Abstract
AIMS
To examine the influence of a pharmaceutical care programme on disease control and health-related quality of life in Type 2 diabetes patients in the United Arab Emirates.
METHODS
A total of 240 Type 2 diabetes patients were recruited into a randomized, controlled, prospective clinical trial with a 12-month follow-up. A range of clinical measures, medication adherence and health-related quality of life (Short Form 36) were evaluated at baseline and up to 12 months. Intervention group patients received pharmaceutical care from a clinical pharmacist, whereas control group patients received their usual care from medical and nursing staff. The primary outcome measure was change in HbA1c. British National Formulary and Framingham scoring methods were used to estimate changes in 10-year coronary heart disease risk scores in all patients.
RESULTS
A total of 234 patients completed the study. Significant reductions (P < 0.001) in mean values (baseline vs. 12 months; 95% confidence interval) of HbA1c[8.5% (8.3, 8.7) vs. 6.9% (6.7, 7.1)], systolic [131.4 mmHg (128.1, 134.7) vs. 127.2 mmHg (124.4, 130.1)] and diastolic blood pressure [85.2 mmHg (83.5, 86.8) vs. 76.3 mmHg (74.9, 77.7)] were observed in the intervention group; no significant changes were noted in the control group. The mean Framingham risk prediction score in the intervention group was 10.56% (9.7, 11.4) at baseline; this decreased to 7.7% (6.9, 8.5) (P < 0.001) at 12 months but remained unchanged in the control group.
CONCLUSIONS
The pharmaceutical care programme resulted in better glycaemic control and reduced cardiovascular risk scores in Type 2 diabetes patients over a 12-month period.
Keywords: cardiovascular risk factors, glycosylated haemoglobin, pharmaceutical care, Type 2 diabetes mellitus
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Pharmaceutical care programmes delivered by pharmacists are known to improve quality of care for both ambulatory and hospitalized patients with a variety of chronic and acute conditions.
Reduction of HbA1c and normalization of blood pressure are key targets for diabetes care programmes, since they are key to reducing diabetes complications.
Good knowledge about disease, medications, diet and exercise requirements can improve the effectiveness of self-management of diabetes.
WHAT THIS STUDY ADDS
In a randomized, controlled clinical trial, a comprehensive pharmaceutical care programme (consisting of patient education and advice on medication adherence, metabolic control and life style) delivered by a clinical pharmacist over a 12-month period, significantly improved glycaemic control and health-related quality of life in Type 2 diabetes patients attending a military hospital outpatient clinic in the United Arab Emirates (UAE).
A significant reduction in HbA1c was important in the reduction of the 10-year coronary heart disease risk scores (by British National Formulary and Framingham methods) seen in patients who received the present care programme.
The outcomes of this study advocate an increased role for clinical pharmacists in the healthcare system in the UAE.
Introduction
Diabetes mellitus is a chronic metabolic disease that directly affects well being and poses a high morbidity risk [1]. Most patients with Type 2 diabetes have a combination of risk factors, including abdominal obesity, high triglycerides, low high-density lipoprotein-cholesterol (HDL-C) levels and hypertension [2,3]. The long-term vascular complications associated with Type 2 diabetes account for the majority of morbidity and mortality in patients [4]. The Hypertension in Diabetes Study Group revealed a sevenfold increase in risk of mortality in Type 2 diabetic patients with hypertension compared with non-diabetic, normotensive patients [5]. The co-existence of Type 2 diabetes and hypertension carries significantly increased risk of coronary heart disease (CHD) and renal disease [6].
Pharmaceutical care (PC) programmes developed and implemented by pharmacists have been found useful in improving the quality of care of both ambulatory and hospitalized patients with various diseases such as hypertension [7], asthma [8], dyslipidaemia [9], heart failure [10] and tuberculosis [11]. Considering the complications of Type 2 diabetes and its high prevalence, many disease management strategies have been developed and implemented in various clinical settings across the world. Together with hospital-based clinician-monitored programmes, pharmacist-led community/hospital-based PC programmes can be devised in an attempt to achieve better glycaemic, metabolic and blood pressure control in this patient group [12].
The latter involves working closely with the patient in designing, implementing and monitoring therapeutic plans to produce improved therapeutic outcomes through a reduction of medicine-related problems [13]. The primary means of improving disease management in diabetic patients are via prudent pharmacological therapy and through lifestyle changes, both of which require significant cooperation and participation from patients [14]. Concerning pharmacological therapy, the aims are to optimize the patient's medication and, second, to ensure that the patient is able and willing to adhere to the prescribed treatments [15]. Such adherence can be assisted by improved patient awareness of their disease state and also by the application of a self-monitoring programme [16]. The concept of PC delivery in Type 2 diabetes is well explored [[17–21]. However, many of these studies have limitations, such as small sample size [16], nonrandomized design [22] and lack of consideration of glycosylated haemoglobin (HbA1c) [23].
According to the Centre for Arab Genomic Studies, the United Arab Emirates (UAE) has a high and increasing prevalence of diabetes in its native population. A national survey conducted jointly by the World Health Organization and the UAE Ministry of Health between 1998 and 2000 indicated a 19.6% prevalence of diabetes mellitus among its nationals. Furthermore, it has been revealed by recent studies in the UAE that prevalence of diabetes is higher in people aged ≥60 years [24].
In the UAE, clinical pharmacy services are at an early stage of development. The overall aim of the present study was to investigate, via a randomized controlled clinical trial, the effect of a pharmacist-led PC programme on disease control and health-related quality of life in Type 2 diabetes patients within the UAE. Specific objectives of the study were
to measure the impact of the PC programme on the quality of care of Type 2 diabetic patients as measured by a range of clinical and humanistic outcomes. The primary outcome measure chosen was HbA1c (reduction) by the end of the 12-month study period
to evaluate the impact of PC (at 12 months' follow-up) on 10-year risk of CHD (intervention group vs. control group).
Methods
Study design
The study was a randomized, controlled, longitudinal, prospective clinical trial with a 12-month patient follow-up. The study protocol was approved by the Research Ethical Committee, Faculty of Medicine, Emirates University, UAE. The study site was Zayed Military Hospital, UAE, a 400-bed facility. Patients were recruited from the general medical wards and from endocrinology and medical outpatient clinics.
Sample size
A sample size calculation, based on published data on the variability of HbA1c in Type 2 diabetes patients [25], indicated that to detect an absolute difference of >1% in HbA1c, with α= 0.05 and a power of 0.90 (90%), a sample size of 104 patients in each of the control and intervention groups was required. Based on these data, to ensure sufficient statistical power and to account for ‘drop-outs’ during the study, a target sample size of 240 patients (120 control and 120 intervention) was selected. The target population was recruited over a period of approximately 1 year.
Study subjects
Patients with Type 2 diabetes mellitus who fulfilled the entrance criteria (i.e. confirmed diagnosis of Type 2 diabetes mellitus by a hospital consultant, receiving oral hypoglycaemic therapy, hospital consultant consented to patient entering trial, patient provided written informed consent to their participation in the research) and had no exclusion criteria present (i.e. secondary forms of hypertension, serum creatinine >184 mmol l−1, macroalbuminuria >300 mg 24 h−1, history of cerebrovascular accidents, convulsive disorder, diabetic proliferative retinopathy or diabetic autonomic neuropathy) were identified for inclusion in the study. Patients who were willing to participate were provided with additional written information and asked to sign the study consent form. If patients themselves were unable to sign the consent form, their next of kin or caregivers were asked to sign on their behalf.
After recruitment, patients were randomly assigned to one of two groups: intervention group or control group. The group allocations were carried out using restricted randomization [26] with both groups being matched as closely as possible for gender and presence of hypertension i.e. diastolic blood pressure ≥90 mmHg (hypertensive) or <90 mmHg (normotensive) [27].
Baseline measurements and assessments
After randomization, each patient was interviewed face-to-face (for approximately 20 min) by the research pharmacist and/or clinical pharmacy staff and a chart review undertaken to obtain details on demographics, family history of diabetes, medications being used, diabetes symptoms, frequency of daily home blood glucose monitoring, diabetes and medication knowledge, adherence to medication and lifestyle advice and to record baseline values of body weight and body mass index (BMI), fasting blood glucose, HbA1c, systolic and diastolic blood pressure, serum total cholesterol, serum creatinine, serum HDL-C, serum low-density lipoprotein-cholesterol (LDL-C) and serum triglycerides [9]. Patients were also asked to self-complete questionnaires on health-related quality of life [Short Form (SF) 36][28]. Arabic versions of the questionnaires were used when the patient was unable to read/understand English.
Pharmaceutical care interventions
For all patients randomized to the intervention group, the research pharmacist had discussions with their physicians regarding drug therapy and, if necessary, treatment modification was recommended, e.g. more intensive management of hypertension or simplification of dosage regimens if deemed appropriate [29], taking account of the latest American Diabetes Association (ADA) recommendations [30].
Patients who were randomized to the intervention group were educated on their illness and their medication in a structured fashion, including discussion on risk of diabetes complications, proper dosage, side-effects and storage of medications, healthy lifestyle and management of diabetes mellitus signs and symptoms through self-monitoring [31].
A printed leaflet to assist with the education programme was developed and the patient was given a copy to take home. Supplementary leaflets containing information about hypertension and hyperlipidaemia were also given to the patients if they suffered from these conditions. The educational advice was reinforced when patients came to the hospital pharmacy to collect their prescribed medicines on their monthly schedule.
In addition, behavioural modification aspects of the PC intervention involved advice on the following: self-monitoring of glycaemic control (patients were encouraged to monitor their blood glucose levels three times per day, to record these values and bring a record book to all subsequent appointments); physical exercise (this involved initiation of an exercise plan that could be incorporated into the patient's daily schedule, after taking into consideration their level of fitness, e.g. 1-h walk daily; diet (the patient was assisted with the identification of dietary behaviour that adversely influences blood glucose control, lipid levels, weight management, and of the times of day when the patient was most vulnerable to overeating, and given improved understanding of the relative effects of certain food choices on blood glucose control); medication adherence (patients were asked about any problems that they had encountered with regard to taking their medication and were offered education and practical help to encourage them to take the medicines prescribed for them by their physician); and smoking cessation (patients were encouraged to stop smoking by advising them about the danger of smoking to health, with emphasis on the increased dangers of smoking in diabetic patients).
The patients randomized to the control group received their normal care (from medical and nursing staff), but did not receive the clinical pharmacy service, i.e. did not receive pharmacist input into treatment plans or patient education; the patients did, however, receive advice on self-monitoring their blood glucose by medical or nursing staff.
Outcome measures
Both groups of patients were asked to return to the hospital outpatient clinic at the scheduled appointment intervals followed by the hospital (4-month intervals) to allow follow-up assessments. All patients (intervention and control group) were assessed as per initial baseline assessment at their scheduled clinic visits (4, 8 and 12 months) by pharmacy staff. Where subjective measures were used, i.e. adherence and health-related quality of life, a standard protocol for questionnaire administration was used to reduce potential bias. The latter outcome measures were only assessed at baseline and 12 months.
Scores for medication knowledge were obtained from answers given by patients when asked to name their prescribed diabetes medicines, the daily dosage, the strength and purpose of each medicine and any significant adverse effects that could result from each medicine. Each correct answer was awarded one mark, with no marks awarded if the patient did not know or gave an incorrect answer. A percentage score was calculated by adding all the marks together, dividing by the maximum possible score and multiplying by 100. Medication knowledge was graded as good (when scored ≥50%) or poor (when scored <50%) [10].
Regarding self-reported adherence to medication, those patients who reported forgetting doses, intentionally missing or taking extra doses were classed as non-adherent [10]. No account was taken of intelligent non-adherence, i.e. when a patient decides for good reason, for example, to take an extra dose or miss a dose. Adherence to lifestyle advice (diet, exercise, smoking, alcohol intake) was scored. Each positive parameter answer was awarded one mark, with no marks awarded if the patient had not made the suggested lifestyle adjustment. A percentage score was calculated by adding all the marks together, dividing by the maximum possible individualized score and multiplying by 100. Adherence with lifestyle adjustment was graded as poor adherence (if score was <75%) or adherence (if the score was ≥75%).
Furthermore, a 10-year risk assessment was carried out for all patients using British National Formulary (BNF) prediction charts [32] and the Framingham scoring method [[33,34] at baseline and at the end of the study period. These methodologies take account of age, gender, smoking status, total cholesterol, HDL and systolic blood pressure.
Data analysis
Statistical analyses were performed using the SPSS package v. 13 (SPSS Inc., Chicago, IL, USA). A per protocol approach was used. Data were summarized as (means ± SD), geometric means or mean differences with 95% confidence intervals (CI). Area under the curve values (AUC) were used as a summary measure to compare parameters, which were assessed at 4, 8 and 12 months. A P-value of <0.05 was considered statistically significant. Two-sample comparisons were made using Student's t-tests for normally distributed variables or Mann–Whitney U-tests for non-normally distributed data (0 and 12 months). Comparisons of proportions were carried out using χ2, Fisher's exact or McNemar's tests.
Results
A total of 240 patients were recruited in the study. Out of 120 patients recruited to each group, 117 completed the study, i.e. three patients dropped out in each group (four patients left the UAE to go to their home country and two patients left their military work and were no longer eligible to receive military hospital services). Figure 1 illustrates the flow of patients through the study and describes various stages at which data were collected. The age, gender, duration of diabetes and family history of diabetes for the two groups are presented in Table 1. Statistical analyses indicated that the groups were well matched (P > 0.05 in all cases). Table 2 indicates medications used for diabetes and other concomitant diseases such as hypertension and or hyperlipidaemia in the study population.
Figure 1.
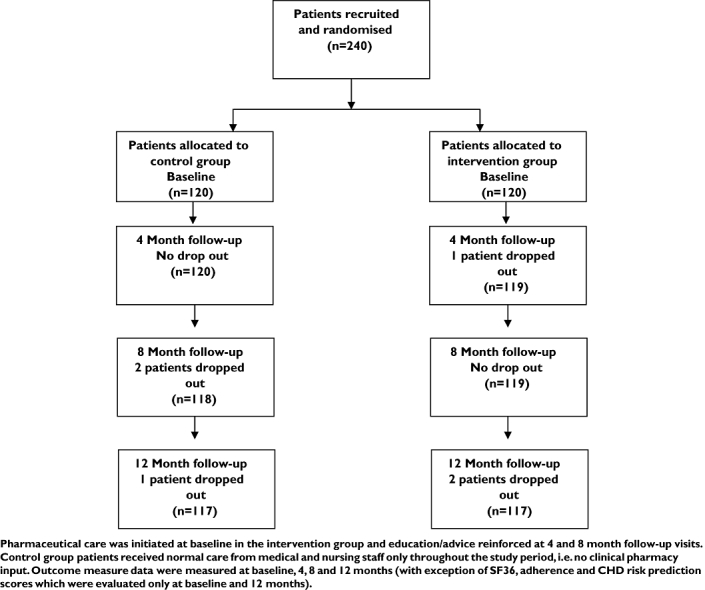
Flow chart relating to various phases of randomized controlled clinical trial
Table 1.
Baseline demographics of the study participants
| Intervention | Control group | ||
|---|---|---|---|
| Variable | group (n = 120) | (n = 120) | |
| Gender | Male | 84 (70%) | 82 (68.3%) |
| Female | 36 (30%) | 38 (31.7%) | |
| Mean age (years) | 48.7 ± 8.2 | 49.9 ± 8.3 | |
| Age group (years) | 35–50 | 74 (61.7%) | 70 (58.3%) |
| 51–65 | 43 (35.8%) | 47 (39.2%) | |
| >65 | 3 (2.5%) | 3 (2.5%) | |
| Duration of diabetes (years) | 6.1 ± 2.9 | 6.2 ± 2.7 | |
| Family history of diabetes | Yes | 53 (44.2%) | 45 (37.5%) |
| No | 67 (55.8%) | 75 (62.5%) |
Values are given as geometric mean (percent of total participants of intervention/control group) or geometric mean ± standard deviation.
Table 2.
Medications used for diabetes and other concomitant diseases such as hypertension and/or hyperlipidaemia in the study population
| Medicine used (daily doses) | No. of patients | % Patients | |
|---|---|---|---|
| Oral hypoglycaemic drugs | Glibenclamide (5 mg) | 115 | 47.9 |
| Gliclazide (80 mg) | 112 | 46.6 | |
| Metformin (500 mg) | 159 | 66.3 | |
| Rosiglitazone (4 mg) | 12 | 5.0 | |
| ACE inhibitors | Lisinopril (10 mg), Perindopril (4 mg), Enalapril (10 mg), Captopril (25 mg) | 74 | 30.8 |
| Angiotensin IIA antagonist | Valsartan (80 mg) | 7 | 2.9 |
| Others Antihypertensive drugs | Calcium channel blocker, (Amlodipine 5 mg, Nifedipine 10 mg); Diuretics (Indapamide, 2.5 mg); β-blocker (Atenolol, 100 mg) | 42 | 17.5 |
| Statins | Simvastatin, Pravastatin, Atorvastatin, Fluvastatin | 71 | 29.6 |
| Analgesics or nonsteroidal anti-inflammatory drugs | 14 | 20.9 | |
| Herbal products or cough syrups | 22 | 32.8 | |
| OTC drugs | Antacids or laxatives | 2 | 3 |
| Vitamins | 29 | 43.3 | |
| Total patients taking OTC drugs | 67 | 27.9 |
Clinical outcome measures
No intervention approach or data collection instrument presented any major difficulties during the study. Changes in key clinical variables over 12 months in the two groups are shown in Table 3, including statistically significant changes in the area under the curve (AUC; summary measure) for the outcomes measured at 0, 4, 8 and 12 months. BMI was used to categorize patients into normal BMI (<25 kg m−2), overweight (25–30 kg m−2) and obese (>30 kg m−2). Data over the study period indicated that the number of the intervention and control group patients who were placed in these three categories did not vary over time (P > 0.05). Mean BMI decreased significantly in the intervention group over time (baseline vs. 12-month values; P < 0.005; Table 3), with no corresponding change in the control group (P > 0.05).
Table 3.
Values of clinical parameters at various time intervals in intervention and control groups
| Baseline | 4 months | 8 months | 12 months | AUC values for IG | AUC values for CG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical parameter | IG | CG | IG | CG | IG | CG | IG | CG | for IG | foir CG | P-value * | P-value ** | P-value *** |
| BMI (kg m−2) | 28.34 | 27.98 | – | – | – | – | 27.29 | 27.99 | – | – | 0.545 | 0.004 | – |
| (27.55, 29.13) | (27.09, 28.86) | (26.57, 28.02) | (27.15, 28.83) | ||||||||||
| Fasting blood glucose (mmol l−1) | 10.83 | 10.26 | 8.21 | 10.05 | 7.91 | 9.89 | 7.78 | 9.48 | 100.55 | 120.63 | 0.113 | <0.001 | <0.001 |
| (10.28, 11.38) | (9.82, 10.70) | (7.88, 8.53) | (9.63, 10.47) | (7.65, 8.17) | (9.44, 10.34) | (7.50, 8.06) | (9.04, 9.91) | (97.96, 103.14) | (116.84, 124.43) | ||||
| HbA1c (%) | 8.5 | 8.4 | 7.6 | 8.0 | 7.1 | 8.4 | 6.9 | 8.3 | 88.6 | 101.3 | 0.405 | <0.001 | 0.003 |
| (8.3, 8.7) | (8.2, 8.6) | (7.4, 7.8) | (7.8, 8.2) | (6.9, 7.2) | (8.2, 8.6) | (6.7, 7.1) | (8.1, 8.5) | (87.0, 90.2) | (98.8, 103.8) | ||||
| Systolic blood pressure (mmHg) | 131.4 | 132.6 | 124.0 | 130.7 | 126.8 | 132.2 | 127.2 | 132.1 | 1485.9 | 1563.7 | 0.638 | <0.001 | 0.028 |
| (128.1, 134.7) | (129.0, 136.2) | (121.4, 126.6) | (127.5, 133.9) | (124.4, 129.3) | (129.6, 135.3) | (124.4, 130.1) | (130.8, 135.1) | (1436.9, 1534.9) | (1510.7, 1616.7) | ||||
| Diastolic blood pressure (mmHg) | 85.2 | 83.9 | 78.4 | 83.7 | 77.4 | 82.1 | 76.3 | 84.1 | 922.2 | 1000.1 | 0.338 | <0.001 | <0.001 |
| (83.5, 86.8) | (82.0, 85.8) | (77.2, 79.6) | (82.0, 85.5) | (76.3, 78.6) | (80.3, 84.0) | (74.9, 77.7) | (82.4, 85.8) | (893.3, 951.1) | (987.7, 1015.5) | ||||
| Serum total cholesterol (mmol l−1) | 5.26 | 5.27 | 4.65 | 5.33 | 4.49 | 5.24 | 4.47 | 5.32 | 55.98 | 63.51 | 0.937 | <0.001 | <0.001 |
| (5.06, 5.45) | (5.07, 5.47) | (4.51, 4.78) | (5.11, 5.55) | (4.35, 4.62) | (5.05, 5.44) | (4.33, 4.61) | (5.12, 5.52) | (54.72, 57.24) | (61.65, 65.37) | ||||
| Serum HDL-C (mmol l−1) | 1.20 | 1.19 | 1.30 | 1.21 | 1.29 | 1.17 | 1.32 | 1.20 | 15.46 | 14.29 | 0.652 | <0.01 | 0.002 |
| (1.16, 1.25) | (1.13, 1.24) | (1.24, 1.37) | (1.16, 1.26) | (1.25, 1.35) | (1.12, 1.23) | (1.27, 1.38) | (1.14, 1.25) | (14.92, 16.00) | (13.78, 14.81) | ||||
| Serum LDL-C (mmol l−1) | 3.55 | 3.48 | 3.11 | 3.51 | 3.08 | 3.59 | 3.04 | 3.61 | 37.84 | 42.66 | 0.531 | <0.001 | <0.001 |
| (3.37, 3.74) | (3.31, 3.64) | (2.99, 3.24) | (3.33, 3.69) | (2.94, 3.21) | (3.42, 3.75) | (2.92, 3.16) | (3.44, 3.78) | (36.68, 39.01) | (41.08, 44.23) | ||||
| Serum triglycerides (mmol l−1) | 1.60 | 1.55 | 1.34 | 1.58 | 1.26 | 1.61 | 1.25 | 1.74 | 15.88 | 19.53 | 0.616 | <0.001 | <0.001 |
| (1.46, 1.74) | (1.43, 1.67) | (1.24, 1.44) | (1.46, 1.71) | (1.18, 1.35) | (1.46, 1.76) | (1.17, 1.33) | (1.61, 1.87) | (14.96, 16.80) | (18.25, 20.81) |
P-values
when baseline values of IG and CG are compared
when 12-month values of IG and CG are compared
when AUCs for IG and CG are compared.
Data are geometric mean (CI). AUC, area under the curve; CG, control group; IG, intervention group; BMI, body mass index; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol.
Diabetes knowledge and medication adherence
The assessment of medication knowledge is an important outcome measure in evaluating the effectiveness of diabetes education programmes [35]. The analysis of data showed that 60.8% (n = 73) of intervention group patients and 64.2% (n = 77) of control group patients had poor medication knowledge at baseline. However, at 12 months, 47% (55 out of 117) of the intervention group patients had poor knowledge compared with 64.1% (75 out of 117) in the control group, indicating a positive impact on medication knowledge of intervention group patients.
Non-adherence (self-reported) with prescribed medications was 48.3% in the intervention group at baseline and 49.1% in the control group. At the 12-month assessment, these values were reduced to 21.4 and 32.5%, respectively.
χ2 analyses were used to compare self-reported adherence with the lifestyle adjustments (1-h walk daily, carbohydrate-restricted diet and cessation of smoking and alcohol) between the intervention group and control group patients at baseline and at 12 months. At baseline the number of intervention group and control group patients who were deemed to be adherent (≥75% score) with recommended lifestyle adjustments was approximately the same (85 vs. 81, respectively; P > 0.05). At the 12-month assessment, an increase in the number of intervention group patients who reported adherence (n = 95) was observed, whereas there was the opposite effect in the control group (n = 75). Overall medication knowledge, medication adherence and lifestyle adherence were significantly higher at the 12-month assessment in the intervention patients when compared with control group patients (P < 0.05).
Ten-year CHD risk using BNF and Framingham prediction scores
The mean (CI) Framingham prediction scores were 10.6 (9.7, 11.4) at baseline for the intervention group and 11.4 (10.6, 12.2) for the control group. At the 12-month assessment the value decreased to 7.7 (6.9, 8.5; P < 0.001) in the intervention group but remained unchanged at 11.5 (10.5, 12.3) in the control group (P > 0.05). The BNF risk prediction indicated a marked increase in the number of patients at low risk (63.3–85.5%) in the intervention group at 12 months (Table 5). Patients at moderate risk also decreased from 36.7 to 13.7% in the intervention group over the study period. Correspondingly, there was a slight reduction in the number of patients in the low-risk group from 65.0 to 59.0% and a slight increase in the moderate-risk group from 31.7 to 37.6% in the control group patients. A similar approach in CHD risk score comparison at baseline and over a period of 12 months to assess the effect of a PC programme in Type 2 diabetes patients has been reported by the Australian Fremantle Diabetes Study [36].
Table 5.
Classification and comparison of patients for 10-year coronary heart disease (CHD) risk scores calculated by British National Formulary (BNF) and Framingham methods
| BNF 10-year CHD risk prediction method | ||||||
|---|---|---|---|---|---|---|
| No. of patients in different risk categories at baseline | No. of patients in different risk categories at 12 months | |||||
| <15% | 15–30% | >30% | <15% | 15–30% | >30% | |
| Group | (mild) | (moderate) | (severe) | (mild) | (moderate) | (severe) |
| Intervention (n (%)) | 76 (63.3%) | 44 (36.7%) | 0 | 100 (85.5%) | 16 (13.7%) | 1 (0.9%) |
| Control (n (%)) | 78 (65.0%) | 38 (31.7%) | 4 (3.3%) | 69 (59.0%) | 44 (37.6%) | 4 (3.4%) |
| P-value * | 0.125 | <0.001 | ||||
| Framingham 10-year CHD risk prediction method | ||||||
|---|---|---|---|---|---|---|
| No. of patients in different risk categories at baseline | No. of patients in different risk categories at 12 months | |||||
| <10% | 10–20% | >20% | <10% | 10–20% | >20% | |
| (mild) | (moderate) | (severe) | (mild) | (moderate) | (severe) | |
| Intervention (n (%)) | 58 (48.3%) | 60 (50%) | 2 (1.7%) | 80 (68.4%) | 37 (31.6%) | 0 |
| Control (n (%)) | 48 (40%) | 71 (59.2%) | 1 (0.8%) | 43 (36.8%) | 74 (63.2%) | 0 |
| P-value * | 0.392 | <0.001 | ||||
When comparing control and intervention groups.
Discussion
The present study was designed to measure the impact of a PC programme on a wide range of clinical and humanistic outcomes related to the different aspects of healthcare in patients with Type 2 diabetes mellitus. The broad range of data in the present study allowed comprehensive assessment of the potential benefits of the intervention. Enhanced patient outcomes were noted in the intervention group, e.g. a reduction in HbA1c and fasting blood glucose levels, improvement in health-related quality of life as measured by SF36, decreased systolic and diastolic blood pressure, improved adherence to the prescribed medication and lifestyle advice and a reduction in CHD risk factors. All intervention approaches (e.g. diabetic education booklet) and data collection instruments were without any major difficulties during the research study.
Main findings of the study
An important outcome of the study was a significant reduction in HbA1c levels in intervention group patients. Although decreases in HbA1c in this study were better than earlier data reported by Berringer et al. [37], the decrease in mean fasting blood glucose in the intervention group did not reach the ADA target goal (5–7.2 mmol l−1). The Diabetes Control and Complications Trial Research Group (DCCT) study reported a 1.90% reduction in HbA1c levels [38] in the intervention group compared with 1.66% in the present study; however, the DCCT study involved younger patients with Type 1 diabetes.
Based on well-established epidemiological data [39], the improvements achieved in HbA1c values, if maintained, have the potential for major health gains in Type 2 diabetic patients. The impact achieved in the cohort of patients in this UAE study was comparable to improvements achieved by other researchers, using a range of intervention approaches. In the present research an important finding was that more patients in the intervention group (45.4%) than in the control group (30.3%) achieved the ADA target goal for HbA1c of <7% at the 12-month assessment (P < 0.0213). Corresponding data from the McWhorter and Oderda [40] study, conducted in a Utah community health centre, indicated that 38.4% of patients in the intervention group and 27.7% in the control group achieved the ADA target over 6 months (P < 0.0412).
The improvements in HbA1c in the present study were probably due to improved adherence to prescribed medication and lifestyle modifications [15]. The Australian Fremantle Diabetes Study [36] showed that pharmacist participation in patient care can significantly improve HbA1c independent of pharmacotherapeutic changes. This latter community-based study involved 180 Type 2 diabetic patients whose HbA1c was decreased by a mean of 0.5% over 12 months from a baseline of 7.5%, whereas there was no change in the control group. Another long-term study [22] showed a reduction in mean HbA1c levels from 7.5 to 7.1% over 4 years. Taken together with the results of the present study, it is clear that PC can result in significant patient benefit in a range of environments over a range of intervention durations.
A positive impact on blood glucose levels was seen in the present study. Earlier work by Berringer et al. [37] evaluated the effects of a PC model on outcomes of self-monitored blood glucose (SMBG), SMBG frequency, and medication adherence rates for patients with diabetes at two independent community pharmacies in Richmond (USA). In the first setting, average morning blood glucose values (n= 27) decreased from 9.9 to 8.8 mmol l−1, from baseline to 6 months, respectively (P= 0.07). In the second setting, blood glucose values (n= 23) decreased from 9.94 to 8.32 mmol l−1 from baseline and 12 months (P < 0.05). There was no statistical difference in SMBG frequency. The mean fasting blood glucose level in the present study decreased dramatically from 10.8 to 7.8 mmol l−1 in the intervention patients, whereas it decreased from 10.3 to 9.5 mmol l−1 in the control patients. The change in fasting blood glucose level in the control group was 0.8 mmol l−1 and 3.0 mmol l−1 in the intervention group at the 12-month assessment, i.e. exceeded the drop reported by Berringer et al. [37].
Irons et al. reported a retrospective cohort analysis of the clinical effectiveness of a physician–pharmacist collaborative drug therapy management diabetes programme in which they concluded that pharmacist-managed diabetes care was effective in improving glycaemic control and was not associated with an increased risk of hypoglycaemic events or unscheduled diabetes-related clinic visits [41].
The research data (Table 3) revealed that there were significant differences over time between the two groups with regard to blood pressure control (systolic and diastolic). There was no evidence that the improvements in the intervention group were due to improved prescribing, since patients in both groups were prescribed a similar range of effective antihypertensive medication. These outcomes were therefore probably due to better adherence to medications and lifestyle advice [42–47]. In the present research an important finding was that more patients in the intervention group (33.6%) than in the control group (25.4%) achieved target blood pressure (systolic and diastolic) values < 130/80 as recommended in hypertension guidelines [3,48]. These UAE results were an improvement on those reported by McFarlane et al., where a target blood pressure of <130/80 mmHg was achieved in 28% of their study patients [49]. Improved adherence to medication and lifestyle advice was also likely to be the main factor in improving lipid profiles.
The improvement in health-related quality of life may in part be attributed to the increased contact of diabetic patients with the clinical pharmacist, but is also likely to be associated with improved adherence to lifestyle advice. The results of the present study lend support to the use of the SF36 as an outcome for evaluating health education programmes in patients with diabetes, as suggested by Brown et al. [50]. The results of the present study indicate that PC interventions can have a positive impact on how diabetes patients are able to cope with daily activities [36,51,52].
Good knowledge about medications, diet, exercise, self-monitoring of blood glucose and treatment modifications is necessary in the effective self-management of diabetes [53]. However, knowledge alone does not guarantee requisite behaviour modifications or effective self-management. The assessment of diabetes-related knowledge is an important outcome measure in diabetes education programmes [54–56]. The present results indicate that the intervention group patients achieved greater medication knowledge during the 12-month assessment period. A study by McWhorter et al. [57] has confirmed that more patients who were provided care, such as education about their disease and medication by the pharmacist, reached the ADA HbA1c target goal of <7%.
Researchers have estimated that, in general, the rate of non-adherence to prescribed medications ranges from 25 to 50% [58]. Non-adherence has been implicated as a major cause of unnecessary hospitalization of patients with diabetes or unscheduled diabetes-related clinic visits [41]. Patient counselling and education are essential for improving outcomes, including patient adherence [52]. The intensive education by the clinical pharmacist in the present study improved adherence (self-reported) at the 12-month assessment period.
Epidemiological analysis (UKPDS) links a 0.5% HbA1c reduction to an estimated 7% reduction in the risk of myocardial infarction and an estimated 12% reduction in risk of stroke [38]. The outcomes of the 10-year CHD risk predictions using BNF and Framingham prediction scores showed a clear positive impact of the present intervention in reducing the risk of CHD in intervention group patients. The present study has paved the way towards integration of clinical pharmacy services into overall healthcare delivery to Type 2 diabetes mellitus patients in the UAE.
Conclusions
The present study has clearly demonstrated the value of comprehensive PC provision to this patient group and provides evidence for negotiation with healthcare managers in the UAE regarding increased pharmacy staffing levels within the hospital service. The main strengths of the study are the diverse outcome measures used and the low drop-out rate. However, it covered a period of only 12 months. Furthermore, consideration must be given to the differences that can occur between a statistically significant difference and a clinically important difference. There is a need therefore to conduct longer studies to see if these improvements can be sustained and true clinical benefits demonstrated. Furthermore, the study was carried out in a UAE military hospital, where adherence to advice may be high; generalizability of the intervention methodology needs to be tested in other clinical settings to include evaluation of the economic impact of the service.
Competing interests
None declared.
The authors thank Dr Gordon Cran (Department of Epidemiology and Public Health, Queen's University Belfast, UK) for his statistical support.
REFERENCES
- 1.Koopmanschap M. Coping with Type II diabetes: the patient's perspective. Diabetologia. 2002;45:S18–22. doi: 10.1007/s00125-002-0861-2. [DOI] [PubMed] [Google Scholar]
- 2.Abuissa H, Bel DS, O’Keefe JH., Jr Strategies to prevent type 2 diabetes. Curr Med Res Opin. 2005;21:1107–14. doi: 10.1185/030079905X50606. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL., Jr Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 4.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–19. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Hypertension in Diabetes Study Group. Hypertension in Diabetes Study (HDS): II. Increased risk of cardiovascular complications in hypertensive type 2 diabetic patients. J Hypertens. 1993;11:319–25. doi: 10.1097/00004872-199303000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Viberti G, Wheeldon NM. Microalbuminuria reduction with Valsartan in patients with type 2 diabetes mellitus. A blood pressure-independent effect (MARVAL) Circulation. 2002;106:672–8. doi: 10.1161/01.cir.0000024416.33113.0a. [DOI] [PubMed] [Google Scholar]
- 7.Garcao JA, Cabrita J. Evaluation of a pharmaceutical care program for hypertensive patients in rural Portugal. J Am Pharm Assoc (Wash) 2002;42:858–64. doi: 10.1331/108658002762063691. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Martin G, Joo I, Sanchez I. Evaluation of the impact of a pharmaceutical care program in children with asthma. Patient Educ Couns. 2003;49:13–18. doi: 10.1016/s0738-3991(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 9.Paulos CP, Nygren CE, Celedon C, Carcama CA. Impact of a pharmaceutical care program in a community pharmacy on patients with dyslipidemia. Ann Pharmacother. 2005;39:939–43. doi: 10.1345/aph.1E347. [DOI] [PubMed] [Google Scholar]
- 10.Sadik A, Yousif M, McElnay JC. Pharmaceutical care of patients with heart failure. Br J Clin Pharmacol. 2005;60:183–93. doi: 10.1111/j.1365-2125.2005.02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark PM, Karagoz T, Apikoglu-Rabus S, Izzettin FV. Effect of pharmacist-led patient education on adherence to tuberculosis treatment. Am J Health Syst Pharm. 2007;64:497–505. doi: 10.2146/ajhp050543. [DOI] [PubMed] [Google Scholar]
- 12.Wermeille J, Bennie M, Brown I, McKnight J. Pharmaceutical care model for patients with type 2 diabetes: integration of the community pharmacist into the diabetes team – a pilot study. Pharm World Sci. 2004;26:18–25. doi: 10.1023/b:phar.0000013465.24857.a8. [DOI] [PubMed] [Google Scholar]
- 13.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes. Am J Med. 2005;118:27S–34S. doi: 10.1016/j.amjmed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 14.McClean MT, McElnay JC, Andrews J. The importance of patient education and patient involvement in the treatment of diabetes. Pharm J. 2000;265:108–10. [Google Scholar]
- 15.Krass I, Susan J, Carlene ST, Armour CL. Impact on medication use and adherence of Australian pharmacists’ diabetes care services. J Am Pharm Assoc. 2005;45:33–40. doi: 10.1331/1544345052843093. [DOI] [PubMed] [Google Scholar]
- 16.Lerman I. Adherence to treatment: the key for avoiding long-term complications of diabetes. Arch Med Res. 2005;36:300–6. doi: 10.1016/j.arcmed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Coast-Senior EA, Kroner BA, Kelley CL, Trilli LE. Management of patients with type 2 diabetes by pharmacists in primary care clinics. Ann Pharmacother. 1998;32:636–41. doi: 10.1345/aph.17095. [DOI] [PubMed] [Google Scholar]
- 18.Cioffi ST, Caron MF, Kalus JS, Hill P, Buckley TE. Glycosylated hemoglobin, cardiovascular, and renal outcomes in a pharmacist-managed clinic. Ann Pharmacother. 2004;38:771–5. doi: 10.1345/aph.1D359. [DOI] [PubMed] [Google Scholar]
- 19.Choe HM, Mitrovich S, Dubay D, Hayward RA, Krein SL, Vijan S. Proactive case management of high-risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial. Am J Manag Care. 2005;11:253–60. [PubMed] [Google Scholar]
- 20.Suppapitiporn S, Chindavijak B, Onsanit S. Effect of diabetes drug counselling by pharmacist, diabetic disease booklet and special medication containers on glycemic control of type 2 diabetes mellitus: a randomized controlled trial. J Med Assoc Thai. 2005;88:S134–41. [PubMed] [Google Scholar]
- 21.Brooks AD, Rihani RS, Derus CL. Pharmacist membership in a medical group's diabetes health management program. Am J Health Syst Pharm. 2007;64:617–21. doi: 10.2146/ajhp060095. [DOI] [PubMed] [Google Scholar]
- 22.Cranor CW, Bunting BA, Christensen DB. The Asheville project: long-term clinical and economic outcomes of a community pharmacy diabetes care program. J Am Pharm Assoc. 2003;43:173–84. doi: 10.1331/108658003321480713. [DOI] [PubMed] [Google Scholar]
- 23.Baran RW, Crumlish K, Patterson H, Shaw J, Erwin WG, Wylie JD, Duong P. Improving outcomes of community dwelling older patients with diabetes through pharmacist counselling. Am J Health Syst Pharm. 1999;56:1535–9. doi: 10.1093/ajhp/56.15.1535. [DOI] [PubMed] [Google Scholar]
- 24.Taher ES. Diabetes in the United Arab Emirates and other Arab countries: need for epidemiological and genetic studies. 2004. p. 75. Genetic disorders in the Arab world: UAE.
- 25.Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. The Diabetes care from diagnosis research team. BMJ. 1998;317:1202–8. doi: 10.1136/bmj.317.7167.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gore S. Assessing clinical trials – restricted randomisation. BMJ (Clin Res Ed) 1981;282:2114–17. doi: 10.1136/bmj.282.6282.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO/ISH Guidelines subcommittee: World Health Organisation. International Society of Hypertension; guidelines for the management of hypertension. J Hypertens. 1999;17:151–83. [PubMed] [Google Scholar]
- 28.Brown GC, Brown MM, Sharma S. Quality of life associated with diabetes mellitus in an adult population. J Diabet Complications. 2000;14:18–24. doi: 10.1016/s1056-8727(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharyya A. Treatment of type 2 diabetes mellitus. Hospital Pharmacist. 2001;8:10–16. [Google Scholar]
- 30.American Diabetes Association. Standards of medical care in diabetes [consensus Statement. Diabetes Care. 2005;28:S4–36. [PubMed] [Google Scholar]
- 31.American Diabetes Association. Self-monitoring of blood glucose [consensus Statement. Diabetes Care. 1996;19:S62–6. [Google Scholar]
- 32.Wood DA, De Backer G, Faergeman O. Prevention of coronary heart disease in clinical practice. Recommendations of the second joint task force of the European Society of Cardiology, European Atherosclerosis Society and European Society of Hypertension. Eur Heart J. 1998;19:1434–503. [Google Scholar]
- 33.Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) Expert Panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 34.Brindle P, Emberson J, Lampe F. Predictive accuracy of the Framingham coronary risk score in British men: prospective cohort study. BMJ. 2003;327:1267–70. doi: 10.1136/bmj.327.7426.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedges LV, Brown SA. Predicting metabolic control in diabetes: a pilot study using meta-analysis to estimate a linear model. Nurs Res. 1994;43:362–8. [PubMed] [Google Scholar]
- 36.Clifford RM, Davis WA, Batty KT, Davis TM. Effect of a pharmaceutical care program on vascular risk factors in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2005;28:771–6. doi: 10.2337/diacare.28.4.771. [DOI] [PubMed] [Google Scholar]
- 37.Berringer R, Shibley MC, Cary CC, Pugh CB, Powers PAG, Rafi JA. Outcomes of a community pharmacy-based diabetes monitoring program. J Am Pharm Assoc. 1999;39:791–7. [PubMed] [Google Scholar]
- 38.Diabetes Control and Complications Trial Research Group (DCCT) The effects of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 39.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 40.McWhorter LS, Oderda GM. Providing diabetes education and care to underserved patients in a collaborative practice at a Utah Community Health Center. Pharmacotherapy. 2005;25:96–109. doi: 10.1592/phco.25.1.96.55623. [DOI] [PubMed] [Google Scholar]
- 41.Irons BK, Lenz RJ, Anderson SL, Wharton BL, Habeger B, Anderson HG., Jr A retrospective cohort analysis of the clinical effectiveness of a physician–pharmacist collaborative drug therapy management diabetes clinic. Pharmacotherapy. 2002;22:1294–300. doi: 10.1592/phco.22.15.1294.33476. [DOI] [PubMed] [Google Scholar]
- 42.Laakso M. Benefits of strict glucose and blood pressure control in type II diabetes: lessons from the UK prospective Diabetes Study. Circulation. 1999;99:461–2. doi: 10.1161/01.cir.99.4.461. [DOI] [PubMed] [Google Scholar]
- 43.English GN, Poirier S. Optimizing adherence to pharmaceutical care plans. J Am Pharm Assoc. 2000;40:475–85. [PubMed] [Google Scholar]
- 44.Boule NG, Weisnagel SJ, Lakka TA. Effects of exercise training on glucose homeostasis: the HERITAGE family study. Diabetes Care. 2005;28:108–14. doi: 10.2337/diacare.28.1.108. [DOI] [PubMed] [Google Scholar]
- 45.Tessier D, Menard J, Fulop T. Effects of aerobic physical exercise in the elderly with type 2 diabetes mellitus. Arch Gerontol Geriatr. 2000;31:121–32. doi: 10.1016/s0167-4943(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 46.Solberg LI, Jay R, Desai JR, O’Connor PJ, Bishop DB, Devlin HM. Diabetic patients who smoke: are they different? Ann Fam Med. 2004;2:26–32. doi: 10.1370/afm.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerveny JD, Leder RD, Weart CW. Issues surrounding tight glycaemic control in people with type 2 diabetes mellitus. Ann Pharmacother. 1998;32:896–905. doi: 10.1345/aph.17375. [DOI] [PubMed] [Google Scholar]
- 48.American Diabetes Association. Hypertension management in adults with diabetes. Diabetes Care. 2004;27:65–7. doi: 10.2337/diacare.27.2007.s65. [DOI] [PubMed] [Google Scholar]
- 49.McFarlane SI, Castro J, Kaur J, Shin JJ, Kelling D, Farag A, Simon N, El-Atat F, Sacerdote A, Basta E, Flack J, Bakris G, Sowers JR. Control of blood pressure and other cardiovascular risk factors at different practice settings: outcomes of care provided to diabetic women compared to men. J Clin Hypertens. 2005;7:73–80. doi: 10.1111/j.1524-6175.2005.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown GC, Brown MM, Sharma S. Quality of life associated with diabetes mellitus in an adult population. J Diabetes Complications. 2000;14:18–24. doi: 10.1016/s1056-8727(00)00061-1. [DOI] [PubMed] [Google Scholar]
- 51.Clifford RM, Batty KT, Davis TM, Davis W, Stein G, Stewart G, Plumridge RJ. A randomised controlled trial of a pharmaceutical care programme in high-risk diabetic patients in an outpatient clinic. Int J Pharm Pract. 2002;10:85–9. [Google Scholar]
- 52.Posey LM. Proving that pharmaceutical care makes a difference in community pharmacy. J Am Pharm Assoc. 2003;43:136–9. doi: 10.1331/108658003321480669. editorial. [DOI] [PubMed] [Google Scholar]
- 53.Speight J, Bradley C. The ADKnowl: identifying knowledge deficits in diabetes care. Diabet Med. 2001;8:626–33. doi: 10.1046/j.1464-5491.2001.00537.x. [DOI] [PubMed] [Google Scholar]
- 54.Nicolucci A, Ciccarone E, Consoli A, Di Martino G, La Penna G, Latorre A, Pandolfi A, Vitacolonna E, Capani F. Relationship between patients’ practice-oriented knowledge and metabolic control in intensively treated type 1 diabetes patients: results of the validation of the knowledge and practices diabetes questionnaire. Diabetes Nut Metab. 2000;5:276–83. [PubMed] [Google Scholar]
- 55.Daly A, Kulkarni K, Boucher J. The new credential: advanced diabetes management. J Am Diet Assoc. 2001;101:940–3. doi: 10.1016/S0002-8223(01)00233-4. [DOI] [PubMed] [Google Scholar]
- 56.Mensing C, Boucher J, Cypress M, Weinger K, Mulcahy K, Barta P, Hosey G, Kopher W, Lasichak A, Lamb B, Mangan M, Norman J, Tanja J, Yauk L, Wisdom K, Adams C. National standards for diabetes self-management education. Diabetes Care. 2003;26:S149–56. doi: 10.2337/diacare.26.2007.s149. [DOI] [PubMed] [Google Scholar]
- 57.McWhorter LS, Fermo JD, Bultemeier NC, Oderda GM. National survey of pharmacist certified diabetes educators. Pharmacotherapy. 2002;22:1579–93. doi: 10.1592/phco.22.17.1579.34131. [DOI] [PubMed] [Google Scholar]
- 58.Vermeire E, Hearnshaw HP, Van Royen P, Denekens J. Patients adherence to treatment: three decades of reseach. A comprehensive review. J Clin Pharm Ther. 2001;26:331–42. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]


