Abstract
AIMS
The aim was to explore the role of CYP2C19 polymorphism in short-term rabeprazole-based triple therapy against Helicobacter pylori infection.
METHODS
Patients with H. pylori infection were tested for CYP2C19 genotype as poor metabolizers (PMs) or extensive metabolizers (EMs, homozygous EM or heterozygous EM) and given rabeprazole for 7 days. Antibiotics (clarithromycin and amoxicillin) were given on days 1–4, days 4–7, or days 1–7. A direct link model with an effect compartment was used in the population pharmacokinetic–pharmacodynamic analysis. The status of H. pylori infection was evaluated.
RESULTS
Rabeprazole clearance was lower in CYP2C19 PMs than in EMs (with average values of 10.7 vs. 16.8 l h−1 in PMs and EMs, respectively), resulting in higher plasma levels in the former group. The values of EC50 and keo of gastrin response increased with multiple doses of rabeprazole. The keo values were lower in CYP2C19 PMs than in EMs on day 1 (0.012 vs. 0.017 × 10−4 l min−1), and higher than in EMs on day 4 (0.804 vs. 0.169 × 10−4 l min−1) of rabeprazole treatment. The predicted gastrin-time profile showed a higher response in CYP2C19 PMs than in EMs on days 4 and 7. Helicobacter pylori was eradicated in all CYP2C19 PMs except in one patient infected by a resistant strain. In contrast, in CYP2C19 EMs the eradication rates ranged from 58 to 85%.
CONCLUSIONS
CYP2C19 genotypes play a role in H. pylori eradication therapy. Rabeprazole-based short-term triple therapy may be applicable in CYP2C19 PMs for H. pylori eradication.
Keywords: CYP2C19 polymorphism, Helicobacter pylori, population PK/PD, rabeprazole
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Asians have a higher percentage of CYP2C19 poor metabolizers (PMs) and a high percentage of Helicobacter pylori infection compared with Whites.
Although rabeprazole has been suggested to be least affected by CYP2C19 genotype, the pharmacokinetic properties of rabeprazole have been shown to correlate with the CYP2C19 genotype.
Short-term (i.e. <7 days) rabeprazole-based triple therapy has been suggested for its use in eradicating H. pylori, whereas the eradication rate has been reported variously as 90–27% without CYP2C19 genotyping.
WHAT THIS STUDY ADDS
Population pharmacokinetic–pharmacodynamic analysis showed that the plasma rabeprazole levels, the values of keo (the first-order rate constant for drug dissipation from the effect compartment), and the predicted gastrin-time profile were higher in CYP2C19 PMs than in extensive metabolizers (EMs) after multiple dosing.
Helicobacter pylori was eradicated in all CYP2C19 PMs except in one patient infected by a resistant strain, whereas the eradication rates ranged from 58 to 85% in CYP2C19 EMs.
Introduction
Helicobacter pylori is closely related to many gastrointestinal diseases, including chronic active gastritis, peptic ulcers, gastric carcinoma and gastric mucosa-associated lymphoid tissue lymphoma [1]. Eradication of H. pylori is important for reducing the relapse rate of ulcers and risk of carcinogenesis. Triple therapy with a proton pump inhibitor (PPI) and two antibiotics is considered a standard treatment for H. pylori infection. When the drugs are given at the recommended doses, the eradication rates range from 70 to 85% for a duration of treatment of 7–14 days [2,3]. Few studies have compared the cost effectiveness of these different lengths of treatment [4]. Nonetheless, short-term (i.e. <7 days) triple therapy has been reported to increase patient compliance and reduce costs and adverse effects [[5–7], but the evidence is limited and the rationale needs to be elucidated.
In an acidic environment, PPIs are protonated to form sulphenamides, which are covalently bound with cysteine residues in the gastric proton pump to inhibit proton secretion [8]. Most PPIs are mainly metabolized by CYP2C19, which is known to exhibit polymorphism in both its genotype and phenotype with much higher percentage of poor metabolizers (PMs) in Asians [[9,10]. Among the PPIs, rabeprazole has been demonstrated to have a high degree of activity against H. pylori. One reason is the higher pKa for the conversion of rabeprazole to the sulphenamide, which means it is converted at a higher pH than other PPIs, resulting in more rapid proton pump inhibition [11]. Furthermore, rabeprazole and its thio-ether metabolite inhibit the motility and urease activity of H. pylori[[12,13]. Given the better pharmacological profile of rabeprazole, a shorter regimen has been suggested for its use in eradicating H. pylori; however, the findings are controversial and the eradication rate using rabeprazole–amoxicillin–clarithromycin for 3–4 days has been reported variously as 90–27% [5]. One reason for the variation in these results could be that the CYP2C19 genotypes of the enrolled subjects were not determined in these studies. Although rabeprazole has been suggested to be the PPI least affected by CYP2C19 genotype [14], the eradication rate of a rabeprazole regimen has been shown to correlate with the CYP2C19 genotype [15]. The pharmacokinetic properties, including the area under the curve (AUC), maximal plasma concentration (Cmax) and clearance (CL) of rabeprazole were all found to be significant different between CYP2C19 PMs and CYP2C19 extensive metabolizers (EMs) [16]. Higher plasma gastrin levels were also observed in CYP2C19 PMs than in EMs after taking rabeprazole 20 mg b.i.d. for 4 days [17]. Since the extent and duration of intragastric acid inhibition have been proven to be important in H. pylori eradication and ulcer healing [[14,18], the higher plasma concentrations of rabeprazole and gastrin in CYP2C19 PMs may contribute to a better clinical outcome.
In the present study, population pharmacokinetic–pharmacodynamic (PK–PD) analysis was used to explore the role of CYP2C19 genotype in rabeprazole-based triple therapy on days 1, 4 and 7 of treatment. In addition, the clinical outcome of H. pylori eradication was evaluated in patients receiving rabeprazole with different antibiotic regimens for 1 week.
Materials and methods
Study design
Patients with H. pylori-positive ulcer or gastritis were recruited at the National Taiwan University Hospital. Patients who met any of the following conditions were excluded: (i) pregnant or nursing woman; (ii) serious concomitant illness; (iii) malignant tumour of any kind; (iv) serious bleeding during the course of this ulcer; (v) previous gastric surgery; and (vi) taking bismuth compounds or PPIs within 30 days prior to pretreatment endoscopy. Ulcer and H. pylori status were evaluated by endoscopy, histology, culture and the 13C-urea breath test. The study was approved by the Ethical Practices Committee of the National Taiwan University Hospital (Study ID number: 920505, NTUH93S060).
These patients were randomly assigned to one of the three study groups after giving their written informed consent. Rabeprazole 20 mg twice daily was given orally for 7 days to all three groups. Amoxicillin 1 g and clarithromycin 500 mg were also given twice daily on days 1–4 of rabeprazole treatment (group A), days 4–7 (group B), or days 1–7 (group C). The CYP2C19 genotype of each participant was analysed by the polymerase chain reaction-based restriction fragment length polymorphism (PCR-RFLP) method.
For PK–PD analysis, to avoid the patients having to be present all day, they were randomly assigned to one of four groups and, on days 1, 4 or 7 of rabeprazole treatment, 10-ml aliquots of venous blood were drawn 30 min before drug administration and at the time points of 0.5, 2, 4 and 8 h (group 1), 0.5, 1, 3 and 6 h (group 2), 1, 3, 6 and 10 h (group 3), or 2, 4, 8 and 10 h (group 4). The blood was centrifuged at 800 g for 10 min within 15 min of collection and 200 µl of plasma was taken for gastrin analysis and the rest of the sample placed in a storage tube containing 350 µl of a 1% aqueous solution of diethylamine for plasma rabeprazole concentration analysis. All samples were frozen at −80 °C until analysis.
Endoscopy and biopsy
During each endoscopic examination, four biopsy specimens were obtained from the antrum and four from the body for histology, bacterial culture, and the Campylobacter-like organism (CLO) test. Two of the four pairs of specimens were processed with haematoxylin and eosin and Warthin–Starry silver stain. Another pair was placed in brain heart infusion broth for transportation, then ground up and streaked onto an agar plate with selective medium [19]. The plates were incubated at 37 °C under micro-aerophilic conditions (5% O2, 10% CO2, 85% N2) for 3–7 days. Helicobacter pylori was identified by its characteristic biotyping as Gram-negative, but oxidase-, catalase-and urease-positive. The fourth pair of specimens was tested using a CLO test kit (Delta West Pty Ltd., Bentley, Western Australia), the colour of which changes from yellow to purple if H. pylori is present.
Evaluation of H. pylori status and antibiotic resistance
Helicobacter pylori status was considered positive when (i) at least two of the above three tests were positive or (ii) only the bacterial culture test was positive. The E-test (AB Biodisk, Solna, Sweden) was used to evaluate the amoxicillin and clarithromycin resistance of H. pylori, which was considered to be resistant to antibiotics when the minimum inhibitory concentration value was ≥1 mg L−1[19]. Helicobacter pylori eradication was evaluated using a 13C-urea breath test before, and at 4–6 weeks after, rabeprazole treatment, performed as described previously [17].
CYP2C19 genotyping
DNA was isolated from peripheral leucocytes in blood samples by a standard method [20]. Genetic polymorphism of the CYP2C19*2 (CYP2C19 m1) and CYP2C19*3 (CYP2C19 m2) alleles was identified by PCR-RFLP as described previously [[21,22]. Patients were identified as homozygous extensive metabolizers (HomEM; wt/wt), heterozygous extensive metabolizers (HetEM; wt/m1 or wt/m2) or poor metabolizers (PM; m1/m1 or m1/m2) according to CYP2C19 genotypes.
Determination of plasma concentrations of rabeprazole and gastrin
Plasma concentrations of rabeprazole were determined by high-performance liquid chromatography as described previously [23]. Plasma gastrin concentrations were determined by radioimmunoassay using ICN kits (ICN Pharmaceuticals, Costa Mesa, CA, USA) according to the manufacturer's protocol, as in a previous study [17].
Population PK–PD analysis
Population PK–PD analysis was performed using the NONMEM program (version V, level 1.1). To establish the PK and PD models, the data were analysed according to the day of rabeprazole treatment (i.e. pooled data for day 1, day 4, or day 7) given that the dosing of antibiotics is irrelevant to the PK–PD properties of rabeprazole. A naive-pooled data analysis (i.e. pool data for all subjects and fit single ‘naive’ individual) was performed to obtain the initial values for population PK–PD analysis. PK properties were estimated before the link to PD analysis. For PK analysis, first-order conditional estimation and a PREDPP subroutine, ADVAN2, were used for a one-compartment model with first-order absorption (ka) and a lag time (Alag). For PD analysis, a first-order method and a PREDPP subroutine, ADVAN6, were used after the estimation of the PK parameters. On the basis of the counterclockwise hysteresis loop observed in the concentration–effect profile of rabeprazole [17], a direct link model with an effect compartment was used [24]. The concentration in the effect compartment (Ce) is described by Equation 1
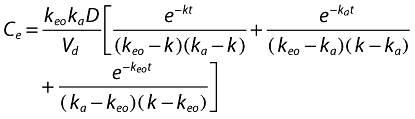 |
(1) |
where keo is the first-order rate constant for drug dissipation from the effect compartment,ka the absorption constant,Vd the volume of distribution, and D the dose of rabeprazole. The PD response is described by Equation 2
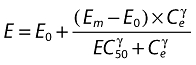 |
(2) |
where E is the gastrin levels after dosing,E0 the baseline gastrin levels,Em the maximal gastrin levels, EC50 the rabeprazole concentration to produce half Em, and γ the slope factor of the concentration–response curve. The base and final models for the PK and PD analysis are listed in Table 1, in which θ represents the predicted population mean of the parameters, η the random effect with a mean of zero and a variance of ω2, and BSA the body surface area estimated using Mosteller's formula [25].
Table 1.
Parameters used in the population pharmacokinetic–pharmacodynamic (PK–PD) analysis for rabeprazole *
| Base PK model | Final PK model |
|---|---|
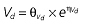 |
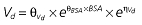 |
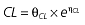 |
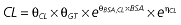 |
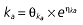 |
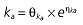 |
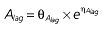 |
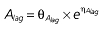 |
| Base PD model | Final PD model |
|---|---|
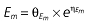 |
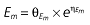 |
 |
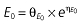 |
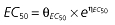 |
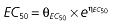 |
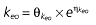 |
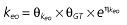 |
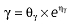 |
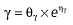 |
Vd, volume of distribution; CL, clearance; ka, absorption constant; Alag, absorption lagged time; Em, maximal effect; E0, baseline effect; EC50, concentration when half Em achieved; keo, elimination constant for the effect compartment; BSA, body surface area; γ, slope; GT, CYP2C19 genotype; θx, population mean of parameter x; ηx, intersubject variability of parameter x.
Statistical analysis
The equivalence of demographic information among various treatment groups or genotypes was revealed by χ2 independence test or t-test or anova. The comparisons of predicted PK–PD parameters among various days or genotypes were analysed by the two-sided t-test or anova. Fisher's exact test or the χ2 test was used to analyse clinical outcome, only those subjects who completed the study being included in the analysis. A P-value < 0.05 was considered to be statistically significant.
Results
Subject characteristics
Forty-eight subjects, 33 men and 15 women, aged 18–74 years were enrolled and assigned randomly to one of the three study groups A, B and C (antibiotics on days 1–4, 4–7, or 1–7, respectively) (Table 2). Nine cases were CYP2C19 PMs and 39 EMs (including 18 HomEM and 21 HetEM). The prevalence rates of CYP2C19 HomEM, HetEM and PM were about 38, 44 and 18%, respectively. One subject with EM genotype in group B was excluded from the PK–PD analysis due to the lack of a blood sample. One subject with EM genotype in group C was excluded from the clinical outcome estimation because she failed to attend for the after-treatment 13C-urea breath test evaluation.
Table 2.
Demographic data for the patients on the different treatment protocols (groups A, B, and C)*
| A | B | C | |
|---|---|---|---|
| No. | 16 | 16 | 16 |
| CYP2C19 (HomEM/HetEM/PM) | 5/8/3 | 5/7/4 | 8/6/2 |
| Age (years) | 53.5 (26–73) | 47.6 (18–72) | 53.3 (37–74) |
| Gender (% M/F) | 62.5/37.5 | 81.3/18.8 | 62.5/37.5 |
| Height (cm) | 165.3 (158–180) | 166.5 (156–176) | 165.2 (155–174) |
| Weight (kg) | 63.1 (49–81) | 65.2 (49–85) | 63.3 (49–77) |
| BSA (m2) | 1.70 (1.47–1.94) | 1.73 (1.48–2.00) | 1.70 (1.50–1.86) |
Rabeprazole treatment from day 1 to day 7, antibiotics (amoxicillin and clarithromycin) being given on days 1–4 (group A), days 4–7 (group B), or days 1–7 (group C). The values in parentheses indicate the data range. HomEM, homozygous extensive metabolizers; HetEM, heterozygous extensive metabolizers; PM, poor metabolizers; BSA, body surface area.
The χ2 independence tests revealed that the gender structure and genotype structure were not significantly different among three study groups, with P-values of 0.418 and 0.605, respectively. The gender structure was not significantly different (P-value = 0.285) between PM and EM groups. The two-way anova showed that the means of age were not significantly different among three groups (P-value = 0.424) or between two genotypes (P-value = 0.962), and no significant differences on the interaction of study groups and genotypes (P-value = 0.370). There was no significant difference in the demographic data for the patients categorized by treatment group (Table 2) or CYP2C19 genotype (except the age between HomEM and HetEM on day 7) or rabeprazole treatment day (Table 3).
Table 3.
Demographic data for the patients categorized according to rabeprazole treatment day
| Day 1 | Day 4 | Day 7 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CYP2C19 | HomEM | HetEM | PM | HomEM | HetEM | PM | HomEM | HetEM | PM |
| No. | 13 | 14 | 5 | 10 | 15 | 7 | 13 | 13 | 6 |
| Age (years) | 55.7 | 51.6 | 52.4 | 54.9 | 46.1 | 53.9 | 57.2 | 44.5* | 48.8 |
| (26–74) | (37–65) | (44–62) | (26–73) | (18–65) | (37–72) | (44–74) | (18–60) | (37–72) | |
| Gender (% M/F) | 69/31 | 64/36 | 40/60 | 80/20 | 73/27 | 57/43 | 69/31 | 77/23 | 67/33 |
| Height (cm) | 165.4 | 165.3 | 165.0 | 166.7 | 165.7 | 165.4 | 164.1 | 167.7 | 165.8 |
| (155–180) | (156–177.5) | (160–169) | (157–180) | (156–177.5) | (160–170) | (155–172.5) | (156–176) | (160–170) | |
| Weight (kg) | 65.2 | 63.2 | 58.2 | 66.2 | 63.1 | 63.4 | 65.0 | 64.9 | 61.2 |
| (55–77.1) | (49–81) | (49.1–62) | (52–77.1) | (49–81) | (49–85) | (52–77) | (52–74) | (49–85) | |
| BSA (m2) | 1.73 | 1.70 | 1.63 | 1.75 | 1.70 | 1.70 | 1.72 | 1.74 | 1.67 |
| (1.55–1.94) | (1.47–1.90) | (1.50–1.71) | (1.51–1.94) | (1.47–1.90) | (1.48–2.00) | (1.51–1.89) | (1.50–1.87) | (1.48–2.00) | |
P < 0.05, day 7 HomEM vs. HetEM. The values in parentheses indicate the data range. HomEM, homozygous extensive metabolizers; HetEM, heterozygous extensive metabolizers; PM, poor metabolizers; BSA, body surface area.
Population PK–PD analysis
In the PK analysis (Table 4), addition of the CYP2C19 genotype and the BSA of the subjects improved the fit compared with the base model, and these were therefore included in the final PK model. For the PK parameters, there was no difference among the different sampling days in subjects with the same CYP2C19 genotype (Table 4). The total clearance of rabeprazole in CYP2C19 PMs was significantly lower than that in CYP2C19 EMs on days 1, 4 and 7 of treatment (P < 0.05). In all treatment days, the average values of rabeprazole clearance were 10.7 and 16.8 l h−1 in PMs and all EMs, respectively. The clearance estimated in HomEM was not different from that in HetEM. The volume of distribution (Vd) and the absorption rate constant (ka) were comparable between patients with the different CYP2C19 genotypes. Accordingly, higher plasma rabeprazole concentrations were observed in CYP2C19 PMs than in EMs after either a single dose (i.e. day 1) or multiple doses (i.e. day 4 and day 7) of rabeprazole treatment.
Table 4.
Estimated pharmacokinetic parameters for the patients categorized by rabeprazole treatment day
| Day 1 | Day 4 | Day 7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HomEM | HetEM | All EM | PM | HomEM | HetEM | All EM | PM | HomEM | HetEM | All EM | PM | |
| CL (l h−1) | 16.8* | 14.8* | 15.8* | 9.75 | 18.6* | 17.7* | 18.0* | 12.6 | 17.8 | 15.7 | 16.7* | 9.87 |
| SD | 2.61 | 3.39 | 3.15 | 2.96 | 4.04 | 3.99 | 3.94 | 3.20 | 8.52 | 5.39 | 6.99 | 2.05 |
| K (1 h−1) | 0.72 | 0.63 | 0.68 | 0.50 | 0.69* | 0.72* | 0.71* | 0.53 | 0.75 | 0.67 | 0.71* | 0.49 |
| SD | 0.16 | 0.18 | 0.17 | 0.25 | 0.07 | 0.07 | 0.07 | 0.08 | 0.32 | 0.14 | 0.25 | 0.08 |
| V (l) | 24.7 | 25.1 | 22.7 | |||||||||
| SD | 10.1 | 4.71 | 3.82 | |||||||||
| Ka (1/h) | 2.26 | 1.91 | 3.55 | |||||||||
| SD | 1.76 | 0.88 | 6.40 | |||||||||
| Alag (h) | 1.33 | 1.71 | 1.44 | |||||||||
| SD | 0.27 | 0.86 | 0.30 | |||||||||
Significant difference (P < 0.05) from PM. HomEM, homozygous extensive metabolizers; HetEM, heterozygous extensive metabolizers; EM, extensive metabolizers; PM, poor metabolizers.
The baseline serum gastrin levels were 37.1 ± 35.4, 29.0 ± 22.9 and 20.8 ± 21.9 pg ml−1, respectively, for HomEM, HetEM and PM (P = 0.717). Since HomEM and HetEM did not differ in their PK properties, they are pooled as EM (compared with PM) for further PD analysis (Table 5). As a result, the maximal effect (Emax) was decreased and the EC50 increased after multiple doses of rabeprazole, indicating that a higher rabeprazole concentration may be required to achieve the same effect after multiple doses. The mean estimated keo value from the effect compartment also increased with multiple doses. It was noted that the keo value was lower in CYP2C19 PMs than in EMs on day 1, and higher than in EMs on day 4 of rabeprazole treatment. A higher keo value indicates a shorter delay before the rabeprazole-induced gastrin response. When the gastrin response of rabeprazole was predicted according to Equation 2 as a function of time, CYP2C19 PMs showed a better response than EMs on days 4 and 7, and the gastrin concentration increased after multiple doses of rabeprazole (Figure 1).
Table 5.
Estimated pharmacodynamic parameters for the patients categorized by rabeprazole treatment day
| Day 1 | Day 4 | Day 7 | ||||
|---|---|---|---|---|---|---|
| All EM | PM | All EM | PM | All EM | PM | |
| E0 | 42.3 | 40.3 | 42.6 | |||
| (pg ml−1) | (23.6) | (55.7) | (6.3) | |||
| Emax | 293.4‡ | 289.8§ | 252.5‡§ | |||
| (pg ml−1) | (104.0) | (1.8) | (3.5) | |||
| EC50 | 0.2†‡ | 56.0†§ | 34.8†§ | |||
| (ng ml−1) | (0.1) | (2.4) | (1.3) | |||
| Keo × 104 | 0.017*†‡ | 0.012* | 0.169*†§ | 0.804* | 7.975‡§ | 9.412 |
| (l min−1) | (0.001) | (0.002) | (0.019) | (0.104) | (18.667) | (16.055) |
| γ | 2.0003†‡ | 0.2603†§ | 0.4478‡§ | |||
| (0.0006) | (0.00001) | (0.0098) | ||||
P < 0.05 between all EMs and PMs on day 1 and day 4.
P < 0.05 between day 1 and day 4.
P < 0.05 between day 1 and day 7.
P < 0.05 between day 4 and day 7. The values in parentheses indicate the standard deviation. EM, extensive metabolizers; PM, poor metabolizers.
Figure 1.
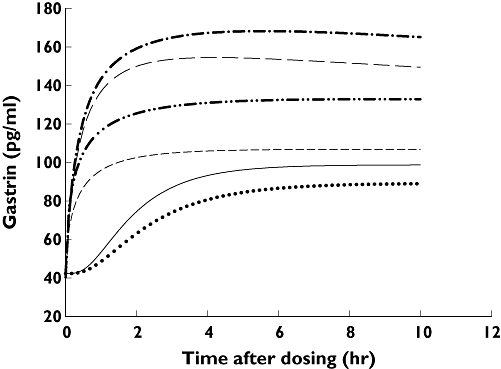
Changes in the plasma gastrin concentration in CYP2C19 poor metabolizers and all extensive metabolizers on different days of rabeprazole treatment. The plots were generated from the pharmacokinetic–pharmacodynamic parameters predicted by nonmem. Day-1 EM ( ); Day-1 PM (
); Day-1 PM ( ); Day-4 EM (
); Day-4 EM ( ); Day-4 PM (
); Day-4 PM ( ); Day-7 EM (
); Day-7 EM ( ); Day-7 PM (
); Day-7 PM ( )
)
Clinical outcome (H. pylori eradication)
Forty-seven subjects completed the evaluation of eradication. Of these,H. pylori infection was successfully eradiated in 36 (Table 6). The sensitivity of H. pylori to clarithromycin played an important role in the eradication rate. Subjects infected by clarithromycin-sensitive H. pylori had significantly higher eradication rates than those infected by clarithromycin-resistant H. pylori (83.3% vs. 20%,P = 0.008; all sampling days combined). There was no significant difference in eradication rate among the different treatment groups, even after stratification for clarithromycin sensitivity (Table 6). However, we do observe the decreasing P-value (P-value decrease from 0.491 to 0.108) for the testing of equal eradication rates among three treatment groups when we considered clarithromycin sensitivity only. It was also noted that H. pylori infection was successfully eradicated in all CYP2C19 PMs, independent of the regimen, except in one patient infected by a resistant strain. In contrast, the eradication rate ranged from 71 to 80% in HomEM and from 43 to 100% in HetEM, depending on the treatments. No significant difference was observed in HomEM and HetEM for H. pylori eradication in each group. Without stratification of HomEM and HetEM, the cure rate in all EMs ranged from 58 to 85%. Despite the higher cure rate in PMs, due to the limited number no statistically significant difference was observed between CYP2C19 PMs and EMs in each treatment group.
Table 6.
Effects of CYP2C19 genotype and clarithromycin resistance on the eradication rate
| EM † | PM | |||||
|---|---|---|---|---|---|---|
| Group | H. pylori eradication * | HomEM | HetEM | All EM | p-value (HomEM, HetEM, PM) | |
| A | S | 4 (0) | 6 (1) | 10 (1) | 2 (0) | 0.99 |
| F | 1 (1) | 2 (1) | 3 (2) | 1 (1) | ||
| B | S | 4 (0) | 3 (0) | 7 (0) | 4 (0) | 0.17 |
| F | 1 (0) | 4 (0) | 5 (0) | 0 (0) | ||
| C | S | 5 (0) | 6 (0) | 11 (0) | 2 (0) | 0.60 |
| F | 2 (1) | 0 (0) | 2 (1) | 0 (0) | ||
S, Success; F, Failure.
The values in parentheses indicate the number of subjects infected by clarithromycin-resistant H. pylori. HomEM, homozygous extensive metabolizers; HetEM, heterozygous extensive metabolizers; EM, extensive metabolizers; PM, poor metabolizers.
The incidence of adverse effects was 12.5, 12.5 and 25% in groups A, B and C, respectively (Table 7). Although adverse events in groups A and B were less frequent, the difference was not significant. The symptoms were mild and did not result in discontinuation of treatment. They included a bad taste, loose stools, dizziness, abdominal distress, and nausea. Drug compliance was 100% in groups A and B and 95% in group C.
Table 7.
Adverse effects in each treatment group
| Group | A (n = 16) | B (n = 16) | C (n = 16) |
|---|---|---|---|
| No. of adverse events | 2 | 2 | 8 |
| Bad taste | 2 | 1 | 4 |
| Loose stools/diarrhoea | 0 | 1 | 2 |
| Dizziness | 0 | 0 | 1 |
| Abdominal distress | 0 | 0 | 1 |
| Nausea | 0 | 0 | 1 |
| Cases with adverse effects (%) | 2 (12.5) | 2 (12.5) | 4 (25) |
Discussion
CYP2C19 is one of the major enzymes in PPI metabolism. The PK–PD properties of PPIs such as omeprazole, lansoprazole or pantoprazole are related to the CYP2C19 genotype [9]. It is now known that the efficacy of omeprazole-based H. pylori therapy is dependent on the CYP2C19 genotype. Omeprazole-based therapies with duration >7 days give significantly higher eradication rates in CYP2C19 PMs than in HomEM [26]. Other studies also found a median intragastric pH < 4 in CYP2C19 HomEM treated with multiple doses of omeprazole for >7 days [[9,27]. Rabeprazole is considered to be more active against H. pylori than most PPIs [[11–13]. The ability of rabeprazole to maintain the intragastric pH above 4 in CYP2C19 HomEM is better than that of omeprazole [28]. Although rabeprazole metabolism has been considered to be less affected by the CYP2C19 genotype, CYP2C19 PMs have a significantly higher plasma rabeprazole concentration and stronger inhibitory activity on acid secretion [[16,28–31]. Furthermore, the concentration of rabeprazole thioether, the major metabolite of rabeprazole with anti-H. pylori activity, is higher in CYP2C19 PM [[14,32].
In the present study, the population PK analysis showed that CYP2C19 PMs had a significant lower clearance than EMs (HomEM and HetEM), resulting in higher plasma rabeprazole levels in CYP2C19 PMs. However, there was no different in PK properties of rabeprazole between HomEM and HetEM. Since the pharmacological activity of PPIs is known to be dose-dependent [14], the higher plasma concentration might result in a better response. To confirm this, further PD analysis was performed using gastrin as a surrogate marker of the intragastric pH [33]. The results showed a correlation between plasma levels of rabeprazole and gastrin based on a direct link model. A greater and more rapid gastrin response was observed in CYP2C19 PMs on days 4 and 7 of rabeprazole treatment, reflecting stronger acid inhibition. In terms of clinical outcome, although the sample size might limit the power for statistical analysis and clarithromycin resistance had a great effect on H. pylori eradication, it was noted that H. pylori was successfully eradicated in all CYP2C19 PMs infected by clarithromycin-sensitive H. pylori regardless of the regimen they received. CYP2C19 PMs tended to have better treatment outcomes under both standard (i.e. 7 days) and short-term H. pylori therapies.
Short-term triple therapy is not regularly used for H. pylori treatment because of the inconsistency in the reported eradiation rates; however, this may be partly due to the failure to report the CYP2C19 genotypes of the subjects [[5–7,34–36]. The successful H. pylori eradication in CYP2C19 PMs seen in the present study suggests the possible use of short-term triple therapy in such patients. In addition, amoxicillin and clarithromycin used in triple therapy are unstable at a pH < 4 [37]. The faster and better control of the intragastric pH in CYP2C19 PMs suggests that short-term rabeprazole-based triple therapy may be useful in such patients. Our PK–PD analysis results also suggest a higher gastrin response in both CYP2C19 PMs and EMs after multiple doses of rabeprazole. This is in accordance with the potent and long-acting inhibition of gastric acid secretion by rabeprazole [38]. When the next dose is given, acid secretion is still inhibited by the previous dose and a relatively small proportion of the acid secretion inhibition is contributed by the next dose. This phenomenon is reflected by the increased EC50 and decreased Emax seen after multiple doses. Although CYP2C19 PMs tended to have a better response than EMs, the sufficiently high intragastric pH achieved in both groups after multiple doses of rabeprazole may partially minimize the difference in H. pylori eradication rate, as observed with therapies longer than 7 days [26].
Taken together, the PK–PD study showed a better response in CYP2C19 PMs after multiple dosing. Also, a tendency of better clinical outcome was observed in CYP2C19 PMs along with a decrease in the number of adverse events with short-term therapy. Thus, the CYP2C19 genotype plays a role in H. pylori eradication therapy, and short-term rabeprazole-based triple therapy may be applicable in CYP2C19 PMs for H. pylori eradication. The possibility of the use of short-term triple therapy in CYP2C19 PMs may provide further advantages, such as increased compliance, reduced cost and less drug resistance.
Competing interests
None to declare.
Supported by a grant from the National Taiwan University Hospital (NTUH. 93S060). The authors thank Mr Hong-Long Wang for his critical assistance with the statistical analysis.
REFERENCES
- 1.Moss SF, Sood S. Helicobacter pylori. Curr Opin Infect Dis. 2003;16:445–51. doi: 10.1097/00001432-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Am J Gastroenterol. 1998;93:2330–8. doi: 10.1111/j.1572-0241.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 3.Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 4.Calvet X, Gene E, Lopez T, Gisbert JP. What is the optimal length of proton pump inhibitor-based triple therapies for H. pylori? A cost-effectiveness analysis. Aliment Pharmacol Ther. 2001;15:1067–76. doi: 10.1046/j.1365-2036.2001.01031.x. [DOI] [PubMed] [Google Scholar]
- 5.Gisbert JP, Khorrami S, Calvet X, Pajares JM. Systematic review: rabeprazole-based therapies in Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003;17:751–64. doi: 10.1046/j.1365-2036.2003.01450.x. [DOI] [PubMed] [Google Scholar]
- 6.Vakil N, Schwartz HJ, Lanza FL, Nardi L, Hahne W, Barth J. A prospective, controlled, randomized trial of 3-, 7-, and 10-day rabeprazole-based triple therapy for H. pylori eradication in the USA. Gastroenterology. 2002;122(Suppl.)(4):A65. [Google Scholar]
- 7.Luth S, Teyssen S, Kolbel CB, Singer MV. 4-day triple therapy with rabeprazole, amoxicillin and clarithromycin in the eradication of Helicobacter pylori in patients with peptic ulcer disease – a pilot study. Z Gastroenterol. 2001;39:279–85. doi: 10.1055/s-2001-12873. [DOI] [PubMed] [Google Scholar]
- 8.Sachs G, Shin JM, Vagin O, Lambrecht N, Yakubov I, Munson K. The gastric H,K-ATPase as a drug target: past, present and future. J Clin Gastroenterol. 2007;41:S226–42. doi: 10.1097/MCG.0b013e31803233b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klotz U, Schwab M, Treiber G. CYP2C19 polymorphism and proton pump inhibitors. Basic Clin Pharmacol Toxicol. 2004;95:2–8. doi: 10.1111/j.1600-0773.2004.pto950102.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein JA, Ishizaki T, Chiba K, de Morais SM, Bell D, Krahn PM, Evans DA. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics. 1997;7:59–64. doi: 10.1097/00008571-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Besancon M, Simon A, Sachs G, Shin JM. Sites of reaction of the gastric H,K-ATPase with extracytoplasmic thio reagents. J Biol Chem. 1997;272:22438–46. doi: 10.1074/jbc.272.36.22438. [DOI] [PubMed] [Google Scholar]
- 12.Tsutsui N, Taneike I, Ohara T, Goshi S, Kojio S, Iwakura N, Matsumaru H, Wakisaka-Saito N, Zhang HM, Yamamoto T. A novel action of the proton pump inhibitor rabeprazole and its thioether derivative against the mobility of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:3069–73. doi: 10.1128/aac.44.11.3069-3073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuchiya M, Imamura L, Park JB, Kobashi K. Helicobacter pylori urease inhibition by rabeprazole, a proton pump inhibitor. Biol Pharm Bull. 1995;18:1053–6. doi: 10.1248/bpb.18.1053. [DOI] [PubMed] [Google Scholar]
- 14.Williams MP, Pounder RE. Review article: the pharmacology of rabeprazole. Aliment Pharmacol Ther. 1999;13(Suppl.)(3):3–10. doi: 10.1046/j.1365-2036.1999.00019.x. [DOI] [PubMed] [Google Scholar]
- 15.Inaba T, Mizuno M, Kawai K, Yokota K, Oguma K, Miyoshi M, Take S, Okada H, Tsuji T. Randomized open trial for comparison of proton pump inhibitors in triple therapy for Helicobacter pylori infection in relation to CYP2C19 genotype. J Gastroenterol Hepatol. 2002;17:748–53. doi: 10.1046/j.1440-1746.2002.02790.x. [DOI] [PubMed] [Google Scholar]
- 16.Horai Y, Kimura M, Furuie H, Matsuquma K, Irie S, Koga Y, Nagahama T, Murakami M, Matsui T, Yao T, Urae A, Ishizaki T. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther. 2001;15:793–803. doi: 10.1046/j.1365-2036.2001.00980.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin CJ, Yang JC, Uang YS, Chern HD, Wang TH. Time-dependent amplified pharmacokinetic and pharmacodynamic responses of rabeprazole in cytochrome P450 2C19 poor metabolizers. Pharmacotherapy. 2003;23:711–9. doi: 10.1592/phco.23.6.711.32177. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Yamade M, Ikuma M, Watanabe H, Ohashi K, Hishida A, Ishizaki T. Treatment strategy to eradicate Helicobacter pylori infection: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert Opin Pharmacother. 2007;8:2701–17. doi: 10.1517/14656566.8.16.2701. [DOI] [PubMed] [Google Scholar]
- 19.Yang JC, Yang KC, Hsu CT, Wang CS, Kuo CF, Wang TH. A multicenter study on eradication of Helicobacter pylori infection in patients with duodenal ulcer by lansoprazole-antibiotics combined therapy. J Microbiol Immunol Infect. 1999;32:1–8. [PubMed] [Google Scholar]
- 20.Sambrook J, Russell DW. Molecular Cloning: a Laboratory Manual. 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2001. pp. 6.8–6.10. [Google Scholar]
- 21.Morais SMF, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594–8. [PubMed] [Google Scholar]
- 22.Morais SMF, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–22. [PubMed] [Google Scholar]
- 23.Nakai H, Shimamura Y, Kanazawa T, Yasuda S, Kayano M. Determination of a new H+-K+ ATPase inhibitor (E3810) and its four metabolites in human plasma by high-performance liquid chromatography. J Chromatogr B. 1994;660:211–20. doi: 10.1016/0378-4347(94)00270-3. [DOI] [PubMed] [Google Scholar]
- 24.Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25:358–71. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- 25.Mosteller RD. Simplified calculation of body surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 26.Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH. The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta-analysis. Am J Gastroenterol. 2006;101:1467–75. doi: 10.1111/j.1572-0241.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 27.Sagar M, Tybring G, Dahl ML, Bertilsson L, Seensalu R. Effects of omeprazole on intragastric pH and plasma gastrin are dependent on the CYP2C19 polymorphism. Gastroenterology. 2000;119:670–6. doi: 10.1053/gast.2000.16515. [DOI] [PubMed] [Google Scholar]
- 28.Shirai N, Furuta T, Moriyama Y, Okochi H, Kobayashi K, Takashima M, Xiao F, Kosuge K, Nakagawa K, Hanai H, Chiba K, Ohashi K, Ishizaki T. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15:1929–37. doi: 10.1046/j.1365-2036.2001.01108.x. [DOI] [PubMed] [Google Scholar]
- 29.Ieiri I, Kishimoto Y, Okochi H, Momiyama K, Morita T, Kitano M, Morisawa T, Fukushima Y, Nakagawa K, Hasegawa J, Otsubo K, Ishizaki T. Comparison of the kinetic disposition of and serum gastrin change by lansoprazole versus rabeprazole during an 8-day dosing scheme in relation to CYP2C19 polymorphism. Eur J Clin Pharmacol. 2001;57:485–92. doi: 10.1007/s002280100342. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto M, Furuta T, Shirai N, Kajimura M, Hishida A, Sakurai M, Ohashi K, Ishizaki T. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2004;76:290–301. doi: 10.1016/j.clpt.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Sugimoto M, Furuta T, Shirai N, Nakamura A, Kajimura M, Hishida A, Ohashi K, Ishizaki T. Comparison of an increased dosage regimen of rabeprazole versus a concomitant dosage regimen of famotidine with rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotypes. Clin Pharmacol Ther. 2005;77:302–11. doi: 10.1016/j.clpt.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda S, Horai Y, Tomono Y, Nakai H, Yamato C, Manabe K, Kobayashi K, Chiba K, Ishizaki T. Comparison of the kinetic disposition and metabolism of E3810, a new proton pump inhibitor, and omeprazole in relation to S-mephenytoin 4′-hydroxylation status. Clin Pharmacol Ther. 1995;58:143–54. doi: 10.1016/0009-9236(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 33.Williams MP, Blanshard C, Millson C, Sercombe J, Pounder RE. A placebo-controlled study to assess the effects of 7-day dosing with 10, 20 and 40 mg rabeprazole on 24-h intragastric acidity and plasma gastrin in healthy male subjects. Aliment Pharmacol Ther. 2000;14:691–9. doi: 10.1046/j.1365-2036.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 34.Gambaro C, Bilardi C, Dulbecco P, Iiritano E, Zentilin P, Mansi C, Usai P, Vigneri S, Savarino V. Comparable Helicobacter pylori eradication rates obtained with 4-and 7-day rabeprazole-based triple therapy: a preliminary study. Dig Liver Dis. 2003;35:763–7. doi: 10.1016/s1590-8658(03)00458-4. [DOI] [PubMed] [Google Scholar]
- 35.Isomoto H, Furusu H, Morikawa T, Mizuta Y, Nishiyama T, Omagari K, Murase K, Inoue K, Murata I, Kohno S. 5-day vs. 7-day triple therapy with rabeprazole, clarithromycin and amoxicillin for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:1619–23. doi: 10.1046/j.1365-2036.2000.00892.x. [DOI] [PubMed] [Google Scholar]
- 36.Wong BC, Wong WM, Yee YK, Hung WK, Yip AWC, Szeto ML, Li KF, Lau P, Fung FMY, Tong TSM, Lai KC, Hu WHC, Yuen MF, Hui CK, Lam SK. Rabeprazole-based 3-day and 7-day triple therapy vs. omeprazole-based 7-day triple therapy for the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2001;15:1959–65. doi: 10.1046/j.1365-2036.2001.01118.x. [DOI] [PubMed] [Google Scholar]
- 37.Erah PO, Goddard AF, Barrett DA, Shaw PN, Spiller RC. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrob Chemother. 1997;39:5–12. doi: 10.1093/jac/39.1.5. [DOI] [PubMed] [Google Scholar]
- 38.Ohning GV, Barbuti RC, Kovacs TO, Sytnik B, Humphries TJ, Walsh JH. Rabeprazole produces rapid, potent, and long-acting inhibition of gastric acid secretion in subjects with Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:701–8. doi: 10.1046/j.1365-2036.2000.00774.x. [DOI] [PubMed] [Google Scholar]


