Abstract
AIMS
To compare adverse drug reaction (ADR) reports from patients and health professionals after the broadcast of a Dutch television consumer programme about the benefits and risks of statins.
METHODS
We performed a quantitative and qualitative analysis on patients' and health professionals' reports of ADRs to statins. These reports were received by the Netherlands Pharmacovigilance Centre Lareb between March 2007 and August 2007. Quantitative data consisted of patient age and gender, number of received reports and characteristics of the report (most frequently reported ADRs, seriousness, drug discontinuation and outcome of the reported reaction). Open text fields in the ADR reporting form were categorized and a content analysis was carried out.
RESULTS
Media attention led to a peak in patient reporting of ADRs but not in reporting by health professionals. There were no differences between patient and health professional reports in seriousness of the ADRs and drug cessation. Patients reported nonrecovery more often than health professionals. The TV programme is mentioned as a reason for drug discontinuation in almost 30 reports. Patients often felt that they did not receive sufficient information and that their concerns were not adequately addressed by healthcare professionals.
CONCLUSIONS
Media attention affects drug use and ADR reporting by patients. Patient reports can provide additional information, making them a useful source of information next to health professional reports. Content analysis provides vital insights into the impact of statins on daily life, and patients' concerns about adverse reactions should be recognized in reports to national pharmacovigilance centres.
Keywords: adverse drug reactions, consumers, media exposure, patients, pharmacovigilance, statins
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
More and more countries accept patient reporting in their spontaneous reporting systems.
Media exposure about drugs can influence patients and health professionals.
Evaluation of patient reporting of adverse drug reactions is needed to provide further evidence of its benefits.
WHAT THIS STUDY ADDS
Patient reporting compared with reporting by health professionals after media attention about statins.
Patient reports provide additional insight into impact on daily life, the patient–health professional relationship and general worries next to health professionals' reports.
Introduction
The acceptance of patient reporting of adverse drug reactions (ADRs) to spontaneous reporting systems and their contribution to pharmacovigilance is still a subject of discussion [1]. Although in several countries patients have the possibility of reporting ADRs, few publications exist about the contribution that patients' reports have in daily practice [1]. The Netherlands Pharmacovigilance Centre Lareb has had favourable experiences with patient reporting; reports from patients usually contain sufficient medical information to be of use to pharmacovigilance [2,3]. The number of patient reports in the Netherlands has risen each year since 2003 when patient reporting was first introduced [2,3]. In 2007 the number of patient reports to the Netherlands Pharmacovigilance Centre Lareb rose with 74% compared with 2006. Evaluation of patient reporting of ADRs is needed to provide further evidence of its benefits [1].
Patients are interested in the safety aspects of drugs [4]. In the Netherlands the TV programme Radar, broadcast on 5 March 2007, aimed to give attention to serious ADRs experienced by some patients taking statins and to question the preventive use of these drugs [5]. There is no evidence in the literature that before this broadcast Dutch patients had specific concerns about statins. The Netherlands Pharmacovigilance Centre Lareb was mentioned as the reporting centre for ADRs during the broadcasts of the programme. Patients who contacted either the programme makers or Lareb were asked to fill in the electronic patient reporting form on the Lareb website. This led to a large increase in patient reporting about statins.
The potential behavioural influence of the media on patients and health professionals can be great [4,6]. The influence of the third-generation pill controversy on the number of prescriptions of these oral contraceptives can be seen as an example of this phenomenon [7]. In the UK, the BBC broadcast the programme Panorama –‘Secrets of Seroxat’ in 2002, followed by two more Panorama documentaries about the antidepressant paroxetine. The programme Secrets of Seroxat attracted a record response in 2002, including 1374 e-mails to the programme makers [8] and a 10-fold increase of calls about paroxetine to a national medication helpline in the month after the programme [9]. The media publicity impact on prescribing of paroxetine and the notification of suspected ADRs have been analysed by Martin et al.10]. Reports of adverse reactions to paroxetine appeared to increase after the publicity about the drug [10]. Prescribing of paroxetine and other selective serotonin reuptake inhibitors declined [10].
In the Netherlands, the Radar programme in 2007 was followed by concerned reactions from Dutch medical bodies and the Dutch Heart Foundation. They worried that the programme would increase the number of patients that stopped taking their statins, with or without consulting their general practitioner (GP) first. The Foundation for Pharmaceutical Statistics (http://www.sfk.nl) calculated the number of patients who stopped taking statins in the Netherlands, finding that the number rose from 30 000 to 41 000 per month after the programme was broadcast, an increase of almost 35%. However, a trend in the number of drug cessations could already be seen a few months before the Radar programme was broadcast. Furthermore, the number of patients starting statin therapy decreased from almost 41 000 in January 2007 to 27 000 in April 2007 [11]. The effects of the broadcast were temporary, however; in September 2007 the number of patients starting or stopping with statin use had returned to their old levels [12].
For the comparison of patients' and health professional reports both quantitative and qualitative methods have been used in the past. A quantitative study of the data can provide information about the extent of reporting by both groups and the frequency of particular outcomes. In addition, qualitative analysis might describe the impact of illness and treatment in the content of everyday life [13]. A qualitative study can enable the investigation of attitudes on sensitive topics [13] such as the patient–health professional relationship.
A quantitative analysis of 3 years' experience with ADR reporting by patients to the Netherlands Pharmacovigilance Centre Lareb was published in 2008 [3]. A more qualitative comparison between ADR reports from health professionals and patients was made by Medawar and Herxheimer in 2002 [14]. They investigated the risk of dependence and suicidal behaviour with paroxetine through analysis of Yellow Card reports sent to the UK Medicines and Healthcare products Regulatory Agency [14].
This study aimed to compare patient and health professional reports of ADRs to statins, following the broadcast of a consumer programme about the benefits and risks of statins. In order to find if additional information is present in the patient reports about statins in the Lareb database, we will use a content analysis of the reports next to quantitative data.
Methods
All ADR reports about statins received by the Netherlands Pharmacovigilance Centre Lareb in the period after the broadcast, between 1 March and 1 August 2007 were taken into account in the analysis. Consumer reports were compared with reports from health professionals (GPs, specialist doctors and pharmacists). We categorized open text fields in the ADR reporting form and analysed these data in addition to the quantitative analysis. Reports from pharmaceutical companies were excluded because the nature of these reports is often not comparable to the spontaneous reports from patients and health professionals.
Data collection
All reports of ADRs were obtained through the ADR reporting form of the Netherlands Pharmacovigilance Centre Lareb.
Health professionals can choose between reporting electronically or on paper, patients can only report electronically. The content of the electronic form and the paper form is identical. Each report used in this analysis, either from a patient or a health professional, was medically assessed for causality and seriousness on an individual basis by a Lareb assessor, a medical doctor or pharmacist specially trained for this purpose.
Quantitative analysis
The number of received reports and characteristics of the reports were analysed quantitatively. Report characteristics were compared on the basis of most frequently reported ADRs, seriousness, drug withdrawal and outcome of the reported ADRs. Patient demographics (age and gender) were recorded in the analysis.
The drugs in our database are divided according to the Anatomical Therapeutic Chemical (ATC) classification of the World Health Organization [15]. For statins as a group the ATC begins with C10AA and for the fixed combination simvastatin/ezetimib the ATC begins with C10AX.
ADRs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) coding system, which refers to a group of MedDRA terms belonging to a System Organ Class (SOC) [16,17]. MedDRA is a multi-axial terminology meaning that a Preferred Term (PT) may be linked to more than one SOC. A MedDRA term may be attributed to multiple system organ classes. Each PT is assigned a primary SOC to avoid ‘double counting’ while retrieving information from all SOCs [18]. For this analysis the primary SOC has been used.
The seriousness of the reports was categorized in our database according to the criteria formulated by the Council for International Organizations of Medical Sciences (CIOMS), namely death, life-threatening factors, hospitalization or prolongation of hospitalization, disability/incapacity, congenital anomaly/birth defect and other ADRs considered serious by the reporter [19].
In order to establish possible differences between reports from patients and healthcare professionals we used a Pearson χ2 test to detect differences in gender, seriousness, drug withdrawal and outcome between patient and health professional reports. Significance was based on χ2 test: P < 0.05. A t-test was used to detect differences in age. SPPS 16.0 was used for statistic analysis (SPSS Inc., Chicago, IL, USA).
Content analysis
Content analysis requires the creation of a list of categories derived from the data collected, and then systematically coding into these categories [13,20]. The data used in this case are derived from the ADR reports sent to the Netherlands Pharmacovigilance Centre Lareb. In the reports both patients and health professionals can choose to provide additional information about the report in a special open text field of the reporting form. The information in this field of the report was used for content analysis. During the initial assessment of the reports and the reviewing of the reports during Lareb's weekly scientific meeting, possible themes for analysis were selected. By reading and rereading the set of reports, categories were identified; namely the impact on daily life of the ADRs, the patient–healthcare professional relationship and impact of the TV programme on drug withdrawal. The first two authors were involved in the process of validating the categories. Specific comments about the Radar programme were also collected. The ‘cutting and pasting’ of data into categories was done manually. We illustrate some points in this study by adding relevant patient quotes.
Results
Quantitative analysis
Patients submitted 265 reports about statins to Lareb concerning 780 ADRs from March 2007 to August 2007. Health professionals submitted 111 reports involving 172 ADRs about statins in the same period. This means that each patient report contained, on average, three ADRs, whereas health professionals' reports had 1.5 ADRs. The total number of reports in this period was 833 for patients and 1609 for health professionals (Table 1).
Table 1.
Reports received between 1 March 2007 and 1 August 2007
| Analysed reports about statins | All reports | |
|---|---|---|
| Patient reports | 265 | 833 |
| Heath professional reports | 111 | 1609 |
| Total | 376 | 2442 |
The peak in the number of patient reports about statins, in reaction to the period around the broadcast, is shown in Figure 1.
Figure 1.
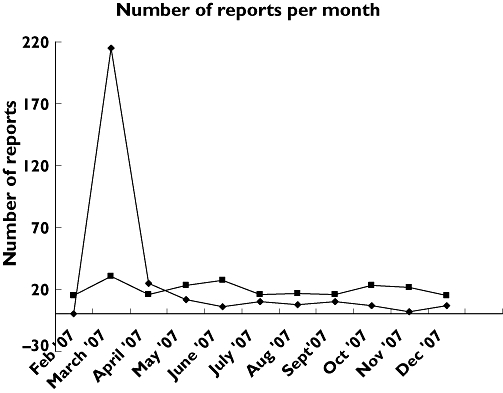
Number of reports about statins per month in a 1-year period. Patients ( ); HP (
); HP ( )
)
Patients who reported directly to Lareb were younger than those in reports by health professionals (57.3 years vs. 61.9 years) (t-test P < 0.001).
In the patient reports, 64% of patients were male, compared with 52% in the reports from health professionals, a statistically significant difference (χ2 test: P < 0.05).
Each report can consist of multiple ADRs. Most of the ADRs reported by both patients and health professionals refer to the SOC musculoskeletal and connective tissue disorders (308 of the total of 780 ADRs for the patient reports vs. 38 of the total of 172 ADRs for the health professional reports) (Table 2). The percentage is significantly higher for patients. Patients also reported more ADRs in the SOC psychiatric disorders (58 patient reports vs. five health professional reports). Health professionals reported more skin and subcutaneous tissue disorders and respiratory disorders than patients did.
Table 2.
Top 10 of reports in a System Organ Class (SOC) for patients' reports compared with health professional (HP) reports
| SOC name | % Patient reports | % HP reports | χ2 test: P-values | |
|---|---|---|---|---|
| 1 | Musculoskeletal and connective tissue disorders | 39.49 | 22.09 | <0.001 |
| 2 | Nervous system disorders | 13.46 | 18.02 | NS |
| 3 | General disorders and administration site conditions | 12.44 | 13.37 | NS |
| 4 | Gastrointestinal disorders | 8.33 | 9.88 | NS |
| 5 | Psychiatric disorders | 7.44 | 2.91 | <0.05 |
| 6 | Skin and subcutaneous tissue disorders | 5.26 | 12.79 | <0.001 |
| 7 | Eye disorders | 2.05 | 3.49 | NS |
| 8 | Renal and urinary disorders | 2.05 | 0.58 | NS |
| 9 | Reproductive system and breast disorders | 1.54 | 2.33 | NS |
| 10 | Respiratory, thoracic and mediastinal disorders | 1.54 | 5.23 | NS |
NS, non-significant.
There is some overlap in the top 10 of most frequently reported ADRs by patients and health professionals (Tables 3 and 4).
Table 3.
Top 10 of most frequently reported adverse drug reactions (ADR) for patients
| ADR | Number of reports | ADR % on total patient reports |
|---|---|---|
| Myalgia | 137 | 51.7 |
| Arthralgia | 43 | 16.2 |
| Muscle spasms | 43 | 16.2 |
| Fatigue | 42 | 15.8 |
| Muscular weakness | 37 | 14.0 |
| Depression | 22 | 8.3 |
| Paraesthesia | 22 | 8.3 |
| Headache | 17 | 6.4 |
| Dizziness | 16 | 6.0 |
| Insomnia | 15 | 5.7 |
Table 4.
Top 10 of most frequently reported adverse drug reactions (ADR) for health professionals (HP)
| ADR | Number of reports | ADR % on total HP reports |
|---|---|---|
| Myalgia | 16 | 14.4 |
| Muscle spasms | 7 | 6.3 |
| Memory impairment | 6 | 5.4 |
| Alopecia | 5 | 4.5 |
| Paraesthesia | 5 | 4.5 |
| Oedema peripheral | 4 | 3.6 |
| Fatigue | 4 | 3.6 |
| Dizziness | 4 | 3.6 |
| Depression | 3 | 2.7 |
| Erectile dysfunction | 3 | 2.7 |
The most frequently reported ADR for both patients and health professionals was myalgia. However, when we looked at myalgia as a proportion of the total number of reports, we found that more than half of the patients' reports were of myalgia, compared with <15% in reports by health professionals. Arthralgia was the second most frequently reported ADR by patients (16.2% of reported reactions), whereas this ADR is not in the top 10 for health professionals.
In total, patients reported 40 serious ADRs and health professionals 13 serious ADRs (15.1% of the cases vs. 11.7%), not statistically significant (χ2 test: P > 0.05).
Among the investigated reports, there were 17 additional patient reports and one health professional report initially marked as serious by the reporter, where the nature of the report did not imply seriousness according to the CIOMS criteria or where there was insufficient medical information for the assessment. In these cases the reports were recoded as nonserious by Lareb's medical assessors using their medical and scientific judgement in order to prevent inconsistencies in the database [21].
Of the patients who reported an ADR, 62% reported that they had stopped using the statin involved. Of the health professionals' reports, 55% mentioned drug discontinuation. The number of drug cessations did significantly differ between patients and health professionals.
The outcome of reaction was known in 86% of all cases. Patients reported nonrecovery from the ADRs significantly more often than was shown in the health professionals' reports (χ2 test: P < 0.001). For patient reports the percentage of nonrecovery of ADRs was 44.3% and for health professional reports 26.6%.
Content analysis
In general, the additional information about the impact on daily life of the ADRs, the patient–healthcare professional relationship and impact of the TV programme on drug withdrawal was much scarcer in the health professionals' reports than in the patients' reports. Approximately 10% of the health professionals' reports provided information about the designated categories of interest, compared with >50% of the patients' reports.
Impact on daily life
More than one-third of patients gave detailed information about the severity of their adverse reaction and the implications for their daily life activities. This can be illustrated with some of the descriptions patients gave about their ADRs
‘I used up to eight different lipid lowering drugs and every time the reaction was so bad that I could hardly dress myself, sometimes could not go up the stairs, could not sleep any more due to the pain in my arms and shoulders and couldn't even hold a cup. Now I've stopped and it will take a while for everything to recover.’
‘During the Radar programme I recognized more and more adverse drug reactions. To keep my weight under control I used to visit gym classes for senior citizens, did fitness training and walked for many kilometres. During the use of simvastatin, I became more and more immobilized and could not leave the house. The quality of my life was reduced severely. Now I'm walking again and feel a lot happier.’
A comment that was made several times was ‘The health benefit of the drug does not measure up to the adverse reactions … ’. One of the patients concluded his report with the following: ‘I'd rather die a couple of years earlier without adverse reactions and pain but with a high quality of life than live longer with these adverse drug reactions’.
In the reports by health professionals, the descriptions about the impact on daily life were much scarcer and less elaborate.
Impact of the TV programme on drug withdrawal
Almost 30 patients explicitly mentioned that they stopped taking their medication as a result of the TV programme. The TV programme is mentioned only twice in health professionals' reports. The impact of the programme on drug cessation is further illustrated by the following patient quotes
‘I recognized myself completely in the words of another patient from the Radar programme. To be sure I stopped taking simvastatin. I mentioned these complaints to my GP, but they were just ignored. Since I've stopped the complaints have obviously decreased.’
‘I recognized myself in the complaints of those patients in the TV programme Radar and then quit taking pravastatin.’
‘After seeing the TV show Radar everything became clear. I had already been going to a physiotherapist for quite a while, with little result. Now I've stopped taking simvastatin and me and my physiotherapist can see the results.’
Patient–healthcare professional relationship
Almost 80 patients gave information about the relationship with their health professional (GP, pharmacist or specialist) in their report. Many patients had already discussed their adverse reactions with a health professional before sending a report to Lareb. The patient–health professional interaction was often judged as unsatisfactory by the patient. Some quotes illustrate this
‘My cardiologist has refused, since yesterday, to counsel me any further because I made the choice not to take atorvastatin. He also said my complaints were a matter of coincidence and disputed that my reactions were of a serious nature. According to him my complaints are minimal and of transient nature.’
‘The General Practitioners' (GP) assistant told me sharply that I'm not to decide for myself whether I take a drug or not.’
‘The specialist was convinced that atorvastatin was a well tested drug but I did not agree. The GP remained silent; he would not take part in our discussion.’
‘I cannot play sports anymore and the GP takes no complaint seriously unless it is visual.’
However, in many cases patients also indicate that they discuss everything concerning their health and medication with a health professional. One patient mentions: ‘My advice is go to your pharmacist when you are using multiple medications and have unexplainable physical problems. After all, the pharmacist has studied about medicines for years. I'm very grateful to my pharmacist for making my life a bit more bearable again’.
Health professionals gave information about their relation with the patient only twice, both of them positive experiences: ‘The specialist, patient and we [pharmacy] all agree that the statin is the most likely cause of the reaction …’ and ‘We [pharmacy] made a deal with the patient to report adverse drug reactions’.
Discussion
The peaks in patient reports of statins after the broadcasts of the TV programme Radar show that media attention can influence reporting. After a couple of weeks the number of reports from patients was back to the baseline level. The peak in reporting was almost not seen in the reports by health professionals.
In the analysis of the impact of media attention around the possible adverse effects of paroxetine on clinical practice in England, a similar phenomenon was seen. Media attention was associated with sharp, but short-term peaks of spontaneous reporting of ADRs related to paroxetine [10].
There were no significant differences between patient and health professional reports in seriousness and the number of drug cessations. However, medical seriousness may differ from patients' views on what constitutes a serious problem.
Patients reported nonrecovery more often than health professionals. Nonrecovery means that the adverse reaction has persistent or permanent effects, even when the drug has been changed or stopped. The less frequently reported recovery by patients could be explained by different views about ‘recovery’. For example: for a hepatic reaction, a physician could judge a full recovery as normalization of hepatic enzymes, whereas a patient might not feel recovered because of persistent fatigue.
Health professionals reported less about musculoskeletal and connective tissue disorders than patients did. An explanation could be that because these effects are described in the summary of product characteristics of various statins, health professionals might not be inclined to report them to a pharmacovigilance centre but focus on other ‘unknown’ adverse reactions. Alternatively, it may be that patients perceive musculoskeletal reactions as serious problems that need reporting, whereas healthcare professionals consider it too trivial to report.
Content analysis of the data showed that the patient reports following the programme Radar provided more insight into impact on daily life, patient–health professional relationship and general worries caused by the TV programme than health professionals' reports. Differences in motivation for reporting an ADR between health professionals and patients could be a factor explaining this.
The patients who reported directly about statins were younger than patients in the reports by health professionals; the greater number of reports from young people could be caused by the easy availability of electronic reports, and underreporting from older people who are less used to the internet. In an earlier comparison of all patient and health professional reports in a 3-year period, the age difference between the groups was not seen, however [3].
Edwards has described that reports from patients and health professionals must be regarded as their concerns, even though they may not be ‘clinically validated’[22]. Although most of the patients' reports are not considered as serious according to the CIOMs criteria, the impact of an ADR on the patient's daily life can be profound [23]. The different point of view of patients, compared with health professionals, is an interesting starting point for obtaining valuable information, e.g. about severity of a reaction or impact on daily life.
In 2007 a study by Golomb et al. assessed patient opinions of how physicians responded when patients presented with possible ADRs. Patients reported that they, and not their physician, usually initiated the discussion about a possible relation between the drug and symptoms. Physicians were more likely to deny than affirm the possibility of a relation, even for symptoms with strong literature support of a drug connection [24]. Poor communication or a reluctance to give patients the information that they want could be factors here.
It is important to be aware that reporting of ADRs stimulated by media exposure of specific safety issues can be a possible source of bias in a spontaneous reporting system [25]. The strong response to programmes about drug safety issues, in the UK [8–10] and the Netherlands, makes it clear that patients want to be involved in issues concerning their health and medication.
Conclusion
Media attention about drugs and their ADRs can influence drug use and the reporting of ADRs by patients. In the Netherlands, patient reports provide additional information by reporting about different categories of adverse reactions or providing information about the impact of ADRs on daily life and the patient–health professional relationship. Patients' concerns about drugs and ADRs should be translated into knowledge by routine reporting to national pharmacovigilance centres.
Competing interests
None to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Appendix S1
Patient Reporting Form
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol. 2007;63:148–56. doi: 10.1111/j.1365-2125.2006.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Grootheest AC, Passier JL, van Puijenbroek EP. Direct reporting of side effects by the patient: favourable experience in the first year. Ned Tijdschr Geneeskd. 2005;149:529–33. [PubMed] [Google Scholar]
- 3.de Lange J, van Hunsel F, Passier A, de Jong-van den Berg L, van Grootheest K. Adverse drug reaction reporting by patients in the Netherlands: three years of experience. Drug Saf. 2008;31:515–24. doi: 10.2165/00002018-200831060-00006. [DOI] [PubMed] [Google Scholar]
- 4.van Grootheest K, de Jong-van den Berg L. Patients' role in reporting adverse drug reactions. Expert Opin Drug Saf. 2004;3:363–8. doi: 10.1517/14740338.3.4.363. [DOI] [PubMed] [Google Scholar]
- 5.Sheldon T. Television show questions statins to boost ratings, Dutch doctors claim. BMJ. 2007;334:604–5. doi: 10.1136/bmj.39157.755718.DB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waller PC, Evans SJ, Beard K. Drug safety and the media. Br J Clin Pharmacol. 2006;61:123–6. doi: 10.1111/j.1365-2125.2005.02528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong-van den Berg L, Tobi H, Bijker B, van den Berg P. Influence of the third generation pill controversy on prescriptions for oral contraceptives among first time users: population based study. BMJ. 2003;326:254. doi: 10.1136/bmj.326.7383.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medawar C, Herxheimer A, Bell A, Jofre S. Paroxetine,Panorama and user reporting of ADRs: consumer intelligence matters in clinical practice and post-marketing drug surveillance. Int J Risk Saf Med. 2002;15:161–9. [Google Scholar]
- 9.Taylor D, Stewart S, Connolly A. Antidepressant withdrawal symptoms – telephone calls to a national medication helpline. J Affect Disord. 2006;95:129–33. doi: 10.1016/j.jad.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Martin RM, May M, Gunnell D. Did intense adverse media publicity impact on prescribing of paroxetine and the notification of suspected adverse drug reactions? Analysis of routine databases, 2001–2004. Br J Clin Pharmacol. 2006;61:224–8. doi: 10.1111/j.1365-2125.2005.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foundation for Pharmaceutical Statistics. Statins: starting and stopping. Pharm Weekbl. 2007;142:17. [Google Scholar]
- 12.Foundation for Pharmaceutical Statistics. Radar effect temporary. Pharm Weekbl. 2008;143:13. [Google Scholar]
- 13.Britten N, Jones R, Murphy E, Stacy R. Qualitative research methods in general practice and primary care. Fam Pract. 1995;12:104–14. doi: 10.1093/fampra/12.1.104. [DOI] [PubMed] [Google Scholar]
- 14.Medawar C, Herxheimer A. A comparison of adverse drug reaction reports from professionals and users, relating to risk of dependence and suicidal behaviour with paroxetine. Int J Risk Saf Med. 2004;16:5–19. [Google Scholar]
- 15.WHO Collaborating Centre for Drug Statistics Methodology. The ATC/DDD system. [accessed 11 February 2008]. Available at http://www.whocc.no/atcddd/
- 16.Brown EG. Effects of coding dictionary on signal generation: a consideration of use of MedDRA compared with WHO-ART. Drug Saf. 2002;25:445–52. doi: 10.2165/00002018-200225060-00009. [DOI] [PubMed] [Google Scholar]
- 17.Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA) Drug Saf. 1999;20:109–17. doi: 10.2165/00002018-199920020-00002. [DOI] [PubMed] [Google Scholar]
- 18.International Conference on Harmonisation (ICH) MedDRA® term selection: points to consider. [accessed 18 April 2008]. Available at http://www.meddramsso.com/MSSOWeb/document_library/TermSelPTC_R3.8.pdf.
- 19.CIOMS Working Group IV. Benefit–risk Balance for Marketed Drugs: Evaluating Safety Signals. Geneva: CIOMS Working Group IV; 1998. [Google Scholar]
- 20.Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ. 2000;320:114–6. doi: 10.1136/bmj.320.7227.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borja J, Donado E, Souto M. Seriousness of adverse events: medical judgment is important. BMJ. 2004;329:1405. doi: 10.1136/bmj.329.7479.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards IR. Spontaneous reporting – of what? Clinical concerns about drugs. Br J Clin Pharmacol. 1999;48:138–41. doi: 10.1046/j.1365-2125.1999.00000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frankenfeld C. Serious’ and ‘severe’ adverse drug reactions need defining. BMJ. 2004;329:573. doi: 10.1136/bmj.329.7465.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golomb BA, McGraw JJ, Evans MA, Dimsdale JE. Physician response to patient reports of adverse drug effects: implications for patient-targeted adverse effect surveillance. Drug Saf. 2007;30:669–75. doi: 10.2165/00002018-200730080-00003. [DOI] [PubMed] [Google Scholar]
- 25.De Bruin ML, van Puijenbroek EP, Egberts AC, Hoes AW, Leufkens HG. Non-sedating antihistamine drugs and cardiac arrhythmias – biased risk estimates from spontaneous reporting systems? Br J Clin Pharmacol. 2002;53:370–4. doi: 10.1046/j.1365-2125.2002.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


