Abstract
Ghrelin (Grln) is a peptide hormone that is predominantly produced in the stomach and stimulates appetite and induces growth hormone (GH) release. We have previously reported that ghrelin is also expressed in T cells and exerts prothymic and anti-inflammatory effects. However, the biologic relevance of T cell–derived ghrelin remains to be determined. Here, we report that acylated-bioactive ghrelin is expressed in human T cells and preferentially segregates within the lipid raft domains upon TCR ligation. The RNA interference (RNAi)–mediated down-regulation of ghrelin in primary human T cells activates IkB, and increases Th1 cytokines and IL-17 secretion. Ghrelin expression declines with increasing age in spleen and T cells and exogenous ghrelin administration in old mice reduces proinflammatory cytokines. These findings demonstrate that ghrelin functions in an autocrine and paracrine capacity to regulate proinflammatory cytokine expression in human and murine T cells and may contribute in regulating “inflamm-aging.”
Introduction
Ghrelin, originally thought to be stimulator of GH axis1 and food intake2 also exerts potent inhibitory effects on proinflammatory mediators via its action on T cells, monocytes,3–5 and endothelial cells.6 Recent evidence suggests that leptin, which inhibits food intake,7 can promote inflammation3,8 and is also produced from T cells.8,9 Interestingly, leptin neutralization in T cells with monoclonal antibodies promotes regulatory T cell (Treg) proliferation9 and protects against experimental autoimmune encephalomyelitis (EAE).8
Many previous reports suggest that apart from cytokines and chemokines, T cells may also express certain hormones.10,11 Emerging evidence supports this long-held view that ligands and receptors of neuroendocrine origin may also directly regulate immune function.3,9 We have recently shown that ghrelin is also expressed and secreted by T cells3 and its expression declines in the thymus with age.12 In addition, mice with ablation of ghrelin and ghrelin-receptor (GHSR) display accelerated thymic involution, whereas ghrelin supplementation promotes thymopoiesis in aged mice12 and protects against sepsis.3 In this study, we tested the hypothesis that the bioactive ghrelin within T cells serves as a regulator of proinflammatory cytokines and investigated whether T cell–expressed ghrelin is functionally significant.
Methods
Human subjects and T-cell isolation
Pheresis packs were prepared from 6 healthy male donors between the age of 30 and 45 years age and T cells were isolated as described previously.3 For primary T cells, leukapheresis packs were acquired from healthy human volunteers from whom informed consent was obtained in accordance with the Declaration of Helsinki.
Mice and ghrelin infusions
Balb/c mice were maintained under the specific pathogen-free conditions of the National Institute on Aging (NIA) animal facility using protocols approved by the animal care and use committee of the NIA Intramural Research Program located in Baltimore, MD. We used 20- to 24-month-old mice for ghrelin infusions using osmotic minipumps as described previously.12
Transfections and T-cell culture
Primary human T cells were transfected with control and ghrelin-specific siRNAs (Dharmacon, Lafayette, CO) using electroporation (Amaxa Biosystems, Gaithersburg, MD) as described previously.13 The transfected cells were cultured in RPMI-1640 medium with 10% FCS for 48 hours and washed and transferred to anti-CD3/28–coated plates and cultured for 24 hours in AIM-V serum-free medium (Invitrogen, Carlsbad, CA).3
Lipid raft isolation and Western blot
Lipid rafts were isolated from primary T cells and dot blot was performed as described previously.13 The control and ghrelin siRNA–transfected T cells were lysed in radioimmunoprecipitation assay (RIPA) buffer and used for Western blot as previously described.13,14 The acyl ghrelin antibody was from Linco (Billerica, MA) and pIkB and IkB antibodies were from Cell Signaling (Beverly, MA) and Santa Cruz Biotechnology (Santa Cruz, CA).
Cytokine and ghrelin estimations
The cytokines were analyzed using multiplex bead-based assays according to the manufacturer's instructions (Biorad Laboratories, Hercules, CA) and ghrelin was measured using enzyme-linked immunosorbent assay (ELISA; Phoenix Pharmaceuticals, Burlingame, CA).
Real-time PCR, flow cytometry, and immunofluorescence microscopy
The total RNA was extracted from 4 to 6 spleens from each age group and used for real-time polymerase chain reaction (PCR) analysis. The reverse-transcription (RT)–PCR, cellular staining, and fluorescence-activated cell sorting (FACS) analysis were performed as described previously.3,12,14
Statistics
The results are expressed as the mean plus or minus SEM. The differences between the means and the effects of treatments were determined by one-way ANOVA using Tukey test, which protects the significance (P < .05) of all pair combinations.
Results and discussion
Ghrelin expression and function in human T cells
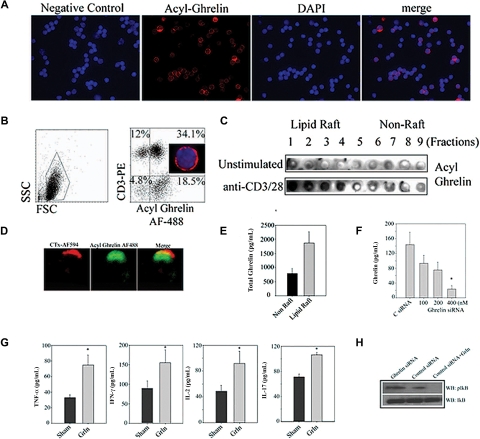
Ghrelin is a 28–amino acid acylated peptide that is predominantly produced from gut but is also known to be widely expressed in various cell and tissue subtypes including immune cells.3,12,15,16 Given that exogenous ghrelin regulates T-cell function,3,5,12 we sought to determine whether T cell–expressed ghrelin is functionally significant. Human T cells constitutively expressed acylated ghrelin with cytoplasmic as well as membrane localization (Figure 1A). The nonacylated or des-acyl form of ghrelin circulates in 3 to 5 times higher concentrations than the bioactive acylated ghrelin peptide.17 Presence of acylated ghrelin in T cells clearly suggests a potential functional role of this peptide. We next quantified the acylated ghrelin expression by FACS and observed that greater than 70% of the human T cells express active ghrelin protein (Figure 1B). The acylated ghrelin was found to localize in lipid raft domains in activated T cells (Figure 1C,D). Quantitation of ghrelin in subcellular compartment of T cells by ELISA revealed that compared with cytoplasmic fractions, significantly higher concentration (P < .05) of ghrelin was detected in lipid rafts (Figure 1E). Our present findings suggest that acylated ghrelin may efficiently interact with GHSR within lipid rafts upon TCR ligation.3 Acylated proteins have a proclivity to localize in sphingolipid-rich membrane microdomains,18 hence it is likely that unique acyl moiety in ghrelin may promote its trafficking to lipid rafts.
Figure 1.
Ghrelin expression and function in T cells. (A) The purified human T cells were stained with incubated with control IgG and did not show nonspecific binding, whereas acylated ghrelin was found to be expressed in T cells. Nuclei are labeled with DAPI and acyl ghrelin was visualized by anti–guinea pig acyl ghrelin antibody conjugated to Alexa Fluor-594. Greater than 50% of the cells are labeled for acyl ghrelin (red). Given that these images are acquired using an epifluorescent microscope, certain dim acyl ghrelinlo cells appear negative in the merge with DAPI. Thus, in the merge image distribution may appear less that 50%. The FACS analysis of peripheral blood mononuclear cells (PBMCs) for acylated ghrelin (B) allows the laser to pick MFIs of varying ghrelin expression (including weakly stained cells) on T cells. Images were acquired by Spot Advanced software on a Zeiss Axiovert S100 Microscope under 100× objective (Carl Zeiss, Thornwood, NY). (B) The human PBMCs were double labeled with anti-CD3 antibody conjugated to phycoerythrin and stained with acyl ghrelin conjugated to Alexa Fluor-488 and analyzed on FACS Calibur (Becton Dickinson, San Jose, CA). A representative plot from duplicate runs of 3 healthy donors is shown. Control antibody staining was not significant in any donor tested. Here, 46% of the PBMCs labeled positive for CD3 and 34% of the PBMCs expressed CD3+ and acylated ghrelin indicating that greater than 70% of the T cells express acylated ghrelin. Approximately 18% of the CD3− ghrelin-positive cells most likely represents B and natural killer (NK) cells and monocytes. (C) Primary human T cells were stimulated by plate-bound anti-CD3 and -CD28, and lipid raft fractions were analyzed for acyl ghrelin expression by dot blot analysis. (D) GM1+ lipid rafts in activated T cells were visualized by cholera toxin conjugated to Alexa Fluor-594; the activated human T cells display polarized expression of acyl ghrelin and colocalized with lipid raft. (E) The lipid raft and cytoplasmic fractions were pooled and equalized for protein, and ghrelin was analyzed using ELISA. (F) Ghrelin siRNA causes significant reduction in ghrelin production from primary human T cells. (G) Ghrelin knockdown in activated T cells increases proinflammatory cytokine secretion. We failed to observe any influence of the siRNA on T-cell proliferation or cell viability and thus we believe these variables have no influence on the cytokine inhibition observed (data not shown). (H) Total protein lysates from control and ghrelin siRNA–transfected cells were analyzed for phosphorylation of IkB. Control siRNA–transfected cells were treated with 100 ng/mL ghrelin for 5 minutes.
We next examined the functional relevance of ghrelin expression in T cells using RNA interference. We observed that compared with control scrambled siRNA sequences, the ghrelin-specific siRNAs caused significant knockdown of ghrelin protein expression in primary human T cells (Figure 1F). The activated T cells where ghrelin expression was reduced displayed a significant increase in TNFα, IFNγ, and IL-2 (Figure 1G) with no changes in cellular proliferation (data not shown). Interestingly, ghrelin knockdown led to significant increase in IL-17 production from T cells (Figure 1G). These findings raise the possibility of a potential role of endogenous ghrelin in regulation of Th17 cell function. Similarly, acylated ghrelin treatment of anti-CD3 mAb-activated human T cells resulted in inhibition of TNFα, IL-6, and IL-17 production after 48 hours, whereas pretreatment with the GHS-R antagonist, D-Lys-GHRP6, abrogated this inhibitory effect (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Given that NFkB is a key transcription factor important for regulation of inflammation,19 we next examined the impact of ghrelin knockdown on a regulator of NFkB signaling, IkB. We observed that compared with the control-transfected T cells, ghrelin knockdown caused increased phosphorylation of IkB, whereas ghrelin supplementation inhibited pIkB (Figure 1H, Figure S1B). These results suggest ghrelin expression in T cells may play an important role in regulating the activation of NFkB. These data suggest that T cell–derived ghrelin may play a key role in regulating the basal and activation- induced proinflammatory cytokine secretion.
Age-related reduction in ghrelin expression and inflammation
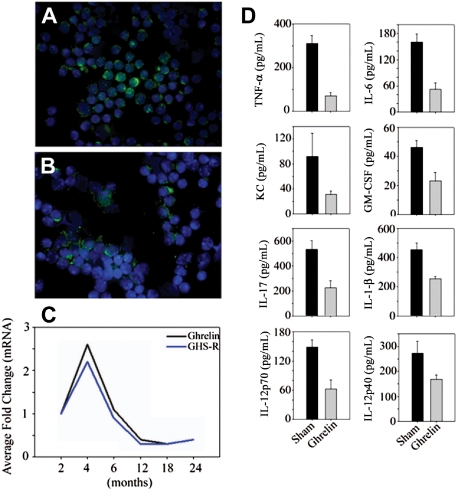
Although circulating ghrelin levels are not significantly reduced in 10-month-old mice, caloric restriction—a prolongevity intervention—enhances ghrelin levels in middle-aged animals.20 Interestingly, intrathymic ghrelin expression declines with age and is associated with age-related thymic involution.12 Thus the reduction of T cell–derived ghrelin in a tissue microenvironment may play a role in regulating the inflammatory cytokines. We observed that quite similar to thymus,12 ghrelin and GHSR mRNA expression increases at 4 months and then progressively declines with age (Figure 2A-C). Given that RNAi-mediated reduction of ghrelin in T cells resulted in an increase in proinflammatory cytokines, we hypothesized that ghrelin infusion in aging may reduce the proinflammatory mediators. Ghrelin was infused in the old mice using subcutaneously implanted osmotic minipumps for a period of 2 weeks. Compared with sham-infused mice, ghrelin led to a significant reduction in IL-6, TNF-α, IL-1β, KC, GMCSF, IL-12, and IL-17 in the serum of aging mice.
Figure 2.
Age-related reduction in ghrelin expression and inflammation. Nonfractionated splenic T cells from (A) 2-month-old and (B) 24-month-old BALB/C mice were separated using negative selection and labeled with ghrelin; nuclei were counterstained with DAPI. (C) The ghrelin mRNA expression in spleen was studied by real-time PCR analysis. The threshold amplification values (Ct) of 4 to 6 mice per group were collapsed and normalized to GAPDH and are presented as fold change. (D) Ghrelin was infused at a concentration of 1.25 μg/hour (levels corresponding to fasting ghrelin peripheral concentration of young mice) for 2 weeks (n = 8), whereas control BALB/c mice were implanted with osmotic pumps containing the PBS vehicle alone. The circulating cytokines were measured using a bead based multiplex assay kit in serum of 20-month-old mice. In all cases, the differences between sham control– and ghrelin-treated mice were significantly different (P < .05). In contrast to the studies by Xia et al,21 we failed to observe any inhibition of T-cell proliferation or alterations in T-cell viability in response to ghrelin treatment that may account for the inhibitory effects of ghrelin on cell activation (data not shown).
Taken together, our results suggest that ghrelin expression in T cells plays an important role in inhibiting proinflammatory cytokines. These observations raise the possibility that ghrelin expression in tissue microenvironments may regulate local inflammatory state. Given that ghrelin inhibits inflammation, increases food intake, and promotes thymic function, our findings suggest that synthetic ghrelin agonists may have potential therapeutic value in age-related inflammatory diseases.
Acknowledgments
We thank Tara Blake and Gary Collins in the Laboratory of Immunology for expert technical assistance. We also thank Dr Robert Wersto and the members of NIA Flow Cytometry Laboratory as well as Karen Madara and the members of the Pheresis Unit for their assistance in this project.
This research was entirely supported by the Intramural Research Program of the NIA, National Institutes of Health.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.D.D. and D.D.T. designed and supervised the research at NIA and wrote and edited the paper; V.D.D. conducted the experiments; H.Y. performed microscopy and contributed to discussions and data analysis; A.C.-J. and B.B.G. isolated the lipid rafts and performed the blots; and K.P. verified some of the cytokine data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dennis D. Taub, Clinical Immunology Section, Laboratory of Immunology, National Institute on Aging-Intramural Research Program, NIH, 5600 Nathan Shock Dr, Baltimore, MD 21224; e-mail taubd@grc.nia.nih.gov.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 3.Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288:486–492. doi: 10.1152/ajpendo.00196.2004. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 6.Li WG, Gavrila D, Liu X, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–2226. doi: 10.1161/01.CIR.0000127956.43874.F2. [DOI] [PubMed] [Google Scholar]
- 7.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 8.De Rosa V, Procaccini C, La Cava A, et al. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest. 2006;116:447–455. doi: 10.1172/JCI26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Rosa V, Procaccini C, Calì G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Dixit VD, Mielenz M, Taub DD, Parvizi N. Leptin induces growth hormone secretion from peripheral blood mononuclear cells via a protein kinase C- and nitric oxide-dependent mechanism. Endocrinology. 2003;144:5595–5603. doi: 10.1210/en.2003-0600. [DOI] [PubMed] [Google Scholar]
- 11.Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 12.Dixit VD, Yang H, Sun Y, et al. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri B, Dixit VD, Ghosh MC, et al. CXCL12-induced partitioning of flotillin-1 with lipid rafts plays a role in CXCR4 function. Eur J Immunol. 2007;37:2104–2116. doi: 10.1002/eji.200636680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixit VD, Weeraratna AT, Yang H, et al. Ghrelin and growth hormone secretagogue receptor constitute a novel autocrine pathway in astrocytoma motility. J Biol Chem. 2006;281:16681–16690. doi: 10.1074/jbc.M600223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4284–4291. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 16.Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988–2994. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 17.Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K. Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem. 2003;278:64–70. doi: 10.1074/jbc.M205366200. [DOI] [PubMed] [Google Scholar]
- 18.McCabe JB, Berthiaume LG. N-terminal acylation confers localization to cholesterol, sphingolipid-enriched membranes but not to lipid rafts/caveolae. Mol Biol Cell. 2001;12:3601–3617. doi: 10.1091/mbc.12.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Youm YH, Nakata C, Dixit VD. Chronic caloric restriction induces forestomach hypertrophy with enhanced ghrelin levels during aging. Peptides. 2007;28:1931–1936. doi: 10.1016/j.peptides.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Q, Pang W, Pan H, Zheng Y, Kang JS, Zhu SG. Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regul Pept. 2004;122:173–178. doi: 10.1016/j.regpep.2004.06.016. [DOI] [PubMed] [Google Scholar]