Abstract
Over the last several decades, the advances in molecular genetics have elucidated kidney cancer gene pathways. Kidney cancer is a heterogeneous disorder. Each specific type of kidney cancer has its own histologic features, gene, and clinical course. Insight into the genetic basis of kidney cancer has been learned largely from the study of the familial or hereditary forms of kidney cancer. Extirpative surgery is currently the treatment of choice for kidney cancer that is confined to the kidney. Treatment for advanced or metastatic kidney cancer is a formidable challenge with the traditional therapies currently available. However, investigation of the mendelian single-gene syndromes, like von Hippel Lindau (VHL: VHL gene), hereditary papillary renal carcinoma (HPRC: c-Met gene), Birt-Hogg-Dubé (BHD: BHD gene), and hereditary leiomyomatosis renal cell cancer (HLRCC: fumarate hydratase gene) provides an opportunity to develop pathway specific therapies. Advances in molecular therapeutics offer novel treatment options for patients with advanced disease.
Keywords: Renal cell carcinoma, gene, VHL, BHD, HPRC, HLRCC
Introduction
It is estimated that 54,390 men and women in the United States will be diagnosed with kidney cancer, while 13,010 will die of their disease in 2008. This number can also be expressed as 1 in 72 men and women will be diagnosed with cancer of the kidney during their lifetime and this incidence continues to rise. (1) With the increased use of computed tomography and other imaging modalities, kidney tumors are being diagnosed at earlier stages, often as incidental lesions. The increased incidence cannot be explained entirely by the wider use of imaging. (2) Although extirpative surgery is often curative, 30% of patients will present with metastases at the time of initial diagnosis. In addition, 30% of initially organ-confined disease will develop metastases during follow-up at variable intervals. (3) Treatment of metastatic disease remains a formidable challenge in this day and age. Elucidation of the genetics basis of kidney cancer has given way to exciting new advances in molecular therapeutics for the treatment of metastatic kidney cancer.
Renal cell carcinomas are adenocarcinomas derived from renal tubular epithelium. Histologically, there are 5 subtypes: conventional (clear cell) (70–80%), chromophile (papillary) (10–15%), chromophobe (3–5%), collecting duct (1%), and unclassified (1%). Papillary is further classified into type 1 (5%) and type 2 (10% of cases) based upon further genetic alterations, histologic, and genetic criteria. (4;5) (4) (6) Renal cell carcinoma can be hereditary as well as sporadic or non-hereditary. While sporadic RCC is often a solitary lesion and most commonly present in individuals in their 60’s, inherited forms tend to be multifocal, bilateral and have an early onset. Each subset has marked different clinical courses, is caused by different genes, and may have different responses to new therapeutics modalities.
In the early 1980s, the search to identify a loss of heterozygosity in one of the alleles of a cancer gene was initiated, with the hope that loss of heterozygosity would indicate the presence of a cancer gene at that location. A tumor suppressor gene requires loss of function or inactivation of one or both of the genes, often by mutation of one allele combined with deletion, or loss, of the second allele; whereas, an oncogene is activated by one change in the gene. In the initial studies, loss of segments at chromosome 3 in tumor tissue, suggested that a cancer gene for kidney cancer was present at this location. (7) Dr Knudson’s “two hit” theory postulates that most tumor suppressor genes encode proteins responsible for the negative regulation of cellular growth. (8) A mutation that causes a loss of the function in one these proteins will lead to tumorigenesis or uninhibited cell growth. As the gene of interest for clear cell kidney cancer was too large to localize with the technology that was available at the time, an approach focusing on a hereditary form of kidney cancer, von Hippel Lindau, was used as the model of investigation. The hope was that the gene involved in hereditary kidney cancer may involve the same gene as in the sporadic form of kidney cancer.
von Hippel Lindau (VHL)
Von Hippel Lindau is a hereditary form of kidney cancer that is inherited in an autosomal dominant pattern with an incidence of 1 in 36,000 live births.(4) Clinical manifestations include the risk for development of renal cell carcinoma, pheochromocytoma, pancreatic cysts, endolymphatic sac tumors, papillary cystadenomas of the epididymis, retinal angiomas, and hemangioblastomas of the brain stem, cerebellum, or spinal cord. The tumors are highly vascular. With the recent improvement in the treatment of the central system manifestation, metastatic renal cell carcinoma has become the most common cause of mortality in these patients. The renal cell carcinoma is of the conventional clear cell type and may be solid or cystic. Patients are at risk to develop up to 600 microscopic tumors and over 1000 cysts per kidney.(9)
Consistent loss of the short arm of chromosome 3 in VHL associated kidney tumors was identified (10). Genetic linkage analysis was utilized to help identify the VHL gene which was identified on the short arm of chromosome 3p. (11) The loss of the single normal allele of the VHL gene in the kidney cancer cell samples was critical and suggested that there was an inherited gene at this location which was associated with the development of VHL-associated clear cell kidney cancer. Germline mutations on the VHL gene are identified in nearly 100% of VHL families. (12)
The different clinical manifestations of VHL can be associated with the location and type of the VHL gene mutation (13;14). Penetrance of the traits is far from complete, and for some, such as pheochromocytomas, they tend to be clustered in certain families but do not occur in others. (15) Maranchie, et. al., identified a significantly higher incidence of renal cell carcinoma in patients with partial germline VHL mutations versus those with complete VHL gene mutations. (16) It is now known that with mutation analysis (e.g. insertion, deletion, missense or nonsense) and the location (e.g. codon position) of the mutation, correlations can be made to the phenotype, i.e., the extent of involvement of the various organ systems affected by VHL. The VHL gene mutation has also been found in sporadic clear cell renal cell carcinoma using polymerase chain reaction (PCR) amplification. (17;18)
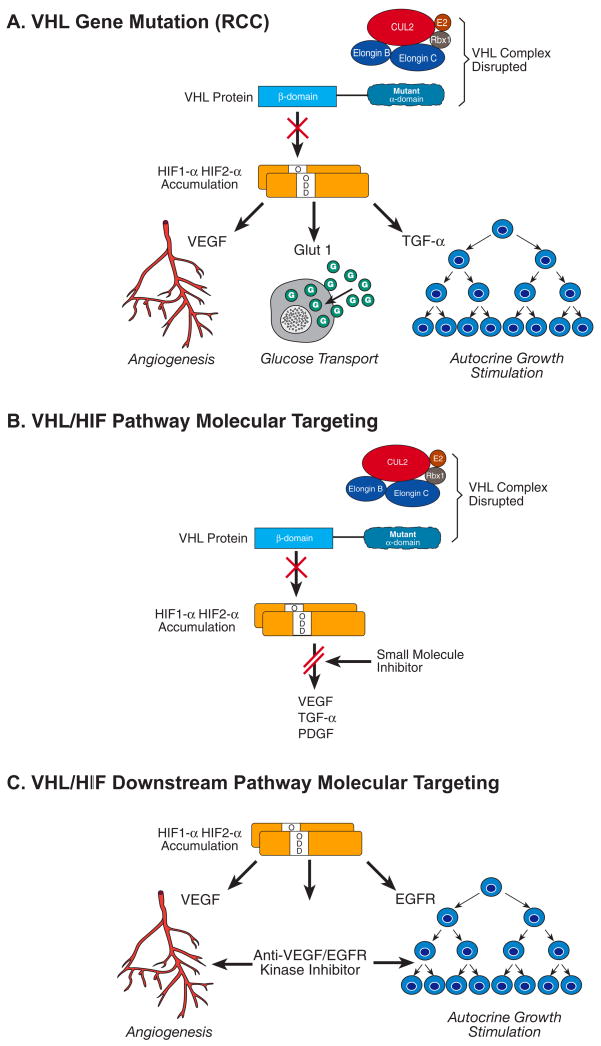
The function of the VHL gene has been evaluated extensively. It is a small gene that encodes 854 nucleotides on three exons and is responsible for encoding the VHL protein. (11) The VHL protein forms a complex with proteins including elongin C, elongin B and Cul-2 (19) (20), (21) and targets the alpha subunits of the hypoxia inducible factors, such as HIF-α and HIF-2α, which are instrumental to ubiquitin-mediated degradation (22) (23). There are multiple downstream genes, such as Glut 1 (glucose transport), vascular endothelial growth factor (VEGF, blood vessel growth), epidermal growth factor (EGF), transforming growth factor (TGF-α), for which HIF regulates. The expression of these genes increased in clear cell carcinoma when the VHL gene is inactivated (Fig. 1). Many of the receptors for HIF-regulated genes are the targets for the new targeted therapeutic approaches for clear cell carcinoma.(24)
Fig. 1.
The VHL gene complex targets hypoxia-inducible factors (HIF) for ubiquitin-mediated degradation. When there is a mutation in the VHL gene in clear cell kidney cancer, in either the elongin binding or the HIF binding domain (A), HIF is not degraded and this protein over-accumulates. Increased HIF levels lead to increased transcription of a number of downstream pathway genes that are thought to be important in kidney cancer, such as vascular endothelial growth factor (VEGF), the glucose transporter, GLUT1, and transforming growth factor _ (TGF_) (B). Targeted approaches to therapy currently include tyrosine kinase inhibitors that target the downstream gene receptors (C). Other approaches being developed to target the VHL-HIF pathway in clear cell kidney cancer include the development of small molecules or other agents which block HIF transcription. Reprinted from Linehan et al. [54].
Hereditary Papillary Renal Carcinoma (HPRC)
Hereditary papillary renal carcinoma is an autosomal dominant hereditary cancer syndrome in which affected individuals are at risk of developing bilateral, multifocal type 1 papillary renal carcinoma, often at a late age of onset (50 to 70 years). (25) To date the kidney is the only organ to be affected in HPRC patients. The tumors are most often well differentiated; however, they are malignant and can metastasize. HPRC is a highly penetrant disease in which affected individuals are highly likely to develop bilateral, multifocal type 1 papillary kidney cancer. In the early reports this disease was described as having a late onset,(26;27); however, recently an early onset form of this disease has been described.(28) Radiographic interpretation of these lesions is difficult often due to their small size and poor enhancement on computed tomography which is attributed to their hypovascularity. Due to their hypovascularity, HPRC kidney tumors (and all type 1 papillary kidney tumors) may easily be misinterpreted as renal cysts. However, computed tomography is the imaging modality of choice as a screening tool due to its greater sensitivity in detecting these lesions. (29) Renal ultrasound may be very helpful in differentiating between a cyst and solid mass in a patient at risk for HPRC. Genetic evaluation of tumors in these families did not demonstrate abnormality on chromosome 3. Three years after describing the syndrome, researchers identified the gene responsible for HPRC on chromosome 7q31. (26;27) In this particular disease process activation of a proto-oncogene is the inciting event, not inactivation of a tumor suppressor gene, as is the case with VHL. Missense mutations in the tyrosine kinase domain of the Met proto-oncogene at 7q31 are responsible for constitutive activation of the MET protein in HPRC. The MET transmember protein is found at a receptor site for hepatocyte growth factor (also known as scatter factor). (30) Upon activation by hepatocyte growth factor, MET tyrosine phosphorylation induces the proliferation and differentiation of epithelial and endothelial cells, cell branching and invasion. (31) In sporadic papillary renal cell carcinoma, trisomy of chromosome 7, which contains HGF/SF and MET, occurs in 95% of patients. (32) In HPRC kidney tumors there is also trisomy of chromosome 7 and a non-random duplication of the chromosome bearing the mutated Met gene, implicating this event in tumorigenesis.(33) Molecular targeting approaches are being developed to inhibit the interaction of HGF and its receptor, and suppression of the downstream signaling cascade of activated c-MET. (5) These agents could then be used as a potential therapy for papillary renal cell carcinoma.
Birt Hogg Dube (BHD)
Birt-Hogg-Dubé is a hereditary cancer syndrome with an autosomal dominant inheritance pattern, in which affected persons are at risk to develop cutaneous fibrofolliculomas, pulmonary cysts, spontaneous pneumothoraces, and renal tumors. (34) The renal lesions are predominately derived from the distal nephron, and are bilateral and multifocal. The renal tumors are primarily chromophobe renal cell carcinomas (33%), hybrid tumors (50%) and oncocytomas (5%). Additionally, multifocal oncocystosis is evident in the surrounding normal renal parenchyma in 50%. Other forms of renal cell carcinoma have been identified in this patient population, to include clear cell carcinoma. (35)
Genetic linkage analysis was performed and used to localize(36) and subsequently to identify the BHD gene to 17p11.2.(37) The gene product is folliculin, and is truncated as a result of insertions, deletions or nonsense mutations.(38) Further investigations have demonstrated the BHD gene has the characteristics of a tumor suppressor gene. (39) A high frequency of mutations in the wild type allele was found when DNA of 77 renal tumors from 12 patients with germline BHD mutations were analyzed, thus providing the second “inactivating hit”. Mutation analysis of the BHD gene in the germline of kindreds suspected of being affected with BHD provide an opportunity for accurate diagnosis of this disease.
Hereditary Leiomyomatosis Renal Cell Cancer (HLRCC)
A fourth inherited renal cell carcinoma syndrome, hereditary leiomyomatosis renal cell cancer (HLRCC) was described in 2001 by Launonen and colleagues. (40) Patients are at risk to develop cutaneous and uterine leiomyomas and an aggressive type of kidney cancer that is often confused with type 2 papillary or collecting duct RCC.(41) The renal tumors in this syndrome are often solitary and unilateral, and they are more likely to be aggressive and lethal if allowed to progress. Most patients will develop cutaneous leiomyomas, and in the case of women, most will have uterine fibroids at a young age, and may have had a hysterectomy prior to formal diagnosis of HLRCC. Penetrance for RCC is lower than for the cutaneous and uterine manifestations, with only a minority (20–35%) of patients developing RCC.
The HLRCC gene was mapped to a region on 1q42–44, and is the site of the fumarate hydratase gene. (42) Fumarate hydratase is an essential enzyme for the conversion of fumarate to malate in the Krebs cycle. The loss of FH function and impediment of the Krebs cycles impairs the Kreb cycle therefore giving way to glycolic metabolism and upregulation of HIF and HIF inducible transcripts (Fig. 2). (43) Subsequent germline mutations were found in 52/56 (93%) in HLRCC families. (44) Elevation of HIF and inactivation of fumarate hydratase are potential molecular therapeutic targets for treatment of this aggressive type of kidney cancer.
Fig. 2.
Hereditary leiomyomatosis and renal cell cancer pseudohypoxia. Fumarate accumulates due to loss of FH activity. Rising fumarate levels inhibit HPH with concomitant upregulation of hypoxia response genes VEGF and GLUT-1. Reprinted from Sudarshan, et al.(53)
Treatment
Clinical management of patients with localized hereditary renal cancer has several goals: namely, preservation of renal function and prevention of metastatic disease. The tumors in these syndromes tend to be multifocal, bilateral and recurrent. Lessons learned from nephron-sparing surgery techniques applied to such patient with VHL can be translated into clinical management of sporadic multifocal renal cell carcinoma. Outcomes of 108 VHL patients whose renal lesions were less than 3cm were followed closely with serial imaging and were compared to 73 VHL patients with tumors larger than 3cm. Patients with lesions less than 3 cm did not develop metastatic disease. In contrast, 20 of the 73 (27%) of the patient with lesions larger than 3cm developed metastases. Median follow for both groups was greater than 5 years. (45) (46) Regardless, surgical extirpation remains the current standard for the treatment of localized renal cell carcinoma. The surgical options are many, and include, but are not limited to, open, laparoscopic, and robotic surgery, in addition to ablative techniques.
Due to the advances in the molecular and genetic biology of renal cell carcinoma, a paradigm shift has occurred in the treatment of patients with advanced renal cell carcinoma. While immunotherapeutic treatments such as interleukin-2 offer the only form of therapy which is associated with complete response, the majority of patients do not respond to this type therapy.(47) The identification of the VHL gene and its pathway has provided the foundation for targeted therapy for this disease.
Bevacizumab, a recombinant human monoclonal antibody to VEGF, has been shown to decrease angiogenesis in renal cell carcinoma by binding and neutralizing VEGF. (48) Other targeted agents, such as sunitinib, a small molecule tyrosine kinase inhibitor of VEGFR-2 and platelet derived growth factor (PDGFR-B), provide a rational approach in the treatment of clear cell kidney cancer. In a single arm, phase II trial of 106 cytokine refractory metastatic clear cell RCC patients, 34% of patients experienced a partial response with a median progression free survival of 8.3 months.(49) A phase III trial, conducted by Motzer, et. al., demonstrated in previously untreated metastatic RCC patients, progression free survival was 11 months versus 5 months in favor of Sunitinib over interferon alpha. (50)
Sorafenib is a Raf kinase inhibitor in addition to an inhibitor of VEGFR-2, VEGFR-3, and PDGFR-B. In a phase II, randomized discontinuation study, sorafenib was associated with a prolonged progression free survival, compared to placebo in patients with metastatic RCC. (51) A subsequent Phase III randomized trial demonstrated a significant prolongation of progression free survival versus placebo in patients with advanced clear cell renal cell carcinoma in whom had previously failed therapy. (52)
Conclusion
In summary, kidney cancer is a heterogeneous disease. As research continues and our understanding of the molecular genetics of renal cell carcinoma expands, improvement in the diagnosis, prevention and treatment of these malignancies is likely. Agents that target the VHL/HIF pathway in patients with advanced clear cell carcinoma have had encouraging results. Combination of therapies, i.e., use of agents that target multiple aspects of the pathway, are currently being studied.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Reference List
- 1.SEER Cancer Statistics Review, 1975–2005. NCI. 2007 (November 2007 SEER data submission. Available from URL: http://seer.cancer.gov/csr/1975_2005/results_merged/sect_01_overview.pdf and http://seer.cancer.gov/csr/1975_2005/results_merged/sect_11_kidney_pelvis.pdf).
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. Journal of the American Medical Association. 1999 May 5;281(17):1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Uchida K, Miyao N, Masumori N, Takahashi A, Oda T, Yanase M, et al. Recurrence of renal cell carcinoma more than 5 years after nephrectomy. Int J Urol. 2002 Jan;9(1):19–23. doi: 10.1046/j.1442-2042.2002.00418.x. [DOI] [PubMed] [Google Scholar]
- 4.Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003 Dec;170(6 Pt 1):2163–72. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 5.Linehan WM, Vasselli J, Srinivasan R, Walther MM, Merino MJ, Choyke P, et al. Genetic Basis of Cancer of the Kidney: Disease-Specific Approaches to Therapy. Clin Cancer Res. 2004 Sep 15;10(18):6282S–9S. doi: 10.1158/1078-0432.CCR-050013. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs G, Emanuel A, Neumann HP, Kung HF. Cytogenetics of renal cell carcinomas associated with von Hippel-Lindau disease. Genes Chromosomes Cancer. 1991 Jul;3(4):256–62. doi: 10.1002/gcc.2870030404. [DOI] [PubMed] [Google Scholar]
- 7.Zbar B, Brauch H, Talmadge C, Linehan WM. Loss of alleles of loci on the short arm of chromosome 3 in renal cell carcinoma. Nature. 1987;327:721–4. doi: 10.1038/327721a0. [DOI] [PubMed] [Google Scholar]
- 8.Knudson AG. Genetics of human cancer. Annu Rev Genet. 1986;20:231–51. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- 9.Walther MM, Lubensky IA, Venzon D, Zbar B, Linehan WM. Prevalence of microscopic lesions in grossly normal renal parenchyma from patients with von Hippel-Lindau disease, sporadic renal cell carcinoma and no renal disease: clinical implications. J Urol. 1995 Dec;154(6):2010–4. [PubMed] [Google Scholar]
- 10.Tory K, Brauch H, Linehan WM, Barba D, Oldfield E, Filling-Katz M, et al. Specific genetic change in tumors associated with von Hippel-Lindau disease. J Natl Cancer Inst. 1989;81:1097–101. doi: 10.1093/jnci/81.14.1097. [DOI] [PubMed] [Google Scholar]
- 11.Latif F, Tory K, Gnarra JR, Yao M, Duh F-M, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–20. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 12.Stolle C, Glenn GM, Zbar B, Humphrey JS, Choyke P, Walther MM, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat. 1998;12(6):417–23. doi: 10.1002/(SICI)1098-1004(1998)12:6<417::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Chen F, Kishida T, Yao M, Hustad T, Glavac D, Dean M, et al. Germline mutations in the von Hippel-Lindau disease tumor suppressor gene: correlations with phenotype. Hum Mutat. 1995;5(1):66–75. doi: 10.1002/humu.1380050109. [DOI] [PubMed] [Google Scholar]
- 14.Ong KR, Woodward ER, Killick P, Lim C, MacDonald F, Maher ER. Genotype-phenotype correlations in von Hippel-Lindau disease. Hum Mutat. 2007 Feb;28(2):143–9. doi: 10.1002/humu.20385. [DOI] [PubMed] [Google Scholar]
- 15.Neumann HPH, Zbar B. Renal cysts, renal cancer and von Hippel-Lindau disease. Kidney Int. 1997;51(1):16–26. doi: 10.1038/ki.1997.3. [DOI] [PubMed] [Google Scholar]
- 16.Maranchie JK, Afonso A, Albert PS, Kalyandrug S, Phillips JL, Zhou S, et al. Solid renal tumor severity in von Hippel Lindau disease is related to germline deletion length and location. Hum Mutat. 2004 Jan;23(1):40–6. doi: 10.1002/humu.10302. [DOI] [PubMed] [Google Scholar]
- 17.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Gen. 1994 May;7(1):85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 18.Shuin T, Kondo K, Torigoe S, Kishida T, Kubota Y, Hosaka M, et al. Frequent somatic mutations and loss of heterozygosity of the von Hippel-lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994;54:2852–5. [PubMed] [Google Scholar]
- 19.Duan DR, Pause A, Burgess WH, Aso T, Chen DY, Garrett KP, et al. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995 Sep 8;269(5229):1402–6. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- 20.Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG., Jr Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269:1444–6. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 21.Pause A, Lee S, Worrell RA, Chen DY, Burgess WH, Linehan WM, et al. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proceedings of the National Academy of Sciences USA. 1997 Mar 18;94(6):2156–61. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iliopoulos O, Jiang C, Levy AP, Kaelin WG, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proceedings of the National Academy of Sciences USA. 1996;93(20):10595–9. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brugarolas J, Kaelin WG., Jr Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell. 2004 Jul;6(1):7–10. doi: 10.1016/j.ccr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Linehan WM. Editorial: Kidney Cancer: A Unique Opportunity for the Development of Disease Specific Therapy. J Urol. 2002 Dec;168:2411–2. doi: 10.1016/S0022-5347(05)64156-0. [DOI] [PubMed] [Google Scholar]
- 25.Zbar B, Tory K, Merino MJ, Schmidt L, Glenn GM, Choyke P, et al. Hereditary papillary renal cell carcinoma. J Urol. 1994 Mar;151(3):561–6. doi: 10.1016/s0022-5347(17)35015-2. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt L, Duh FM, Chen F, Kishida T, Glenn GM, Choyke P, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Gen. 1997 May;16(1):68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt L, Junker K, Weirich G, Glenn GM, Choyke P, Lubensky IA, et al. Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene. Cancer Res. 1998 April 15;58:1719–22. [PubMed] [Google Scholar]
- 28.Schmidt LS, Nickerson ML, Angeloni D, Glenn GM, Walther MM, Albert PS, et al. Early onset Hereditary Papillary Renal Carcinoma: germline missense mutations in the tyrosine kinase domain of the Met proto-oncogene. J Urol. 2004 Oct;172(4 Part 1 Of 2):1256–61. doi: 10.1097/01.ju.0000139583.63354.e0. [DOI] [PubMed] [Google Scholar]
- 29.Choyke PL, Walther MM, Glenn GM, Wagner JR, Venzon DJ, Lubensky IA, et al. Imaging Features of Hereditary Papillary Renal Cancers. Journal of Computer Assisted Tomography. 1997;21(1997):737–41. doi: 10.1097/00004728-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991 Feb 15;251(4995):802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 31.Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem. 2003 Feb 1;88(2):408–17. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs G. Molecular cytogenetics of renal cell tumors. Adv Cancer Res. 1993;62:89–124. doi: 10.1016/s0065-230x(08)60316-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang Z, Park WS, Pack S, Schmidt L, Pak E, Pham T, et al. Trisomy 7 -harboring non-random duplication of the mutant MET allele in hereditary papillary renal carcinomas. Nat Gen. 1998;20(September):66–9. doi: 10.1038/1727. [DOI] [PubMed] [Google Scholar]
- 34.Zbar B, Alvord WG, Glenn GM, Turner M, Pavlovich CP, Schmidt L, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dube syndrome. Cancer Epidemiol Biomarkers Prev. 2002 Apr;11(4):393–400. [PubMed] [Google Scholar]
- 35.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, et al. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol. 2002 Dec;26(12):1542–52. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt LS, Warren MB, Nickerson ML, Weirich G, Matrosova V, Toro JR, et al. Birt-Hogg-Dube syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. Am J Hum Genet. 2001 Oct;69(4):876–82. doi: 10.1086/323744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn GM, Turner ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell. 2002 Aug;2(2):157–64. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dube syndrome. Am J Hum Genet. 2005 Jun;76(6):1023–33. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vocke CD, Yang Y, Pavlovich CP, Schmidt LS, Nickerson ML, Torres-Cabala CA, et al. High Frequency of Somatic Frameshift BHD Gene Mutations in Birt-Hogg-Dube-Associated Renal Tumors. J Natl Cancer Inst. 2005 Jun 15;97(12):931–5. doi: 10.1093/jnci/dji154. [DOI] [PubMed] [Google Scholar]
- 40.Launonen V, Vierimaa O, Kiuru M, Isola J, Roth S, Pukkala E, et al. Inherited Susceptibility to uterine leiomyomas and renal cell cancer. Proceedings of the National Academy of Sciences USA. 2001 Mar 13;98(6):3387–2. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merino MJ, Torres-Cabala C, Pinto P, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007 Oct;31(10):1578–85. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 42.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Gen. 2002 Apr;30(4):406–10. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 43.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005 Aug;8(2):143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Toro JR, Nickerson ML, Wei MH, Warren MB, Glenn GM, Turner ML, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003 Jul;73(1):95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walther MM, Choyke PL, Glenn GM, Lyne JC, Rayford W, Venzon D, et al. Renal Cancer in Families with Hereditary Renal Cancer: Prospective Analysis of a Tumor Size Threshold for Renal Parenchymal Sparing Surgery. J Urol. 1999;161(5):1475–9. doi: 10.1016/s0022-5347(05)68930-6. [DOI] [PubMed] [Google Scholar]
- 46.Duffey BG, Choyke PL, Glenn GM, Grubb RL, Venzon D, Linehan WM, et al. The Relationship Between Renal Tumor Size and Metastases in Patients with von Hippel-Lindau Disease. J Urol. 2004 Jul;172(1):63–5. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 47.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. Journal of Clinical Oncology. 2003 Aug 15;21(16):3127–32. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003 Jul 31;349(5):427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006 Jun 7;295(21):2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 50.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007 Jan 11;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 51.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology. 2006 Jun 1;24(16):2505–12. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 52.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007 Jan 11;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 53.Sudarshan S, Linehan WM, Neckers L. HIF and fumarate hydratase in renal cancer. Br J Cancer. 2007;96(3):403–407. doi: 10.1038/sj.bjc.6603547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linehan WM, Zbar B. Focus on kidney cancer. Cancer Cell. 2004;6:223–8. doi: 10.1016/j.ccr.2004.09.006. [DOI] [PubMed] [Google Scholar]