Abstract
Dopamine modulation of neuronal activity during memory tasks identifies a nonlinear inverted-U shaped function. Both the dopamine transporter (DAT) and dopamine D2 receptors (encoded by DRD2) critically regulate dopamine signaling in the striatum and in prefrontal cortex during memory. Moreover, in vitro studies have demonstrated that DAT and D2 proteins reciprocally regulate each other presynaptically. Therefore, we have evaluated the genetic interaction between a DRD2 polymorphism (rs1076560) causing reduced presynaptic D2 receptor expression and the DAT 3′-VNTR variant (affecting DAT expression) in a large sample of healthy subjects undergoing blood oxygenation level-dependent (BOLD)-functional magnetic resonance imaging (MRI) during memory tasks and structural MRI. Results indicated a significant DRD2/DAT interaction in prefrontal cortex and striatum BOLD activity during both working memory and encoding of recognition memory. The differential effect on BOLD activity of the DAT variant was mostly manifest in the context of the DRD2 allele associated with lower presynaptic expression. Similar results were also evident for gray matter volume in caudate. These interactions describe a nonlinear relationship between compound genotypes and brain activity or gray matter volume. Complementary data from striatal protein extracts from wild-type and D2 knock-out animals (D2R−/−) indicate that DAT and D2 proteins interact in vivo. Together, our results demonstrate that the interaction between genetic variants in DRD2 and DAT critically modulates the nonlinear relationship between dopamine and neuronal activity during memory processing.
Keywords: working memory, recognition memory, fMRI, dopamine, transport, D2, receptor
Introduction
The relationship between dopamine levels and neuronal activity in prefrontal cortex and in striatum (Alexander et al., 1986; Goldman-Rakic, 1996) describes a nonlinear function (inverted-U) (Seamans and Yang, 2004). In other words, there is a critical range of dopamine stimulation within which neuronal activity is more focused, i.e., less neuronal activity for better behavioral performance (Mattay et al., 2003). Above or below this critical range of dopamine stimulation, neuronal activity becomes more diffuse and behavioral performance deteriorates (Mattay et al., 2003). Earlier studies have demonstrated that this relationship is strongly modulated by dopamine D1 receptors (Seamans and Yang, 2004). However, several additional mechanisms regulate dopamine levels, including D2 receptor signaling as well as reuptake by the dopamine transporter (DAT). Studies in animals and in humans strongly implicate D2 receptors and the DAT in regulating working memory performance and prefronto-striatal neuronal activity (Arnsten et al., 1995; Kimberg et al., 2001; Mozley et al., 2001; Glickstein et al., 2002; Cropley et al., 2006; Kellendonk et al., 2006; Mehta and Riedel, 2006; Chou et al., 2007). Recent studies focusing on the relationship between D2 and DAT have also shown that DAT activity is regulated by D2 receptors (Meiergerd et al., 1993; Parsons et al., 1993; Dickinson et al., 1999; Mortensen and Amara, 2003). Mice genetically engineered to lack the D2 receptor display reduced activity of DAT (Dickinson et al., 1999). D2 agonist and antagonist agents modify the kinetics of dopamine reuptake by DAT (Kimmel et al., 2001). Moreover, DAT is also regulated by dopamine D2 receptors through a direct protein–protein interaction which facilitates the recruitment of intracellular DAT to the cell surface, thereby enhancing dopamine clearance (Lee et al., 2007). Finally, this physical interaction appears to be more specific for presynaptic D2 receptors (Bolan et al., 2007), which is supported by results showing that mice lacking DAT have complete loss of function of presynaptic D2 receptors (Jones et al., 1999).
By a mechanism of alternative splicing, the D2 receptor gene (DRD2) encodes two molecularly distinct isoforms, D2S and D2L. D2L acts mainly at postsynaptic sites and D2S serves presynaptic autoreceptor functions (Khan et al., 1998; Usiello et al., 2000). The D2S isoform appears to be involved in regulation of prefronto-striatal synaptic plasticity associated with long-term potentiation, centrally implicated in the physiology of memory (Centonze et al., 2004). Consistently, we have demonstrated that a novel intronic DRD2 polymorphism (rs1076560, G>T) of the D2 receptor affects both relative expression of D2S in prefrontal cortex/striatum and activity of the striato-thalamic-prefrontal pathway during working memory in healthy subjects (Zhang et al., 2007) and in patients with schizophrenia (Bertolino et al., 2008a).
A functional variable number of tandem repeat (VNTR) polymorphism in the 3′ untranslated region of the DAT gene has been described (Vandenbergh et al., 1992). Alleles of this polymorphism range from 3 to 11 repeats, with the 9- and 10-repeat alleles by far the most common (Vandenbergh et al., 1992). As compared with the 9-repeat allele, the 10-repeat allele has been associated with increased gene expression both in vitro (Mill et al., 2002; VanNess et al., 2005) and in vivo (Heinz et al., 2000). Consistently, several studies have reported that the 10-repeat allele is associated with more focused cortical activity during memory and attention in healthy subjects as well as in patients with ADHD (Fossella et al., 2002; Cornish et al., 2005; Bertolino et al., 2006a; Johanson et al., 2006; Schott et al., 2006; Caldú et al., 2007).
Based on this previous evidence, we used functional magnetic resonance imaging (fMRI) in healthy subjects to explore the relationship of DRD2 and DAT functional polymorphisms with brain activity during working memory. To confirm the working memory results in another cognitive setting known to involve the prefronto-striatal-thalamic-prefrontal circuit, we also evaluated the interaction of these two genotypes with brain activity during encoding of recognition memory. Our hypothesis was based on the physiological role of dopamine in regulating neuronal signal-to-noise ratios in this pathway, on earlier blood oxygenation level-dependent (BOLD) fMRI studies (Bertolino et al., 2006a; 2008a,b; Zhang et al., 2007), on known trophic action of dopamine on neurons and synapses (Nieoullon, 2002), and on earlier in vitro experiments demonstrating direct interaction between D2 and DAT. Thus, we hypothesized that these two genes interact in modulating the nonlinearity of cortical and subcortical activity in humans during memory performance and in modulating gray matter volume (assessed respectively with BOLD fMRI and Voxel-Based-Morphometry). Moreover, to further substantiate the possibility that the effects in humans might depend on a direct interaction between these two proteins, we analyzed this possibility by coimmunoprecipitation. Importantly, using mouse striata from wild-type (WT) and DRD2 knock-out mice, we were able to show a physical interaction between DAT and D2 proteins in vivo.
Materials and Methods
Subjects
We studied one hundred forty two healthy subjects (57 males, mean age 27.4 ± 7.3). The present study was approved by the local intramural research board at the University of Bari. Moreover, after complete description of the study to the subjects, written informed consent was obtained. For additional demographics, see Tables 1, 2.
Table 1.
Demographics and behavioral performance of the N-back working memory sample
| Total N (males) | Age | IQ | Handedness | Working memory test (2-back).Correct responses (%) | Working memorytest (2-back).Reaction time (ms) | |
|---|---|---|---|---|---|---|
| DRD2 | ||||||
| GG | 110 (37♂) | 27.7 (±7.4) | 113.5 (±13.0) | 0.75 (±0.43) | 67.7 (±16.7) | 523.2 (±248.9) |
| GT | 32 (19♂) | 28.4 (±7.9) | 111.4 (±14.7) | 0.73 (±0.32) | 69.4 (±15.7) | 499.7 (±246.0) |
| DAT | ||||||
| 10/10 repeat | 51 (21♂) | 28.6 (±7.1) | 115.7 (±10.8) | 0.70 (±0.43) | 70.2 (±15.4) | 517.0 (±254.8) |
| 9 repeat carriers | 91 (35♂) | 27.4 (±7.6) | 111.6 (±14.4) | 0.76 (±0.38) | 66.9 (±17.0) | 518.1 (±244.7) |
| DRD2-DAT | ||||||
| GG-10/10 repeat | 41 (13♂) | 28.6 (±7.1) | 117.9 (±9.6) | 0.72 (±0.43) | 69.4 (±16.3) | 507.3 (±261.5) |
| GG-9 repeat carriers | 69 (24♂) | 27.1 (±7.5) | 111.0 (±14.0) | 0.76 (±0.42) | 66.7 (±17.0) | 533.0 (±242.4) |
| GT-10/10 repeat | 10 (8♂) | 28.4 (±7.6) | 107.0 (±11.5) | 0.62 (±0.43) | 73.2 (±11.3) | 553.9 (±235.4) |
| GT-9 repeat carriers | 22 (11♂) | 28.4 (±8.2) | 114.1 (±15.6) | 0.78 (±0.25) | 67.6 (±17.3) | 473.3 (±252.2) |
Results are mean ± SD.
Table 2.
Demographics and behavioral performance of the recognition memory sample
| Total N (males) | Age | IQ | Handedness | Recognition memory test (encoding).Correct responses (%) | Recognition memory test (encoding).Reaction time (ms) | |
|---|---|---|---|---|---|---|
| DRD2 | ||||||
| GG | 82 (23♂) | 26.2 (±5.9) | 112.6 (±11.4) | 0.7 (±0.47) | 94.8 (±3.9) | 1129.5 (±187.3) |
| GT | 20 (9♂) | 26.5 (±5.8) | 112.5 (±12.2) | 0.7 (±0.36) | 92.5 (±5.5) | 1170.6 (±221.5) |
| DAT | ||||||
| 10/10 repeat | 38 (10♂) | 27.5 (±5.3) | 113.7 (±10.4) | 0.66 (±0.46) | 94.6 (±3.9) | 1140.4 (±192.2) |
| 9 repeat carriers | 64 (22♂) | 25.6 (±6.1) | 112.1 (±12.0) | 0.73 (±0.44) | 94.2 (±4.6) | 1136.1 (±196.8) |
| DRD2-DAT | ||||||
| GG-10/10 repeat | 33 (7♂) | 27.0 (±5.6) | 114.5 (±9.7) | 0.62 (±0.49) | 94.7 (±3.9) | 1113.3 (±182.6) |
| GG-9 repeat carriers | 49 (16♂) | 25.7 (±6.2) | 111.7 (±12.1) | 0.76 (±0.46) | 94.8 (±3.9) | 1140,0 (±191.7) |
| GT-10/10 repeat | 5 (3♂) | 30.2 (±2.2) | 109.0 (±5.7) | 0.87 (±0.16) | 94.2 (±3.7) | 1303.3 (±183.1) |
| GT-9 repeat carriers | 15 (6♂) | 25.5 (±6.0) | 113.2 (±11.9) | 0.70 (±0.39) | 92.0 (±6.0) | 1123.2 (±220.0) |
Results are mean ± SD.
Genotype determination
SNP rs1076560 of DRD2 and the 3′ VNTR DAT genotypes were determined as described previously (Bertolino et al., 2006a, 2008b; Zhang et al., 2007).
3′ VNTR DAT.
Genotyping of the DAT1 40-bp repeat (VNTR) polymorphism in the 3′ untranslated region (rs# 28363170) was determined using forward 5′-TGTGGTGTAGGGAACGGCCTGAG-3′ and reverse 5′-CTTCCTGGAGGTCACGGCTCAAGG-3′ primers. DNA amplification by PCR of the 40-base pair repeat alleles was performed as described previously (Szekeres et al., 2004). PCR products were separated by 4% agarose gel electrophoresis, visualized by ultraviolet transillumination and fragment sized by comparison with Invitrogen 100bp DNA ladder. To confirm the results obtained with agarose gel electrophoresis, genomic DNA fragments were PCR-amplified using fluorescent labeled forward primer, resolved on an ABI Prism 3100 DNA Sequencer (Applied Biosystems) and analyzed with Genotyper software.
DRD2.
SNP rs1076560 was analyzed with allele-specific PCR primers as described (Papp et al., 2003) or SNaPshot [Applied Biosciences (ABI)] (Zhang et al., 2005).
As in several previous studies (Heinz et al., 2000; Jacobsen et al., 2000; Cheon et al., 2003; Durston et al., 2005; van Dyck et al., 2005; Gilbert et al., 2006; Schott et al., 2006; Laucht et al., 2007), since the number of subjects homozygous for the 9-repeat allele of the DAT was very small, we grouped all subjects carrying at least one 9-repeat allele in one group, 9-repeat carriers. After genotype determination, the groups were divided based on DRD2, DAT, and DRD2-DAT genotypes. The Ns were as follows: DRD2 (GG = 110, GT = 32); DAT (9-repeat carriers = 91, 10/10-repeat = 51); DRD2-DAT (GT 9-repeat carriers = 22, GG 9-repeat carriers = 69, GT 10/10-repeat = 10, GG 10/10-repeat carriers = 41). No DRD2 TT subjects were observed in this sample. The allelic distribution of both genes was in Hardy Weinberg equilibrium (DRD2 df 1, χ2>2.1, p > 0.1, DAT df 1, χ2 = 1.2, p > 0.2).
Functional imaging tasks and acquisition parameters
N-back working memory paradigm.
The genotype groups in this sample were matched for all demographic variables except gender (p < 0.05). During fMRI, all subjects completed a blocked paradigm of the N-back task with a 2-back working memory condition and a nonmemory guided control condition 0-back (Bertolino et al., 2004). This paradigm has been extensively used to evaluate activity of prefrontal cortex and striatum. “N-back” refers to how far back in the sequence of stimuli the subject had to recall. The stimuli consisted of numbers (1–4) shown in random sequence and displayed at the points of a diamond-shaped box. There was a visually paced motor task which also served as a nonmemory guided control condition (0-back) that simply required subjects to identify the stimulus currently seen. In the working memory condition, the task required recollection of a stimulus seen two stimuli (2-back) previously while continuing to encode additionally incoming stimuli. As in earlier studies in which we used this paradigm, both numerical and spatial information together were used to guide subjects response to the task. Performance data were recorded as the number of correct responses (accuracy) and as reaction time.
Each subject was scanned using the same MR scanner with a gradient-echo echo planar imaging (EPI) sequence using the following parameters: 20 contiguous slices echo time = 30 ms, repetition time = 2000 ms; field of view 24 cm; matrix 64 × 64 (Bertolino et al., 2004, 2006a,b, 2008a). We used a simple block design in which each block consisted of eight alternating 0-back and 2-back conditions (each lasting 30 s), obtained in 4 min and 8 s, 120 whole-brain fMRI volumes. The first four scans at the beginning of each time series were acquired to allow the signal to reach a steady state and were not included in the final analysis.
Recognition memory paradigm.
102 subjects of the original sample performed this additional paradigm. The genotype groups in this sample were matched for all demographic variables, including sex (all p > 0.1). The fMRI paradigm consisted of the encoding (“indoor” or “outdoor”) and subsequent retrieval (“new” or “old”) of novel, complex scenes, a task that has consistently been shown to produce activation of the prefrontal cortex and of the striatum in human neuroimaging experiments (Hariri et al., 2003; Bertolino et al., 2006c). Stimuli of neutral valence were presented in a blocked paradigm that provides robust power and sensitivity for BOLD signal change in the hippocampal region. Four encoding blocks were followed by four retrieval blocks in an interleaved design with a passive rest condition, resulting in a total of 16 blocks. Each block was 20 s. long, producing a total scan time of 5.33 min. During encoding blocks, subjects viewed six images, presented serially for 3 s each, and determined whether each image represented an indoor or outdoor scene (Hariri et al., 2003). An equal number of indoor and outdoor scenes were presented in each encoding block. All scenes were of neutral emotional valence and were derived from the International Affective Picture System (Lang et al., 1997). During subsequent retrieval blocks, subjects again viewed six images, presented serially for 3 s each, and determined whether each scene was new or old. In each retrieval block, half the scenes were old (i.e., presented during the encoding blocks) and half were new (i.e., not presented during the encoding blocks). The order of indoor and outdoor scenes as well as new and old scenes were randomly distributed throughout the encoding and retrieval blocks, respectively. During the interleaved rest blocks, subjects were instructed to fixate on a centrally presented cross-hair. Before the beginning of each block, subjects viewed a brief (2 s) instruction: “Indoor or Outdoor?”, “New or Old?”, or “Rest”. However, because of the blocked paradigm, the retrieval phase is actually a mixture between encoding and retrieval: subjects in this phase view an equal number of new and old stimuli. The new stimuli are likely to engage encoding mechanisms in the hippocampus, and this activity would be mixed in with any retrieval related activity. Therefore, because of this limitation, we decided not to analyze the retrieval data [for review, see Schacter and Wagner (1999); Squire et al. (2004)]. During scanning, all subjects responded by button presses with their right hand, allowing determination of behavioral accuracy and reaction time.
Each subject was scanned using a GE Signa 3T scanner (General Electric). BOLD functional images were acquired with a gradient-echo echo planar imaging (EPI) sequence and covered 24 axial slices (4 mm thick, 1 mm gap) that began at the cerebral vertex and encompassed the entire cerebrum and the majority of the cerebellum (repetition time/echo time, 2000/28 ms; field of view, 24 cm; matrix, 64 × 64).
BOLD–fMRI image analysis. Preprocessing and statistical analyses of the fMRI data
Analysis of the fMRI data was completed using statistical parametric mapping (SPM5; http://www.fil.ion.ucl.ac.uk/spm). Images for each subject were realigned to the first volume in the time series to correct for head motion (< 2.5 mm of translation, <1.50 rotation), spatially normalized into a standard stereotactic space (Montreal Neurological Institute, MNI, template) using a 12 parameter affine model and smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter, set at 10 mm full-width at half-maximum. Voxelwise signal intensities were ratio normalized to the whole-brain global mean. For each experimental condition, a box car model convolved with the hemodynamic response function (HRF) at each voxel was modeled. Predetermined condition effects at each voxel were calculated using a t statistic, producing a statistical image for the contrasts of 2-back versus 0-back (N-back) and of encoding versus rest (recognition memory). All these individual contrast images were then used in second-level random effects models to determine task-specific regional responses at the group level for the entire sample.
To detect the association of DRD2 and DAT genotypes and fMRI activation in the working memory and recognition memory cortical and subcortical networks, the contrast images of all subjects were included in whole-brain image analyses for all predetermined condition effects using second-level random effects models for ANCOVA analyses within SPM5 covarying for gender. For recognition memory data, ANOVA was used instead because the genotype groups were matched for all demographic variables. Because of earlier fMRI studies of working memory and recognition memory (Bertolino et al., 2006a,c; 2008a,b; Zhang et al., 2007) demonstrating main effects of these two genes, a statistical threshold of p < 0.005, k = 3, uncorrected, was used to evaluate main effects of DRD2 and DAT. However, the same statistical threshold with a further false discovery rate (FDR) small volume correction for multiple comparisons (Meyer-Lindenberg et al., 2008) [using a 10-mm-radius sphere centered around the coordinates in caudate, putamen, and prefrontal cortex published in previous studies (p = 0.05) (Hofer et al., 2007; Koch et al., 2007; Tan et al., 2007)] was used to identify significant responses for the interaction between the two genes in these anatomical regions. These coordinates included: x −36, y 9, z 59; x −36, y 30, z 23; x 20, y −3, z 17; x −18, y 3, z 15; x 8, y 12, z 8 (Tan et al., 2007); x −18, y 3, z 15 (Hofer et al., 2007); x 42, y 12, z 36 (Koch et al., 2007). Because we did not have a priori hypotheses regarding the activity of brain regions outside of the prefrontal cortex and striatum, we used a statistical threshold of p = 0.05, corrected for multiple comparisons across all voxels, for these whole-brain comparisons. All fMRI results are reported in Montreal Neurological Institutes (MNI) coordinates system.
Voxel-based morphometry acquisition and processing
Eighty six subjects of the original sample were included in these analyses. Again, genotype groups in this sample were matched for all demographic variables except gender (p < 0.05). 3D structural MRI images were obtained on a 3T GE MR scanner using a T1-weighted SPGR sequence (TR/TE/NEX = 25/3/1; flip angle, 6°; matrix size, 256 × 256; field of view, 25 × 25 cm) with 124 sagittal slices (slice thickness = 1.3 mm, in-plane resolution of 0.94 × 0.94). VBM analysis was performed using a unified segmentation protocol (Ashburner and Friston, 2005) implemented in SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Briefly, this protocol involves a number of fully automated preprocessing steps, including: extraction of brain, spatial normalization into stereotactic MNI space, segmentation into gray and white matter and CSF compartments, correction for volume changes induced by spatial normalization (modulation), and smoothing with a 8 mm full width at half maximum (FWHM) isotropic Gaussian kernel.
Global effects of DRD2 and DAT genotypes on gray matter volume were evaluated using an ANCOVA in SPM5. We controlled for potential confounds in our statistical model including linear and quadratic expansions of age (Büchel et al., 1996), gender, and total brain volume. Because of our strong a priori hypothesis, we used a region of interest (ROI) approach using the “Human aal atlas” within the Wake Forest University PickAtlas 1.04 (http://www.fmri.wfubmc.edu/cms/). Statistical nonstationary inference (Hayasaka et al., 2004) was performed at the cluster level at p < 0.05 corrected within the caudate ROI by using the ns toolbox implemented in SPM5 to avoid increased false positive rate due to nonstationary nature of structural images (http://fmri.wfubmc.edu/cms/NS-General). VBM data are reported with reference to the MNI standard space within SPM5.
Membrane preparations, Western blotting, and immunoprecipitation analyses
Mouse striatal extracts from D2R−/− (Baik et al., 1995) and wild-type (WT) littermates were rapidly dissected and proteins were extracted as previously described (Tirotta et al., 2008), with minor modifications. Briefly, striata were homogenized on ice with a polyethylene pestle, in a Membrane Extraction Buffer (MEB 50 mm TRIS, pH 7.5, 150 mm NaCl, 5 mm EDTA, pH 8.0), with protease and phosphatase inhibitors mixture (Sigma). Samples were sedimented by centrifugation (40 min, 70,000 × g, 4°C), and the pellets were resuspended in MEB supplemented with 10 mm 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and gently rotated at 4°C for 40 min. The solubilized fractions were finally centrifuged, to remove the insoluble material (10 min, 18,000 × g at 4°C). Supernatants represent solubilized membrane extracts.
For immunoprecipitation (IP) assays, 500 μg of membrane extracts were incubated for 4 h with 20 μl of protein-A/G Sepharose (GE Lifesciences) at 4°C, in 500 μl of PBS supplemented by protease and phosphatase inhibitors mixture and centrifuged at 3000 × g for 5 min. The supernatants were incubated overnight at 4°C with 5 μg of the appropriate antibody (anti-DAT # s.c.-14002, from Santa Cruz Biotechnology; or mouse monoclonal anti-D2R raised against a peptide corresponding to the mouse D2R sequence from aa 309 to 322 (Doi et al. 2006). Sepharose beads were washed four times by centrifugation in MEB buffer, then resuspended in Laemmli sample buffer and resolved on SDS 12% PAGE. As negative control, immunoprecipitations were performed using either normal mouse or rabbit IgG. For Western blots, 50 μg of membrane extracts were loaded per lane and separated on 12% SDS-PAGE. Proteins were transferred to polyvinylidene fluoride (PVDF) membrane (Millipore). Nonspecific binding was blocked in 5% nonfat dry milk in 0.05% Tween 20 in PBS for 1 h, at room temperature. Immunocomplexes were revealed by using appropriate peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch) along with a chemiluminescent reagent (SuperSignal West-Pico, Pierce).
Results
Demographics and behavioral memory performance
ANOVAs and χ2 tests indicated that there were no significant differences between genotype groups in any demographic variable (all p > 0.1), with the exception of gender which was unequally distributed for DRD2 genotype and for DRD2-DAT compound genotypes (respectively, χ2 = 6.5, df 1, p = 0.01, χ2 = 10.1, df 3, p = 0.01).
Separate ANOVAs on behavioral working memory and encoding of recognition memory performance did not demonstrate any statistically significant main effect or interaction of the two polymorphisms on either accuracy or reaction time during working memory or encoding of recognition memory (all F(1,137)<2, all p > 0.15) thus allowing us to examine the effect of complex genotypes on brain activity independent of behavioral variation. Covarying these analyses for gender did not modify the results. For additional results, see also Tables 1, 2.
Neuroimaging results of the N-back working memory paradigm
Main effect of task
Consistent with prior reports (Callicott et al., 1999, 2000; Bertolino et al., 2004, 2006a), a pattern of brain regions was activated during working memory. These anatomical regions included bilateral prefrontal cortex, bilateral parietal cortex, the anterior cingulate, the head of the caudate, the putamen and bilateral cerebellum in both groups.
Genotype main effects and interaction during working memory
ANOVA of the main effect of DRD2 genotype revealed several clusters in which GT subjects had greater activity than GG subjects. These areas included the middle and inferior frontal gyri, the anterior cingulate and the right putamen (Table 3). The inverse contrast did not reveal any significant difference. ANOVA of the main effect of DAT genotype revealed several clusters in left middle and inferior frontal gyri in which 9-repeat carriers had greater activity than 10/10-repeat subjects (Table 3). The inverse contrast did not reveal any statistically significant difference. These main effects of DRD2 and DAT are internally consistent as both GT DRD2 genotype and the 9-repeat DAT genotype are expected to lead to enhanced dopamine release.
Table 3.
Statistics and Montreal Neurological Institute coordinates for the effects of DRD2 and DAT genotypes as well as for their interaction on brain activity during working memory
| Region (Brodmann's area) | Talairach coordinates (x, y, z) | k | Z value | p |
|---|---|---|---|---|
| Main effect of DRD2 | ||||
| Right inferior frontal gyrus (BA 45) | 59, 23, 6 | 25 | 3.30 | 0.000a |
| Right middle frontal gyrus (BA 10) | 30, 52, −3 | 7 | 2.74 | 0.003a |
| Right putamen | 26, 7, −6 | 3 | 2.61 | 0.004a |
| Right anterior cingulate (BA 32) | 15, 26, 19 | 8 | 2.9 | 0.002a |
| Main effect of DAT | ||||
| Left middle frontal gyrus (BA 6) | −41, 6, 51 | 8 | 3.18 | 0.001a |
| Left middle frontal gyrus (BA 8/9) | −49, 20, 37 | 11 | 2.83 | 0.002a |
| Left inferior frontal gyrus (BA 47) | −45, 22, −7 | 7 | 3.09 | 0.001a |
| Interaction between DAT and DRD2 | ||||
| Left middle frontal gyrus (BA 6) | −38, 6, 51 | 15 | 4.17 | 0.019b |
| Left middle frontal gyrus (BA 9) | −38, 26, 26 | 19 | 3.21 | 0.019b |
| Left caudate body | −8, 0, 7 | 32 | 3.04 | 0.019b |
| Right caudate body | 11, −6, 18 | 14 | 2.95 | 0.019b |
| Interaction between DAT and DRD2 | ||||
| Precuneus (BA 31) | 26, −72, 21 | 6 | 3.48 | 0.000a |
| Left superior frontal gyrus (BA 9) | −34, 49, 33 | 7 | 3.31 | 0.000a |
| Left middle frontal gyrus (BA 10) | −38, 52, 11 | 9 | 3.23 | 0.001a |
| Left cingulate gyrus (BA 32) | −11, 24, 34 | 19 | 3.21 | 0.001a |
| Right superior temporal gyrus (BA 39) | 38, −53, 24 | 4 | 3.19 | 0.001a |
| Left inferior frontal gyrus (BA 47) | −45, 22, −7 | 17 | 3.19 | 0.001a |
| Left superior temporal gyrus (BA 22) | −63, −35, 16 | 3 | 3.00 | 0.001a |
aUncorrected;
bFDR-corrected.
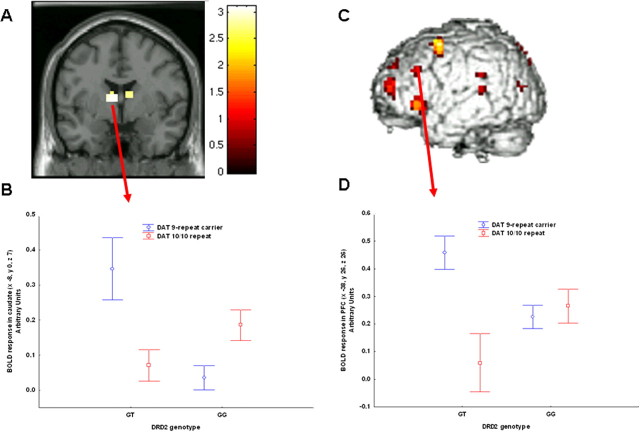
We next addressed the question whether DRD2 and DAT interact with each other. We expected that the genotypes potentially associated with putative intermediate levels of dopamine would show more focused activity for a similar level of behavioral performance (Williams and Castner, 2006). ANOVA revealed several locales with a statistically significant interaction between the two genotypes, including the caudate bilaterally and the left middle frontal gyrus (Table 3, Fig. 1). Analysis of the BOLD signal change outside of SPM revealed an interaction in the right and left head of the caudate (Fig. 1A,B) (left caudate F(1,137) = 9.1, p = 0.002; post hoc with Fisher LSD: GT 9-carrier repeat>GT 10/10 repeat, p = 0.02; GG 10/10 repeat>GG 9-carrier repeat, p = 0.01; right caudate F(1,137) = 9.2, p = 0.002; post hoc: GT 9-carrier repeat>GT 10/10 repeat, p = 0.03; GG 10/10 repeat>GG 9-carrier repeat, p = 0.009) such that subjects with 9-repeat genotype of the DAT have greater activity in the context of DRD2 GT genotype, whereas the opposite is true in the context of the GG DRD2 genotype.
Figure 1.
A, Coronal MRI section through the caudate nuclei indicating locales with DRD2-DAT genotype interaction on BOLD response during working memory (image thresholded at p < 0.005, uncorrected); B, mean ± SE of BOLD response in caudate of the interaction between DRD2 and DAT genotypes. C, Three-dimensional rendering indicating the interaction between DRD2-DAT genotypes on cortical working memory; D, mean ± SE confidence intervals of BOLD response of the interaction between DRD2-DAT genotype in left middle frontal gyrus activity during working memory.
A polynomial regression also revealed a nonlinear relationship between compound genotype and BOLD signal in right and left head of the caudate (respectively, R2 = 0.09, F = 7.46, p = 0.0008; R2 = 0.09, F = 6.99, p = 0.001) (Fig. 2A). Similarly, in the left middle frontal gyrus subjects with 9-carrier repeat genotype had greater activity based on GT DRD2 genotype (Fig. 1C,D) F(1,137) = 7.3, p = 0.007; post hoc: GT 9-carrier repeat>GT 10/10 repeat p = 0.005; GG 10/10 repeat>GG 9-carrier repeat p = 0.5). A polynomial regression also revealed a nonlinear relationship between compound genotype and BOLD signal in left DLPFC (R2 = 0.054, F = 4.0, p = 0.01) (Fig. 2B).
Figure 2.
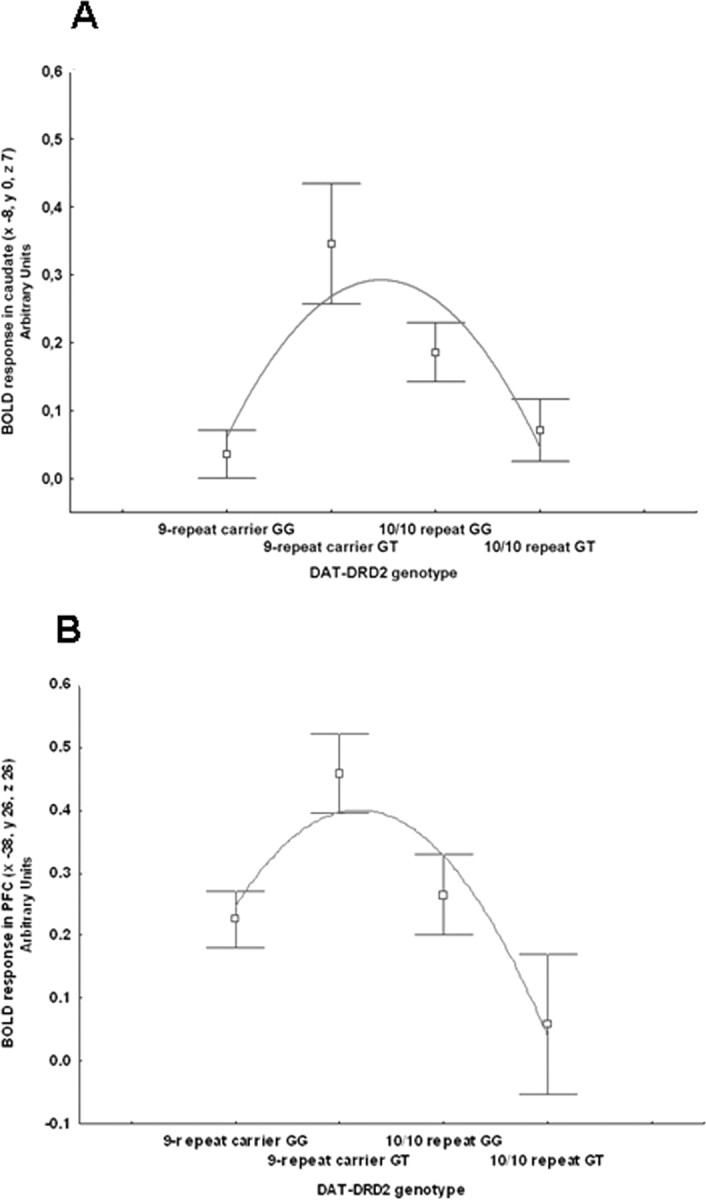
A, B, Mean ± SE of BOLD response in caudate (A) and in middle frontal gyrus (PFC; B) during working memory in compound genotype groups. Since we expected that the genotypes potentially associated with putative intermediate levels of dopamine would show more focused activity for a similar level of behavioral performance, genotype groups are ordered from putative less dopamine reuptake (DAT 9-carriers have less DAT expression) and release (DRD2 GG subjects have greater presynaptic D2 mRNA) to greater reuptake and release (DAT 10–10 repeat DRD2 GT subjects). The relationship between compound genotype and BOLD response is nonlinear. Genotypes on the y-axis are ordered to reflect the nature of our hypothesis about the relationship between putative levels of dopamine stimulation determined by compound genotypes and BOLD activity for a given level of performance. Thus, genotypes at the two extremes of the curve are hypothesized to have intermediate levels of dopamine stimulation and more efficient BOLD activity (less activity for a given behavioral performance).
Additional results are reported in Table 3.
Neuroimaging results of the recognition memory paradigm
Main effect of task
Consistent with prior reports (Hariri et al., 2003; Bertolino et al., 2006c, 2008b), we found significant bilateral activation of the prefrontal cortex (including dorsolateral prefrontal and ventrolateral prefrontal cortex), and the striatum (including the head of the caudate and putamen).
Genotype main effects and interaction during recognition memory
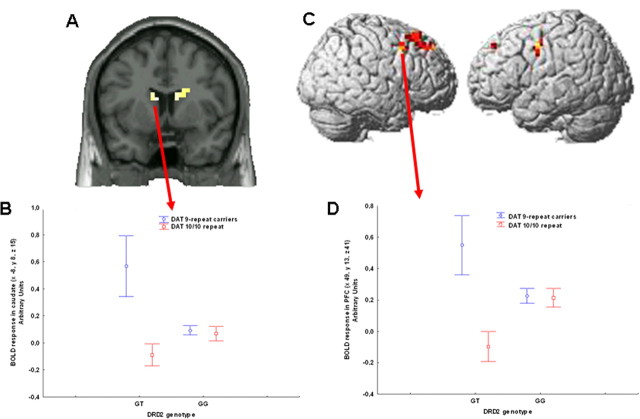
ANOVA of the main effect of DRD2 genotype revealed that GT subjects had statistically significant greater activity than GG in the left putamen and right inferior frontal gyrus (Table 4). The inverse contrast did not reveal any significant difference. ANOVA of the main effect of DAT genotype revealed that carriers of the 9-repeat genotype had statistically significant greater activity than 10/10-repeat subjects bilaterally in the caudate, and in right inferior and middle frontal gyri as well as in left middle frontal gyrus (Table 4). The inverse contrast did not reveal any statistically significant difference. ANOVA also revealed several locales with a statistically significant interaction between the two genotypes bilaterally in the caudate and in right middle frontal gyrus. Analysis of the BOLD signal change in the left head of the caudate revealed an interaction (Fig. 3A,B) (F(1,103) = 6.1, p = 0.01; post hoc with Fisher LSD: GT 9-carrier repeat>GT 10/10 repeat p = 0.004; GG 10/10 repeat vs GG 9-carrier repeat p = 0.5). Analysis of the BOLD signal change in middle frontal gyrus revealed another interaction (Fig. 3C,D) (F(1,103) = 4.3, p = 0.03; post hoc: GT 9-carrier repeat>GT 10/10 repeat p = 0.03; GG 10/10 repeat vs GG 9-carrier repeat p = 0.7).
Table 4.
Statistics and Montreal Neurological Institute coordinates for the effects of DRD2 and DAT genotypes as well as for their interaction on brain activity during encoding of recognition memory
| Region (Brodmann's area) | Talairach coordinates (x, y, z) | k | Z value | p |
|---|---|---|---|---|
| Main effect of DRD2 | ||||
| Left putamen | −22, 5, 14 | 6 | 3.10 | 0.001a |
| Right inferior frontal gyrus (BA 47) | 48, 22, −1 | 6 | 2.88 | 0.002a |
| Main effect of DAT | ||||
| Thalamus, medial dorsal nucleus | −8, −18, 11 | 54 | 3.57 | 0.000a |
| Left precentral gyrus (BA 6) | −55, −2, 42 | 21 | 3.38 | 0.000a |
| Inferior frontal gyrus (BA 47) | −19, 10, −16 | 4 | 3.20 | 0.001a |
| Supramarginal gyrus (BA 40) | 41, −46, 34 | 3 | 3.10 | 0.001a |
| Thalamus | 4, −4, 8 | 17 | 2.92 | 0.002a |
| Claustrum | 30, 18, −1 | 8 | 2.84 | 0.002a |
| Interaction between DAT and DRD2 | ||||
| Right caudate body | 11, 7, 17 | 15 | 3.06 | 0.047b |
| Left caudate body | −8, 8, 15 | 2 | 3.00 | 0.047b |
| Right middle frontal gyrus (BA 8) | 49, 13, 41 | 10 | 2.83 | 0.047b |
| Interaction between DAT and DRD2 | ||||
| Inferior frontal gyrus (BA 47) | −22, 10, −16 | 11 | 3.24 | 0.001a |
| Precuneus (BA 19) | 26, −78, 35 | 4 | 3.19 | 0.001a |
| Precentral gyrus (BA 6) | −41, −5, 42 | 13 | 3.10 | 0.001a |
| Precentral gyrus (BA 4) | 59, −19, 42 | 4 | 3.08 | 0.001a |
| Thalamus, medial dorsal nucleus | 4, −21, 8 | 5 | 3.00 | 0.001a |
| Caudate body | 19, −9, 24 | 4 | 2.88 | 0.002a |
| Caudate tail | 26, −30, −17 | 3 | 3.11 | 0.001a |
| Inferior frontal gyrus (BA 45) | 49, 19, 9 | 5 | 2.84 | 0.002a |
| Inferior frontal gyrus (BA 13) | 30, 14, −10 | 8 | 2.76 | 0.003a |
aUncorrected;
bFDR-corrected.
Figure 3.
A, Coronal MRI section through the caudate nuclei indicating locales with DRD2-DAT genotype interaction on BOLD response during encoding of recognition memory (image thresholded at p < 0.005, uncorrected); B, mean ± SE of BOLD response of the interaction between DRD2 and DAT genotypes. C, Three-dimensional rendering indicating the interaction between DRD2-DAT genotypes on cortical activity during encoding of recognition memory; D, mean ± SE of BOLD response of the interaction between DRD2-DAT genotype in right middle frontal gyrus activity during encoding of recognition memory.
A polynomial regression also revealed a nonlinear relationship between compound genotype and BOLD signal in the head of the caudate and in middle frontal gyrus (respectively, R2 = 0.072, F = 4.05, p = 0.02, R2 = 0.048, F = 2.6, p = 0.07) (Fig. 4A,B).
Figure 4.
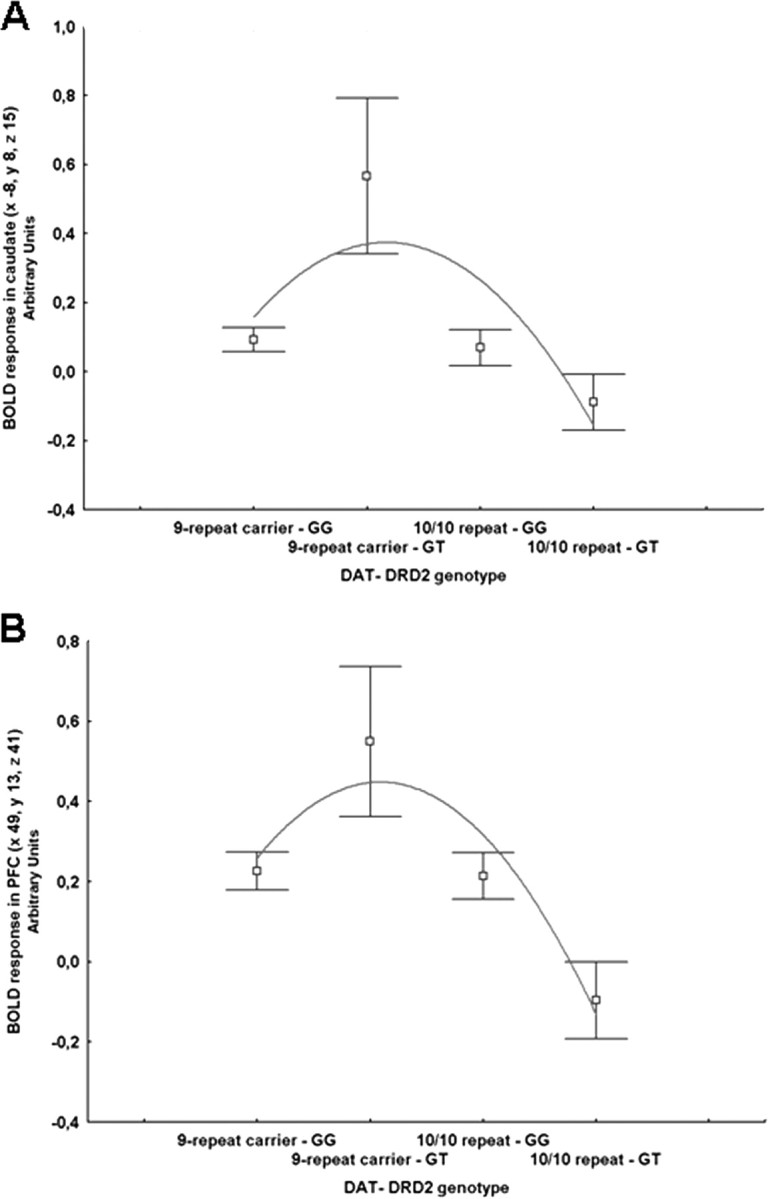
A, B, Mean ± SE of BOLD response in caudate (A) and in middle frontal gyrus (PFC; B) during encoding of recognition memory in compound genotype groups ordered from putative less dopamine reuptake (DAT 9-carriers have less DAT expression) and release (DRD2 GG subjects have greater presynaptic D2 mRNA) to greater reuptake and release (DAT 10–10 repeat DRD2 GT subjects). The relationship between compound genotype and BOLD response is nonlinear.
Voxel-based morphometry
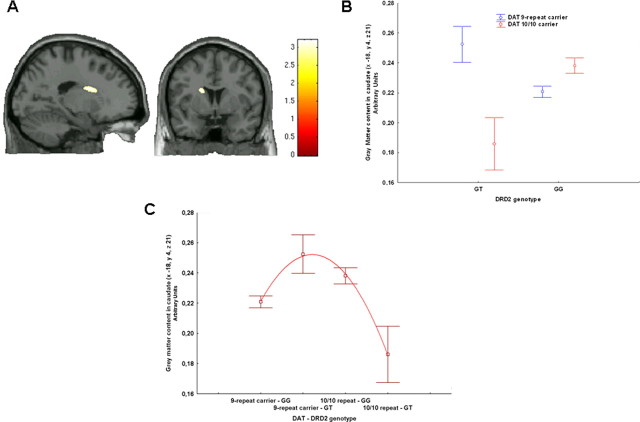
ANOVA demonstrated a significant effect of DRD2 genotype on caudate gray matter volume. GG subjects had greater gray matter volume in right caudate compared with GT subjects (x 11, y 1, z 17, k = 377, Z = 2.84, p = 0.04). No statistically significant main effect of DAT genotype was found. However, ANOVA demonstrated a statistically significant interaction between DRD2 and DAT genotypes in the left and right caudate (respectively, x −18, y 5, z 19, k = 567, Z = 3.1, p = 0.01; x 12, y 9, z 14, k = 37, Z = 2.63, p = 0.06) (Fig. 5A,B). Extraction of gray matter content from the left caudate cluster confirmed the interaction (F(1,82) = 16.2, p = 0.0001; post hoc with Fisher LSD: GT 9-carrier repeat>GT 10/10 repeat, p = 0.001; GG 10/10 repeat>GG 9-carrier repeat, p = 0.01). Polynomial regression also revealed a nonlinear relationship between compound genotype and gray matter in the caudate (respectively, R2 = 0.18, F = 9.46, p = 0.0001) (Fig. 5C).
Figure 5.
A, Sagittal and coronal MRI sections through the caudate nuclei indicating locales with DRD2-DAT genotype interaction on gray matter content (image thresholded at p < 0.005, uncorrected); B, mean ± SE of gray matter content of the interaction between DRD2 and DAT genotypes; C, mean ± SE of gray matter content in caudate in compound genotype groups ordered from putative less dopamine reuptake (DAT 9-carriers have less DAT expression) and release (DRD2 GG subjects have greater presynaptic D2 mRNA) to greater reuptake and release (DAT 10–10 repeat DRD2 GT subjects). The relationship between compound genotype and gray matter content is nonlinear.
Direct interaction between dopamine D2 receptors and DAT proteins in vivo
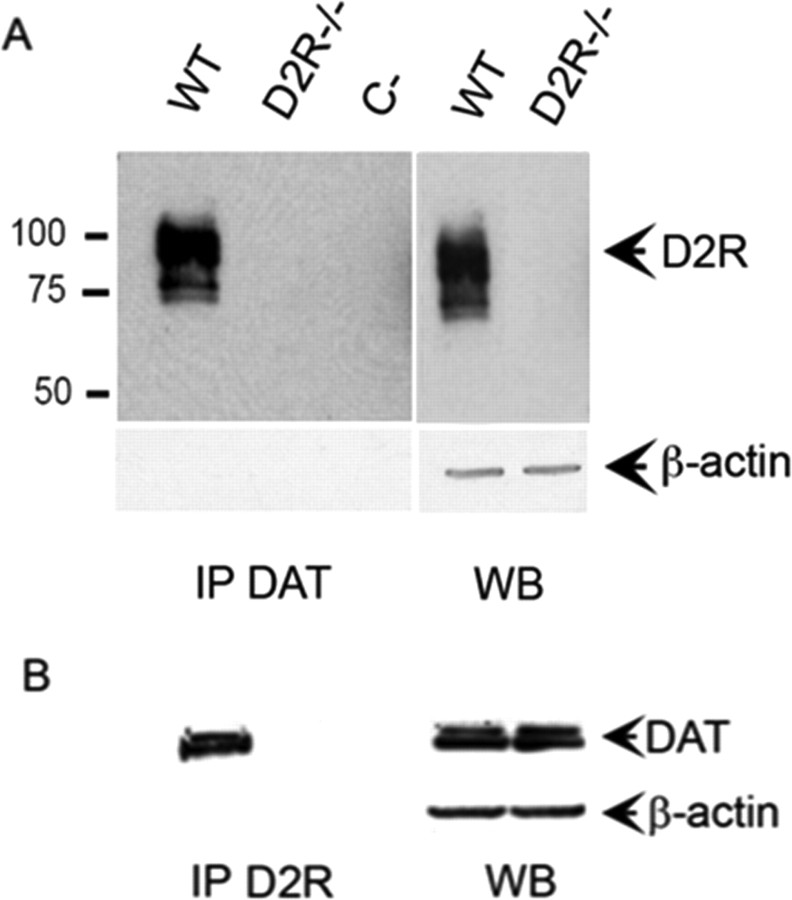
Previous data indicate that reduction of D2 receptors or DAT strongly influence neuronal activity as well as the presence of an interaction between the different genotypes. Importantly, a direct protein-protein interaction between DAT and D2 has been recently demonstrated. This interaction promotes the recruitment of DAT to the plasma membrane and regulates DAT function in transfected cells as well as in primary culture of rat midbrain neurons (Lee et al., 2007). Disruption of DAT/D2 interaction has been postulated to decrease dopamine uptake (Lee et al., 2007). We thus assessed whether these two proteins might physically interact in vivo. To do this we used newly generated anti-D2 receptor antibodies (Doi et al., 2006; Tirotta et al., 2008) to perform immunoprecipitation analyses from striatal mouse extracts. As control of our experiments, we used D2 receptor knock-out striatal extracts. Importantly, we found that D2 receptors interact with DAT in vivo (Fig. 6A). Indeed, using either DAT antibodies (IP DAT) (Fig. 6A) or D2 receptor antibodies (IP D2) (Fig. 6B), we were able to respectively immunoprecipitate either D2 (Fig. 6A) or DAT (Fig. 6B) from striatal extracts of wild-type (WT) animals. This interaction is abolished in the brain of knock-out animals (D2R−/−) in which immunoprecipitation analyses, using either the D2 or DAT specific antibodies, did not detect the presence of a band, as expected (Fig. 6), further confirming the specificity of the antibodies.
Figure 6.
Immunoprecipitation experiments show D2R-DAT interaction in vivo. Striatal membranes were prepared from WT and D2R−/− mice. Proteins (500 μg) were used for immunoprecipitation experiments using either DAT or D2R specific antibodies. A, Samples were immunoprecipitated with anti-DAT and reveled with anti-D2R antibodies (first 2 lanes). Note the presence of immunoprecipitated D2R only in WT striata but not from D2R−/− striatal membranes; C, control experiments were performed using normal IgG rabbit. Western blots (WB) were performed using 50 μg striatal membranes showing the presence of D2R only in WT extracts. B, Same as in A, but proteins were precipitated with anti-D2R antibody and revealed with anti-DAT antibody; C, control experiments were performed using normal IgG mouse. Note that DAT was precipitated only from WT extracts. Western blots, as in A, were revealed using anti-DAT antibody showing presence of DAT in WT and D2R−/− extracts. Actin was used as internal control of loaded quantities of striatal extracts in A and B.
Discussion
Consistent with earlier functional imaging studies, with the known distribution of dopamine projections and of D2S and DAT in the synapse, our results suggest a robust interaction between DRD2 rs1076560 genotype and the 3′ VNTR polymorphism of the DAT. More specifically, DRD2 and DAT genotypes have independent effects on activity of brain areas involved in the working memory network with DRD2 heterozygotes and DAT 9-repeat carriers having greater activity. Moreover, these two genotypes interact in modulating response of the cortical and subcortical working memory network, including the striatum and the dorsolateral prefrontal cortex. The data obtained strongly suggest that the effect of DAT alleles is especially manifest in the context of DRD2 GT genotype. Also, these two genotypes identify similar main effects and interactions in the striatum and dorsolateral prefrontal cortex during encoding of recognition memory, providing replication of the effects in the context of another cognitive paradigm. Consistent with earlier literature, these interactions identify nonlinear relationships between putative dopamine levels and brain activity. As a further demonstration of the functional effects of these polymorphisms, these two genotypes also interacted in determining gray matter volume of the caudate. Once again, the effect of DAT is mostly evident in the context of DRD2 GT genotype. Moreover, to strengthen these results in humans and the in vitro earlier results of a physical D2-DAT interaction, we also demonstrate that these two proteins indeed interact in vivo as indicated by the immunoprecipitation analyses from striatal mouse extracts providing a possible molecular mechanism of the effects in humans.
The present results are consistent with earlier studies from our group and others indicating that DRD2 and DAT 3′ VNTR genotypes have independent effects on activity of the striatum and of the prefrontal cortex during different cognitive challenges (Smolka et al., 2005; Bertolino et al., 2006a, 2008b; Yacubian et al., 2007; Zhang et al., 2007). DRD2 rs1076590 heterozygotes and DAT 9-repeat carriers have greater activity in these brain areas compared with the other genotypes. Moreover, this is the first study addressing the interaction between these two genotypes on in vivo measures of brain activity. The molecular mechanisms responsible for the effects we have measured in vivo with BOLD fMRI in striatum and in prefrontal cortex may be several. D2 receptors are abundantly expressed in the striatum, where the D2S isoform is mostly presynaptic and inhibits dopamine release. Thus, DRD2 GT genotype associated with less D2S may increase dopamine levels in the striatum thus increasing its activity. DATs in the striatum are also found presynaptically and are responsible for dopamine reuptake. Similar to DRD2, the effect of DAT 9-carrier genotype may be associated with less dopamine reuptake, greater dopamine levels and, eventually, greater striatal activity. As for the interaction between the two genes, recent evidence has clearly demonstrated that D2 receptors and DATs are often coupled in presynaptic terminals and they reciprocally regulate each other. Therefore, the interaction we demonstrate in the striatum can be easily understood in that the DRD2 GT genotype is associated with reduced expression of presynaptic D2 receptors which might induce less presynaptic inhibition and greater release of dopamine. Similarly, the 9-repeat allele of the DAT, associated with lower expression of DATs, very likely induces diminished reuptake of dopamine and thereby greater striatal activity. Our data further suggest that the effect of DRD2 GT genotype might be compensated by the 10-repeat allele of the DAT, or, conversely, that the effect of DRD2 GG genotype is compensatory of the 9-repeat allele of the DAT. These results are also consistent with the VBM data in striatum suggesting an interaction of these two genes on gray matter volume. This effect can be explained by the known trophic action of dopamine on neurons and synapses (Nieoullon, 2002).
Interestingly, a physical interaction between DAT and D2 has been reported and the responsible regions have been identified in in vitro studies (Lee et al., 2007). These findings are nicely complemented by our immunoprecipitation results which provide in vivo evidence that, in the mouse brain, D2 receptors interact with DAT as demonstrated by the reciprocal ability of D2 and DAT antibodies to immunoprecipitate either DAT or D2, respectively in WT striatal extracts. The specificity of this interaction is indicated by the total absence of DAT or D2 in immunoprecipitates from D2 receptor knock-out mice. Disruption of this interaction has been postulated to decrease dopamine uptake (Lee et al., 2007). Thus, absence or reduction of D2 might prevent/impair the formation of this complex, leading to aberrant DA release and reuptake. In agreement with this contention, blockade of DAT activity by cocaine stimulates an outstanding elevation of the extracellular dopamine levels, in the striatum of D2R−/− mice, which is well above that evoked in WT mice (Rouge-Pont et al., 2002).
Explanation of the interaction of these two genetic variants in prefrontal cortex may be more complex. D2 receptors in prefrontal cortex are also found presynaptically on dopamine terminals (Pickel et al., 2002) modulating dopamine release and D2S seem to be expressed relatively more abundantly in prefrontal cortex (Zhang et al., 2007). In the cortex, DATs tend to be found on nonvaricose axon segments of small diameter which make symmetric synapses with GABA neurons (Sesack et al., 1998; Lewis et al., 2001), with a distribution similar to that of some D2 receptors (Haber et al., 1995; Pickel et al., 2002; Negyessy and Goldman-Rakic, 2005). Thus, both D2 receptors and DATs may act through GABA neurons to indirectly modulate prefrontal pyramidal neuron firing (Goldman-Rakic, 1996). An alternative explanation of this interaction in prefrontal cortex is that the cortico-striato-thalamic-cortical is critical for cognition and memory (Alexander et al., 1986). An important modulator of this circuit is dopamine. Specifically, greater release of dopamine in the striatum increases activity of the whole network (Tisch et al., 2004). Thus, it is possible that the effect of DRD2 and DAT genotypes as well as their interaction might be associated with modulation of dopamine release in the striatum which would increase activity in the whole cortico-striato-thalamic-cortical circuit (Tisch et al., 2004).
Like in earlier studies in healthy humans which used other genes to study dopamine signaling (Yacubian et al., 2007; Bertolino et al., 2008b), in the present study the interaction between DRD2 and DAT genotypes identifies nonlinear relationships of dopamine signaling with neuronal activity in the caudate and in prefrontal cortex during working memory. This interaction between the two genes is consistent with a large series of experiments suggesting a nonlinear inverted-U shaped relationship between prefrontal dopamine levels and prefrontal neuronal activity (Williams and Castner, 2006; Vijayraghavan et al., 2007). Our data are consistent with these earlier studies investigating dopamine modulation of prefrontal neuronal activity. Moreover, together with previous studies (Yacubian et al., 2007; Bertolino et al., 2008b), the present results suggest that dopamine modulation of neuronal activity may follow a nonlinear dose–response curve also in other brain areas, including the striatum. Our data also extend earlier studies suggesting that this nonlinear inverted-U relationship is not exclusively determined by D1 receptor signaling but also includes D2 receptors and DATs. In this regard, we note that the profile of the curve may change if changing the order of compound genotypes on the y-axis. However, we had preselected the genotype order to reflect the hypothesis that genotypes putatively associated with intermediate levels of dopamine (those in which the effect of DRD2 would be compensated by the DAT effect or vice versa) would be more efficient (less BOLD activity for a given level of behavioral performance). This hypothesis is consistent with several other studies in the literature [for review, see Seamans and Yang (2004); Williams and Castner (2006)] indicating that too much or too little dopamine activity is detrimental for memory and it is associated with reduced efficiency.
Some potential limitations of the present data have to be discussed. The working memory and VBM samples were not matched for gender, and we covaried the relative analyses for this variable to control for the possibility that gender modulates the interaction between genotypes on brain activity or gray matter volume. Therefore, we cannot fully discount the possibility of a gender effect on the variables we have measured. However, this was not the main purpose of our study and exploring a three-way interaction would have probably required an even larger number of subjects to achieve sufficient statistical power.
Some of the imaging findings are lateralized. One reason for these results may be the nature of cognitive requests of task paradigms. For example, in several of our earlier papers with the N-back paradigm findings were either lateralized or they were statistically more robust in left DLPFC (Bertolino et al., 2004; 2006a,b; 2008a). Therefore, a likely reason for lateralization in DLPFC is the verbal versus nonverbal nature of the two memory tasks.
Another limitation of the present study is that we have not measured dopamine receptors and transporters directly in humans. Therefore, the effects demonstrated with BOLD fMRI are not necessarily related to dopamine levels or release. Our results might be related to plasticity mechanisms associated with different molecular pathways present at the same time but not necessarily related to these two genotypes. However, the immunoprecipitation results in the animal experiments at the very least suggest that these interactions are possible in vivo.
In conclusion, the data of the present study suggest a dopamine-related mechanism modulating activity of the prefronto-striatal network during memory performance. This genetically modulated mechanism may be important to determine brain phenotypes found in several disorders implicating dopamine dysregulation of prefronto-striatal networks, including schizophrenia, drug abuse, and Parkinson's disease.
Footnotes
This work was supported by European Community Grant LSHM-CT-2004-005166 and University of California, Irvine funds to E.B. This work was also supported in part by grants from the National Institute on Drug Abuse (DA022199) and General Medical Sciences (GM61390) (W.S.). We are thankful to Riccarda Lomuscio, Rita Masellis, and Tiziana Quarto, and Drs. Antonio Rampino, Miriam Rizzo, Barbara Gelao, Luciana Lobianco, and Apostolos Papazacharias for help with acquisition of the data.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, Altamura M, Nappi G, Papa S, Callicott JH, Mattay VS, Bellomo A, Scarabino T, Weinberger DR, Nardini M. Interaction of COMT Val108/158 Met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, Rubino V, Rampino A, Sinibaldi L, Caforio G, Petruzzella V, Pizzuti A, Scarabino T, Nardini M, Weinberger DR, Dallapiccola B. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006a;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Petruzzella V, Latorre V, Rubino V, Dimalta S, Torraco A, Blasi G, Quartesan R, Mattay VS, Callicott JH, Weinberger DR, Scarabino T. Prefrontal dysfunction in schizophrenia controlling for COMT Val(158)Met genotype and working memory performance. Psychiatry Res. 2006b;147:221–226. doi: 10.1016/j.pscychresns.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, Caforio G, Petruzzella V, Kolachana B, Hariri A, Meyer-Lindenberg A, Nardini M, Weinberger DR, Scarabino T. Prefrontal-hippocampal coupling during memory processing is modulated by COMT Val158Met genotype. Biol Psychiatry. 2006c;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Caforio G, Blasi G, Rampino A, Romano R, Di Giorgio A, Taurisano P, Papp A, Pinsonneault J, Wang D, Nardini M, Popolizio T, Sadee W. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain. 2008a doi: 10.1093/brain/awn248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Di Giorgio A, Blasi G, Sambataro F, Caforio G, Sinibaldi L, Latorre V, Rampino A, Taurisano P, Fazio L, Romano R, Douzgou S, Popolizio T, Kolachana B, Nardini M, Weinberger DR, Dallapiccola B. Epistasis between dopamine regulating genes identifies a nonlinear response of the human hippocampus during memory tasks. Biol Psychiatry. 2008b;64:226–234. doi: 10.1016/j.biopsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS. D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol. 2007;71:1222–1232. doi: 10.1124/mol.106.027763. [DOI] [PubMed] [Google Scholar]
- Büchel C, Wise RJ, Mummery CJ, Poline JB, Friston KJ. Nonlinear regression in parametric activation studies. Neuroimage. 1996;4:60–66. doi: 10.1006/nimg.1996.0029. [DOI] [PubMed] [Google Scholar]
- Caldú X, Vendrell P, Bartrés-Faz D, Clemente I, Bargalló N, Jurado MA, Serra-Grabulosa JM, Junqué C. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuroimage. 2007;37:1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Centonze D, Usiello A, Costa C, Picconi B, Erbs E, Bernardi G, Borrelli E, Calabresi P. Chronic haloperidol promotes corticostriatal long-term potentiation by targeting dopamine D2L receptors. J Neurosci. 2004;24:8214–8222. doi: 10.1523/JNEUROSCI.1274-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Kim YK, Namkoong K, Kim CH, Lee JD. Dopamine transporter density in the basal ganglia assessed with [123I]IPT SPET in children with attention deficit hyperactivity disorder. Eur J Nucl Med Mol Imaging. 2003;30:306–311. doi: 10.1007/s00259-002-1047-3. [DOI] [PubMed] [Google Scholar]
- Chou YH, Huang WS, Su TP, Lu RB, Wan FJ, Fu YK. Dopamine transporters and cognitive function in methamphetamine abuser after a short abstinence: A SPECT study. Eur Neuropsychopharmacol. 2007;17:46–52. doi: 10.1016/j.euroneuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, Grant C, Cross G, Bentley L, Hollis CP. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10:686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Innis RB, Nathan PJ. Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry. 2006;59:898–907. doi: 10.1016/j.biopsych.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR. Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem. 1999;72:148–156. doi: 10.1046/j.1471-4159.1999.0720148.x. [DOI] [PubMed] [Google Scholar]
- Doi M, Yujnovsky I, Hirayama J, Malerba M, Tirotta E, Sassone-Corsi P, Borrelli E. Impaired light masking in dopamine D2 receptor-null mice. Nat Neurosci. 2006;9:732–734. doi: 10.1038/nn1711. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, Steenhuis MP, Minderaa RB, Buitelaar JK, Kahn RS, van Engeland H. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, Posner MI. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Wang Z, Sallee FR, Ridel KR, Merhar S, Zhang J, Lipps TD, White C, Badreldin N, Wassermann EM. Dopamine transporter genotype influences the physiological response to medication in ADHD. Brain. 2006;129:2038–2046. doi: 10.1093/brain/awl147. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J Neurosci. 2002;22:5619–5629. doi: 10.1523/JNEUROSCI.22-13-05619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Ryoo H, Cox C, Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol. 1995;362:400–410. doi: 10.1002/cne.903620308. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, Rettenbacher MA, Verius M, Golaszewski SM, Felber S, Fleischhacker WW. Neural substrates for episodic encoding and recognition of unfamiliar faces. Brain Cogn. 2007;63:174–181. doi: 10.1016/j.bandc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry. 2000;157:1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology. 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95:7731–7736. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, Lease J, D'Esposito M. Cortical effects of bromocriptine, a D-2 dopamine receptor agonist, in human subjects, revealed by fMRI. Hum Brain Mapp. 2001;12:246–257. doi: 10.1002/1097-0193(200104)12:4<246::AID-HBM1019>3.0.CO;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Joyce AR, Carroll FI, Kuhar MJ. Dopamine D1 and D2 receptors influence dopamine transporter synthesis and degradation in the rat. J Pharmacol Exp Ther. 2001;298:129–140. [PubMed] [Google Scholar]
- Koch K, Wagner G, Nenadic I, Schachtzabel C, Roebel M, Schultz C, Axer M, Reichenbach JR, Sauer H, Schlösser RG. Temporal modeling demonstrates preserved overlearning processes in schizophrenia: an fMRI study. Neuroscience. 2007;146:1474–1483. doi: 10.1016/j.neuroscience.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical report A-6. Gainesville, FL: University of Florida; 1997. International affective picture system (IAPS): technical manual and affective ratings. [Google Scholar]
- Laucht M, Skowronek MH, Becker K, Schmidt MH, Esser G, Schulze TG, Rietschel M. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symptoms among 15-year-olds from a high-risk community sample. Arch Gen Psychiatry. 2007;64:585–590. doi: 10.1001/archpsyc.64.5.585. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F. Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 2007;26:2127–2136. doi: 10.1038/sj.emboj.7601656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Riedel WJ. Dopaminergic enhancement of cognitive function. Curr Pharm Des. 2006;12:2487–2500. doi: 10.2174/138161206777698891. [DOI] [PubMed] [Google Scholar]
- Meiergerd SM, Patterson TA, Schenk JO. D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J Neurochem. 1993;61:764–767. doi: 10.1111/j.1471-4159.1993.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. Neuroimage. 2008;40:655–661. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur J Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- Negyessy L, Goldman-Rakic PS. Subcellular localization of the dopamine D2 receptor and coexistence with the calcium-binding protein neuronal calcium sensor-1 in the primate prefrontal cortex. J Comp Neurol. 2005;488:464–475. doi: 10.1002/cne.20601. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Papp AC, Pinsonneault JK, Cooke G, Sadée W. Single nucleotide polymorphism genotyping using allele-specific PCR and fluorescence melting curves. Biotechniques. 2003;34:1068–1072. doi: 10.2144/03345dd03. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Schad CA, Justice JB., Jr Co-administration of the D2 antagonist pimozide inhibits up-regulation of dopamine release and uptake induced by repeated cocaine. J Neurochem. 1993;60:376–379. doi: 10.1111/j.1471-4159.1993.tb05864.x. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Garzón M, Mengual E. Electron microscopic immunolabeling of transporters and receptors identifies transmitter-specific functional sites envisioned in Cajal's neuron. Prog Brain Res. 2002;136:145–155. doi: 10.1016/s0079-6123(02)36014-x. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci. 2002;22:3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein HG, Tischmeyer W, Gundelfinger ED, Heinze HJ, Düzel E. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci. 2006;26:1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K, Braus DF, Goldman D, Büchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Szekeres G, Kéri S, Juhász A, Rimanóczy A, Szendi I, Czimmer C, Janka Z. Role of dopamine D3 receptor (DRD3) and dopamine transporter (DAT) polymorphism in cognitive dysfunctions and therapeutic response to atypical antipsychotics in patients with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;124B:1–5. doi: 10.1002/ajmg.b.20045. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Catechol-O-methyltransferase Val158Met modulation of prefrontal–parietal–striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirotta E, Fontaine V, Picetti R, Lombardi M, Samad TA, Oulad-Abdelghani M, Edwards R, Borrelli E. Signaling by dopamine regulates D2 receptors trafficking at the membrane. Cell Cycle. 2008;7:2241–2248. doi: 10.4161/cc.7.14.6307. [DOI] [PubMed] [Google Scholar]
- Tisch S, Silberstein P, Limousin-Dowsey P, Jahanshahi M. The basal ganglia: anatomy, physiology, and pharmacology. Psychiatr Clin North Am. 2004;27:757–799. doi: 10.1016/j.psc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46:745–751. [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Gläscher J, Kalisch R, Leuenberger B, Braus DF, Büchel C. Gene-gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci U S A. 2007;104:8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, Sadée W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]