Abstract
Purpose
Detection of micrometastasis in melanoma-draining lymph nodes is important for staging and prognosis. Immunohistochemical staining (IHC) using S-100p-HMB-45–, and MART-1– specfic antibodies is used for detecting metastases in sentinel lymph nodes (SLN). However, improvement in IHC is needed for melanoma micrometastasis detection.
Experimental Design
Paraffin-embedded archival tissue (PEAT) specimens were obtained from 42 non-SLN macrometastases, 42 SLN metastases, and 16 tumor-negative SLNs of 100 melanoma patients who underwent SLN biopsy. PEAT specimens were assessed by IHC with high molecular weight-melanoma-associated antigen (HMW-MAA)– specific monoclonal antibodies (mAb) and with S-100p-, HMB-45–, and MART-1– specific antibodies. Quantitative real-time reverse-transcriptase PCR assay was used for HMW-MAA and MART-1 mRNA detection.
Results
Expression frequency and immunostaining intensity were higher for HMW-MAA than MART-1in nodal macrometastases (P < 0.0001and P < 0.0001, respectively) and micrometastases (P < 0.0001and P = 0.004, respectively). All 52 (100%) macrometastases were positive with HMW-MAA – specific mAbs, whereas 43 (83%) were positive with MART-1– specific mAbs. In a comparison analysis, 23 of 23 (100%) micrometastases were HMW-MAA – positive, whereas 21 (91%) and18 (78%) specimens were S-100p – and HMB-45– positive, respectively. Quantitative real-time reverse-transcriptase PCR analysis of 48 nodal metastases showed HMW-MAA mRNA detection in SLNs with metastases.
Conclusions
HMW-MAA is more sensitive and specific than MART-1, S-100p, and HMB-45 for IHC-based detection of SLN micrometastases. SLN PEAT – based detection specificity of melanoma micrometastases can be improved by IHC with HMW-MAA – specific mAbs.
The most frequent site of metastatic melanoma is the regional tumor-draining lymph node basin. Because the sentinel lymph node (SLN) is the first node in this basin to receive drainage from a primary melanoma, it is most likely the initial target of early metastasis (1). SLN biopsy, a minimally invasive technique to assess the tumor-draining nodal basin, has revolutionized the surgical management of primary malignant melanoma (1–3). This approach allows for a more focused, efficient, and comprehensive pathologic analysis of micrometastatic disease. Immunohistochemical staining (IHC) of SLNs with antibodies for S-100p (a member of the S100 protein family), HMB-45 (human melanoma, black-45), and MART-1 (melanoma antigen recognized by T cells) is reportedly 10% to 30% more sensitive than H&E staining for identification of melanoma micrometastases in lymph nodes (4–7). However, S-100p and HMB-45 antibodies have limited specificity or sensitivity for IHC detection of lymph node micrometastases, respectively. Moreover, the use of multiple antibodies with different specificities is logistically cumbersome in reading and requires a larger number of tissue sections. Even when the logistics and the tissue supply are not problematic, the outcome can be unsatisfactory: ~>20% of patients whose SLNs are negative for tumor presence by IHC will develop recurrent disease (8). These findings suggest that current IHC techniques may fail to detect all clinically significant nodal micrometastases in melanoma patients, thereby understaging patients. Detection of melanoma micrometastasis or isolated tumor cells in the SLN is an important prognostic factor (8).
The limitations of currently used IHC approaches to detect metastatic melanoma cells in SLNs prompted us to evaluate high molecular weight-melanoma-associated antigen (HMW-MAA), also known as the melanoma chondroitin sulfate proteoglycan (9), as an IHC biomarker. This antigen mediates interactions of melanoma cells with the extracellular matrix and seems to play a role in the metastatic potential of melanoma cells (9). HMW-MAA has been shown to promote melanoma invasion upon activation through cytoskeletal rearrangements. HMW-MAA activation can regulate integrin-mediated migration and phosphorylation of focal adhesion kinase (9). Because of its high expression in >80% of primary and metastatic melanoma lesions, with limited interlesional and intralesional heterogeneity, and its restricted distribution in normal tissues, the HMW-MAA has been successfully used as a marker to visualize melanoma lesions with radiolabeled monoclonal antibodies (mAb) and as a target of immunotherapy (9). In this study, we focused on MART-1 for rigorous comparison because the sensitivity/specificity of MART-1–specific mAbs equal or exceeds those of HMB-45–specific mAbs for detection of micrometastatic melanoma in regional lymph nodes (10, 11). Comparison of HMW-MAA IHC to S-100p and HMB-45 was also carried out in SLNs. We have shown that metastatic tumor cells in paraffin-embedded archival tissue (PEAT) SLN can be detected by a quantitative real-time reverse-transcription PCR (qRT) assay (12). We thus compared HMW-MAA and MART-1 as mRNA biomarkers for detection of nodal metastasis in melanoma.
Materials and Methods
Cell lines and lymphocytes
Thirteen human metastatic melanoma cell lines (ME-01, ME-02, ME-05, ME-09, ME-10, ME-13, ME-16, ME-17, ME-18, ME-19, ME-20, ME-35, and ME-36) were grown at 37°C in a 5% CO2 humidified atmosphere, in RPMI 1640 (Life Technologies-Bethesda Research Laboratories) supplemented with 10% fetal bovine serum. Informed consent, approved by Saint John’s Health Center (Santa Monica, CA)/John Wayne Cancer Institute institutional review board, was obtained for all other human specimens used in this study.
Paraffin-embedded tissues
PEAT blocks of nodal specimens were obtained from 100 John Wayne Cancer Institute patients who underwent SLN biopsy for clinically localized malignant melanoma between 1995 and 2006. After preoperative lymphoscintigraphy to identify the tumor-draining lymph node basin(s), isosulfan blue dye (Lymphazurin; Hirsch Industries, Inc.) was injected at the tumor site; SLNs were identified by blue staining and/or by probe-detected uptake of 99m technetium sulfur colloid (1–3). Patients for this study were selected based on availability of PEAT blocks from resected regional nodes, and selection was conducted by the database operator, independently of investigators and biostatisticians.
Fifty-eight SLN and 42 non-SLN PEAT blocks were retrieved. Non-SLN refers to lymph nodes removed by complete node dissection after SLN biopsy was done and determined to be tumor positive. All of these specimens had been initially assessed by a surgical pathologist (R.R.T.) at Saint John’s Health Center: specimens were stained with H&E, and, if negative, SLN tissues were further examined by IHC with S-100p–specific rabbit polyclonal antibody, HMB-45 –specific mAbs, and MART-1–specific mAbs, as previously described (2, 3). Ten SLN and 42 non-SLN specimens contained macrometastases (>2 mm), 32 SLN specimens contained micrometastases (<2 mm), and 16 SLN specimens were negative for tumor presence.
mAbs
The mAbs 763.74, VF1-TP41.2, and VT80.12 that recognize distinct determinants of HMW-MAA were developed and characterized as described (9, 13). mAbs were purified from ascitic fluid by sequential precipitation with caprylic acid and ammonium sulfate (14). The purity of mAbs preparations was assessed by SDS-PAGE; activity was assessed by ELISA with HMW-MAA–positive melanoma cells. A combination of the three mAbs, each at the concentration of 0.5 mg/mL, was used as a probe in IHC assays of PEAT specimens. MART-1–specific mAbs M2-7C10 and horseradish peroxidase–conjugated anti-mouse immunoglobulin xenoantibodies were purchased from GeneTex, Inc. and DakoCytomation, respectively, and used according to the manufacturer’s suggestion.
Immunohistochemistry
IHC was done on 5-μm PEAT sections that had been incubated overnight at 50°C and deparaffinized in xylene. When HMW-MAA–specific mAbs were used as a probe, the CSA II System (CSA II, Biotin-Free Catalyzed Amplification System; DakoCytomation) was modified as follows. For antigen retrieval, tissue sections were treated with 1 mmol/L EDTA (pH 8.0) and heated to the boiling point for 15 min. After three 5-min washes in TBS containing Tween 20 buffer (Tris-HCl/NaCl/Tween 20), endogenous peroxidase was quenched with peroxidase block. Nonspecific binding was blocked by serum-free protein. Tissue sections were then incubated overnight at 4°C with HMW-MAA–specific mAbs at 15 μg/mL. Negative controls were incubated with normal mouse IgG (Santa Cruz Biotechnology) under the same experimental conditions. After washings, tissue sections were incubated for 15 min at room temperature with horseradish peroxide–conjugated anti-mouse immunoglobulin antibodies. After a 15-min incubation with an amplification reagent at room temperature, anti-horseradish peroxidase was applied and incubation was continued for an additional 15 min at room temperature. After development with substrate (VIP Substrate kit; Vector Labs), tissue sections were counter-stained with Gill’s hematoxylin 1× (Fisher Scientific Company) for 1 min at room temperature, dehydrated, and mounted.
Standard procedures were used for IHC with MART-1 mAbs M2-7C10. After deparaffinization, endogenous peroxidase was quenched with peroxidase block (Fisher Scientific). For antigen retrieval, sections were incubated with 10 mmol/L citrate buffer (pH 6.0), at 120°C for 20 min, then cooled to room temperature for 20 min in PBS (Invitrogen Corporation) and exposed to blocking solution (Protein Block Serum-Free; DakoCytomation). Sections were incubated with mAbs M2-7C10 at room temperature for 60 min. After three 5-min rounds of PBS washing, sections were incubated with horseradish peroxidase–labeled anti-mouse immunoglobulin antibodies (DAKO EnVision+ System; DakoCytomation) for 30 min, followed by three 5-min rounds of PBS washing. A 10-min development (AEC + Substrate-Chromogen; DakoCytomation) was followed by three 5-min rounds of PBS washings. Sections were counterstained via Gill’s hematoxylin (Fisher Scientific), and then mounted.
PEAT sections were scored according to the percentage of stained melanoma cells: 100%, 75% to 99%, 50% to 74%, 25% to 49%, 1% to 24%, or 0 (negative). The intensity of staining was scored as strong (+++), intermediate (++), weak (+), or negative (−). The staining of each PEAT section was scored as the average percentage of stained cells based on assessments by three independent observers (Y.G., T.A., and A.T.).
RNA isolation
Total RNA was extracted from cells of the 13 melanoma lines using Tri-Reagent (Molecular Research Center, Inc.), as previously described (12, 15). For nodal assessment, we selected PEAT blocks with sufficient tissue for both IHC and qRT assays. These 58 PEAT blocks comprised of 31 non-SLN and SLN macrometastases, 17 SLN micrometastases, and 10 tumor-negative SLNs. Five 10-μm sections were cut from each PEAT block with a sterile microtome blade and placed in sterile microcentrifuge tubes (Eppendorf; ref. 12). After deparaffinization, specimens were treated with a proteinase K digestion buffer for 3 h before RNA extraction, as previously described (12). Total RNA was extracted, isolated, and purified using a modified RNAWiz (Ambion) extraction method, as previously described (12). RNA was quantified and assessed for purity by UV spectrophotometry and a RIBOGreen detection assay (Molecular Probes; ref. 16).
Primers and reverse transcriptio-PCR
Primer and probe sequences were designed for the qRT assay, as previously described (12). Fluorescence resonance energy transfer probe sequences were designed to enhance the specificity of the assay. Specific primers were designed to sequence at least one exon-exon region. The HMW-MAA primer sequence was as follows: 5′-TGGAAGAACAAAGGTCTCTGG-3′(forward); 5′-GCTGGCCAAGAGATTGGAG-3′(reverse). The HMW-MAA fluorescence resonance energy transfer probe sequence was as follows: 5′-FAM-AGGATCACCGTGGCTGCTCT-BHQ-1–3′. The MART-1 primer sequence was as follows: 5′-AAAACTGTGAACCTGTGGT-3′(forward); 5′-TTCAAGCAAAAGTGTGAGAGA-3′(reverse). The MART-1 fluorescence resonance energy transfer probe sequence was as follows: 5′-FAM-CAGAACAGTCACCA CCACCTTATT-BHQ-1–3′. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primer sequence was as follows: 5′-GGGTGTGAACCATGAGAAGT-3′(forward); 5′-GACTGTGGTCAT-GAG TCCT-3′(reverse). The GAPDH fluorescence resonance energy transfer probe sequence was as follows: 5′-FAM-CAGCAATGCCTCCTG-CACCACCAA-BHQ-1–3′. Expression of the housekeeping gene GAPDH was used as an internal reference for mRNA integrity.
qRT analysis
Reverse-transcriptase reactions were done on 1.0 μg of extracted total RNA using Moloney murine leukemia virus reverse-transcriptase (Promega) with oligo-dT primers, as previously described (12). The qRT assay was done on the iCycler iQ RealTime PCR Detection System (Bio-Rad Laboratories) using 250 ng total RNA per reaction. The PCR mixture consisted of 0.4 μmol/L of each primer, 0.3 μmol/L Taq Man probe, 1 unit of AmpliTaq Gold polymerase (Applied Biosystems), 200 μmol/L each of deoxynucleotide triphosphate, 4.5 mmol/L MgCl2, and Ampli Taq buffer (Applied Biosystems) diluted to a final working volume. Samples were amplified by the following sequence: precycling hold at 95°C for 10 min; 35 cycles of denaturation at 95°C for 1 min; annealing for 1 min at 55°C for GAPDH, at 63°C for HMW-MAA, and at 59°C for MART-1; and extension at 72°C for 1 min. Absolute copy numbers were determined by a standard curve with serial dilutions (106–101 copies) of HMW-MAA, MART-1, and GAPDH cDNA templates. PCR efficiency evaluated from the slopes of the curves was between 95% and 100%. The correlation coefficient for all standard curves was ≥0.99. We confirmed the product size of HMW-MAA, MART-1, and GAPDH by gel electrophoresis, and then optimized the assay conditions for qRT. HMW-MAA mRNA expression was designated as relative mRNA copies (absolute mRNA copies of HMW-MAA/absolute mRNA copies of GAPDH) to compensate for comparison of different assays. Each sample was assayed in triplicate with positive and negative reagent individual and positive and negative marker controls.
Statistical analysis
The Wilcoxon signed-rank test was used to analyze differences in percentage of stained cells and intensity of staining by HMW-MAA – specific mAbs and MART-1 mAbs. The Wilcoxon rank sum test was used to assess differences in HMW-MAA and MART-1 mRNA expression among macrometastases, micrometastases, and tumor-negative tissues. The Fisher’s exact test was used to assess the frequency of HMW-MAA and MART-1 expression in nodal metastases assessed by IHC and qRT. Analysis was done using SAS statistical software (SAS Institute), and all tests were two-sided with a significance level of a P value of <0.05.
Results
IHC studies
Before investigating HMW-MAA expression in nodal tissue by IHC, we tested HMW-MAA mAbs to assess the expression of HMW-MAA in PEAT operative specimens of primary and metastatic melanoma lesions in lung, small intestine, and skin. Then, IHC with HMW-MAA–specific mAbs was optimized for PEAT.
The optimal HMW-MAA–specific mAbs assay was then used to analyze 100 PEAT specimens of nodal tissue: 42 non-SLN macrometastases, 10 SLN macrometastases, 32 SLN micrometastases, and 16 tumor-negative SLNs. As shown in Fig. 1, membrane staining of melanoma cells was specific because HMW-MAA–specific mAbs stained melanoma cells but did not stain surrounding lymphocytes. Furthermore, normal mouse IgG did not stain melanoma cells. Table 1 summarizes the data, in terms of percentage of stained melanoma cells and staining intensity. HMW-MAA–specific mAbs stained melanoma cells in lymph node macrometastases and micrometastases but did not stain tumor-negative lymph nodes.
Fig. 1.
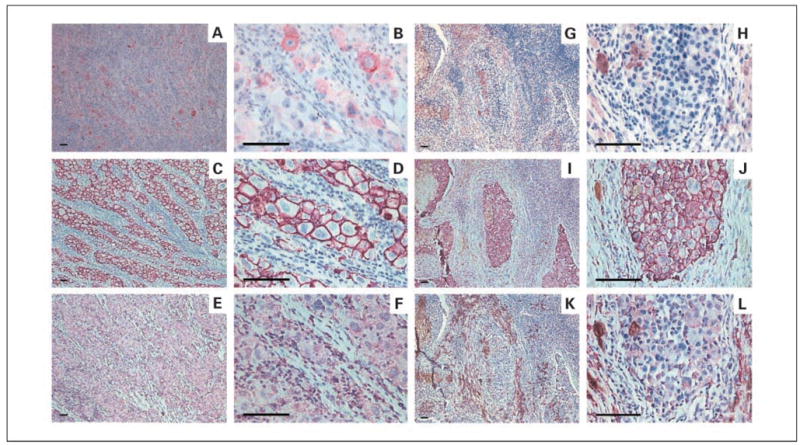
A to F, comparison of MART-1and HMW-MAA expression in SLNs by IHC. A, MART-1 (+; ×100). B, MART-1 (+; ×400). The cells immunoreactive for MART-1show red cytoplasmic staining. C, HMW-MAA (+; ×100). D, HMW-MAA (+; ×400). The cells immunoreactive for HMW-MAA show purple membrane staining. E, normal mouse IgG (−), negative control (×100). F, normal mouse IgG (−; ×400). G to L, comparative IHC between MART-1and HMW-MAA IHC in SLN macrometastasis. G, MART-1 (−; ×100). H, MART-1 (−; ×400). I, HMW-MAA (+; ×100). J, HMW-MAA (+; ×400). K, normal mouse IgG (−; ×100) L, normal mouse IgG (−; ×400). Scale bars, 100 μm.
Table 1.
HMW-MAA IHC analysis of nodal macrometastases and micrometastases
| Intensity of staining in melanoma cells | Percentage of stained melanoma cells in a node* |
|||||
|---|---|---|---|---|---|---|
| 100 | 99–75 | 74–50 | 49–25 | 24–1 | 0 | |
| Strong (+++) | 16 | 0 | 0 | 0 | 0 | 0 |
| Intermediate (++) | 14 | 21 | 9 | 1 | 2 | 0 |
| Weak (+) | 2 | 8 | 6 | 3 | 2 | 0 |
| Negative (−) | 0 | 0 | 0 | 0 | 0 | 0 |
n = 84 nodes.
We performed a pilot study that compared IHC staining with S-100p and HMB-45 antibodies to staining the HMW-MAA mAbs. Table 2A shows results for seven macrometastases: all seven SLN macrometastases stained positive for all three markers. Table 2B shows the results obtained with 23 SLN micrometastases: 21 (91%), 18 (78%), and 23 (100%) micrometastases were stained by S-100p–, HMB-45–, and HMW-MAA–specific antibodies, respectively. These findings indicate that HMW-MAA–specific mAbs are at least as sensitive as S-100p–specific rabbit antibodies and more sensitive than mAbs HMB-45 for detection of SLN micrometastases.
Table 2.
Comparison of HMW-MAA, S-100p, and HMB-45IHC of SLN
| Patient no | Detection of melanoma cells |
||
|---|---|---|---|
| S-100p | HMB45 | HMW-MAA | |
| 1 | + | + | + |
| 2 | + | + | + |
| 3 | + | + | + |
| 4 | + | + | + |
| 5 | + | +/− | + |
| 6 | + | + | + |
| 7 | + | + | + |
| Total | 7/7 (100%) | 7/7 (100%) | 7/7 (100%) |
| 1 | − | +/− | + |
| 2 | + | + | + |
| 3 | − | + | + |
| 4 | + | − | + |
| 5 | + | + | + |
| 6 | + | − | + |
| 7 | + | − | + |
| 8 | + | +/− | + |
| 9 | + | + | + |
| 10 | + | + | + |
| 11 | + | − | + |
| 12 | + | + | + |
| 13 | + | + | + |
| 14 | + | + | + |
| 15 | + | +/− | + |
| 16 | + | +/− | + |
| 17 | + | + | + |
| 18 | + | +/− | + |
| 19 | + | + | + |
| 20 | + | + | + |
| 21 | + | + | + |
| 22 | + | + | + |
| 23 | + | − | + |
| Total | 21/23 (91%) | 18/23 (78%) | 23/23 (100%) |
NOTE: Nodes were analyzed by IHC.
We then compared HMW-MAA–specific mAbs and MART-1 mAbs for standard IHC analysis of SLN tissue. MART-1 mAbs stained the cytoplasm of melanoma cells (Fig. 1). For both macrometastases and micrometastases, the intensity of staining by HMW-MAA–specific mAbs was stronger than the intensity of staining by MART-1 mAbs (Table 3A, P < 0.0001 and Table 3C, P = 0.004, respectively). Of the 52 nodal macro-metastases (SLN and non-SLN), 43 (83%) stained MART-1 positive, but all 52 (100%) stained HMW-MAA positive (Table 3A). Of 32 micrometastases from SLN, 100% stained positive for HMW-MAA but only 22 (69%) for MART-1 (Table 3C). The percentage of melanoma cells stained by HMW-MAA–specific mAbs in macrometastases and micro-metastases was significantly higher than that stained by MART-1 mAbs (Table 3B, P < 0.0001 and Table 3D, P < 0.0001).
Table 3.
Comparison of staining intensity and percentage of melanoma cells stained with HMW-MAA and MART-1 mAbs
| A. In nodal macrometastases* | ||
|---|---|---|
| Staining intensity | HMW-MAA | MART-1 |
| Strong (+++) | 11† (21%) | 1 (2%) |
| Intermediate (++) | 28 (54%) | 20 (38%) |
| Weak (+) | 13 (25%) | 22 (42%) |
| Negative (−) | 0 (0%) | 9 (17%)‡ |
|
B. Comparison of the percentage of melanoma cells stained in nodal macrometastases§ | ||
| Melanoma cells (%) | HMW-MAA | MART-1 |
|
| ||
| 100 | 23|| (44%) | 12 (23%) |
| 99–75 | 17 (33%) | 7 (13%) |
| 74–50 | 7 (13%) | 9 (17%) |
| 49–25 | 2 (4%) | 8 (15%) |
| 24–1 | 3 (6%) | 7 (13%) |
| 0 (negative) | 0 (0) | 9 (17%)‡ |
| >50% | 47 (90%) | 28 (54%) |
|
C. Comparison of the staining intensity of melanoma cells stained in nodal micrometastases¶ | ||
| Staining intensity | HMW-MAA | MART-1 |
|
| ||
| Strong (+++) | 5** | 3 (9%) |
| Intermediate (++) | 19 (59%) | 13 (41%) |
| Weak (+) | 8 (25%) | 6 (19%) |
| Negative (−) | 0 | 10 (31%)‡ |
|
D. Comparison of the percentage of melanoma cells stained in nodal micrometastases†† | ||
| Melanoma Cells (%) | HMW-MAA | MART-1 |
|
| ||
| 100 | 9‡‡ (28%) | 3 (9%) |
| 99–75 | 12 (38%) | 7 (22%) |
| 74–50 | 8 (25%) | 6 (19%) |
| 49–25 | 2 (6%) | 4 (13%) |
| 24–1 | 1 (3%) | 2 (6%) |
| 0 (negative) | 0 | 10 (31%)§§ |
| >50 | 29 (91%) | 16 (50%) |
n = 52 Nodes were analyzed.
Number of nodes with the indicated phenotype.
P < 0.0001.
n = 52 (SLN and non-SLN) were analyzed.
Number of nodes with the indicated phenotype.
n = 32 SLNs were analyzed.
Number of nodes with the indicated phenotype.
n = 32 SLNs were analyzed.
Number of nodes with the indicated phenotype.
P = 0.004.
The majority (>50%) of melanoma cells were stained in 28 (54%) of the 52 macrometastases (SLN and non-SLN) stained by MART-1 mAbs and in 47 (90%) of the 52 stained by HMW-MAA–specific mAbs (Table 3B, P < 0.0001). The majority of cells were stained by MART-1 mAbs in 16 (50%) and by HMW-MAA–specific mAbs in 29 (91%) of the 32 SLN micrometastases (Table 3D, P = 0.0023). Thus, IHC using HMW-MAA–specific mAbs is more sensitive than MART-1 mAbs in terms of the percentage and intensity of staining nodal macrometastases and micrometastases. HMW-MAA–specific mAbs detected occult metastasis not detected by MART-1 mAbs.
qRT HMW-MAA analysis
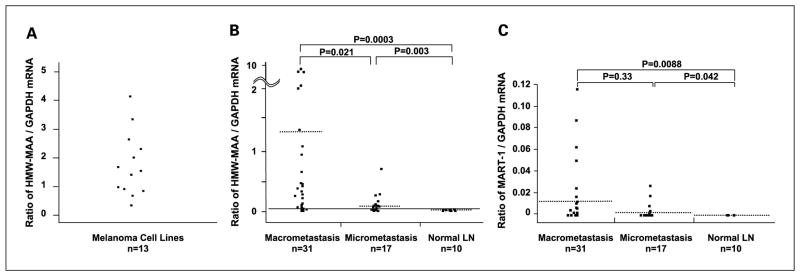
To investigate the potential of HMW-MAA as a molecular biomarker to detect SLN metastasis, we assessed HMW-MAA mRNA by qRT. An optimal qRT assay for HMW-MAA detection was established using melanoma cell lines, and PEAT specimens of clearly identified metastatic melanoma. Initially, qRT assay conditions for melanoma cells were optimized by measurement of HMW-MAA mRNA expression in 13 melanoma lines (Fig. 2A); HMW-MAA mRNA was expressed in all melanoma lines.
Fig. 2.
A, HMW-MAA mRNA expression in melanoma cell lines. HMW-MAA mRNA expression was designated as relative mRNA copies (absolute mRNA copies of HMW-MAA/absolute mRNA copies of GAPDH). B, HMW-MAA mRNA expression in nodal macrometastases and micrometastases. HMW-MAA mRNA expression was designated as relative mRNA copies (absolute mRNA copies of HMW-MAA/absolute mRNA copies of GAPDH). Dotted bars, mean copy numbers. The cutoff for HMW-MAA positivity was 2.95 × 10−2, corresponding to the mean relative HMW-MAA copy number plus1SD in tumor-negative nodes. C, MART-1mRNA expression in macrometastases, micrometastases, and tumor-free nodes. MART-1mRNA expression was designated as relative mRNA copies (absolute mRNA copies of MART-1/absolute mRNA copies of GAPDH). Dotted bars, mean copy numbers.
Expression of HMW-MAA, MART-1, and GAPDH mRNA was assessed in PEAT nodal macrometastases (SLN and non-SLN) and micrometastases (SLN; Fig. 2B and C). An optimal qRT assay for MART-1 detection of SLN metastasis in PEAT had been established previously (12). The range of absolute mRNA copies was 0 to 5.6 × 105 for HMW-MAA, 0 to 7.0 × 103 for MART-1, and 1.1 × 102 to 7.5 × 105 for GAPDH. The relative mRNA copy number for HMW-MAA was significantly (P = 0.0003) higher in 31 macrometastases (median, 0.26) than in tumor-free nodes, significantly (P = 0.0033) higher in 17 micrometastases (median, 0.06) than in tumor-free nodes, and significantly (P = 0.021) higher in macrometastases than in micrometastases (Fig. 2B). When the cutoff for HMW-MAA positivity was established, corresponding to the mean relative HMW-MAA copy number plus one SD in tumor-negative nodes, 32 (67%) of 48 nodal metastases expressed HMW-MAA mRNA. Assessment of the same PEAT specimens showed that MART-1 was expressed in 31 (65%) of 48 nodal metastases. Relative MART-1 copy number was significantly higher in macrometastases (P = 0.0088) and micrometastases (P = 0.042) than in tumor-free nodes (Fig. 2C).
The expression of HMW-MAA and MART-1 in nodal metastases was comparable. In combination, the two markers detected 39 (81%) of 48 nodal metastases. Both HMW-MAA and MART-1 mRNA were detected by qRT, and micrometastases were distinguishable from macrometastases by relative HMW-MAA copy number. These results indicate that the sensitivity of qRT assay is equivalent for HMW-MAA and MART-1, and that the two biomarkers seem to be complementary for qRT detection of nodal metastases.
Discussion
More sensitive and accurate IHC biomarkers to detect occult metastatic melanoma in SLNs may help reduce misdiagnosis of SLN metastasis. In the present study, we assessed HMW-MAA–specific mAbs for IHC assessment of melanoma-draining SLNs. HMW-MAA–specific mAbs detected melanoma cells in all 84 nodal macrometastases or micrometastases. IHC with HMW-MAA–specific mAbs was more sensitive in terms of percentage of stained melanoma cells and staining intensity than IHC with HMB-45 and MART-1 mAbs, commonly used in surgical pathology analysis. Furthermore, HMW-MAA–specific mAbs detected micrometastases that were not detected by MART-1 mAbs. The visualization of IHC staining with HMW-MAA mAbs was more distinct than MART-1 and HMB-45 mAbs. The HMW-MAA mAbs stained the cell surface, whereby the MART-1 and HMB-45 mAbs primarily stained the cytoplasm.
S-100p is a traditional IHC marker used for diagnosis of melanomas. However, S-100p lacks specificity because it can also be detected in Langerhans cells, dendritic cells, macrophages, Schwann cells, and a wide range of tumors (17, 18).
HMB-45 IHC is also used to identify melanoma lesions, but HMB-45 mAbs stains breast carcinomas, plasmacytomas, angiomyolipomas, and pigmented nerve sheath tumors (19). The mean sensitivity of HMB-45 IHC is ~86% (range, 70–100%) in primary melanoma lesions (19–22) and ~72% (range, 43–100%) in melanoma metastases (17, 21, 22). MART-1 (Melan-A) is expressed in the cytoplasm of benign nevus cells and melanoma cells. MART-1 mAbs also stains positive in adrenocortical adenomas and carcinomas, and sex-cord stromal tumors of the ovary (23). In primary melanoma lesions, the mean sensitivity of MART-1 expression is ~84% (range, 75–97%; refs. 18, 21, 22, 24). About 76% (range, 71–81%) of metastatic melanoma lesions express MART-1 (18, 21, 22).
Among the three most common IHC biomarkers used for melanoma diagnosis (S-100p, HMB-45, and MART-1), S-100p has the highest sensitivity (4, 5). However, unlike HMB-45 and MART-1, S-100p has the lowest specificity, and MART-1 is more specific than S-100p and HMB-45 as an IHC biomarker for detection of nodal micrometastases (10, 11).
When the qRT assay was used to compare HMW-MAA and MART-1 as molecular biomarkers of melanoma, the rate of mRNA expression in PEAT SLN micrometastases or macro-metastases was 67% for HMW-MAA and 65% for MART-1. For both markers, mRNA was expressed in lower levels than in the respective IHC analysis. Factors influencing mRNA detection could be related to the age of the tissue, mRNA degradation, mRNA copy number, and time of tissue processing from surgical removal. Although the overall rate of metastasis detection was comparable for both mRNA biomarkers, HMW-MAA mRNA was detected in nodal metastases that did not express MART-1 mRNA. Combining the two markers for qRT assay can increase the sensitivity of mRNA assessment by distinguishing nodal metastases in PEAT SLN specimens in a multimarker format (12). HMW-MAA has potential value as a component of a multimarker probe for qRT assessment of occult metastatic melanoma in lymph nodes.
In summary, IHC analysis by HMW-MAA can increase the accuracy of IHC detection of metastatic melanoma in tumor-draining regional nodes over HMB-45 and MART-1. IHC of HMW-MAA and MART-1 in combination provides higher specificity and sensitivity than S-100p and HMB-45 IHC analysis.
Acknowledgments
Grant support: NIH, NCI Project II P0 CA029605, CA012582, and R01CA10550003 grants.
References
- 1.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 2.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 3.Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230:453–63. doi: 10.1097/00000658-199910000-00001. discussion 463–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochran AJ, Wen DR, Herschman HR. Occult melanoma in lymph nodes detected by antiserum to S-100 protein. Int J Cancer. 1984;34:159–63. doi: 10.1002/ijc.2910340204. [DOI] [PubMed] [Google Scholar]
- 5.Yu LL, Flotte TJ, Tanabe KK, et al. Detection of microscopic melanoma metastases in sentinel lymph nodes. Cancer. 1999;86:617–27. [PubMed] [Google Scholar]
- 6.Gibbs JF, Huang PP, Zhang PJ, Kraybill WG, Cheney R. Accuracy of pathologic techniques for the diagnosis of metastatic melanoma in sentinel lymph nodes. Ann Surg Oncol. 1999;6:699–704. doi: 10.1007/s10434-999-0691-2. [DOI] [PubMed] [Google Scholar]
- 7.Messina JL, Glass LF, Cruse CW, Berman C, Ku NK, Reintgen DS. Pathologic examination of the sentinel lymph node in malignant melanoma. Am J Surg Pathol. 1999;23:686–90. doi: 10.1097/00000478-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Bostick PJ, Chatterjee S, Chi DD, et al. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J Clin Oncol. 1998;16:2632–40. doi: 10.1200/JCO.1998.16.8.2632. [DOI] [PubMed] [Google Scholar]
- 9.Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–96. doi: 10.1615/critrevimmunol.v24.i4.40. [DOI] [PubMed] [Google Scholar]
- 10.Shidham VB, Qi DY, Acker S, et al. Evaluation of micrometastases in sentinel lymph nodes of cutaneous melanoma: higher diagnostic accuracy with Melan-A and MART-1 compared with S-100 protein and HMB-45. Am J Surg Pathol. 2001;25:1039–46. doi: 10.1097/00000478-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Karimipour DJ, Lowe L, Su L, et al. Standard immunostains for melanoma in sentinel lymph node specimens: which ones are most useful? J Am Acad Dermatol. 2004;50:759–64. doi: 10.1016/j.jaad.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi H, Morton DL, Kuo C, et al. Prognostic significance of molecular upstaging of paraffin-embedded sentinel lymphnodes in melanoma patients. J Clin Oncol. 2004;22:2671–80. doi: 10.1200/JCO.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacomini P, Natali P, Ferrone S. Analysis of the interaction between a human high molecular weight melanoma-associated antigen and the monoclonal antibodies to three distinct antigenic determinants. J Immunol. 1985;135:696–702. [PubMed] [Google Scholar]
- 14.Temponi M, Kageshita T, Perosa F, Ono R, Okada H, Ferrone S. Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: comparison with other methods of purification. Hybridoma. 1989;8:85–95. doi: 10.1089/hyb.1989.8.85. [DOI] [PubMed] [Google Scholar]
- 15.Koyanagi K, O’Day SJ, Gonzalez R, et al. Microphthalmia transcription factor as a molecular marker for circulating tumor cell detection in blood of melanoma patients. Clin Cancer Res. 2006;12:1137–43. doi: 10.1158/1078-0432.CCR-05-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto Y, Matsuzaki Y, Kurihara S, et al. A new melanoma antigen fatty acid-binding protein 7, involved in proliferation and invasion, is a potential target for immunotherapy and molecular target therapy. Cancer Res. 2006;66:4443–9. doi: 10.1158/0008-5472.CAN-05-2505. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgibbons PL, Chaurushiya PS, Nichols PW, Chandrasoma PT, Martin SE. Primary mucosal malignant melanoma: an immunohistochemical study of 12 cases with comparison to cutaneous and metastatic melanomas. Hum Pathol. 1989;20:269–72. doi: 10.1016/0046-8177(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 18.Yu CH, Chen HH, Liu CM, et al. HMB-45 may be a more sensitive maker than S-100 or Melan-A for immunohistochemical diagnosis of primary oral and nasal mucosal melanomas. J Oral Pathol Med. 2005;34:540–5. doi: 10.1111/j.1600-0714.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 19.Prasad ML, Jungbluth AA, Iversen K, Huvos AG, Busam KJ. Expression of melanocytic differentiation markers in malignant melanomas of the oral and sinonasal mucosa. Am J Surg Pathol. 2001;25:782–7. doi: 10.1097/00000478-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Gown AM, Vogel AM, Hoak D, Gough F, McNutt MA. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986;123:195–203. [PMC free article] [PubMed] [Google Scholar]
- 21.Blessing K, Sanders DS, Grant JJ. Comparison of immunohistochemical staining of the novel antibody melan-A with S100 protein and HMB-45 in malignant melanoma and melanoma variants. Histopathology. 1998;32:139–46. doi: 10.1046/j.1365-2559.1998.00312.x. [DOI] [PubMed] [Google Scholar]
- 22.Jungbluth AA, Busam KJ, Gerald WL, et al. A103: an anti-melana monoclonal antibody for the detection of malignant melanoma in paraffin-embedded tissues. Am J Surg Pathol. 1998;22:595–602. doi: 10.1097/00000478-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Busam KJ, Iversen K, Coplan KA, et al. Immunoreactivity for A103, an antibody to melan-A (Mart-1), in adrenocortical and other steroid tumors. Am J Surg Pathol. 1998;22:57–63. doi: 10.1097/00000478-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Busam KJ, Chen YT, Old LJ, et al. Expression of melan-A (MART1) in benign melanocytic nevi and primary cutaneous malignant melanoma. Am J Surg Pathol. 1998;22:976–82. doi: 10.1097/00000478-199808000-00007. [DOI] [PubMed] [Google Scholar]