Abstract
Bipolar I disorder (BPI) is among the top 10 most debilitating illnesses globally. In this critical review, we first review recent functional neuroimaging findings in two core domains of pathology in BPI: emotion processing and executive control. These abnormalities in core domains represent potential endophenotypes of the illness. We then show that an emerging number of studies have demonstrated that neuroimaging can help to identify endophenotypic markers whose identification would help to clarify diagnosis and discriminate the depression in BPI from that of unipolar depression and identify at-risk individuals who will subsequently develop the illness (to facilitate early intervention). We end by reviewing the new directions in neuroimaging in BPI, including studies of children with bipolar disorder, potential medication effects, and the use of newer neuroimaging techniques to help us achieve the previously mentioned goals of improving BPI patients' mental well-being.
Introduction
Bipolar I disorder (BPI) affects at least 1% of the population, is associated with increased mortality, and is among the top 10 most disabling illnesses worldwide. The disorder also presents a significant societal cost, with poor clinical and functional outcome, and is associated with a significant suicidality increase [1].
BPI is a disorder in which patients experience episodes of depression and clear manic episodes. When one takes into account those with bipolar II and subthreshold bipolar disorder capturing those with briefer or only treatment-emergent hypomania, lifetime prevalence rates approach 5% [2].
One major issue in diagnosing and treating bipolar disorder is the high rate of misdiagnosis or late diagnosis. In one community sample of diagnosed bipolar disorder patients, approximately 70% had a missed diagnosis. A total of 60% of those were diagnosed with major depressive disorder, with one third going 10 years or more without a correct diagnosis. In addition, these patients had on average 3.5 other diagnoses and saw on average four physicians before receiving the correct diagnosis [3].
Also, the depression associated with bipolar disorder frequently continues to be treated as unipolar depression (UPD). Bipolar depression has been shown to respond poorly to antidepressants [4] and is associated with increased rates of switching to a manic state. As such, once bipolar disorder or UPD is correctly diagnosed, providing effective treatment is a primary concern.
Taken together, these findings highlight two major dilemmas for psychiatrists trying to provide effective treatment for depression: 1) correctly diagnosing depression as belonging to UPD or bipolar disorder and 2) initiating and individualizing treatment as early as possible.
The recently released research agenda for the DSMV stresses an etiologic- and pathophysiologically based diagnostic system, as opposed to the symptomatologic and syndromic approach of the third and fourth editions. For each affected individual, these pathophysiologic processes involve dynamic interplay among genetic, epigenetic, and environmental factors interacting to create a cumulative liability to illness expressed as a phenotype. The greater the number of factors involved in generating an illness, the more complex the phenotype and the more complex any attempt at genetic analysis will be. An endophenotype is an objective marker—often used synonymously with “biomarker” or “vulnerability marker”—that is more closely related to the disorder's underlying pathophysiology than the condition's “fuzzy” symptomatology and therefore can be used to classify and diagnose complex psychiatric diseases. Endophenotypes should be consistently associated with the illness and represent persistent “trait” rather than episodic or “state” features. They also should be found in high-risk individuals such as nonaffected first-degree family members at a higher rate than in the population [5].
Identifying endophenotypic markers for bipolar disorder at this time would seek to serve two main goals: 1) to clarify diagnosis and discriminate the depression in bipolar disorder from that of UPD to treat accordingly and 2) to identify at-risk individuals for early diagnosis with the goal of intervening before illness onset.
Neuroimaging techniques are now some of the most powerful tools we have for identifying endophenotypes. Brain imaging techniques such as MRI, positron emission tomography (PET), and diffusion tensor imaging (DTI) are being used increasingly to directly quantify neural system abnormalities associated with bipolar disorder and UPD. These neural system abnormalities are in turn providing direct evidence of disorder-specific abnormalities in neural systems. These neural systems are linked to core domains of pathology in bipolar disorder and UPD and, therefore, endophenotypes to target the previously described treatment goals.
In this critical review, we scrutinize recent findings from neuroimaging studies examining these core domains of pathology in bipolar disorder and UPD, as well as emerging studies seeking to define biomarkers differentiating the depression in bipolar disorder from that of UPD.
Core domains of pathology in bipolar disorder: impaired emotion processing and executive control
Bipolar disorder exists as a multisystem disorder involving disruptions across several symptom domains [6]. One core domain of pathology is mood instability, which presents as variable states of depression and/or mania or hypomania, as well as other aspects of affective lability, including anxiety and irritability. A second core domain implicated in bipolar disorder is disrupted cognitive function, specifically impaired executive control. This refers to a broad collection of cognitive processes, including attention, working memory, and inhibitory control, that together allow the flexible organization of complex behavior [7]. Executive function disruptions can result in altered concentration, decision making, and memory. These in turn likely will impact upon daily living activities, occupational productivity, and general social function. Executive deficits are likely to further impair the ability to regulate mood [8]: individuals with bipolar disorder may be unable to use appropriate cognitive-control processes such as reappraisal, suppression, or inhibitory processes to regulate and maintain appropriate emotional states [9].
Neural systems underlying emotion processing and executive control
Existing data in healthy individuals point toward distinguishable neural systems for emotion processing and executive control. Functional neuroanatomic investigations in healthy individuals to date implicate a network of subcortical anterior limbic structures for appropriate emotion processing. These include such regions as the amygdala, ventral striatum, subgenual (ventral) cingulate, ventromedial prefrontal cortex, anterior hippocampus, and anterior insula [10].
However, neural systems responsible for coordinating appropriate executive function include lateral prefrontal cortical areas containing dorsolateral (DLPFC) and ventrolateral prefrontal cortex (VLPFC) [11], which are closely interconnected with striatal regions involved in response selection and the hippocampus, a key node in memory encoding and retrieval [12].
Abnormalities in neural systems underlying emotion processing and executive control in bipolar disorder
Relevant findings in bipolar disorder to date have centered around functional and structural neuroimaging studies. We first describe findings from functional neuroimaging studies that have helped to elucidate the nature of emotion processing and executive control neural system abnormalities in bipolar disorder. We then review the most pertinent structural neuroimaging findings in bipolar disorder studies. We end by reviewing the emerging trends and technologies that may increase understanding of neural system abnormalities in bipolar disorder.
Functional Neuroimaging Studies of Bipolar Disorder
Here we review findings from recent functional neuroimaging studies, the majority of which have used functional MRI (fMRI) because of its good spatial and temporal resolution. We focus on studies that have used specific emotion processing and executive control paradigms in examining functional neural abnormalities in bipolar disorder.
Emotion Processing
Most investigations to date have examined individuals in the depressed or manic state, with a few examining euthymic states. Paradigms investigating mood instability and emotion processing have used a variety of stimuli, including emotional scripts, words, and music. One frequently used stimulus is emotional faces. The human face is a complex stimulus, carrying information regarding social (age, identity, gaze, sex) and affective cues that are recognized throughout different races and cultures to represent specific emotions [13].
Remitted bipolar disorder
Few studies have examined neural response to emotional challenges in remitted (euthymic) individuals with bipolar disorder. Studies investigating response to emotional stimuli have demonstrated increased subcortical (amygdala and ventral striatal) activity to mildly happy [14••] and intense fearful expressions [14••,15]. Another study demonstrated increased amygdala activity to happy expressions in a mixed group of remitted and symptomatic bipolar disorder patients [16•]. The largest functional neuroimaging study to date in euthymic bipolar disorder [17•] demonstrated that emotional facial stimuli in an emotional Go/No-Go task resulted in altered emotional modulation of cognitive processing; this then resulted in a ventral limbic activation increase with the task's emotional component.
Other studies using emotional words as opposed to faces have demonstrated widespread decreases in subcortical and prefrontal cortical neural activity in remitted individuals with BPI [18•]. This suggests that emotional facial expressions may be processed distinctly from other emotional stimuli for remitted individuals with bipolar disorder and may be particularly salient as socially relevant environmental stimuli.
Bipolar depression
Studies to date have demonstrated increased subcortical limbic activity to emotional facial expressions in stable bipolar individuals with subsyndromal depression [14••,16•] and to visualized scenes (captioned photographs) in depressed bipolar individuals [19] compared with healthy controls. Also, depressed bipolar disorder patients have demonstrated increased subcortical limbic activity to positive stimuli such as happy faces [20•] when compared with manic bipolar disorder patients and healthy controls. The latter pattern of increased subcortical limbic activity to positive emotional stimuli may distinguish bipolar from unipolar depressed individuals.
Mania
Studies in manic individuals also have demonstrated increased subcortical limbic activity to emotional stimuli, particularly increased subcortical limbic activity to negative emotional stimuli. Relative to healthy individuals, manic bipolar disorder patients demonstrate increased amygdala, insula, and limbic subcortical activity during implicit processing (ie, gender labeling or expression matching) of negative emotional facial expressions and to negative scenes [20•,21,22•,23]. Manic individuals also have demonstrated decreases in subcortical activity [20•,22•] during explicit, emotion labeling of sad faces. The inconsistent findings regarding subcortical activity patterns in manic individuals during implicit emotion processing may result from different levels of attentional engagement in implicit versus explicit emotion-processing tasks.
Executive Control
The second core domain of pathology noted to be altered in those with bipolar disorder is poor cognition, specifically executive dysfunction. Executive function attributes such as working memory and attention have been most frequently examined (as has decision making). Working memory tasks such as the digit-sorting task have been shown to reliably increase DLPFC activity, whereas attentional tasks, including the Stroop interference task and continuous performance task, have implicated DLPFC, dorsal regions of the anterior cingulate gyrus, and ventral prefrontal cortex (VPFC) in healthy controls [24,25]. The majority of functional studies investigating cognition in bipolar disorder have been performed in euthymic individuals; however, data regarding cognition in mood states are emerging.
Remitted bipolar disorder
Euthymic individuals with bipolar disorder have demonstrated poor task performance on executive measures such as the Stroop task [26•], with reduced dorsal and ventral prefrontal cortical [19,27] and dorsal cingulate [28] activity on working memory tasks compared with healthy controls.
Within subcortical regions associated with emotion processing rather than executive function, activity increases have been shown during performance of a working memory task [29], suggesting that bipolar individuals may be using neural systems implicated in emotion processing during nonemotional task performance.
Bipolar depression
Limited studies exist that investigate executive function in bipolar depression. One recent study [30] demonstrated decreased dorsal cingulate activity during a color–word Stroop task and increased striatal activity during a paced motor task in bipolar depressed individuals compared with controls. One study examining remitted patients with subsyndromal depression during an attentional task [31] found decreased VLPFC activity with depression severity correlating negatively with the magnitude of VLPFC decrease (ie, greater depression severity was associated with more normalized VLPFC activity). Similarly, a study [19] comparing neural activity during attentional Stroop task performance in euthymic versus depressed individuals with bipolar disorder showed relative increases in ventrolateral pre-frontal cortical activity in bipolar depressed compared with euthymic individuals. These studies suggest a more complex pattern of functional abnormality in lateral PFC that may distinguish bipolar depression from bipolar remission, with bipolar depression showing more normalized VLPFC activity (Table 1).
Table 1.
Neural activity during emotion processing and cognitive-challenge tasks in BP and UPD
| BP remitted | BP depressed | BP manic | Unipolar depressed | |
|---|---|---|---|---|
| Emotion processing | Increased amygdala and ventral striatal activity to positive [14••,52] and negative stimuli [15]; decreased subcortical activity to emotional words [18•] | Increased amygdala and ventral striatal activity to positive [14••,20•] and negative stimuli [14••,44,45,48] | Increased amygdala and ventral striatal activity to negative stimuli [19,20•,21] | Increased amygdala and ventral striatal activity to negative but not positive stimuli [14••,45] |
| Cognitive control | Decreased DLPFC and VPFC [19,27] | Increased DLPFC and VPFC relative to BP remitted [19,31] | Decreased DLPFC and VPFC [19,21] | Decreased DLPFC [46] |
BP—bipolar disorder; DLPFC—dorsolateral prefrontal cortex; UPD—unipolar depression; VPFC—ventral prefrontal cortex. (Adapted from Phillips and Vieta [9].)
Mania
Mania is what clearly establishes a bipolar disorder diagnosis rather than one of UPD. To fully understand bipolar disorder's pathology and pathophysiology, we must examine functional abnormalities in neural systems that may persist across mania, depression, and remission. Functional studies have demonstrated decreased ventral prefrontal cortical activity during performance of a variety of cognitive control tasks [21]. One study looking at state versus trait characteristics [19] demonstrated a distinct decrease across all mood states in rostral left ventral PFC. In addition, state differences were found, with elevated mood being associated with blunted activity in the right VPFC, whereas depressed mood was associated with an increase in the left VPFC.
Findings from PET Studies in Bipolar Disorder
Although PET is an invasive procedure and has poorer spatial resolution than fMRI, PET has the advantage over fMRI of being able to measure perfusion and/or metabolism. As such, it has been used to measure resting state activity in individuals with bipolar disorder.
Resting state
Resting state PET studies in depressed BPI, bipolar II, and manic individuals have shown increased amygdalar and ventral striatal limbic subcortical activity compared with healthy controls [32-34].
Emotion processing
In remitted and depressed patients with bipolar disorder, decreased blood flow was shown in medial prefrontal cortex during sad mood induction relative to baseline [35], with lateral prefrontal decrease unique to depressed individuals. However, this study did not include a group of comparison healthy individuals. Another study using a decision-making task [36] showed increased dorsal anterior cingulate and decreased orbitofrontal cortex (OFC) activity during mania. OFC in turn has been implicated as important in the regulation of visceromotor and emotional behavioral control.
Executive control
An earlier study using an auditory discrimination continuous performance task (CPT) found that depressed bipolar disorder patients had absolute decreased prefrontal cortex and increased, normalized, subcortical (ventral striatum, thalamus, right amygdala) metabolism compared with healthy controls. Degree of depression correlated negatively with prefrontal and positively with anterior paralimbic subcortical metabolism [37]. A more recent study examining metabolism during the CPT showed that decreased subgenual prefrontal metabolism was associated with slower hit rate reaction time and more omission errors in a depressed bipolar group but not in the control group [38]. Decreased DLPFC metabolism in the bipolar group also was associated with more commission errors, suggesting failure to activate areas important in cognitive control.
Structural neuroimaging findings implicating these core domains
Structural neuroimaging studies in adult BPI broadly support findings from functional neuroimaging studies. In adults, there are findings of enlarged amygdalae [39], decreased dorsal and ventral prefrontal cortices [40], and smaller [41] or no change in hippocampi. Pediatric findings differ slightly.
Taken together, these findings implicate functional and structural abnormalities in distinct, distributed neural systems for emotion processing and executive control in bipolar disorder. We next examine the extent to which functional neuroimaging study findings suggest a distinction between functional abnormalities in neural systems underlying emotion processing and executive control in bipolar disorder versus those reported in UPD.
Neural systems underlying emotion processing and executive control in UPD
Given the diagnostic difficulties inherent in accurately diagnosing the depression of bipolar disorder, we must look to findings that may distinguish individuals with bipolar disorder from those with UPD. The majority of studies in UPD pre- and post-treatment are resting state studies, and neural responses are somewhat discrepant in regard to limbic subcortical and prefrontal activation [42,43]. However, findings from studies using emotional challenge paradigms suggest that similar to bipolar disorder individuals, UPD individuals show increased amygdala and subcortical activity to emotional stimuli [44,45] and decreased lateral prefrontal activity in response to cognitive-control tasks compared with healthy individuals [46] and after exposure to emotional stimuli [47]. However, three findings from neuroimaging studies may distinguish bipolar disorder from UPD.
First, in UPD, there appears to be a state dependence of functional neural abnormalities in that abnormal neural activity amelioration has been shown after remission. For example, abnormally increased amygdala activity to negative emotional faces in UPD individuals is significantly reduced in remission after antidepressant medication administration [44,45,48].
Second, in UPD, unlike BPI, findings suggest that limbic subcortical activation abnormalities may occur in response to some negative, but not positive, emotional stimuli [45] (Table 1). Only one functional neuroimaging study to date has directly compared BPI and UPD individuals [14••], and here BPI remitted distinguished itself from UPD by amygdala activity to positive emotional stimuli (Table 1).
Third, findings indicate that depressed bipolar individuals display increased lateral prefrontal cortex activity relative to remission during attentional-control tasks [19,31]. However, it is unclear whether this is the case in depressed relative to remitted individuals with UPD. Some longitudinal studies, albeit studying individuals at rest rather than during attentional-control tasks [42], indicate a pattern of increased activity in DLPFC in remission relative to depressed state in UPD. The nature of the differential pattern of dorsolateral prefrontal cortical activity during attentional-control tasks in the depressed state and remission therefore may distinguish bipolar and nonbipolar individuals.
New Directions in Bipolar Neuroimaging
As the field of psychiatric neuroimaging develops, several key areas will gain importance: neuroimaging in pediatric bipolar disorder, examining medication effects, emerging technologies, and using neuroimaging to answer key clinical questions.
Pediatric bipolar disorder
Bipolar disorder is increasingly thought to be a complex psychiatric disorder with evidence of long-term neuro-developmental disruption. As such, one must look to examine alterations in these core domains in younger populations. Studies have shown that children and adolescents with bipolar disorder demonstrate poor executive function [49] and emotional regulation [50].
Functional studies investigating these core domains in remitted, euthymic adolescents with bipolar disorder have demonstrated increases in activity within subcortical regions associated with emotion processing rather than working memory or attention during Stroop attentional task performance [51]. Also, a lack of age-related activation increase in VPFC was noted in healthy controls during the task. Another study demonstrated increases in DLPFC and subcortical activation during a working memory task [52].
During an emotional face-processing task, adolescents with bipolar disorder reported greater hostility in neutral faces than did controls and overall demonstrated increased subcortical activity to emotional faces [49] compared with healthy controls. This supports findings of impaired social cognition and response flexibility in euthymic adolescents with bipolar disorder [53]. Another study showed increased subcortical activity to positive stimuli but increased DLPFC activity to negative stimuli in adolescents with bipolar disorder [52].
To date, the majority of neuroimaging investigations in children and adolescents have been structural studies. These have implicated cortical [54] and subcortical [51,52,55] areas as having structural alterations. Studies in adolescent and first-episode bipolar disorder [56] and one longitudinal study of adolescents [55] demonstrate smaller amygdalae and smaller [51] or no change in hippocampus in adolescent bipolar disorder, which differs from the previously described findings of enlarged amygdalae in adults. In examining prefrontal volumes, earlier pediatric studies failed to detect any difference; however, a more recent study in children and adolescents using a more sensitive, voxel-based, morphometric, automated technique showed decreased volume of DLPFC and amygdala and specific striatal structures [54]. Other recent findings indicate decreased VPFC in adolescents with bipolar disorder [57•]. Given the differential findings between children and adolescents with bipolar disorder and adults in key areas such as the amygdala, further study must be done to determine possible differences between those with early- versus late-onset and/or diagnosis and to track change over development, including possible compensatory mechanisms.
BPI Versus Bipolar II Disorder
Most studies to date have focused on BPI; however, emerging data show bipolar disorder to exist on a spectrum affecting up to 5% of individuals [2]. As such, future studies should attempt to define pathophysiology in bipolar II disorder, as did the recent study [33] using PET that demonstrated increased limbic subcortical activity at baseline in bipolar II.
Medication Effects in Neuroimaging
Most neuroimaging studies of bipolar disorder have recruited medicated individuals, as these are more representative of the bipolar population, and it often is clinically unfeasible and ethically problematic to withdraw such individuals from medication. However, surprisingly few bipolar disorder studies have examined associations between psychotropic medication and neural activity. Recent studies have acknowledged this potential confound. However, they have either not examined the association [18•] or subdivided individuals into different medication-related subgroups (medication-free, lithium, anticonvulsive, antipsychotic, antidepressant) [14••,16•,17•]. Studies examining emotion processing demonstrated no significant [17•] or decreased amygdala activity [16•] in medicated versus unmedicated subgroups, whereas those using cognitive-control paradigms reported no significant [19,31] or increased DLPFC activation [26•] in medicated versus unmedicated subgroups.
Other studies examined correlations between chlorpromazine dose equivalents and neural activity in bipolar individuals taking antipsychotics [15,28]. They reported significant negative correlations between dose equivalent and amygdala activity to emotional stimuli in bipolar males [15] and significant positive correlations between antipsychotic dose and dorsal prefrontal cortex activity during selective attention tasks in bipolar adults [28].
Together, these findings indicate that medicated more so than unmedicated bipolar individuals show decreased subcortical limbic activity during emotion processing and increased dorsal prefrontal cortical activity during cognitive-control paradigms (ie, with activity levels in these regions more closely resembling those of healthy individuals). These reports suggest that functional neural abnormalities observed in bipolar individuals likely reflect pathophysiologic processes that may be ameliorated by (rather than being abnormalities secondary to) psychotropic medication. No studies have examined the impact of total psychotropic medication load, reflecting medication dose and variety, upon neural activity in bipolar disorder. Therefore, this remains a major limitation of current neuroimaging studies of the disorder.
Emerging Technologies
We have focused on studies using structural and functional MRI, as well as PET. However, other imaging modalities are being used increasingly. Two modalities are magnetoencephalography (MEG) and DTI. MEG examines the time course of magnetic activity associated with neural activation; however, it lacks adequate spatial localization. It has been used in emotional paradigms to clarify neural activation's time course to emotional facial stimuli [58], but this has yet to be done in bipolar disorder. DTI is a more sensitive MRI probe that investigates the directional element of osmotic diffusion or fractional anisotropy (FA). Disruption of axonal bundle sheath integrity results in FA loss. Whereas other measures have failed to perceive consistent changes in white matter architecture, DTI has demonstrated decreased FA in prefrontal white matter. This suggests changes in pathway integrity in a way that may represent axonal disorganization as opposed to destruction. Therefore, it may represent abnormal neural development [29].
Emerging strategies also will increasingly use multimodal technologies. For example, fMRI or DTI could be used in concert with MEG or PET to provide spatial information in concert with temporal activity measures to elucidate the time course of activation within neural circuits and spatial localization. One [59•] combined MEG and magnetic resonance spectroscopy, a form of MRI using different chemical signals, to look at antidepressant treatment in UPD and demonstrated normalized habituation pattern to auditory stimuli after treatment. Similar studies in bipolar disorder could help to clarify the timing and the location of circuits implicated in bipolar disorder's pathogenesis and to refine treatment-relevant endophenotypes.
Conclusions
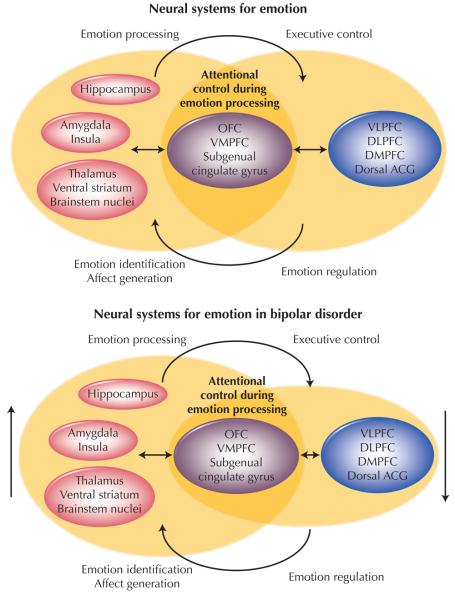
Psychiatric neuroimaging is an emerging field whose capabilities are only beginning to be demonstrated. Studies in bipolar disorder now validate clinical findings of disruptions in core domains of pathology in bipolar disorder. Chiefly, we see altered emotion processing and disrupted executive control. An emerging number of studies also have demonstrated that neuroimaging can help to identify endophenotypes to discriminate between bipolar disorder and UPD. Therefore, it may be a useful technique to help with future diagnosis of these disorders. Functional neuroimaging studies in bipolar disorder should now focus on the functional integrity of neural systems more specifically associated with emotion regulation by using paradigms designed to directly measure these processes. Such paradigms include tasks involving attentional control during emotional stimuli processing that likely recruit lateral prefrontal cortical regions implicated in executive control. They also include those tasks involving decision making about emotional material that recruit OFC [36]. The combination of functional abnormalities in limbic subcortical neural regions implicated in emotion processing together with functional abnormalities in the previously mentioned prefrontal cortical neural regions probably result in the emotional lability and impaired ability to regulate emotion, respectively [8] (Fig. 1).
Figure 1.
Two overlapping neural systems implicated in bipolar disorder. An anterior limbic subcortical system (left) is responsible for emotion processing. Lateral prefrontal cortical regions (right) are implicated in executive control. These two systems interact and overlap in ventral frontal areas such as the orbitofrontal cortex (OFC) that are responsible for decision making about emotional material and attentional control during emotional stimuli processing. Directional findings in bipolar disorder are represented by size of nodes and vertical arrows. DLFPC—dorsolateral prefrontal cortex; DMPFC—dorsomedial prefrontal cortex; Dorsal ACG—dorsal cingulate gyrus; VLPFC—ventrolateral prefrontal cortex; VMPFC—ventromedial prefrontal cortex.
Studies now are beginning to examine neural systems underlying these core domains of pathology in children with bipolar disorder and those at high risk for developing the illness. As new protocols, techniques, and technologies emerge, we now have the possibility of addressing important clinical questions, including the identification of treatment-relevant endophenotypes to create treatment response groupings to meet the long-term goal of rational treatment advances for bipolar disorder.
Acknowledgments
Neither author has a possible conflict of interest, financial or otherwise.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Baldessarini RJ, Tondo L. Suicide risk and treatments for patients with bipolar disorder. JAMA. 2003;290:1517–1519. doi: 10.1001/jama.290.11.1517. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the National Depressive and Manic-depressive Association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64:161–174. [PubMed] [Google Scholar]
- 4.Fagiolini A, Frank E, Cherry CR, et al. Clinical indicators for the use of antidepressants in the treatment of bipolar I depression. Bipolar Disord. 2002;5:277–282. doi: 10.1034/j.1399-5618.2002.01222.x. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 6.Phillips ML, Frank E. Redefining bipolar disorder-toward DSM-V. Am J Psychiatry. 2006;163:1135–1136. doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]
- 7.Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2000;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- 8.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 9.Phillips ML, Vieta E. Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophr Bull. 2007;33:893–904. doi: 10.1093/schbul/sbm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips ML, Drevets WC, Rauch SL, et al. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 11.Robbins TW. Dissociating executive functions of the prefrontal cortex. In: Roberts AC, Robbins TW, Weiskrantz LR, editors. The Prefrontal Cortex: Executive and Cognitive Functions. Oxford University Press; New York: 1998. [Google Scholar]
- 12.Zola-Morgan S, Squire LR, Alvarez-Royo P, et al. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdale. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]
- 13.Ekman P. Facial expression of emotion: an old controversy and new findings. Philos Trans R Soc London B Biol Sci. 1992;335:63–69. doi: 10.1098/rstb.1992.0008. [DOI] [PubMed] [Google Scholar]
- 14••.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. The only functional neuroimaging study to date to directly compare bipolar disorder and UPD during emotion processing. [DOI] [PubMed] [Google Scholar]
- 15.Yurgelun-Todd DA, Gruber SA, Kanayama G, et al. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 16•.Blumberg HP, Donegan NH, Sanislow CA, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. Demonstrated decreased subcortical reactivity to facial expressions in medicated bipolar disorder. [DOI] [PubMed] [Google Scholar]
- 17•.Wessa M, Houenou J, Paillere-Martinot ML, et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. The largest functional neuroimaging study to date in bipolar disorder. [DOI] [PubMed] [Google Scholar]
- 18•.Malhi GS, Lagopoulos J, Sachdev PS, et al. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005;7:58–69. doi: 10.1111/j.1399-5618.2005.00255.x. Demonstrated decreased subcortical limbic activity to emotional words in contrast to other studies showing increased activity to facial expressions. [DOI] [PubMed] [Google Scholar]
- 19.Blumberg HP, Leung HC, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 20•.Chen CH, Lennox B, Jacob R, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. Examined effects of positive, negative, and neutral stimuli using patients with depression and elevated mood. [DOI] [PubMed] [Google Scholar]
- 21.Altshuler L, Bookheimer S, Proenza MA, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 22•.Lennox BR, Calder JR, Lupson J, et al. Behavioral and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol Med. 2004;34:795–802. doi: 10.1017/s0033291704002557. One of the few studies in mania. The authors examined explicit and implicit emotion processing. [DOI] [PubMed] [Google Scholar]
- 23.Malhi GS, Lagopoulos J, Ward PB, et al. Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci. 2004;19:741–754. doi: 10.1111/j.0953-816x.2003.03159.x. [DOI] [PubMed] [Google Scholar]
- 24.Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interaction between emotion and cognition. Cogn Emot. 1998;12:353–385. [Google Scholar]
- 25.Häger F, Volz HP, Gaser C, et al. Challenging the anterior attentional system with a continuous performance task: a functional magnetic resonance imaging approach. Eur Arch Psychiatry Clin Neurosci. 1998;248:161–170. doi: 10.1007/s004060050034. [DOI] [PubMed] [Google Scholar]
- 26•.Strakowski SM, Adler CM, Holland SK, et al. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. Demonstrated relative increases in DLPFC in bipolar disorder related to medication treatment. [DOI] [PubMed] [Google Scholar]
- 27.Monks PJ, Thompson JM, Bullmore ET, et al. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord. 2004;6:550–564. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 28.Gruber SA, Rogowska J, Yurgelun-Todd DA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord. 2004;82:191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Adler CM, Holland SK, Schmithorst V, et al. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 30.Marchand WR, Lee JN, Thatcher GW, et al. A functional MRI study of a paced motor activation task to evaluate frontal-subcortical circuit function in bipolar depression. Psychiatry Res. 2007;155:221–230. doi: 10.1016/j.pscychresns.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Kronhaus DM, Lawrence NS, Williams AM, et al. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 32.Drevets WC, Price JL, Bardgett ME, et al. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 33.Mah L, Zarate CA, Jr, Singh J, et al. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Blumberg HP, Stern E, Martinez D, et al. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- 35.Kruger S, Seminowicz D, Goldapple K, et al. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54:1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- 36.Rubinsztein JS, Fletcher PC, Rogers RD, et al. Decision making in mania: a PET study. Brain. 2001;124:2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- 37.Ketter TA, Kimbrell TA, George MS, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- 38.Brooks JO, 3rd, Wang PW, Strong C, et al. Preliminary evidence of differential relations between prefrontal cortex metabolism and sustained attention in depressed adults with bipolar disorder and healthy controls. Bipolar Disord. 2006;3:248–254. doi: 10.1111/j.1399-5618.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- 39.Altshuler LL, Bartzokis G, Grieder T, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Larson MP, DelBello MP, Zimmerman ME, et al. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 41.Blumberg HP, Kaufman J, Martin A, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 42.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 43.Martin SD, Martin E, Rai SS, et al. Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Arch Gen Psychiatry. 2001;58:641–648. doi: 10.1001/archpsyc.58.7.641. [DOI] [PubMed] [Google Scholar]
- 44.Fu CH, Williams SC, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 45.Surguladze S, Bramer MJ, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in UPD. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Elliott R, Baker SC, Rogers RD, et al. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997;27:931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- 47.Siegle GJ, Steinhauer SR, Thase ME, et al. Can't shake that feeling: fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 48.Sheline YI, Barch DM, Donnelly JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 49.Rich BA, Schmajuk M, Perez-Edgar KE, et al. The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biol Psychiatry. 2005;58:532–539. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blumberg HP, Martin A, Kaufman J, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;7:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 52.Chang K, Adleman NE, Dienes K, et al. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 53.McClure EB, Treland JE, Snow J, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 54.Dickstein DP, Milham MP, Nugent AC, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 55.Blumberg HP, Fredericks C, Wang F, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen BK, Sassi R, Axelson D, et al. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 57•.Blumberg HP, Krystal JH, Bansal R, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. Demonstrated greater age-related decrease in VPFC size in patients with bipolar disorder compared with healthy controls. [DOI] [PubMed] [Google Scholar]
- 58.Streit M, Dammers J, Simsek-Kraues S, et al. Time course of regional brain activations during facial emotion recognition in humans. Neurosci Lett. 2003;342:101–104. doi: 10.1016/s0304-3940(03)00274-x. [DOI] [PubMed] [Google Scholar]
- 59•.Tollkötter M, Pfleiderer B, Soros P, Michael N. Effects of antidepressive therapy on auditory processing in severely depressed patients: a combined MRS and MEG study. J Psychiatr Res. 2006;40:293–306. doi: 10.1016/j.jpsychires.2005.09.003. An excellent example of multimodal imaging. [DOI] [PubMed] [Google Scholar]