Abstract
Nuclear factor-κB (NF-κB) has an important role in multiple myeloma (MM) cell pathogenesis in the context of the bone marrow (BM) microenvironment. In NF-κB signaling cascades, IκB kinase α (IKKα) and IKKβ are key molecules that predominantly mediate noncanonical and canonical pathways, respectively. In this study, we examined the biologic sequelae of the inhibition of IKKα versus IKKβ in MM cell lines. All MM cell lines have constitutive canonical NF-κB activity, and a subset of MM cell lines shows noncanonical NF-κB activity. Adhesion to BM stromal cells further activates both canonical and noncanonical NF-κB activity. IKKβ inhibitor MLN120B blocks canonical pathway and growth of MM cell lines but does not inhibit the noncanonical NF-κB pathway. Although IKKα knockdown induces significant growth inhibition in the cell lines with both canonical and noncanonical pathways, it does not inhibit NF-κB activation. Importantly, IKKα down-regulation decreases expression of β-catenin and aurora-A, which are known to mediate MM cell growth and survival. Finally, IKKβ inhibitor enhances the growth inhibition triggered by IKKα down-regulation in MM cells with both canonical and noncanonical NF-κB activity. Combination therapy targeting these kinases therefore represents a promising treatment strategy in MM.
Introduction
We and others have shown that the bone marrow (BM) microenvironment promotes growth, survival, and drug resistance in multiple myeloma (MM) cells by direct contact of MM cells with components of the BM microenvironment, including BM stromal cells (BMSCs), osteoblasts, osteoclasts, and endothelial cells.1,2 Furthermore, these cells secrete cytokines, which trigger mitogen-activated protein kinase kinase/MAPK, phosphoinositide-3 kinase/Akt, Janus kinase 2/signal transducers and activators of transcription 3, and nuclear factor-κB (NF-κB) signaling cascades in MM cells. We have shown that NF-κB activated by growth factors and/or cytokines modulates expression of cytokines, adhesion molecules, and antiapoptotic proteins in MM cells. Therefore, blockade of NF-κB signaling represents a novel therapeutic strategy in MM.
Previous studies have shown that NF-κB is a Rel family member protein heterodimer composed of p50 (NFκB1) and p65 (RelA), which is inactivated by its association with IκB family inhibitors.3 IκBα therefore has a crucial role in regulating NF-κB activation. After phosphorylation of IκBα protein by IκB kinase (IKK) complex, IκB is targeted for ubiquitination and degradation by the proteasome, thereby allowing translocation of NF-κB into the nucleus where it binds to specific DNA sequences in the promoters of target genes. To examine the biologic sequelae of specific NF-κB inhibition in MM cells, we have used 2 IKKβ inhibitors, PS-11454 and MLN120B.5 In these studies, the effect of IKKβ on growth and survival of MM cells in the context of the BM milieu is only modest. Specifically, we found that NF-κB activity was not inhibited by these agents in MM.1S cells, although significant inhibition was observed in RPMI8226 and INA6 MM cells.
Recent studies have shown the relationship between NF-κB activity and genetic abnormalities in MM,6,7 suggesting the biologic significance of the noncanonical NF-κB pathway in MM pathogenesis. For example, the noncanonical NF-κB pathway is constitutively activated in MM cells with inactivation of tumor necrosis factor (TNF) receptor–associated factor 3,7 suggesting the need to target the noncanonical pathway therapeutically and that inhibition of the canonical pathway alone may be insufficient to block NF-κB activity. Nonetheless, MLN120B inhibits canonical NF-κB pathway and induces growth inhibition in many MM cell lines, suggesting that IKKβ inhibitors may hold promise for the treatment in MM.6
Although previous studies have shown that IKKα and IKKβ play crucial roles in mediating NF-κB activity,8,9 the biologic sequelae of inhibiting each kinase in MM cells have not been demonstrated. In this study, we characterize mechanisms of constitutive canonical vs noncanonical NF-κB activity in MM cells, which can be enhanced by coculture with BMSCs to mediate MM cell growth. Importantly, we demonstrate that inhibition of IKKβ blocks growth only in MM cell lines with canonical NF-κB activity, whereas it activates noncanonical NF-κB cascade in cells with both pathways. Surprisingly, IKKα knockdown significantly inhibits MM cell growth without inhibiting NF-κB activity, suggesting that IKKα targets signaling cascades mediating MM cell proliferation other than NF-κB. Finally, targeting both IKKα and IKKβ inhibits growth of MM cells with both canonical and noncanonical NF-κB activation, suggesting the therapeutic potential of combination inhibitor therapy.
Methods
Cells
MM cell lines were obtained from ATCC (Manassas, VA), the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), or kindly provided by sources and maintained as previously described.10 BM specimens were obtained from patients with MM and mononuclear cells separated by Ficoll-Hypaque density sedimentation. Primary CD138+ plasma cells from MM patients were obtained using negative selection as previously described,11 with Institutional Review Board–approved (Dana-Farber Cancer Institute) informed consent and in accordance with the Declaration of Helsinki protocol. BM mononuclear cells were used to establish long-term BMSC cultures, as previously described.11
Reagents
IKKβ inhibitor MLN120B12 was provided by Millennium Pharmaceuticals (Cambridge, MA), dissolved in dimethyl sulfoxide, stored at −20°C, and diluted in culture medium immediately before use; control media contained less than 0.1% dimethyl sulfoxide. TNF-α was purchased from R&D Systems (Minneapolis, MN). 17-Allylamino-17-demethoxygeldanamycin (17AAG) was obtained from Calbiochem (San Diego, CA).
Cell-growth assay
MM cell growth was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide dye absorbance, as previously described.11 DNA synthesis was measured by [3H]-thymidine (PerkinElmer Life and Analytical Sciences, Waltham, MA) uptake. Cells were pulsed with [3H]-TdR (0.5 μCi/well) during the last 8 hours of culture, as previously described.5 All experiments were performed 3 times in quadruplicate.
Immunoblotting and immunoprecipitation
Protein lysates were obtained by cell lysis in Triton X-100 buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 5 mM ethylenediaminetetraacetic acid, 5 mM NaF, 2 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 5 μg/mL leupeptin, and 5 μg/mL aprotinin). Whole-cell lysates were immunoblotted with antiphospho (p)–IKKα/β, -IKKα, -IKKβ, -IκBα, -p65 NF-κB, and –β-catenin (Cell Signaling Technology, Danvers, MA) antibodies (Abs); as well as -GAPDH, -ARK1 (aurora A), and –α-tubulin Abs (Santa Cruz Biotechnology, Santa Cruz, CA). Nuclear protein lysates were immunoblotted with anti-p65 NF-κB Ab (Cell Signaling Technology); -p50, -p52, -RelB, and –C-Rel Abs (Santa Cruz Biotechnology); as well as –nuclear matrix protein p84 Ab (Abcam, Cambridge, MA). Immunoprecipitation using anti-IKKα and –β-catenin Ab was carried out as described previously.13 Anti-GFP (Cell Signaling Technology) served as a control Ab. The intensity of the blots was digitalized using ImageJ software (National Cancer Institute, Bethesda, MD).
Electrophoretic mobility shift analysis
Electrophoretic mobility shift analysis (EMSA) was carried out for detection of NF-κB activity, as previously described.5 Briefly, nuclear extracts from MM cells were obtained using the Nuclear Extraction Kit (Panomics, Fremont, CA). Double-stranded NF-κB consensus oligonucleotide probe (Promega, Madison, WI) was end-labeled with [γ32P]ATP (10 mCi/mL; PerkinElmer Life and Analytical Sciences). Binding reactions containing 0.035 pmol/μL oligonucleotide and 8 μg nuclear protein were conducted at room temperature for 20 minutes in binding buffer.4 Samples were loaded onto a 4% polyacrylamide gel, transferred to Whatman paper (Whatman, Maidstone, United Kingdom), and visualized by autoradiography. For supershift analysis, anti-p65, RelB, c-Rel (Santa Cruz Biotechnology), p50 (Abcam), or p52 (Rockland Immunochemicals, Gilbertsville, PA) Abs were incubated for 5 minutes before adding the reaction mixtures. The samples were loaded onto a 4% polyacrylamide gel, transferred to Whatman paper (Whatman), and visualized by autoradiography. For BMSC coculture, MM cells were incubated in BMSC-coated flasks for 12 hours. MM cells were then harvested by pipetting and subjected to nuclear protein extraction. Purity of MM cells was confirmed by CD138 expression (> 95%) using flow cytometry.
Immunohistochemistry
Immunohistochemistry was performed on bone marrow from 10 healthy volunteers, 20 persons with monoclonal gammopathy of undetermined significance (MGUS), and 30 patients with MM, as described previously.14 Anti–human p50 (catalog no. 3035), p65 (catalog no. 4764), and p52 (catalog no. 3017) Abs were obtained from Cell Signaling Technology. Anti-CD138 Ab was obtained from BD Biosciences (San Jose, CA). The primary Abs were visualized with the corresponding biotinylated Ab coupled to streptavidin-peroxidase complex (Vector Laboratories, Burlingame, CA). All Abs, conditions, and reactivity were tested with positive control slides.
Real-time quantitative polymerase chain reaction
IKKβ gene expression was examined by real-time polymerase chain reaction (PCR) and normalized to expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as previously described.15 Briefly, total RNA was extracted from untreated or 17AAG (1 μM for 4 hours and 8 hours)–treated MM.1S and RPMI8226 cells using the RNeasy Kit and RNase-Free DNase Set (QIAGEN, Valencia, CA). cDNA was synthesized using the first-strand cDNA Synthesis Kit (Superarray Bioscience, Frederick, MD). Quantitative real-time PCR was carried out in a 7500 Real-time PCR system (Applied Biosystems, Foster City). cDNAs were amplified in 25-μL reactions containing SYBR Green PCR Master Mix (Superarray Bioscience). Amplification was done at 95°C for 10 minutes, followed by 95°C for 15 seconds and 60°C for 1 minute, for a total of 40 cycles. Gene-specific oligonucleotide primers included GAPDH: forward 5′-AATCCCATCACCATCTTCCA-3′; and reverse 5′-TGGACTCCACGACGTACTCA-3′. Primers for survivin were purchased from Superarray Bioscience. Data represent mean plus or minus SD of triplicate wells.
Paracrine MM cell growth
To evaluate growth stimulation and signaling in MM cells adherent to BMSCs, 2 × 104 MM cells were cultured in 96-well BMSC-coated plates. MM cell proliferation was assessed by [3H]-thymidine uptake, as previously described.5
Detection of cytokines in BMSC culture supernatants
Seventy-nine cytokines produced by BMSCs were detected by RayBio Human Cytokine Antibody Array V (RayBiotech, Norcross, GA), according to the manufacturer's instructions.
IKKα knockdown by shRNA
Scrambled and IKKα pLKO shRNA vectors were kindly provided by Dr William Hahn (Dana-Farber Cancer Institute). Recombinant lentivirus was produced by transient transfection of 293T cells following a standard protocol. After 48 hours, MM cells were incubated with culture supernatants from 293T cells containing crude virus for 6 hours and washed with media. A second transfection was repeated on the next day, and 48 hours later, MM cells were counted and examined for cell proliferation and by immunoblotting analysis.
Statistical analysis
Statistical significance of differences observed in drug-treated versus control cultures was determined using the Wilcoxon signed-rank test. The minimal level of significance was P less than .05.
Results
Both canonical and noncanonical NF-κB pathways are activated in MM cell lines
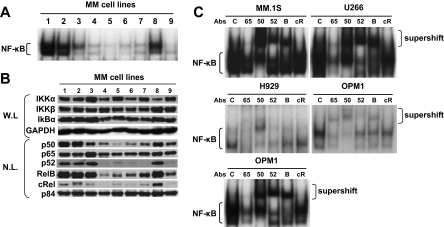
We first examined baseline NF-κB activity in MM cell lines by EMSA. As expected, the intensity of constitutive NF-κB activity was variable (Figure 1A lanes 1-9), with highest NF-κB activity in MM.1S cells (lane 1) and lowest NF-κB activity in H929 cells (lane 5). We next examined expression of proteins mediating canonical and noncanonical NF-κB pathways in MM cell lines. All MM cell lines highly expressed IKKα and IKKβ, as well as p65 (RelA) and p50 (NFκB1) proteins involved in the canonical pathway. Importantly, these cell lines also nuclear expressed p52 (NFκB2) and RelB proteins involved in noncanonical pathway (Figure 1B), suggesting that both canonical and noncanonical NF-κB pathways might be constitutively activated in these MM cell lines. Indeed, recent studies have shown that NF-κB activity is maintained by canonical and noncanonical pathways.16,17 To examine activity of canonical vs noncanonical NF-κB pathway, we next carried out supershift assays using anti-p65 (RelA), -p50, -52, -RelB, and –cRel Abs in MM.1S, U266, RPMI8228, H929, and OPM1 cells. Consistent with protein expression (Figure 1A), intensity of original NF-κB bands were supershifted and/or reduced by p65 and p50 antibodies in RPMI8226 and H929 cells. In addition to p65 and p50, p52, and RelB antibodies also reduced NF-κB intensity in MM.1S, U266, and OPM1 cells (Figure 1C). These data suggest that both the canonical and noncanonical NF-κB pathways are constitutively activated in these MM cell lines.
Figure 1.
Expression of proteins involved in canonical and noncanonical pathways modulating NF-κB activity in MM cell lines. (A) Nuclear extracts from MM cell lines were subjected to EMSA to assess baseline NF-κB activity. Lane 1 indicates MM.1S; lane 2, MM.1R; lane 3, U266; lane 4, INA6; lane 5, H929; lane 6, RPMI8226; lane 7, RPMI-LR5; lane 8, OPM1; and lane 9, OPM2. (B) Immunoblot analysis of IKKs, Rel family member, p50, and p52 proteins in MM cell lines (lanes 1-9 as noted in legend for panel A). Whole-cell lysates (W.L.) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, followed by Western blotting with IKKα, IKKβ, IκB, and GAPDH Abs. Nuclear protein lysates (N.L.) were blotted with p50, p52, p65, RelB, cRel, and p84 nuclear protein Abs. Immunoblots are representative of similar results from 3 experiments. (C) To determine the role of Rel family proteins mediating constitutive NF-κB activity in MM.1S, U266, H929, RPMI8226, and OPM1 cells, supershift assays were carried out using anti-p65,(65), p50(50), p52(52), RelB (B), and c-Rel (cR) Abs. Exposure time of autoradiography varied for each cell line.
The BM microenvironment triggers activation of NF-κB in MM cells
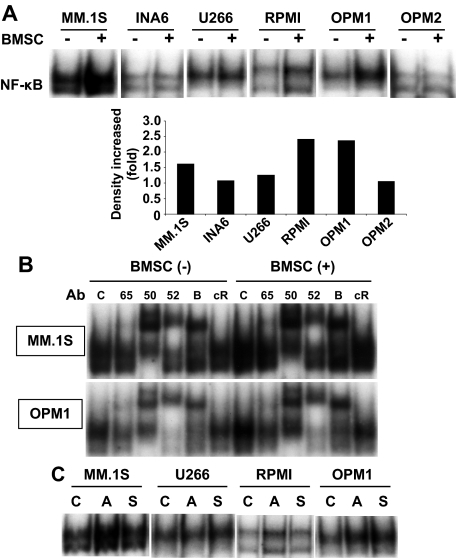
Because BMSCs secrete many cytokines and chemokines that activate NF-κB, we next examined whether NF-κB in MM cells was activated in the presence of BMSCs. BMSCs triggered robust activation of NF-κB in MM.1S and OPM1 cells, but only weak activation in INA-6, U266, RPMI8226, and OPM2 cells (Figure 2A). Cytokine antibody array of supernatants from cultures of MM cells and BMSCs showed that cytokines known to activate NF-κB (ie, TNF-α, interleukin-1β [IL-1β]) were detectable (data not shown). To delineate which NF-κB activation pathway is activated in MM cells by culture with BMSCs, we next performed supershift assay in MM.1S and OPM1 cells cultured with BMSCs. Antibodies targeting both the canonical and noncanonical pathway proteins showed increased supershifted bands induced by BMSC cocultures (Figure 2B), indicating that BMSCs activate both canonical and noncanonical NF-κB pathways. Interestingly, supernatants from BMSC cultures also activated NF-κB in MM.1S, U266, and OPM1 cell lines (Figure 2C).
Figure 2.
NF-κB activation in MM cell lines induced by coculture with BMSCs. (A) Indicated MM cell lines were cultured with BMSCs for 12 hours and NF-κB activity was assessed by EMSA. The intensity of the bands was digitalized by ImageJ software and indicates fold increase compared with nontreated (control) cells after BMSC coculture. (B) To determine the role of Rel family proteins mediating NF-κB activity induced by coculture of MM.1S and OPM1 cells with BMSCs, supershift assays were carried out using anti-p65(65), p50(50), p52(52), RelB (B), and c-Rel (cR) Abs. (C) MM cells were cultured alone (C) as well as with BMSCs (A, adhesion) or with BMSC culture supernatant (S) for 12 hours. Nuclear extracts were subjected to EMSA to assess NF-κB activity. Exposure time of autoradiography varied for each cell line.
p50 and p52 expression in human normal plasma cells and MM cells
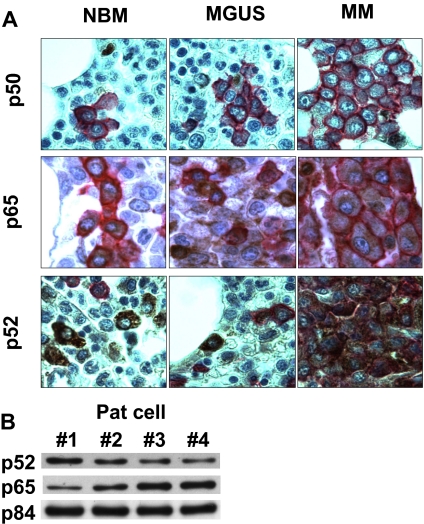
To assess the clinical relevance of canonical and noncanonical NF-κB activity in MM cell lines, we next examined p50 and p65 (canonical) as well as p52 (noncanonical) expression in normal BM (n = 10), MGUS BM, (n = 20), and MM patient tumor cells (n = 30). Tissue microarray immunohistochemistry (TMA-IHC) was used to examine the relative levels and patterns of p50, p65, and p52 expression. In 10 of 10 normal bone marrow (NBM), 20 of 20 MGUS, and 20 of 30 MM samples, p50 was weakly positive in the nucleus/cytoplasm. All samples showed p65 staining predominantly in the cytoplasm. p52 was positive in 8 of 10, 18 of 20, and 30 of 30 in NBM, MGUS, and MM samples, respectively. Staining for p50, p65, and p52 on IHC is mostly cytroplasmic and only weakly nuclear. Representative results of TMA-IHC analysis of NBM, MGUS BM, and MM cells revealed expression of p50, p65, and p52 in MGUS and MM (Figure 3A). As in MM cell lines (Figure 1B), expression of p65 and p52 was also detected by immunoblotting in nuclear extracts from purified MM tumor cells (Figure 3B). These results suggest that both canonical and noncanonical NF-κB pathway may contribute to the total NF-κB activity in both MGUS and MM tumor cells.
Figure 3.
p50 and p52 expression in patient MM cells. (A) Immunohistochemical analysis for p50, p65, and p52 expression was performed on BM tissue microarrays from healthy donors, persons with MGUS, and MM patients. Representative results are shown. CD138 is stained in red; p50 and p52 is stained in brown. Immunohistochemical studies were performed on consecutive serial tissue sections from archival paraffin-embedded bone marrow biopsies from normal individuals (NBM), as well as patients with MGUS and MM. Tissue sections were incubated with either anti-p50, -p65, or -p52 Abs, and immunoreactivity was visualized with peroxidase stains. Histologic micrographs were taken using a Leica DM200 microscope (aperture HC PLANs 10×/22, objective lenses: N PLAN 100×/1.25 oil), and a SPOT Insight QE model camera with SPOT Advanced acquisition software (Diagnostic Instruments, Sterling Heights, MI). (B) Nuclear extracts from patient MM cells were immunoblotted with anti-p52, -p65 Abs. p84 served as a positive control for nuclear protein.
Inhibition of IKKβ does not inhibit NF-κB activity in MM cells with noncanonical NF-κB activity
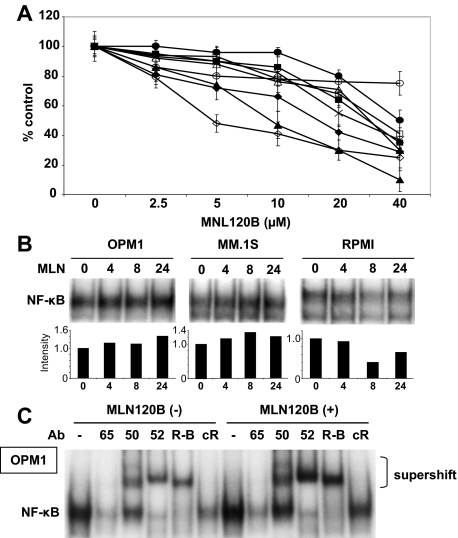
We have previously reported that specific IKKβ inhibitors PS-11454 and MLN120B,5 when used as single agents, show only modest antitumor activities in MM cells. IKKβ is predominantly involved in the canonical NF-κB pathway via triggering phosphorylation and proteasomal degradation of IκBα. Previous reports have also shown that the noncanonical NF-κB pathway can be activated in MM cells.6,7 We therefore hypothesized that MM cell lines with predominantly canonical pathway activation may be more sensitive to IKKβ inhibition than those lines with both canonical and noncanonical pathway activation. Indeed, we found that H929, RPMI8226, RPMI-LR5, and OPM2 cells have primarily canonical activation and are more sensitive to MLN120B than those cell lines (MM.1S, MM.1R, U266, and OPM1) with noncanonical as well as canonical NF-κB activation (Figure 4A). To confirm the association between sensitivity to MLN120B and NF-κB activity in these cell types, we next performed EMSA in RPMI8226 as well as OPM1 and MM.1S cells. Consistent with [3H]-thymidine uptake assay data, MLN120B significantly inhibited NF-κB activity in RPMI8226 cells (Figure 4B). Surprisingly, NF-κB was activated by MLN120B treatment in a time-dependent fashion in OPM1 and MM.1S cells (Figure 4B). To delineate the role of each NF-κB pathway in MLN120B-induced NF-κB activation, we next performed supershift assay in OPM1 cells before and after MLN120B treatment. Importantly, anti-p52 and -RelB Abs showed more significant intensity of the shifted bands after treatment compared with anti-p65 or -p50 Abs (Figure 4C). For example, intensity of total NF-κB was 1.3-fold increased; however, intensity of p52 supershifted band was 2.4-fold enhanced by MLN120B treatment (data not shown). These results suggest that IKKβ inhibitor does not block total NF-κB activity and may activate noncanonical pathway in cells with both canonical and noncanonical pathways.
Figure 4.
Inhibition of IKKβ does not block proliferation of MM cell lines which express both canonical and noncanonical NF-κB pathways. (A) MM.1S (○), MM.1R (■), U266 (□), INA6 (●), H929 (▴), RPMI8226 (♦), RPMI-LR5 (◇), OPM1 (Δ), and OPM2 (×) cells were cultured in the presence of MLN120B (0-40 μM) for 72 hours. Cell proliferation was assessed by 3H-thymidine uptake. (B) OPM1, MM.1S, and RPMI8226 cells were treated with or without MLN120B (5 μM) for the indicated time intervals. Nuclear extracts were subjected to EMSA to assess NF-κB activity. Exposure time of autoradiography varied for each cell line and intensity of the bands were digitalized by ImageJ software and indicated as fold-increase relative to control. (C) To determine the role of Rel family proteins mediating NF-κB activity in OPM1 cells before and after MLN120 treatment, supershift assays were carried out using anti-p65(65), p50(50), p52(52), RelB (R-B), and c-Rel (cR) Abs.
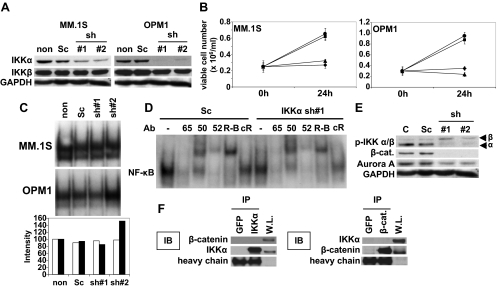
Inhibition of IKKα significantly inhibits MM cell growth
We next similarly examined the biologic significance of IKKα inhibition in MM cells. Because no small molecule inhibitors against IKKα are available, we used shRNA lentivirus transfection system to knockdown IKKα. To define nonspecific effect of this transfection, we used 2 different shRNAs. MM.1S and OPM1 cells were transfected with either scrambled (Sc) or IKKα shRNAs (1 and 2); significant down-regulation in IKKα protein expression was confirmed by Western blot in IKKα, but not in Sc, shRNA transfectants (Figure 5A). Importantly, IKKα shRNA transfected MM.1S (Figure 5B left panel) and OPMI (Figure 5B right panel) cells demonstrated significant growth inhibition compared with nontransfected and scrambled shRNA transfectants. For example, 2.9-, 0.8-, and 1.2-fold increases in viable cell number were observed in scrambled shRNA, IKKα shRNA 1, and IKKα shRNA 2 transfected OPM1 cells, respectively.
Figure 5.
Down-regulation of IKKα triggers significant growth inhibition without altering NF-κB activity. (A) MM.1S and OPM1 cells were transfected with either scrambled (Sc) or 2 different IKKα shRNA. Whole-cell lysates were immunoblotted with anti-IKKα, IKKβ, and GAPDH Abs. (B) Nontransfected (non, ●), scramble-shRNA transfected (Sc, ■), IKKα sh1 (▴), or IKKα sh2 (♦) transfected MM.1S (left panel) and OPM1 (right panel) cells (0.25 × 106/mL) were cultured for 48 hours. Cell viability was determined by trypan blue exclusion. (C) MM.1S and OPM1 cells were transfected with Sc or IKKα shRNAs. Nuclear extracts were subjected to EMSA to assess NF-κB activity. The intensity of the bands was digitalized by ImageJ software and indicated as fold increase relative to nontransfected cells. (D) To determine the role of Rel family proteins mediating NF-κB activity in OPM1 cells before and after IKKα shRNA 1, supershift assays were carried out using anti-p65, p50, p52, and RelB (R-B) Abs. (E) MM.1S cells were transfected with Sc or IKKα shRNAs. Whole-cell lysates were immunoblotted with anti-p-IKKα/β, -β-catenin, -Aurora-A, and -GAPDH Abs. (F) MM.1S cell lysates were immunoprecipitated with anti-GFP, -IKKα, or -β-catenin Abs and then immunoblotted with anti-IKKα or -β-catenin Abs. Whole-cell lysates (W.L.) served as a protein loading control for IKKα and β-catenin.
We next examined the effect of IKKα down-regulation on NF-κB activity in MM.1S and OPM1 cells. Specifically, nuclear extracts were isolated from the same treated cells used for IKKα protein expression (Figure 5A). Surprisingly, there is no association between growth inhibition and NF-κB activity in IKKα shRNA transfected cells (Figure 5C). To examine the effect of IKKα knockdown on canonical and noncanonical NF-κB activation pathways, we performed supershift assays in scrambled versus IKKα shRNA 1 OPM1 transfectants, which indicated that neither canonical nor noncanonical pathway was inhibited by IKKα down-regulation (Figure 5D). These data suggest that partial inhibition of IKKα alone may be insufficient to block either the canonical or noncanonical NF-κB pathways.
We next examined the molecular mechanisms whereby IKKα down-regulation triggered growth inhibition of MM cells without inhibiting NF-κB activity. Interestingly, down-regulation of IKKα enhanced phosphorylation of IKKβ, suggesting compensatory activation of the canonical pathway. Previous studies have shown that IKKα could modulate function and/or expression of β-catenin,18,19 Wnt/β-catenin signaling,20–22 and Aurora-A.23 Moreover, inhibition of Wnt/β-catenin signaling induces significant growth inhibition in MM cells.24 We therefore examined protein expression of β-catenin, as well as its downstream molecule Aurora A, in scrambled vs IKKα shRNA MM.1S transfectants. Importantly, levels of β-catenin and Aurora-A proteins were markedly lower in IKKα shRNA transfectants than in scrambled shRNA transfectants (Figure 5E). To define physical interaction of IKKα with β-catenin, we performed immunoprecipitation; however, neither β-catenin nor IKKα protein coimmunprecipitated with anti-IKKα or –β-catenin (Figure 5F). These results strongly suggest that MM cell growth inhibition induced by IKKα down-regulation is not the result of a decrease of NF-κB activity; and that down-regulation of β-catenin and Aurora A may, at least in part, account for IKKα down-regulation-induced growth inhibition of MM cells.
Dual inhibition of IKKα and IKKβ
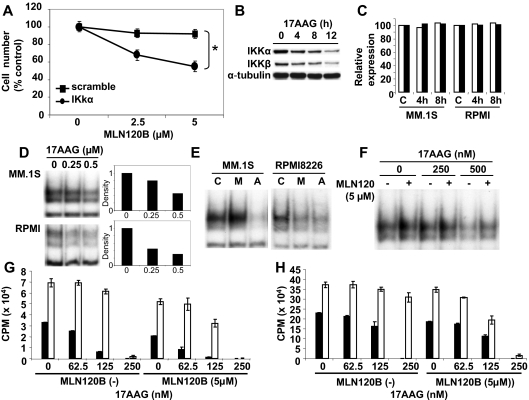
We next examined whether inhibition of both IKKα and IKKβ could augment the growth inhibitory effect in MM cells induced by targeting either IKKα or IKKβ alone. MM.1S cells transfected with scrambled or IKKα shRNA were cultured in the presence or absence of MLN120B (2.5 and 5 μM). Although MLN120B alone did not trigger growth inhibition in scrambled shRNA transfectants, it significantly enhanced growth inhibition in IKKα shRNA transfectant (Figure 6A). Because lentivirus knockdown does not have therapeutic implications and IKKα specific small molecule inhibitors are not available, we used Hsp90 inhibitor 17AAG in this combination strategy. Specifically, we first examined IKKα and IKKβ expression after 17AAG treatment. Consistent with our previous study,25 17AAG down-regulated both IKKα and IKKβ expression in MM.1S cells in a time-dependent fashion (Figure 6B). Similar results were observed in RPMI8226 cells (data not shown). To address the mechanism of this effect, we next measured IKKβ transcription using quantitative real-time PCR before and after 17AAG treatment. IKKβ mRNA level in MM.1S cells was not altered by 17AAG treatment (Figure 6C), suggesting that down-regulation of IKKβ occurred at a posttranscriptional level. Similar results were observed with IKKα (data not shown).
Figure 6.
Dual inhibition of IKKα and IKKβ significantly inhibits MM cell growth. (A) MM.1S cells were transfected with scrambled (■) or IKKα (●) shRNA. Both cells were then cultured in the presence (2.5, 5 μM) or absence of MLN120B for 48 hours, and viable cells were enumerated by trypan-blue exclusion. Percentage growth inhibition was calculated as follows: (cell number after 24 hours with MLN120B treatment/cell number without MLN120B treatment) × 100. *P < .01. (B) MM.1S cells were cultured with 17AAG (1 μM) for 4, 8, and 12 hours. Whole cell lysates were subjected to immunoblotting with anti-IKKα, -IKKβ, and -α-tubulin Abs. (C) MM.1S cells were cultured with 17AAG (1 μM) for 4 hours and 8 hours. mRNA level was examined using real-time quantitative PCR, normalized to expression of GAPDH. (D) MM.1S and RPMI8226 cells were cultured with 17AAG (0.25 and 0.5 μM) for 8 hours. Nuclear extracts were subjected to EMSA. Exposure time of autoradiography varied for each cell line. The intensity of the bands was digitalized by ImageJ software and indicated fold decrease relative to nontreated cells. (E) MM.1S and RPMI8226 cells were cultured with 17AAG (0.5 μM) or MLN120B (5 μM) for 8 hours. Nuclear extracts were subjected to EMSA. (F) MM.1S cells were cultured with 17AAG (250 and 500 nM) in the presence or absence of MLN120B (5 μM) for 8 hours. Nuclear extracts were subjected to EMSA. MM.1S (G) and INA6 (H) cells were cultured for 48 hours in BMSC-coated (□) or noncoated (■) wells in control media or MLN120B (5 μM), with or without 17AAG (62.5, 125, or 250 nM). DNA synthesis was assessed by [3H]-thymidine uptake; data represent mean ( ± SD) of quadruplicate cultures.
Because 17AAG down-regulated both IKKα and IKKβ, we next examined the effect of 17AAG treatment on NF-κB activity. Although MLN120B (Figure 4B) or IKKα shRNA (Figure 5C) did not down-regulate NF-κB, 17AAG markedly inhibited NF-κB activity, in a dose-dependent fashion, in MM.1S cells (Figure 6D). Consistent with MLN120B treatment (Figure 4B), NF-κB activity in RPMI8226 cells was blocked by 17AAG treatment (Figure 6D). Therefore, both MLN120B and 17AAG have different effects on NF-κB activity, depending on the cell line. For example, NF-κB activity was inhibited in RPMI8226, but enhanced in MM.1S cells, by MLN120B. On the other hand, NF-κB activity was not inhibited by 17AAG in both MM.1S and RPMI8226 cells (Figure 6E). Inhibition of constitutive NF-κB activity was further examined by culturing MM.1S cells with (5 μM) or without MLN120B, in the presence (250 or 500 nM) or absence of 17AAG for 8 hours. As expected, enhanced NF-κB triggered by MLN120B in MM.1S cells was significantly blocked by 17AAG (Figure 6F).
Because we have shown that the BM microenvironment confers growth and drug resistance in MM cells,2 we next studied the effect of combined MLN120B and 17AAG treatment on MM cell growth in the BM milieu. Importantly, proliferation of MM.1S (2.1-fold, Figure 6G) and INA6 (1.5-fold, Figure 6H) cells triggered by adherence to BMSCs was modestly inhibited by 5 μM MLN120B; this inhibition was significantly (P < .01) increased by 17AAG in a dose-dependent fashion. These results confirm that the combination of MLN120B and 17AAG remains cytotoxic against MM cells in the context of the BM microenvironment.
Discussion
NF-κB plays a crucial role in the pathogenesis of various cancers.17,26–28 Specifically, NF-κB regulates transcription of proteins mediating cell cycle progression, antiapoptosis, drug resistance, and cytokine/chemokine production.29–31 However, the precise mechanism and role of NF-κB activation in the pathogenesis of MM have not been fully characterized. Importantly, a recent study using high-density oligonucleotide array comparative genomic hybridization and gene expression profiling7 demonstrated dysregulation of NF-κB in approximately 20% of MM patient samples. Therefore, NF-κB represents a promising therapeutic target in many types of cancer, including MM.
The proteasome inhibitor bortezomib is a novel agent that targets NF-κB by preventing proteasomal degradation of IκBα. It demonstrates remarkable anti-MM activities in preclinical in vitro and in vivo models of MM in the BM milieu,32,33 has shown remarkable clinical activity in MM, and was approved by the Food and Drug Administration in 2003, 2005, and 2008 for treatment of relapsed refractory, relapsed, and newly diagnosed MM, respectively.34 Besides bortezomib, a variety of other novel agents with preclinical and/or clinical anti-MM activity, such as thalidomide,35 immunomodulatory derivatives,36 histone deacetylase inhibitors,37 transforming growth factor-β receptor tyrosine kinase inhibitor,38 lysophosphatidic acid acyltransferase-β inhibitor,39 1′-acetoxychavicol acetate,40 and proteasome inhibitor NPI0052,41 all inhibit NF-κB activation, associated with significant inhibition of MM cell growth. However, these agents are not specific NF-κB inhibitors, and the precise molecular mechanisms of NF-κB inhibition by these agents are not delineated.
To characterize the biologic sequelae of specific NF-κB inhibition in MM, we have previously used specific IKKβ inhibitors PS-11454 and MLN120B,12 which completely block TNF-α–induced NF-κB activation, MM cell growth in the context of BMSCs, and IL-6 secretion from BMSCs triggered by MM cell adhesion. However, the direct growth inhibitory effect of these agents against MM cells is modest.4,5 Moreover, recent studies have shown that NF-κB can be activated via both canonical and noncanonical (alternative) pathways.16 Because IKKβ mediates predominantly the canonical pathway, inhibition of IKKβ alone may be insufficient to inhibit total NF-κB activity in MM cells.
Specifically, although the canonical pathway with p50 (NFκB1)/p65 (RelA) heterodimer mediates NF-κB activity, recent studies have revealed the importance of the noncanonical pathway with predominantly p52 (NFκB2)/RelB. In this study, we therefore examined the relative contribution of canonical versus noncanonical pathway mediating NF-κB activation in MM cells. As expected, all MM cell lines examined express p50 and p65 canonical pathway; 4 of 9 MM cell lines also express p52 and RelB. Interestingly, those cell lines that express p50/p65 and p52/RelB have higher NF-κB activity than those cell lines that lack p52/RelB. Supershift assays confirm that both canonical and noncanonical pathways are active in cell lines expressing p50/p65 and p52/RelB, suggesting the importance of noncanonical pathway in constitutive NF-κB activity in these MM cells. These results are consistent with a previous report demonstrating constitutive activation of noncanonical NF-κB pathway in patient MM cells.7
We have shown that cytokines (ie, TNF-α, IL1β, stromal-derived factor-1α, B cell–activating factor) secreted by BMSCs and other components in the BM activate NF-κB in MM cells in vitro2,10,42; however, the relative role of canonical and noncanonical pathway mediating BMSC-induced NF-κB activation in MM cells has not been defined. In this study, we demonstrated that both canonical and noncanonical pathways were activated by culture of MM cells with BMSCs. Furthermore, the intensity of NF-κB activation triggered by BMSCs is higher in MM cells with both canonical and noncanonical pathways than in those MM cells with predominantly canonical pathway. To assess the biologic significance of these in vitro studies demonstrating both canonical and noncanonical NF-κB activation in MM cells triggered by BMSCs, we performed p50, p65, and p52 staining in TMA samples of plasma cells from several healthy donors, persons with MGUS, and MM patients. Consistent with previous studies6,7 and our in vitro studies, the majority of MGUS and MM patient samples express p50, p65, and p52; and immunoblotting confirmed their nuclear localization. These results confirm that both canonical and noncanonical pathways are activated in MGUS and MM patient plasma cells in the BM microenvironment, suggesting that growth, survival, and drug resistance of MM cells conferred in the BM microenvironment are mediated, at least in part, by NF-κB.
Because both canonical and noncanonical NF-κB pathways are activated, we further examined the biologic sequelae of inhibiting of each pathway in MM cell lines. To block the canonical pathway, we used specific IKKβ inhibitor MLN120B.12 We have previously shown that MLN120B has modest anti-MM activity in vitro and in vivo, with differential sensitivity of MM cell lines to MLN120B.5 In this study, we found that MM cell lines with canonical NF-κB activity were more sensitive to MLN120B than those with both canonical and noncanonical NF-κB activation. In our experiments, IKKα shRNA could not completely down-regulate IKKα expression. It therefore remains possible that more complete IKKα knockdown would inhibit NF-κB activation; moreover, it is also possible that IKKα down-regulation triggers compensatory activation of IKKβ. Another recent study has shown growth inhibitory effects of MLN120B in MM cells6 at much higher concentrations than the IC50 between 5 and 10 μM, observed in our study. Interestingly, MM cell lines with activated noncanonical pathway evidenced by nuclear p52 expression (ie, EJM, XG2, KMS18) were also sensitive to MLN120B in this earlier study.6 The differences in sensitivity to MLN120B might be the result of experimental methods (3H-thymidine uptake vs viable cell count). In addition, we show that MLN120B can inhibit the canonical but enhanced noncanonical pathways. This unexpected result suggests the possibility of cross-talk signaling between canonical and noncanonical pathways; ie, IKKα phosphorylates and mediates IKKβ activity.43
IKKα is involved in both canonical and noncanonical NF-κB pathways. In the canonical pathway, a key step is activation of an IKK complex, which is generally maintained by 3 IKK subunits: IKKα and IKKβ act as catalytic subunits, whereas IKKγ acts as a regulatory subunit of the kinase.8 In the noncanonical pathway, IKKα typically is composed of a homodimer and mediates phosphorlation of p52 precursor protein p100.16,44 In this study, we therefore expected that inhibition of IKKα could bock both canonical and noncanonical pathways, associated with MM cell growth inhibition. As expected, IKKα shRNA transfectants showed significant growth inhibition in MM cell lines. Surprisingly, IKKα shRNA transfected cells had even higher NF-κB activity than control vector transfected cells, indicating that the growth inhibitory effect of IKKα down-regulation was independent of NF-κB activity in these cells.
We therefore sought to identify possible molecular mechanisms whereby IKKα down-regulation induced MM cell growth inhibition. We found that IKKα knockdown cells showed significantly lower expression of β-catenin and Aurora-A than control cells. Previous studies have shown that IKKα mediates not only NF-κB activation, but also other pathways, including regulation of chromatin remodeling and cell cycle regulation via modulating Aurora kinase A phosphorylation.23 Wnt signaling plays a crucial role in both development and differentiation; moreover, activation of canonical Wnt pathway results in β-catenin stabilization in human cancers. In MM, Wnt/β-catenin axis plays a role in progression and metastatic potential45; conversely, inhibition of β-catenin by a small molecule inhibitor PKF115-584 induces remarkable anti-MM activity in vitro and in vivo in a MM xenograft mouse model.24 Most recently, anti-MM activities triggered by inhibiting Aurora-A have also been shown.46 Our results therefore suggest that MM cell growth inhibition by IKKα knockdown may be mediated, at least in part, by down-regulation of β-catenin and/or Aurora-A expression in MM cells. Ongoing studies will delineate the molecular mechanisms whereby down-regulation of IKKα triggers decreased expression of these proteins.
Having shown the sequelae of inhibiting canonical versus noncanonical NF-κB activation pathways, we next examined whether simultaneous inhibition of both pathways enhances the growth inhibitory effect. Although IKKα down-regulation triggered significant growth inhibition in MM cells, we found that MLN120B further augmented its effect, specifically in cells with both canonical and noncanonical pathways. To validate the clinical potential of combined inhibition, we used Hsp90 inhibitor 17AAG as an IKK inhibitor.25,47 We confirmed that 17AAG down-regulates both IKKα and IKKβ in MM cells at a posttranscriptional level. As expected, 17AAG significantly inhibited NF-κB activity in both MM.1S cells and RPMI8226 cells. Although Hsp90 has other client proteins involved in MM cell growth, our results suggest that growth inhibition triggered by Hsp90 may be mediated, at least in part, via down-regulating IKKs.
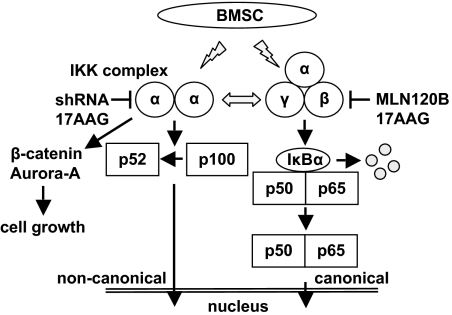
We further studied the sequelae of combined MLN120B and 17AAG treatment in MM cells. As expected, the combination treatment markedly inhibited TNF-α–induced phosphorylation and degradation of IκBα (data not shown). Moreover, induction of noncanonical NF-κB activity by MLN120B was blocked by 17AAG, associated with an enhanced growth inhibitory effect. Because we have shown that the BM microenvironment confers drug resistance on MM cells,2 we further examined the growth inhibitory effect of this combination in MM cells in the context of the BM microenvironment. Importantly, we confirmed that this combination blocked MM cell growth, even in the presence of BMSCs. Moreover, the growth inhibitory effect of this combination treatment was most significant in IL-6–dependent INA6 cells. Because IL-6 production by BMSCs is modulated by NF-κB activity,48 combination treatment strategies targeting both canonical and noncanonical pathways may both target MM cells and overcome growth and cell adhesion-mediated drug resistance49 to conventional therapy conferred by the BM microenvironment (Figure 7). These studies provide the preclinical rationale for combination therapy strategies to improve patient outcome in MM.
Figure 7.
NF-κB activity in MM cells. MM cells express both canonical and noncanonical NF-κB activity. In MM cells, both canonical and noncanonical NF-κB pathways are activated, and there is possible cross-talk signaling between each pathway. BMSCs activate canonical and noncanonical NF-κB pathways in MM cells. Both canonical and noncanonical pathways should be blocked to inhibit total NF-κB activation in the context of the MM microenvironment. IKKα knockdown induces down-regulation of β-catenin and Aurora-A expression independent of NF-κB inhibition.
Acknowledgments
This work was supported by National Institutes of Health (grant nos. SPORE IP50 CA10070, PO-1 78378, and RO-1 CA 50947), the Multiple Myeloma Research Foundation (T.H., D.C.), and the Lebow Family Fund to Cure Myeloma (K.C.A.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.H. performed experiments and wrote the manuscript; D.C. and K.C.A. designed the experiments and wrote the manuscript; T.K., H.I., Y.O., K.P., and A.P. performed experiments; N.R., N.C.M., and P.G.R. designed the experiments; and R.D.C. designed and performed experiments.
Conflict-of-interest disclosure: K.C.A. is receiving research funding from Celgene Corporation and Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Kenneth C. Anderson, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.
References
- 1.Hazlehurst LA, Damiano JS, Buyuksal I, Pledger WJ, Dalton WS. Adhesion to fibronectin via beta1 integrins regulates p27kip1 levels and contributes to cell adhesion mediated drug resistance (CAM-DR). Oncogene. 2000;19:4319–4327. doi: 10.1038/sj.onc.1203782. [DOI] [PubMed] [Google Scholar]
- 2.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin AS., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Hideshima T, Chauhan D, Richardson P, et al. NF-κB as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 5.Hideshima T, Neri P, Tassone P, et al. MLN120B, a novel IKKβ inhibitor, blocks multiple myeloma cell growth in vitro and in vivo. Clin Cancer Res. 2006;12:5887–5894. doi: 10.1158/1078-0432.CCR-05-2501. [DOI] [PubMed] [Google Scholar]
- 6.Annunziata CM, Davis RE, Demchenko Y, et al. Frequent engagement of the classical and alternative NF-κB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keats JJ, Fonseca R, Chesi M, et al. Promiscuous mutations activate the noncanonical NF-κB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 9.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 10.Tai YT, Li XF, Breitkreutz I, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66:6675–6682. doi: 10.1158/0008-5472.CAN-06-0190. [DOI] [PubMed] [Google Scholar]
- 11.Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen D, Nong Y, Morgan JG, et al. A selective small molecule IκB Kinase beta inhibitor blocks nuclear factor κB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes, and mast cells. J Pharmacol Exp Ther. 2006;317:989–1001. doi: 10.1124/jpet.105.097584. [DOI] [PubMed] [Google Scholar]
- 13.Hideshima T, Mitsiades C, Akiyama M, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–1534. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 14.Carrasco DR, Sukhdeo K, Protopopov M, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11:349–360. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hideshima T, Catley L, Raje N, et al. Inhibition of Akt induces significant downregulation of survivin and cytotoxicity in human multiple myeloma cells. Br J Haematol. 2007;138:783–791. doi: 10.1111/j.1365-2141.2007.06714.x. [DOI] [PubMed] [Google Scholar]
- 16.Jost PJ, Ruland J. Aberrant NF-κB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 17.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 18.Lamberti C, Lin KM, Yamamoto Y, et al. Regulation of β-catenin function by the IkappaB kinases. J Biol Chem. 2001;276:42276–44286. doi: 10.1074/jbc.M104227200. [DOI] [PubMed] [Google Scholar]
- 19.Albanese C, Wu K, D'Amico M, et al. IKKα regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol Biol Cell. 2003;14:585–599. doi: 10.1091/mbc.02-06-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derksen PW, Tjin E, Meijer HP, et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci U S A. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiang YW, Endo Y, Rubin JS, Rudikoff S. Wnt signaling in B-cell neoplasia. Oncogene. 2003;22:1536–1545. doi: 10.1038/sj.onc.1206239. [DOI] [PubMed] [Google Scholar]
- 22.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 23.Prajapati S, Tu Z, Yamamoto Y, Gaynor RB. IKKα regulates the mitotic phase of the cell cycle by modulating Aurora A phosphorylation. Cell Cycle. 2006;5:2371–2380. doi: 10.4161/cc.5.20.3359. [DOI] [PubMed] [Google Scholar]
- 24.Sukhdeo K, Mani M, Zhang Y, et al. Targeting the β-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:7516–7521. doi: 10.1073/pnas.0610299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsiades CS, Mitsiades NS, McMullan CJ, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107:1092–1100. doi: 10.1182/blood-2005-03-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karin M, Lin A. NF-κB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Hawke N, Baldwin AS. NF-κB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 28.Van Waes C. Nuclear factor-κB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007;13:1076–1082. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 29.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badrichani AZ, Stroka DM, Bilbao G, Curiel DT, Bach FH, Ferran C. Bcl-2 and Bcl-XL serve an anti-inflammatory function in endothelial cells through inhibition of NF-κB. J Clin Invest. 1999;103:543–553. doi: 10.1172/JCI2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NF-κB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12:73–90. doi: 10.1016/s1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 32.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 33.Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 35.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS., Jr Inhibition of NF-κB activity by thalidomide through suppression of IκB kinase activity. J Biol Chem. 2001;276:22382–22387. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 36.Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 37.Catley L, Weisberg E, Tai YT, et al. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood. 2003;102:2615–2622. doi: 10.1182/blood-2003-01-0233. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi T, Hideshima T, Nguyen AN, et al. TGF-β receptor I kinase inhibitor downregulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. Clin Cancer Res. 2004;10:7540–7546. doi: 10.1158/1078-0432.CCR-04-0632. [DOI] [PubMed] [Google Scholar]
- 39.Hideshima T, Chauhan D, Hayashi T, et al. Anti-tumor activity of lysophosphatidic acid acyltransferase (LPAAT)-β inhibitors, a novel class of agents, in multiple myeloma. Cancer Res. 2003;63:8428–8436. [PubMed] [Google Scholar]
- 40.Ito K, Nakazato T, Xian MJ, et al. 1′-acetoxychavicol acetate is a novel nuclear factor κB inhibitor with significant activity against multiple myeloma in vitro and in vivo. Cancer Res. 2005;65:4417–4424. doi: 10.1158/0008-5472.CAN-05-0072. [DOI] [PubMed] [Google Scholar]
- 41.Chauhan D, Catley L, Li G, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Mitsiades CS, Mitsiades N, Poulaki V, et al. Activation of NF-κB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto Y, Yin MJ, Gaynor RB. IκB kinase α (IKKα) regulation of IKKβ kinase activity. Mol Cell Biol. 2000;20:3655–3666. doi: 10.1128/mcb.20.10.3655-3666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto Y, Gaynor RB. IκB kinases: key regulators of the NF-κB pathway. Trends Biochem Sci. 2004;29:72–79. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Qiang YW, Walsh K, Yao L, et al. Wnts induce migration and invasion of myeloma plasma cells. Blood. 2005;106:1786–1793. doi: 10.1182/blood-2005-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi Y, Reiman T, Li W, et al. Targeting aurora kinases as therapy in multiple myeloma. Blood. 2007;109:3915–3921. doi: 10.1182/blood-2006-07-037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-κB activation. Oncogene. 2004;23:5378–5386. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- 48.Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-κB. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- 49.Landowski TH, Olashaw NE, Agrawal D, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) is associated with activation of NF-κB (RelB/p50) in myeloma cells. Oncogene. 2003;22:2417–2421. doi: 10.1038/sj.onc.1206315. [DOI] [PubMed] [Google Scholar]