisions were defined based on Structure-Probability Maps (Lancaster et al., 1997) and anatomical landmarks (Devinsky et al., 1995; Vogt et al., 1995), as previously described in detail (Pizzagalli et al., 2006). On average, estimates of resting current density were based on 110.7 artifact-free epochs (S.D.: 37.2, range: 37–174). Log-transformed delta current density in BAs 24, 25, and BA32 was not associated with either the total number of artifact-free epochs or the percentage of eyes-open epochs contributing to the individual averages, all rs(39) ≤ .10, p ≥ .52.
fMRI data
The imaging protocol and fMRI processing stream have been described previously (Dillon et al., 2008; Santesso et al., 2008). Briefly, fMRI data were acquired on a 1.5 T Symphony/Sonata scanner (Siemens Medical Systems; Iselin, NJ). During functional imaging, gradient echo T2*-weighted echoplanar images were acquired using the following parameters: TR/TE: 2500/35; FOV: 200 mm; matrix: 64 × 64; 35 slices; 222 volumes; voxels: 3.125 × 3.125 × 3 mm. A high-resolution T1-weighted MPRAGE structural volume was collected for anatomical localization and extraction of structural ROIs using standard parameters (TR/TE: 2730/3.39 ms; FOV: 256 mm; matrix: 192 × 192; 128 slices; voxels: 1.33 × 1.33 × 1 mm). Padding was used to minimize head movement.
Analyses were conducted using FS-FAST (http://surfer.nmr.mgh.harvard.edu) and FreeSurfer (Fischl et al., 2002; Fischl et al., 2004). Pre-processing included motion and slice-time correction, removal of slow linear trends, intensity normalization, and spatial smoothing with a 6 mm FWHM Gaussian filter. A temporal whitening filter was used to estimate and correct for autocorrelation in the noise. Next, a gamma function (intended to model the hemodynamic response) was convolved with stimulus onsets, and the general linear model assessed the fit between the model and the data. Participants with incremental (volume-to-volume) or cumulative head movements greater than 3.75 mm or degrees were removed from the analysis (n = 5). For the remaining participants, motion parameters were included in the model as nuisance regressors.
For this study, the main fMRI results of interest were regression coefficients (beta weights) extracted from four components of the basal ganglia (NAcc, caudate, putamen, and globus pallidus) and the rACC.1 These ROIs were structurally defined by FreeSurfer’s automatic cortical and sub-cortical parcellation algorithms, which are highly reliable and compare favorably to manual methods (Desikan et al., 2006; Fischl et al., 2002; Fischl et al., 2004). For each participant and ROI, mean beta weights were extracted for delivery of monetary gains, monetary penalties, and no-change feedback. For the sake of consistency with prior neuroimaging work, in which anhedonia has been associated with brain activation to actual positive stimuli (Epstein et al., 2006; Harvey et al., 2007; Keedwell et al., 2005), fMRI analyses focused on responses to outcomes. At the request of an anonymous reviewer, mean beta weights were also extracted for reward cues to evaluate the specificity of correlations with anhedonia vis-à-vis consummatory versus anticipatory phases of reward processing.
FreeSurfer’s algorithms also provide volumetric information for each ROI and the total intracranial volume. To adjust for gender and intracranial volume, we z-standardized intracranial volume and the volumes of each of the ROIs within genders and then regressed the z-scores for each ROI on the z-scores for intracranial volume. This regression approach was selected to avoid introducing sex differences due to the larger intracranial volume in males relative to females. All statistical analyses for the volumetric variables were conducted with the residuals derived from these regressions.
Statistical Analyses
fMRI data were analyzed with mixed ANOVAs using Feedback (gain, no-change, penalty) and Gender (male, female) as factors. For basal ganglia regions, Hemisphere (left, right) and Region (NAcc, caudate, putamen, pallidus) were added as additional within-subject factors. The Greenhouse-Geisser correction was used when applicable. Pearson correlations and partial correlations were computed to test the main hypotheses. Differences between dependent correlation coefficients were tested using the formula proposed by Steiger (1980). Results are reported with an alpha-level of 0.05 (two-tailed) unless otherwise noted. In light of prior findings (Epstein et al., 2006; Harvey et al., 2007), the a priori hypotheses of negative correlations between anhedonia and (1) NAcc volume and (2) NAcc response to rewards were tested one-tailed. Primary analyses involved five predicted correlations (anhedonia–NAcc volume, anhedonia–NAcc response to gains, anhedonia–rACC response to gains, anhedonia–resting rACC delta activity, NAcc response to gains–resting rACC delta activity). All other correlations were performed in order to test the specificity of the five main findings; consequently, corrections for multiple testing were not implemented.
Results
Intercorrelations of MASQ and PANAS Scales
As shown in Table 1, the MASQ scales were moderately to highly correlated with each other and with PANAS state negative affect in both sessions. However, mirroring prior observations (Watson and Clark, 1995), only MASQ AD displayed a significant negative correlation with PANAS state positive affect at both sessions. Mean and standard deviation of MASQ AD (weighted by gender) did not differ from the values reported by Watson et al. (1995, Table 1) for a large student sample, t(1112) = 1.28, p = .20, F(40, 1072) = 1.07, p = .35.
Table 1.
Intercorrelations Between MASQ Scales and State Positive and Negative Affect
| Variable | MASQ AD | MASQ GDD | MASQ GDA | MASQ AA |
|---|---|---|---|---|
| MASQ AD | 1.00 | |||
| MASQ GDD | .71*** | 1.00 | ||
| MASQ GDA | .54*** | .78*** | 1.00 | |
| MASQ AA | .43** | .55*** | .56*** | 1.00 |
| PANAS Positive Affect Behavioral Session | −.46** | −.29 | −.08 | −.07 |
| PANAS Negative Affect Behavioral Session | .64** | .75*** | .70*** | .31* |
| PANAS Positive Affect EEG Session | −.30† | −.14 | −.22 | −.11 |
| PANAS Negative Affect EEG Session | .45** | .57*** | .39* | .61*** |
Notes. N = 39 for PANAS correlations at EEG session due to two missing values, N = 41 for the remaining correlations.
EEG = Electroencephalogram.
MASQ = Mood and Anxiety Symptom Questionnaire administered at the behavioral session. AD = Anhedonic Depression; GDD = General Distress: Depressive Symptoms; GDA = General Distress: Anxious Symptoms; AA = Anxious Arousal PANAS = state version of the Positive and Negative Affect Schedule.
p = .06.
p < .05.
p < .01.
p < .001. current density in one of the a priori defined regions of interest in the rACC (BA32, see text for details), and (D) between NAcc response to monetary gains and log-transformed resting delta current density in BA32.
Basal Ganglia and rACC Responses to Monetary Gains and Penalties
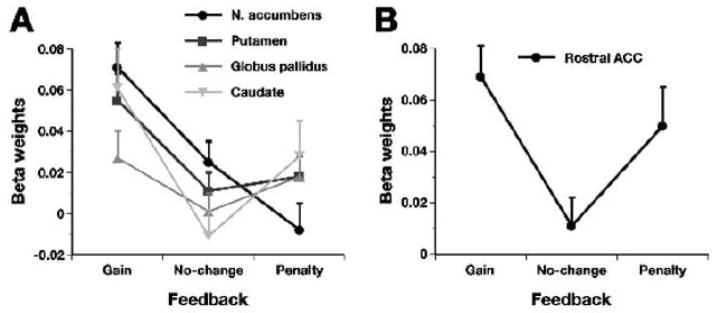
To verify that the basal ganglia were activated by monetary gains in the MID task, we computed a Feedback × Region × Hemisphere × Gender ANOVA. Findings revealed a significant main effect of Feedback, F(2, 51.5) = 8.00, p = .001, and a significant Feedback × Region interaction, F(3.3, 85.6) = 6.97, p = .0003 (see Figure 2A). A priori specified contrasts revealed that all basal ganglia regions were activated more strongly by gains versus no-change feedback, F(1, 26) ≥ 4.43, p ≤ .045. Notably, only the NAcc was associated with reduced activity after penalties relative to no-change feedback, F(1, 26) = 3.83, p = .06. Thus, across hemispheres and gender, the basal ganglia was reliably activated by gains, and only the NAcc showed signs of deactivation after penalties relative to no-change feedback.
Figure 2.
Mean beta weights (and standard errors) in (A) the four basal ganglia regions and (B) the rACC in response to monetary gains, no-change feedback, and monetary penalties (averaged across hemispheres). Note that only the nucleus accumbens (NAcc) showed a trend for smaller responses to penalties relative to no-change feedback.
To evaluate whether the structurally defined rACC ROI was activated by monetary gains, we computed a Feedback × Gender ANOVA and obtained a significant main effect of Feedback, F(1.9, 50.4) = 5.63, p < .007 (Figure 2B). A priori specified contrasts revealed higher activation to gains versus no-change feedback, F(1, 26) = 12.48, p = .002, as well as higher activation to penalties versus no-change feedback, F(1, 26) = 4.18, p = .051.
Functional and Structural Correlates of Anhedonia
Relationship with NAcc responses to gains and penalties
As hypothesized, anhedonia as measured by the MASQ AD was negatively associated with NAcc responses to gains averaged across the two hemispheres, r(26) = −.43, p = .011, one-tailed (see Table 2 and Figure 3A). No significant correlations were observed between MASQ AD and gain-related responses in any of the other four regions of interest (putamen, caudate, pallidus, rACC). Highlighting the specificity of these findings, none of the other MASQ scales correlated significantly with the NAcc responses to gains (see Table 2), and the correlation between MASQ AD and NAcc gain responses remained virtually unchanged after simultaneously partialling out the other three MASQ scales, r(23) = −.35, p = .041, one-tailed. Furthermore, the correlation between MASQ AD and NAcc responses to gains differed significantly from the (non-significant) correlations between MASQ AD and NAcc responses to penalties, r(26) = .25, p = .20, z = 2.41, p = .016, or no-change feedback, r(26) = .11, p = .58, z = 2.30, p = .021. Although not a main focus of the present study, NAcc responses to penalties were positively correlated with MASQ GDA scores (see Table 2), indicating that more anxious participants showed stronger NAcc responses to penalties.2
Table 2.
Correlations between MASQ Scales, Nucleus Accumbens (NAcc) Volume and Responses to Feedback, and Resting Delta Activity in Rostral Anterior Cingulate Regions
| Variable | MASQ AD | MASQ GDD | MASQ GDA | MASQ AA | NAcc Volume | NAcc Gains | NAcc No Change | NAcc Penalties |
|---|---|---|---|---|---|---|---|---|
| NAcc Volume | −.35† | −.13 | −.14 | −.03 | 1.00 | |||
| NAcc Gains | −.43†† | −.29 | −.19 | −.30 | −.12 | 1.00 | ||
| NAcc No Change | .11 | .14 | −.00 | −.33 | −.16 | .26 | 1.00 | |
| NAcc Penalties | .25 | .19 | .40* | .32 | −.06 | −.09 | −.03 | 1.00 |
| Functional ROI (delta) | .52*** | .42** | .25 | .21 | .13 | −.49** | −.07 | .14 |
| BA24 (delta) | .44** | .24 | .08 | .14 | .14 | −.47* | −.06 | .16 |
| BA25 (delta) | .35* | .17 | .01 | .03 | .18 | −.41* | .02 | .05 |
| BA32 (delta) | .54*** | .34* | .21 | .23 | −.00 | −.56** | −.06 | .16 |
Notes. N = 28 for NAcc correlations in the fMRI sample, N = 41 for the remaining correlations. Correlations predicted a priori are highlighted in bold type. Regression analyses were performed on beta weights extracted for each condition (gains, penalties, no-change).
BA24, BA25, and BA32 = log-transformed average delta current density in a priori defined regions of interest in the rACC (see text for details).
MASQ = Mood and Anxiety Symptom Questionnaire administered at the behavioral session (AD = Anhedonic Depression; GDD = General Distress: Depressive Symptoms; GDA = General Distress: Anxious Symptoms; AA = Anxious Arousal).
Functional ROI = log-transformed average delta current density in the functionally defined ROI (i.e., a cluster of 16 contiguous voxels with significant (p < .001) correlations between MASQ AD and log-transformed delta current density, see Figure 1).
NAcc Gains = NAcc responses to monetary gains; NAcc Penalties = NAcc responses to monetary penalties; NAcc Volume = NAcc volume corrected for gender and intracranial volume.
p = .03, one-tailed.
p = .011, one-tailed.
p < .05.
p < .01.
p < .001.
Figure 3.
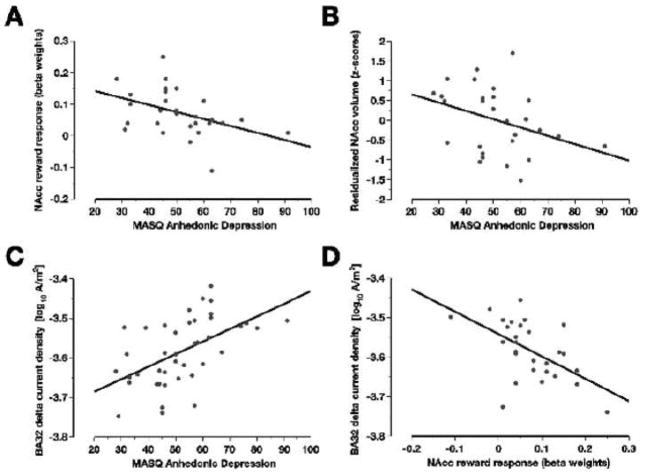
Scatterplots for the correlations (A) between the Anhedonic Depression scale of the Mood and Anxiety Symptom Questionnaire (MASQ AD) and the NAcc response to monetary gains, (B) between MASQ AD and NAcc volume corrected for gender and intracranial volume (see text for details), (C) between MASQ AD and log-transformed resting delta (1.5–6.0 Hz)
Supplementary analyses examining responses to reward cues did not reveal any significant correlations with MASQ AD for the NAcc, r(26) = .12, p = .54, or any of the other four ROIs, |r(26)| ≤ .25, p ≥ .20. In addition, the correlation between MASQ AD and NAcc responses to gains was significantly stronger than the correlation involving NAcc responses to reward cues, z = 2.03, p = .04, indicating that the association was specific to reward consumption rather than anticipation.
Relationship with NAcc volume
As shown in Table 2 and Figure 3B, MASQ AD displayed a negative correlation with NAcc volume (adjusted for gender and intracranial volume) that remained significant after simultaneously partialling out the other three MASQ scales, r(23) = −.38, p = .03, one-tailed. No significant associations were observed between MASQ AD and the adjusted volumes of the other basal ganglia regions, .22 ≥ r(26) ≥ .02, ps ≥ .27. Moreover, NAcc volume and NAcc reward responses to gains were uncorrelated (Table 2), indicating that both variables explained separate components of MASQ AD variance (see below).
Relationship with resting EEG delta current density
The computation of voxel-by-voxel correlations between MASQ AD and log-transformed delta current density identified only one cluster of positive correlations significant at p < 0.001. As shown in Figure 1, this functionally defined ROI (16 contiguous voxels, 5.49 cm3) was located in rACC regions overlapping with areas emerging from fMRI studies of anhedonia and pleasure ratings. Furthermore, MASQ AD was positively correlated with resting delta current density in each of the three a priori defined affective subdivisions of the ACC (BAs 24, 25, and 32; see Figure 3C and Table 2).
Control analyses indicated that this finding was characterized by substantial specificity. First, the MASQ AD scores did not correlate with delta current density in the more dorsal, cognitive subdivisions of the ACC (rs = .12 and .04 for BA24′ and BA32′, respectively), highlighting region-specificity. Second, all significant correlations between MASQ AD and delta current density shown in Table 2 remained significant after simultaneously partialling out the other three MASQ scales, r(36) ≥ .33, p ≤ .042, emphasizing symptom-specificity. In contrast, the correlations between MASQ GDD and delta current density in BA32 and the functionally defined ROI (see Table 2) were no longer significant after partialling out MASQ AD, r(38) = . 09. In addition, the MASQ AD-delta current density correlations remained significant after simultaneously partialling out participants’ ratings of state positive and negative affect during both the MASQ administration and EEG recording, r(33) ≥ .39, p ≤ .021, suggesting that the observed associations were not based on individual differences in affective state during the experimental sessions.3 Finally, as hypothesized, associations between MASQ AD scores and resting EEG activity were strongest for the delta band.4
Relationship Between Resting EEG Delta Current Density and NAcc Responses to Gains
As shown in Table 2, NAcc responses to gains, but not penalties, were negatively correlated with delta current density both in the functionally defined ROI and in the a priori defined rACC subdivisions, rs(26) ≤ −.41, ps ≤ .031. Furthermore, these correlations differed (1.60 ≤ z ≤ 2.62, p ≤ .11) from analogous correlations with NAcc responses either to penalties, rs(26) ≤ .16, ps ≥ .42, or no incentive feedback, rs(26) ≤ .07, ps ≥ .71. Emphasizing the specificity of the association between resting delta activity in the rACC and NAcc responses to gains, no correlations emerged between delta current density in the rACC and either responses to gains in any of the other basal ganglia regions or responses to reward cues in the NAcc.
Controls for potential influences of gender and outliers
All significant correlations in Table 2 remained at least marginally significant (p ≤ .05, one-tailed), when all variables were first standardized within gender and Spearman’s rank correlations instead of Pearson’s correlations were computed. Thus, neither gender differences nor outliers were driving the associations. In addition, none of the significant associations in Table 2 were significantly moderated by gender, indicating that similar correlations were observed for men and women.
Multivariate Model Predicting Anhedonia
To evaluate unique and cumulative contributions of the various physiological variables to anhedonia, NAcc responses to gains, NAcc volume, and resting delta current density in the rACC (functional ROI) were simultaneously entered in a multiple regression predicting MASQ scores. Findings revealed that all three variables were significant predictors of anhedonia (NAcc responses to gains: beta = −.30, p = .05, one-tailed; NAcc volume: beta = −.43, p = .005, one-tailed; resting delta rACC current density: beta = .37, p = .024, two-tailed). Accordingly, components of MASQ AD variance explained by the three variables were at least partially independent, despite the significant association between the two measures of functional activity. Notably, the model explained 45% of the variance in anhedonic symptoms, R2 = .45, F(3, 24) = 6.44, p = .002.
Discussion
This study integrated resting EEG, structural MRI, and fMRI to identify neural correlates of anhedonia, an important endophenotype and vulnerability factor for psychiatric disorders (e.g., Gooding et al., 2005; Hasler et al., 2004; Loas, 1996; Pizzagalli et al., 2005). As hypothesized, we observed (1) a negative association between anhedonia and NAcc responses to reward feedback (i.e., monetary gains), (2) a negative association between anhedonia and NAcc volume, and (3) a positive association between anhedonia and resting EEG delta activity (i.e., low resting activity) in rACC. Contrary to our hypotheses, no correlations between rACC activation to reward feedback and anhedonia emerged. However, resting rACC delta activity was negatively associated with NAcc responses to gains, indicating that the delta rhythm is indeed associated with stimulus-elicited activity in the brain’s reward circuit as suggested by Knyazev (2007). Thus, the present findings provide novel insights into both brain mechanisms associated with anhedonia and functional correlates of EEG delta activity.
Anhedonia and NAcc Structure and Function
Replicating prior work (Epstein et al., 2006; Keedwell et al., 2005), we found a negative correlation between anhedonic symptoms and NAcc responses to positive stimuli (monetary gains) measured at a separate session (on average, more than one month later). Unlike prior studies, the current analyses revealed that this association was specific to anhedonic symptoms (versus anxiety symptoms or general distress, as assessed by the three other MASQ scales), to the NAcc (versus the three other basal ganglia regions), to reward feedback (versus punishment and neutral feedback), and to the consumatory (versus anticipatory) phase of reward processing. These findings show that anhedonia predicts ventral striatal responses to rewarding stimuli not only in depressed patients (Epstein et al., 2006; Keedwell et al., 2005), but also in healthy subjects, and emphasize substantial specificity between reward-related NAcc responses and anhedonia. Providing initial insights into the causal direction underlying this association, Schlaepfer et al. (2008) recently showed that deep brain stimulation in the NAcc increased glucose metabolism in the stimulated region and alleviated anhedonia in three patients with treatment-resistant forms of depression. Taken together, these observations suggest that functional abnormalities in the NAcc play an important role in the manifestation of anhedonia.
Replicating findings by Harvey et al. (2007), we also observed a specific negative association between MASQ AD (and not the other MASQ scales) and NAcc volume. In contrast to the prior study, this association was specific to NAcc and did not extend to other basal ganglia regions (e.g., caudate). Interestingly, the variance in anhedonia accounted for by structural differences in NAcc did not overlap with variance associated with individual differences in NAcc responses to gains. This raises the question whether the structural component represents variance in trait anhedonia, whereas the functional component may be largely based on individual differences in state anhedonia. At least two observations speak against this possibility. First, functional responses to incentives were assessed at a different session, which occurred, on average, more than a month after the MASQ administration. Thus, only mood states of considerable stability could underlie the observed associations. Second, we re-computed the correlations after averaging the MASQ AD scores across the behavioral and EEG sessions. These analyses revealed an increased correlation for NAcc responses to gains, r(26) = −.49, but not for NAcc volume, r(26) = −.20 (compare to values in Table 2). Consequently, it seems more likely that structural and functional differences in the NAcc tap into different aspects of neural reward processing that may nonetheless both be relevant to anhedonia.
In the current study, we are unable to pinpoint these separate aspects. In addition, further work will be needed to decompose the relative contributions of anticipatory versus consummatory aspects of reward processing to anhedonia. In animal work, hedonic “liking” has been associated with NAcc opioid activity, whereas NAcc dopamine appears to be more closely tied to incentive salience (“wanting”) and behavioral activation (Berridge, 2007; Salamone et al., 2007) and both “liking” and “wanting” might arguably be reduced in anhedonia. In our sample, the correlation between anhedonia and NAcc responses was specific to the consummatory (“liking”) rather than anticipatory (“wanting”) phase of reward processing. This finding contrasts with recent data in schizophrenic patients, in which negative symptoms (including anhedonia) have been linked to ventral striatal responses to anticipatory cues in a similar version of the MID task (Juckel et al., 2006a, 2006b). In addition to clear differences in group composition (patients with schizophrenia vs. psychiatrically healthy subjects), differences in task design might explain the discrepancy between the current and Juckel’s findings. Specifically, unlike prior studies, in which 66% of reward trials led to reward feedback (Juckel et al., 2006a, 2006b), in the current study, gains were delivered on 50% of reward trials, and were thus more unpredictable. Because striatal responses have been found to be maximal when rewards are unpredictable (e.g., Delgado, 2007; O’Doherty et al., 2004), the current design might have increased our ability to identify lawful associations between NAcc responses to gains and anhedonia in this psychiatrically healthy sample. Based on these discrepancies, we believe it is premature to conclusively state whether anhedonia is primarily characterized by dysfunction in anticipatory vs. consummatory phases of reward processing. Future studies using a variety of experimental tasks and/or pharmacological manipulations of the dopamine and opioid systems will be needed to elucidate the roles of “wanting” and “liking” in anhedonia.
Anhedonia and rACC Function
In the present study, a positive association between anhedonia and resting EEG delta activity in rACC regions emerged. This association was specific to anhedonia (versus the other MASQ subscores), to rostral (versus dorsal, more cognitive) ACC subregions, and to the delta frequency band (with the exception of similar but weaker correlations in the theta band; see footnote 4). Furthermore, the cluster showing the strongest correlations between delta current density and anhedonia overlaps with regions where correlations between anhedonia/depression and fMRI signal in response to pleasant stimuli have been found in prior work (e.g., Harvey et al., 2007; Keedwell et al., 2005). Given that resting delta oscillations are inversely correlated with resting brain activity across individuals (Niedermeyer, 1993; Pizzagalli et al., 2004; Reddy et al., 1992; Scheeringa et al., 2008), these observations support the hypothesis that anhedonia is associated with tonically decreased brain activity in an area of the brain that has been associated with subjective pleasure ratings in response to stimuli from various modalities (de Araujo et al., 2003; Grabenhorst et al., 2008; Rolls et al., 2008; Rolls et al., 2003). Moreover, it should also be noted that our observation of a positive correlation between anhedonia and delta current density in the subgenual ACC (BA25) emerging from the a priori ROI analyses nicely dovetails with prior findings of higher delta current density (and lower metabolic activity) in BA25 in depressive patients with melancholia (i.e., a subtype of major depression prominently characterized by anhedonia, Pizzagalli et al., 2004).
Taken together, the present findings (1) indicate that anhedonia, rather than general distress, anxiety or other traits and states typically elevated in depression, may be linked to altered brain functioning in rACC, and (2) suggest that anhedonia may not only be characterized by reduced NAcc responsivity to rewards, but also by tonically low resting activity in the rACC. The latter observation is novel but consistent with ample evidence that the rACC figures prominently in the brain’s reward circuitry. It receives dense dopaminergic innervation (Gaspar et al., 1989) and projects to the striatum (in particular the NAcc) and the ventral tegmental area (Haber et al., 2006; Öngür and Price, 2000; Sesack and Pickel, 1992). In rats, stimulation of the rACC increases burst firing patterns in ventral tegmental area dopamine neurons (Gariano and Groves, 1988; Murase et al., 1993), and these burst firing patterns increase dopamine release in the NAcc (Schultz, 1998), which has been implicated in incentive salience and behavioral activation (see above). In humans, the rACC shows activity increases in response to dopamine-inducing drugs (Udo de Haes et al., 2007; Völlm et al., 2004), reduced functional connectivity with striatal areas after dopamine depletion (Nagano-Saito et al., 2008), reduced reward-learning signals in treatment-resistant depression (Kumar et al., 2008), and has been implicated in subjective pleasure reactions (see above) and preference judgments (e.g., Paulus and Frank, 2003).
Critically, the rACC is also considered a key node of the brain’s default network (i.e., a network of interconnected regions activated during resting states and deactivated during engaging tasks, Buckner et al., 2008), and Scheeringa et al. (2008) have demonstrated that frontal midline delta/theta activity is inversely correlated with activity in the default network. Thus, viewed from this perspective, the present findings suggest an association between anhedonia and reduced activity in the default network, which is thought to “facilitate flexible self-relevant mental explorations – simulations – that provide a means to anticipate and evaluate upcoming events before they happen” (Buckner et al., 2008, p. 2). Depressive patients underestimate the occurrence of positive stimuli presented to them (e.g., Pause et al., 2003) and anticipate fewer positive outcomes in the near future (MacLeod and Salaminiou, 2001; MacLeod et al., 1997; Miranda and Mennin, 2007; Moore et al., 2006). These observations raise the intriguing possibility that reduced resting activity in the rACC node of the default network may underlie difficulty with positive future-oriented mentation (i.e., underestimation of positive events in the past along with deficits in imagining positive scenarios for the future). Future studies will be needed to test this speculation.
Although the rACC was also reliably activated by reward feedback in the MID task, we did not observe the expected positive association between reward responses in this area and anhedonia (Harvey et al., 2007; Keedwell et al., 2005). We note, however, that positive associations between anhedonia/depression and rACC responses to positive stimuli have been reported most consistently in the context of overall rACC deactivations to emotional stimuli, with healthy controls and individuals low in anhedonia showing the most pronounced deactivations (Gotlib et al., 2005; Grimm et al., 2008; Harvey et al., 2007). It is therefore possible that individuals with anhedonic symptoms do not display task-induced deactivations in this node of the brain’s default network due to their abnormally low activity in this area under rest. This novel hypothesis, which might also explain the seemingly paradoxical positive associations between anhedonia and rACC reward responses observed in some studies (Harvey et al., 2007; Keedwell et al., 2005), could be readily tested in studies combining fMRI measures of task-related deactivation and PET or EEG measures of resting activity.
Rostral ACC Delta Activity and NAcc Reward Responses
The robust and specific negative correlations observed between delta current density in the more rostral, affective subdivisions of the ACC and the NAcc response to gains constitute novel evidence in healthy humans for the hypothesis that the EEG delta rhythm is associated with reward processing in the ventral striatum (Knyazev, 2007). The direction of this effect is consistent with animal data demonstrating that dopamine release in the NAcc is associated with decreased delta activity (Chang et al., 1995; Ferger et al., 1994; Kropf and Kuschinsky, 1993; Leung and Yim, 1993; Luoh et al., 1994) and with a recent report of increased event-related delta activity in pre-symptomatic Huntington’s disease, a neurological disorder associated with marked reductions in striatal dopamine D1 and D2 receptor density (Beste et al., 2007). Specificity of the effect to rACC and NAcc constitutes further support for the hypothesized role for delta as an index of neural reward processing.
As described above, the rACC is itself an important node of the brain’s reward circuitry and anatomical studies in monkeys have demonstrated that rACC regions preferentially project to the NAcc versus other striatal regions (Haber et al., 2006). Though providing strong evidence for a link between delta and reward, the present findings from resting EEG data do not speak to the precise functions of delta activity in reward processing. Cohen, Elger, and Fell (2008) have recently reported that frontal midline delta activity decreases during anticipation of loss and win feedback and increases in response to the feedback itself, particularly to unexpected win feedback. These data suggest opposite changes in delta activity in the anticipatory and consummatory phases of reward processing and indicate how investigators could capitalize on the superior temporal resolution of the EEG to probe individual differences in the dynamics of neural reward processing.
Limitations and Conclusions
Apart from several strengths (e.g., use of multiple neuroimaging techniques, larger sample size than previous studies), we should also note some important limitations. First, because our sample primarily consisted of young undergraduates, it remains to be seen whether the present findings will generalize to other, more heterogeneous samples. Second, although we took several precautions to control for potential state influences on the observed association between anhedonia and the resting EEG (assessment at separate sessions, partialling of state affect), we cannot rule out that state affect contributed to the present findings. Studies with repeated assessments of resting EEG could provide interesting information on the relative importance of state and trait contributions to the variance in rACC delta activity (Hagemann et al., 2002). Third, studies with concurrent measurement of resting EEG and PET in sufficiently large samples are clearly warranted to bolster our interpretation of LORETA estimates of delta current density in the rACC as an inverse indicator of brain activity in this region, given that the coupling of (low) delta and regional glucose metabolism may not be as tight in clinical samples (Pizzagalli et al., 2004). Fourth, although the five correlations tested in the primary analyses were predicted a priori based on previous findings and/or theoretical arguments, the current findings await replication due to the lack of correction for multiple comparisons. Finally, as with all correlational studies, the present findings do not imply causality, or even a causal direction. Accordingly, it is currently unknown whether reduced NAcc volume, for example, is a vulnerability factor for or a consequence of anhedonia. Future studies using longitudinal designs, experimental manipulations of striatal and medial PFC activity (e.g., Schlaepfer et al., 2008), and/or focusing on the molecular genetics of reward processing (e.g., Kirsch et al., 2006) will be needed to investigate more refined hypothesis about the neurobiological substrates of anhedonia.
Nonetheless, using a multi-modal neuroimaging approach, we showed that anhedonia is correlated with weaker NAcc responses to monetary gains, reduced NAcc volume, and increased resting EEG delta activity (i.e., reduced resting brain activity) in rACC regions in a sample of young volunteers. Collectively, these three physiological measures explained 45% of variance in anhedonic symptoms. Both anhedonia and the regions of the brain’s reward system implicated in the present study have been linked to several severe psychiatric disorders, including depression and schizophrenia. Thus, our findings provide further support for the conceptualization of anhedonia as a promising endophenotype and vulnerability factor for these disorders, and suggest that further studies on the neural basis of anhedonia in healthy individuals may help overcome the limitations of the current psychiatric nosology and offer important insights into pathophysiology.
Acknowledgments
This research was supported by grants from NIMH (R01 MH68376) and NCCAM (R21 AT002974) awarded to DAP. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH, NCCAM, or the National Institutes of Health. Dr. Pizzagalli has received research support from GlaxoSmithKline and Merck & Co., Inc. for projects unrelated to this research. Jan Wacker was supported by a grant from the G.-A.-Lienert-Stiftung zur Nachwuchsförderung in Biopsychologischer Methodik during his stay at the Department of Psychology, Harvard University.
The authors would like to thank Jeffrey Birk and Elena Goetz for their skilled assistance, Allison Jahn, Kyle Ratner, and James O’Shea for their contribution at early stages of this research, Decklin Foster for technical support, and Nancy Brooks and Christen Deveney for their role in the recruitment of this sample.
Footnotes
In an alternative analysis, we obtained average beta weights for spherical ROIs with an 8 mm radius centered on the approximate location of the peak correlation between anhedonia and the BOLD response to positive stimulation in the left and right ventromedial PFC (x = ± 8, y = 44, z = −7) as reported by Harvey et al. (2007) and Keedwell et al. (2005). The results were very similar to those reported here for the rACC.
Highlighting the specificity of this link, this correlation differed from the non-significant associations observed between MASQ GDA and NAcc responses to gains, r(26) = −.19, p = .34, z = 2.07, p = .038, and no-change feedback, r(26) = −.00, p = .99, z = 1.71, p = .087, and remained significant after simultaneously partialling the other three MASQ scales, r(23) = .41, p = .041. In spite of this promising specificity, the correlation between MASQ GDA and NAcc responses to monetary penalties should be interpreted cautiously, because it was not predicted and would not reach statistical significance after correction for multiple testing.
Two participants had missing data in at least one of their state positive and negative affect ratings and therefore could not be included in this analysis.
Similar but somewhat smaller correlations emerged between MASQ AD scores and theta current density, rs(39) = .35, .30, and .45, for BAs 24, 25, and 32, respectively, p ≤ .06. Moreover, with the sole exception of a correlation between MASQ AD and beta1 current density in BA32, r(39) = .33, p = .035, no significant associations were observed between MASQ AD and current density in these areas in any of the other EEG frequency bands.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Saft C, Yordanova J, Andrich J, Gold R, Falkenstein M, Kolev V. Functional compensation or pathology in cortico-subcortical interactions in preclinical Huntington’s disease? Neuropsychologia. 2007;45:2922–2930. doi: 10.1016/j.neuropsychologia.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chang AY, Kuo TB, Tsai TH, Chen CF, Chan SH. Power spectral analysis of electroencephalographic desynchronization induced by cocaine in rats: correlation with evaluation of noradrenergic neurotransmission at the medial prefrontal cortex. Synapse. 1995;21:149–157. doi: 10.1002/syn.890210208. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Fell J. Oscillatory Activity and Phase-Amplitude Coupling in the Human Medial Frontal Cortex during Decision Making. J Cogn Neurosci. 2008 doi: 10.1162/jocn.2008.21020. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45:36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Ferger B, Kropf W, Kuschinsky K. Studies on electroencephalogram (EEG) in rats suggest that moderate doses of cocaine or d-amphetamine activate D1 rather than D2 receptors. Psychopharmacology (Berl) 1994;114:297–308. doi: 10.1007/BF02244852. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, McKenna PJ, Frith CD, Grasby PM, Friston KJ, Dolan RJ. Brain activations in schizophrenia during a graded memory task studied with functional neuroimaging. Arch Gen Psychiatry. 1998;55:1001–1008. doi: 10.1001/archpsyc.55.11.1001. [DOI] [PubMed] [Google Scholar]
- Gariano RF, Groves PM. Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulate cortices. Brain Res. 1988;462:194–198. doi: 10.1016/0006-8993(88)90606-3. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol. 1989;279:249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Matts CW. Clinical status of at-risk individuals 5 years later: further validation of the psychometric high-risk strategy. J Abnorm Psychol. 2005;114:170–175. doi: 10.1037/0021-843X.114.1.170. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37:111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Roehrs TA. Mu-opioid self-administration vs passive administration in heroin abusers produces differential EEG activation. Neuropsychopharmacology. 2005;30:212–221. doi: 10.1038/sj.npp.1300596. [DOI] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered Negative BOLD Responses in the Default-Mode Network during Emotion Processing in Depressed Subjects. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, Bartussek D. Does resting electroencephalograph asymmetry reflect a trait? an application of latent state-trait theory. J Pers Soc Psychol. 2002;82:619–641. [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12703:767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, Waldeck T, Geraci M, Roiser JP, Neumeister A, Meyers N, Charney DS, Drevets WC. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry. 2008;65:521–531. doi: 10.1001/archpsyc.65.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RG. Pleasure and brain activity in man. Deep and surface electroencephalograms during orgasm. Journal of Nervous and Mental Disease. 1972;154:3–18. doi: 10.1097/00005053-197201000-00002. [DOI] [PubMed] [Google Scholar]
- Ito H, Kawashima R, Awata S, Ono S, Sato K, Goto R, Koyama M, Sato M, Fukuda H. Hypoperfusion in the limbic system and prefrontal cortex in depression: SPECT with anatomic standardization technique. J Nucl Med. 1996;37:410–414. [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Knutson B, Kienast T, Gallinat J, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006a;187:222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006b;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Reuter M, Mier D, Lonsdorf T, Stark R, Gallhofer B, Vaitl D, Hennig J. Imaging gene-substance interactions: the effect of the DRD2 TaqIA polymorphism and the dopamine agonist bromocriptine on the brain activation during the anticipation of reward. Neuroscience Letters. 2006;405:196–201. doi: 10.1016/j.neulet.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav Rev. 2007;31:377–395. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kropf W, Kuschinsky K. Effects of stimulation of dopamine D1 receptors on the cortical EEG in rats: different influences by a blockade of D2 receptors and by an activation of putative dopamine autoreceptors. Neuropharmacology. 1993;32:493–500. doi: 10.1016/0028-3908(93)90175-3. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC. Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin A, Grace AA. Physiological properties of rat ventral pallidal neurons recorded intracellularly in vivo. J Neurophysiol. 1996;75:1432–1443. doi: 10.1152/jn.1996.75.4.1432. [DOI] [PubMed] [Google Scholar]
- Leung LS, Yim CY. Rhythmic delta-frequency activities in the nucleus accumbens of anesthetized and freely moving rats. Can J Physiol Pharmacol. 1993;71:311–320. doi: 10.1139/y93-049. [DOI] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- Luoh HF, Kuo TB, Chan SH, Pan WH. Power spectral analysis of electroencephalographic desynchronization induced by cocaine in rats: correlation with microdialysis evaluation of dopaminergic neurotransmission at the medial prefrontal cortex. Synapse. 1994;16:29–35. doi: 10.1002/syn.890160104. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod AK, Salaminiou E. Reduced positive future-thinking in depression: Cognitive and affective factors. Cognition and Emotion. 2001;15:99–107. [Google Scholar]
- MacLeod AK, Tata P, Kentish J, Jacobsen H. Retrospective and prospective cognitions in anxiety and depression. Cognition and Emotion. 1997;11:467–479. [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lewis PJ, Regenold W, Wagner HN., Jr Paralimbic hypoperfusion in unipolar depression. J Nucl Med. 1994;35:929–934. [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: some conjectures. Bull Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- Michel CM, Henggeler B, Brandeis D, Lehmann D. Localization of sources of brain alpha/theta/delta activity and the influence of the mode of spontaneous mentation. Physiological Measurement. 1993;14(Suppl 4A):A21–26. doi: 10.1088/0967-3334/14/4a/004. [DOI] [PubMed] [Google Scholar]
- Michel CM, Lehmann D, Henggeler B, Brandeis D. Localization of the sources of EEG delta, theta, alpha and beta frequency bands using the FFT dipole approximation. Electroencephalogr Clin Neurophysiol. 1992;82:38–44. doi: 10.1016/0013-4694(92)90180-p. [DOI] [PubMed] [Google Scholar]
- Miranda R, Mennin DS. Depression, generalized anxiety disorder, and certainty in pessimistic predictions about the future. Cognitive Therapy and Research. 2007;31:71–82. [Google Scholar]
- Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, Suckling J, Poon L, Simmons A, Andrew C, Sharma T. Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport. 2003;14:177–182. doi: 10.1097/00001756-200302100-00003. [DOI] [PubMed] [Google Scholar]
- Moore AC, MacLeod AK, Barnes D, Langdon DW. Future-directed thinking and depression in relapsing-remitting multiple sclerosis. British Journal of Health Psychology. 2006;11:663–675. doi: 10.1348/135910705X85781. [DOI] [PubMed] [Google Scholar]
- Mülert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, Moller HJ, Hegerl U, Pogarell O. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98:215–225. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28:3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E. Sleep and EEG. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalograpghy: Basic Principles, Clinical Applications, and Related Fields. Williams & Wilkins; Baltimore, MD: 1993. pp. 153–166. [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005;30:821–832. doi: 10.1038/sj.npp.1300667. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, Koukkou M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. 1999;90:169–179. doi: 10.1016/s0925-4927(99)00013-x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Frank LR. Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport. 2003;14:1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- Pause BM, Raack N, Sojka B, Goder R, Aldenhoff JB, Ferstl R. Convergent and divergent effects of odors and emotions in depression. Psychophysiology. 2003;40:209–225. doi: 10.1111/1469-8986.00023. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional Neuroanatomy of Emotion: A Meta-Analysis of Emotion Activation Studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J Psychiatr Res. 2009;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;325(9):393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity predicts degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting Anterior Cingulate Activity and Abnormal Responses to Errors in Subjects With Elevated Depressive Symptoms: A 128-Channel EEG Study. Hum Brain Mapp. 2006;27:185–201. doi: 10.1002/hbm.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rado S. Psychoanalysis of Behavior: Colelcted Papers. Vol. 1. Grune and Stratton; New York: 1956. [Google Scholar]
- Reddy RV, Moorthy SS, Mattice T, Dierdorf SF, Deitch RD., Jr An electroencephalographic comparison of effects of propofol and methohexital. Electroencephalogr Clin Neurophysiol. 1992;83:162–168. doi: 10.1016/0013-4694(92)90030-l. [DOI] [PubMed] [Google Scholar]
- Reid MS, Flammino F, Howard B, Nilsen D, Prichep LS. Topographic imaging of quantitative EEG in response to smoked cocaine self-administration in humans. Neuropsychopharmacology. 2006;31:872–884. doi: 10.1038/sj.npp.1300888. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA. Warm pleasant feelings in the brain. Neuroimage. 2008;41:1504–1513. doi: 10.1016/j.neuroimage.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, de Araujo IE. Different representations of pleasant and unpleasant odours in the human brain. European Journal of Neuroscience. 2003;18:695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Dillon DG, Birk JL, Holmes AJ, Goetz E, Bogdan R, Pizzagalli DA. Individual differences in reinforcement learning: Behavioral, electrophysiological, and neuroimaging correlates. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int J Psychophysiol. 2008;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signals of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Scott JC, Cooke JE, Stanski DR. Electroencephalographic quantitation of opioid effect: comparative pharmacodynamics of fentanyl and sufentanil. Anesthesiology. 1991;74:34–42. doi: 10.1097/00000542-199101000-00007. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Maguire RP, Jager PL, Paans AM, den Boer JA. Methylphenidate-induced activation of the anterior cingulate but not the striatum: a [15O]H2O PET study in healthy volunteers. Hum Brain Mapp. 2007;28:625–635. doi: 10.1002/hbm.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Pedersen TH, Hartvig H, Egander A, Clemmensen K, Rasmussen NA, Andersen F, Gjedde A, Rosenberg R. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand. 2002;106:35–44. doi: 10.1034/j.1600-0447.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Völlm BA, de Araujo IE, Cowen PJ, Rolls ET, Kringelbach ML, Smith KA, Jezzard P, Heal RJ, Matthews PM. Methamphetamine activates reward circuitry in drug naive human subjects. Neuropsychopharmacology. 2004;29:1715–1722. doi: 10.1038/sj.npp.1300481. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Depression and the melancholic temperament. European Journal of Personality. 1995;9:351–366. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]